Leaching of Waste Pharmaceutical Blister Package Aluminium in Sulphuric Acid Media
Abstract
1. Introduction
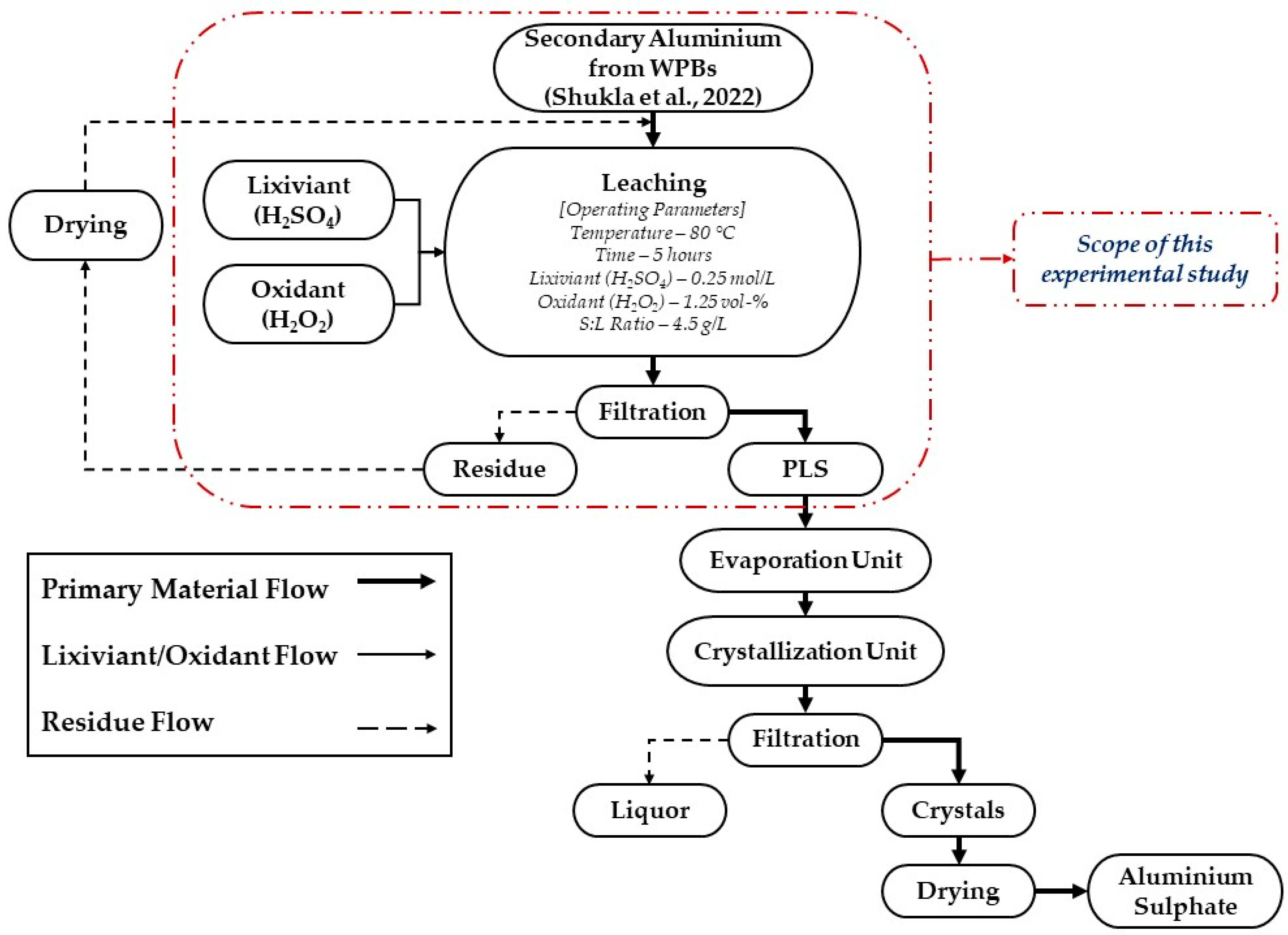
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Leaching Experiments
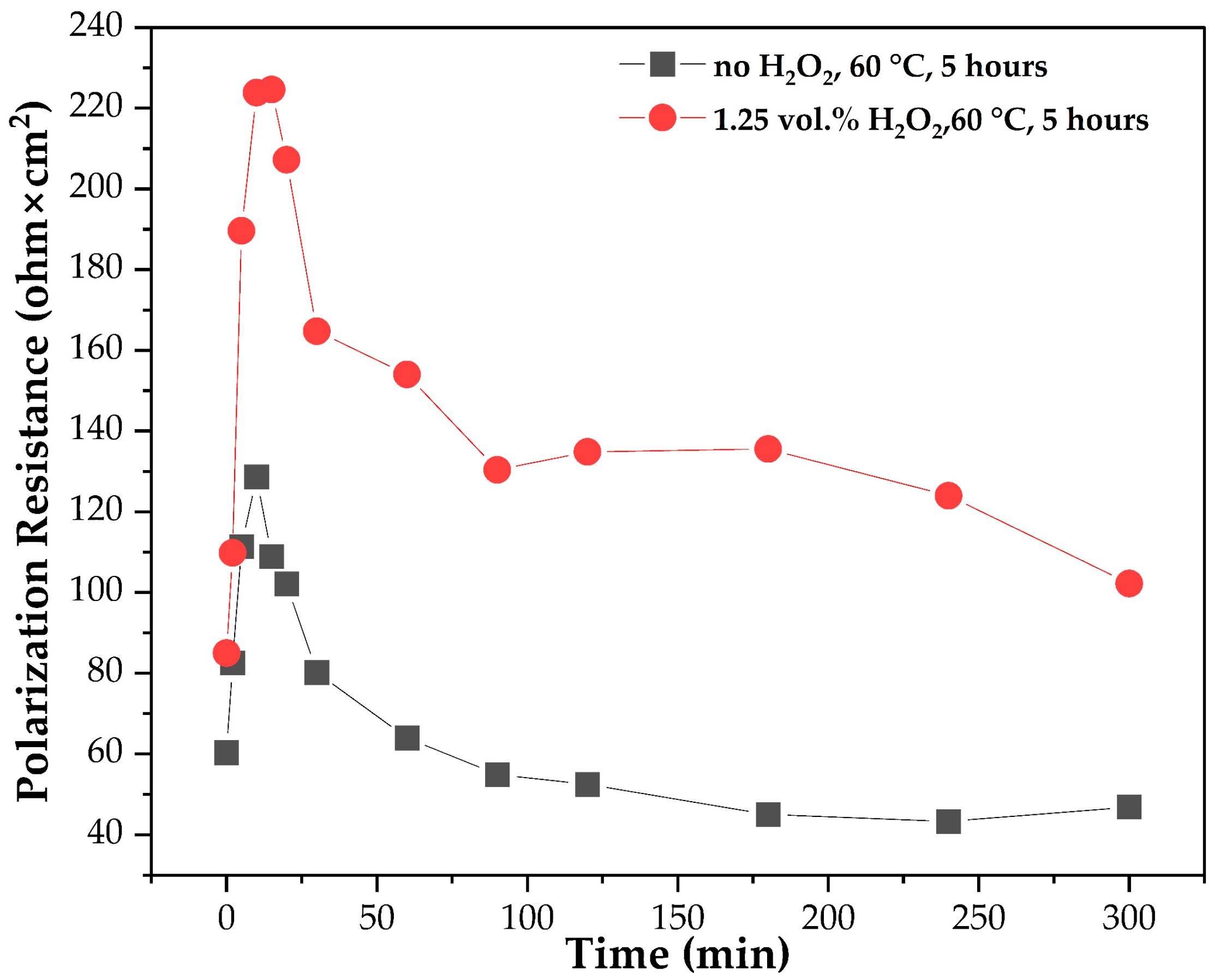
2.2.2. Electrochemical Experiments
3. Results
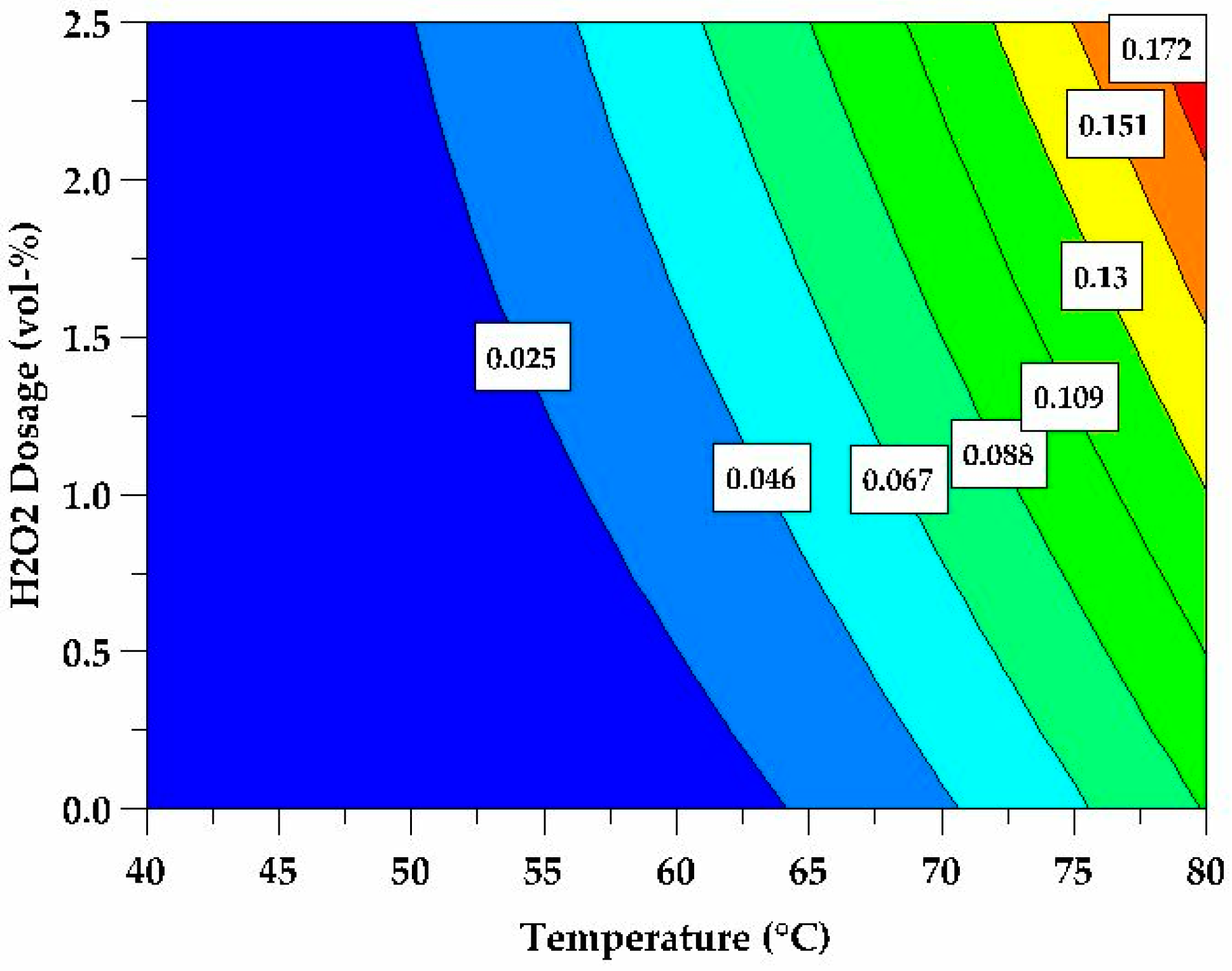
3.1. Effect of Leaching Parameters (Acidity, Temperature, Time, Use of H2O2 and S:L Ratio) on Aluminium Extraction
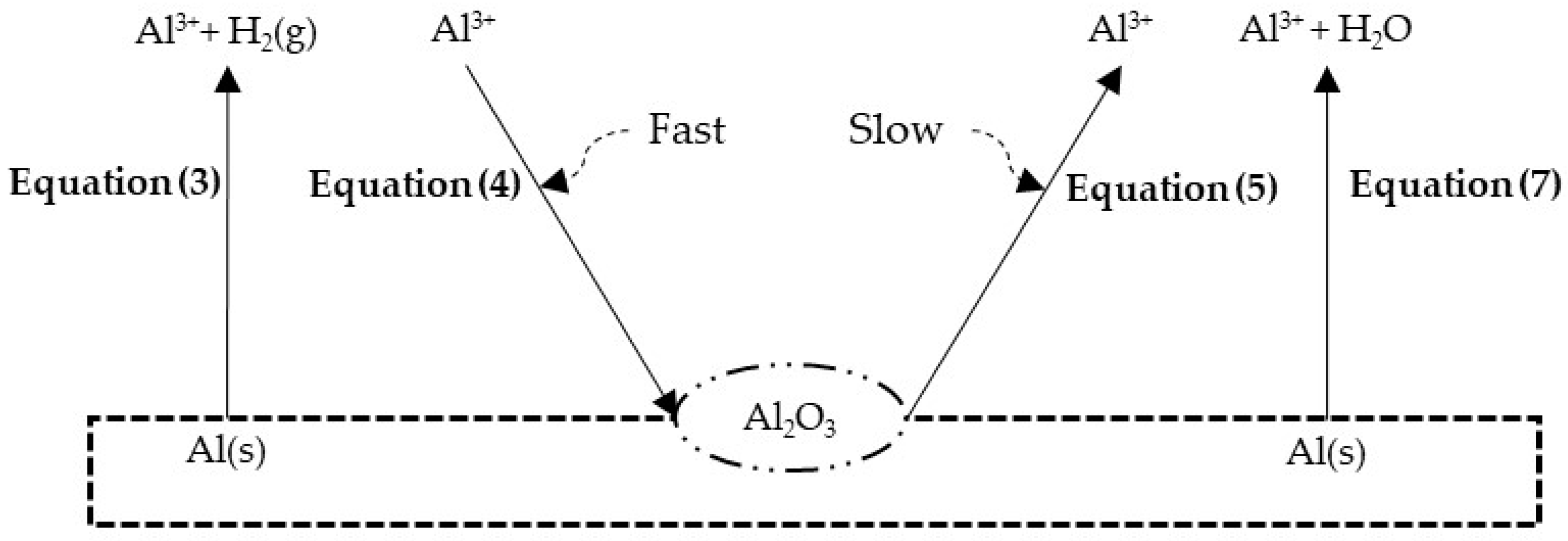
3.2. Leaching Mechanism
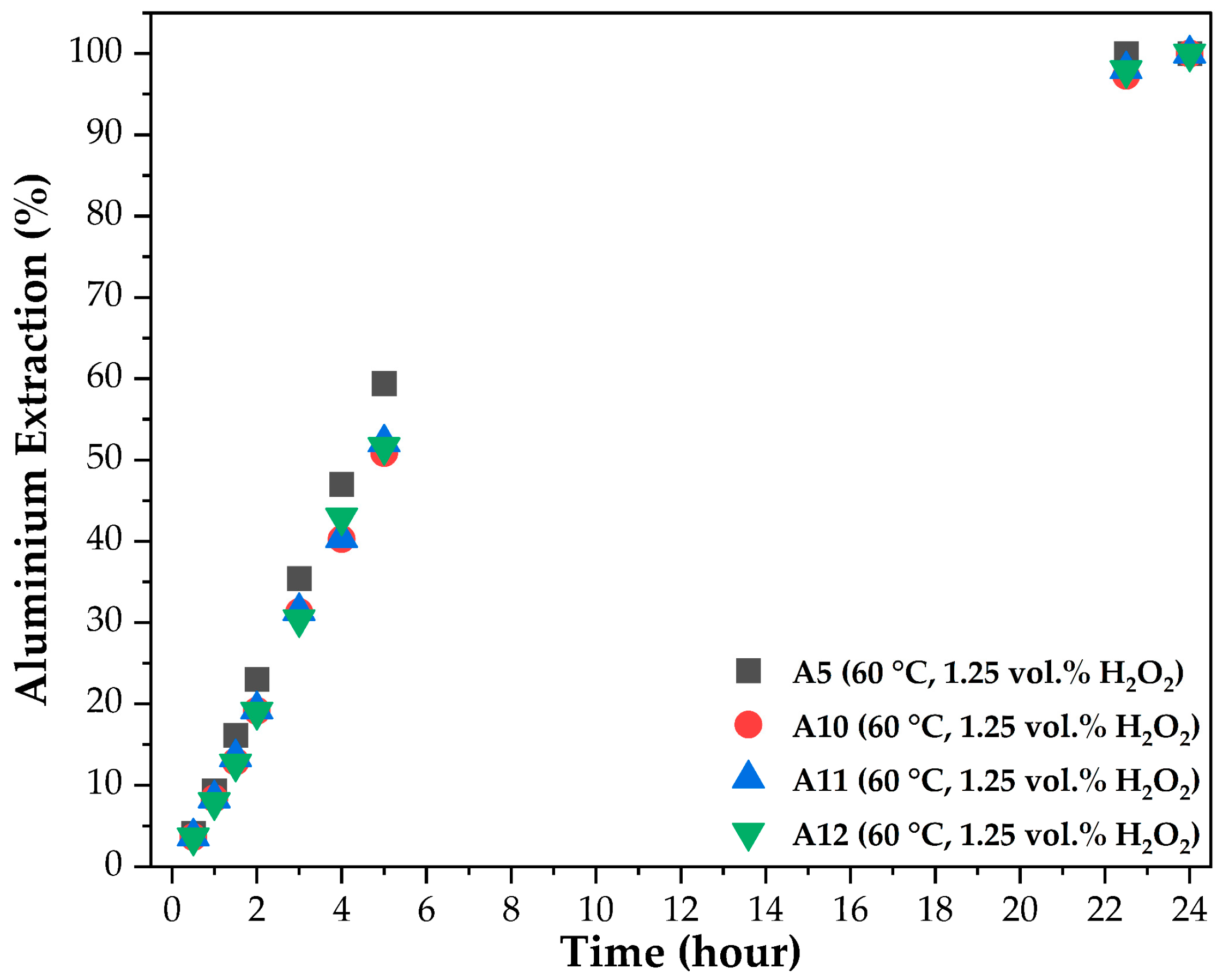
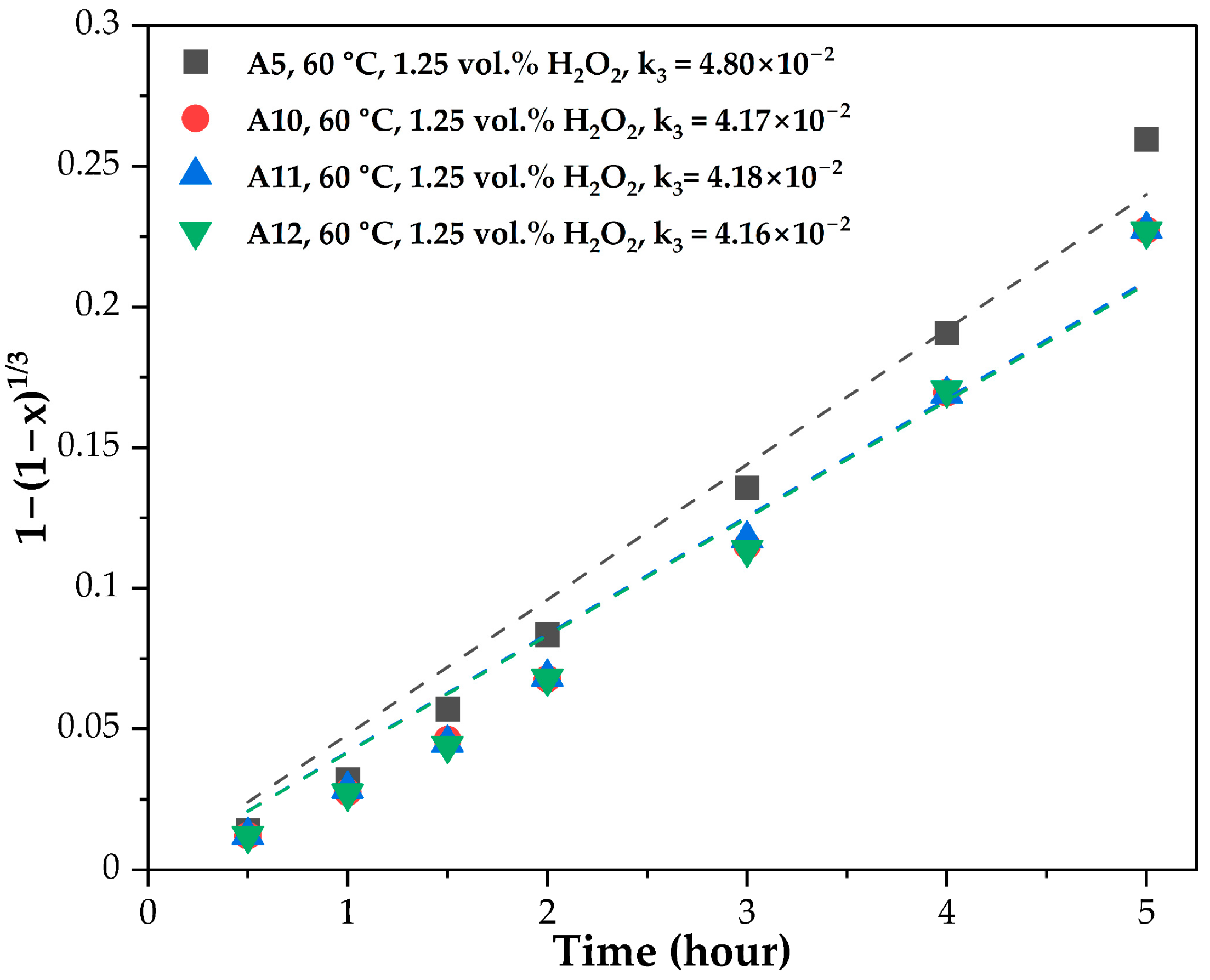
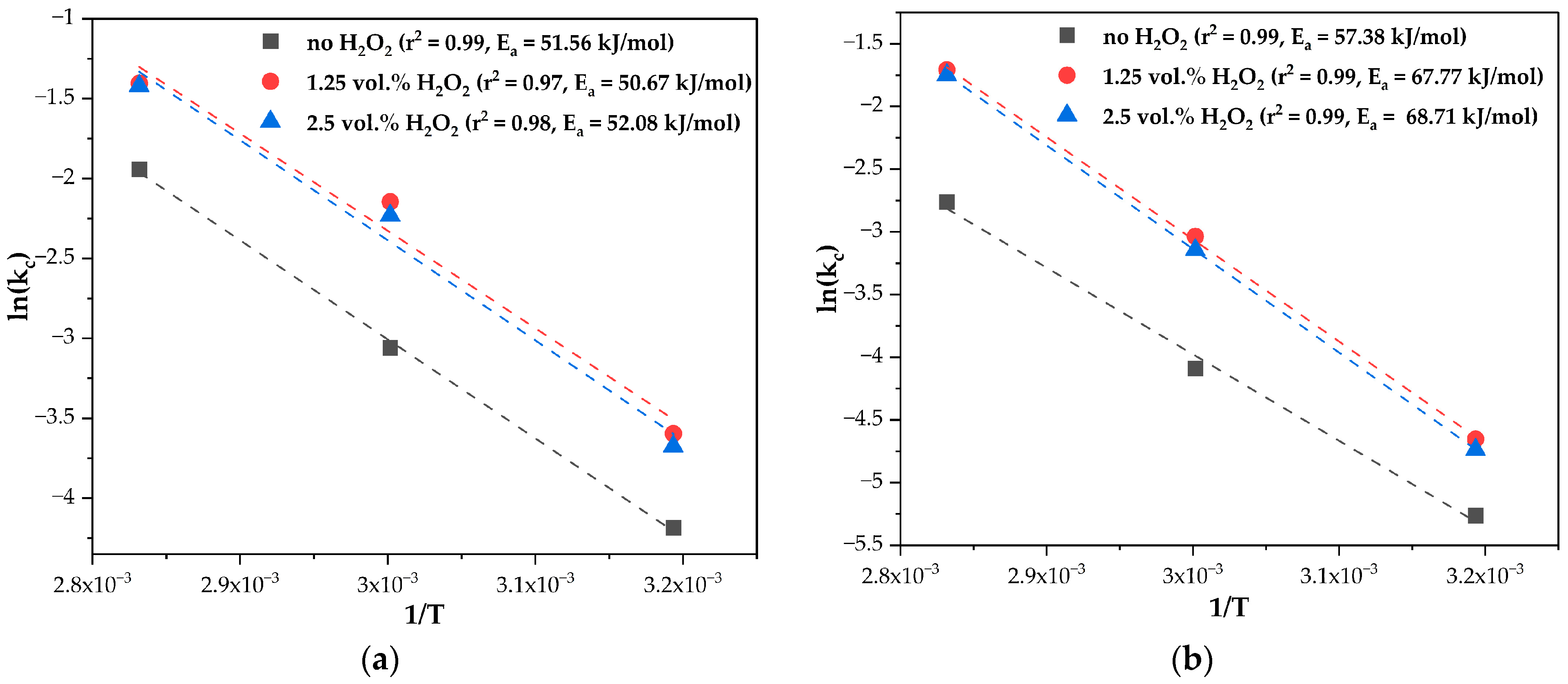
3.3. Leaching Kinetics
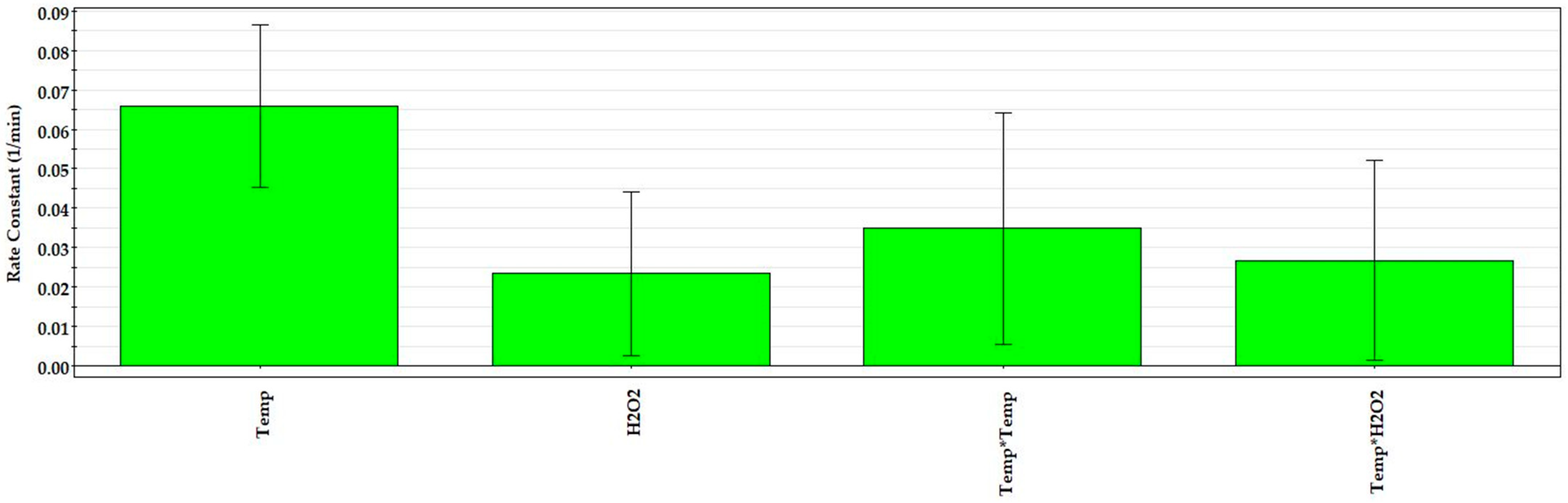
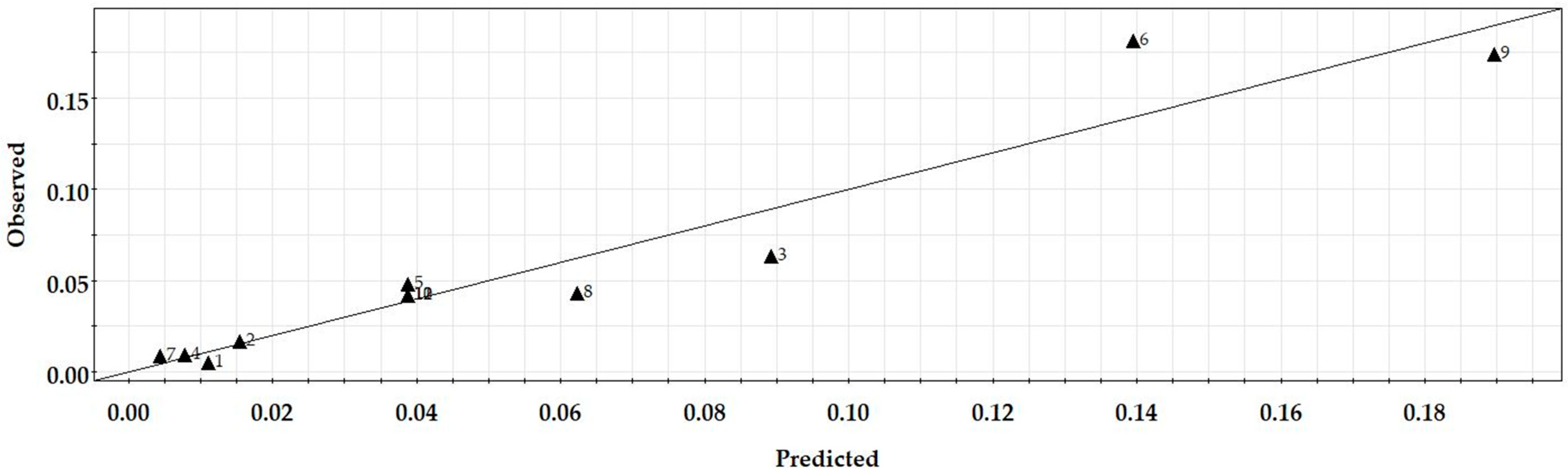
3.4. Regression Modelling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Primary Aluminium Production—International Aluminium Institute. Available online: https://international-aluminium.org/statistics/primary-aluminium-production/ (accessed on 2 May 2023).
- OECD Global Forum on Environment Focusing on Sustainable Materials Management. Available online: http://www.oecd.org/environment/resourceproductivityandwaste/46194971.pdf (accessed on 2 May 2023).
- Circular Aluminium Action Plan: A Strategy for Achieving Aluminium’s Full Potential for Circular Economy by 2030. Available online: https://european-aluminium.eu/wp-content/uploads/2022/08/european-aluminium-circular-aluminium-action-plan.pdf (accessed on 2 May 2023).
- Sun, Y.; Huang, X.; Liu, C.; Zhou, M.; Zhang, X. Impurity Iron Separation from Molten Secondary Aluminum by Pulsed Electric Current. J. Alloys Compd. 2022, 934, 167903. [Google Scholar] [CrossRef]
- Habashi, F. Handbook of Aluminum: Volume 2: Alloy Production and Materials Manufacturing; Totten, G.E., MacKenzie, D.S., Eds.; CRC press: Boca Raton, FL, USA, 2003; Volume 2, pp. 38–40. ISBN 978-0824708962. [Google Scholar]
- Kondo, M.; Maeda, H.; Mizuguchi, M. The Production of High-Purity Aluminum in Japan. JOM 1990, 42, 36–37. [Google Scholar] [CrossRef]
- Padamata, S.K.; Yasinskiy, A.; Polyakov, P. A Review of Secondary Aluminum Production and Its Byproducts. JOM 2021, 73, 2603–2614. [Google Scholar] [CrossRef]
- de Oliveira, D.P.; Costa, J.S.R.; Oliveira-Nascimento, L. Sustainability of Blisters for Medicines in Tablet Form. Sustain. Chem. Pharm. 2021, 21, 1–7. [Google Scholar] [CrossRef]
- Dalal, S.P.; Dalal, P.; Motiani, R.; Solanki, V. Experimental Investigation on Recycling of Waste Pharmaceutical Blister Powder as Partial Replacement of Fine Aggregate in Concrete. Resour. Conserv. Recycl. Adv. 2022, 14, 200076. [Google Scholar] [CrossRef]
- Shukla, S.; Halli, P.; Khalid, M.K.; Lundström, M. Waste Pharmaceutical Blister Packages as a Source of Secondary Aluminum. JOM 2022, 74, 612–621. [Google Scholar] [CrossRef]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The Ecoinvent Database Version 3 (Part I): Overview and Methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Huijbregts, M.A.J.; Steinmann, Z.J.N.; Elshout, P.M.F.; Stam, G.; Verones, F.; Vieira, M.; Zijp, M.; Hollander, A.; van Zelm, R. ReCiPe2016: A Harmonised Life Cycle Impact Assessment Method at Midpoint and Endpoint Level. Int. J. Life Cycle Assess. 2017, 22, 138–147. [Google Scholar] [CrossRef]
- Wang, C.Q.; Wang, H.; Liu, Y.N. Separation of Polyethylene Terephthalate from Municipal Waste Plastics by Froth Flotation for Recycling Industry. Waste Manag. 2015, 35, 42–47. [Google Scholar] [CrossRef]
- Gente, V.; la Marca, F.; Lucci, F.; Massacci, P. Electrical Separation of Plastics Coming from Special Waste. Waste Manag. 2003, 23, 951–958. [Google Scholar] [CrossRef]
- Gente, V.; la Marca, F.; Lucci, F.; Massacci, P.; Pani, E. Cryo-Comminution of Plastic Waste. Waste Manag. 2004, 24, 663–672. [Google Scholar] [CrossRef]
- Agarwal, V.; Halli, P.; Helin, S.; Tesfaye, F.; Lundström, M. Electrohydraulic Fragmentation of Aluminum and Polymer Fractions from Waste Pharmaceutical Blisters. ACS Sustain. Chem. Eng. 2020, 8, 4137–4145. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Liu, Y. Separation of Aluminum and Plastic by Metallurgy Method for Recycling Waste Pharmaceutical Blisters. J. Clean. Prod 2015, 102, 378–383. [Google Scholar] [CrossRef]
- Wang, C.-Q.; Wang, H.; Gu, G.-H.; Fu, J.-G.; Liu, Y.-N. Kinetics and Leaching Behaviors of Aluminum from Pharmaceutical Blisters in Sodium Hydroxide Solution. J. Cent. South Univ. 2015, 22, 4545–4550. [Google Scholar] [CrossRef]
- Yousef, S.; Mumladze, T.; Tatariants, M.; Kriūkienė, R.; Makarevicius, V.; Bendikiene, R.; Denafas, G. Cleaner and Profitable Industrial Technology for Full Recovery of Metallic and Non-Metallic Fraction of Waste Pharmaceutical Blisters Using Switchable Hydrophilicity Solvents. J. Clean. Prod. 2018, 197, 379–392. [Google Scholar] [CrossRef]
- Nieminen, J.; Anugwom, I.; Kallioinen, M.; Mänttäri, M. Green Solvents in Recovery of Aluminium and Plastic from Waste Pharmaceutical Blister Packaging. Waste Manag. 2020, 107, 20–27. [Google Scholar] [CrossRef] [PubMed]
- N,N-Dimethylcyclohexylamine|C8H17N—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/N_N-Dimethylcyclohexylamine (accessed on 2 May 2023).
- Tsakiridis, P.E. Aluminium Salt Slag Characterization and Utilization—A Review. J. Hazard. Mater. 2012, 217–218, 1–10. [Google Scholar] [CrossRef]
- Amer, A.M. Extracting Aluminum from Dross Tailings. JOM 2002, 54, 72–75. [Google Scholar] [CrossRef]
- Feng, C.; Liu, Y.; Sun, M.; Zhang, W.; Zhang, J.; Wang, S. Investigation of Aluminum Gate CMP in a Novel Alkaline Solution. J. Semicond. 2016, 37, 016002. [Google Scholar] [CrossRef]
- Sadawy, M.M. Effect of Al2O3 Additives on the Corrosion and Electrochemical Behavior of Steel Embedded in Ordinary Portland Cement Concrete. Am. J. Mater. Res. 2014, 1, 53–58. [Google Scholar]
- Dorella, G.; Mansur, M.B. A Study of the Separation of Cobalt from Spent Li-Ion Battery Residues. J. Power Sources 2007, 170, 210–215. [Google Scholar] [CrossRef]
- Klyuchnikova, N.; Lumar’, E. Use of Cermet Binder to Obtain Mullite-Corundum Material. Glass Ceram. 2015, 72, 18–20. [Google Scholar] [CrossRef]
- Ferreira, D.A.; Prados, L.M.Z.; Majuste, D.; Mansur, M.B. Hydrometallurgical Separation of Aluminium, Cobalt, Copper and Lithium from Spent Li-Ion Batteries. J. Power Sources 2009, 187, 238–246. [Google Scholar] [CrossRef]
- Chernyaev, A.; Zou, Y.; Wilson, B.P.; Lundström, M. The Interference of Copper, Iron and Aluminum with Hydrogen Peroxide and Its Effects on Reductive Leaching of LiNi1/3Mn1/3Co1/3O2. Sep. Purif. Technol. 2022, 281. [Google Scholar] [CrossRef]
- Lewis, T.J. The Corrosion of Aluminium in Concentrated Hydrogen Peroxide. J. Appl. Chem. 2007, 11, 405–413. [Google Scholar] [CrossRef]
- Perry, R.H.; Green, D.W.; Maloney, J.O. Perry’s Chemical Engineers’ Handbook; McGraw-Hill: San Francisco, CA, USA, 1984; p. 121. ISBN 0070498415. [Google Scholar]
- Shreve, R.N.; Austin, G.T. Shreve’s Chemical Process Industries, 5th ed.; Austin, G.T., Ed.; McGraw-Hill: Noida, India, 1984; p. 357. ISBN 9781259029455. [Google Scholar]
- Chow, C.W.K.; van Leeuwen, J.A.; Fabris, R.; Drikas, M. Optimised Coagulation Using Aluminium Sulfate for the Removal of Dissolved Organic Carbon. Desalination 2009, 245, 120–134. [Google Scholar] [CrossRef]
- Birnin-Yauri, A.U.; Aliyu, M. Synthesis and Analysis of Potassium Aluminium Sulphate (Alum) from Waste Aluminium Can. Int. J. Adv. Res. Chem. Sci. 2014, 1, 1–6. [Google Scholar]
- Barik, S.P.; Park, K.H.; Parhi, P.K.; Park, J.T.; Nam, C.W. Extraction of Metal Values from Waste Spent Petroleum Catalyst Using Acidic Solutions. Sep. Purif. Technol. 2012, 101, 85–90. [Google Scholar] [CrossRef]
- Lisińska, M.; Saternus, M.; Willner, J. Research of Leaching of the Printed Circuit Boards Coming from Waste Mobile Phones. Arch. Metall. Mater. 2018, 63, 143–147. [Google Scholar] [CrossRef]
- Chernyaev, A.; Wilson, B.P.; Lundström, M. Study on Valuable Metal Incorporation in the Fe–Al Precipitate during Neutralization of LIB Leach Solution. Sci. Rep. 2021, 11, 23283. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; Wiley: Bengaluru, India, 1998; p. 580. ISBN 978-0-471-25424-9. [Google Scholar]
- Ashraf, M.; Zafar, Z.I.; Ansari, T.M. Selective Leaching Kinetics and Upgrading of Low-Grade Calcareous Phosphate Rock in Succinic Acid. Hydrometallurgy 2005, 80, 286–292. [Google Scholar] [CrossRef]
- Abdel-Aal, E.A. Kinetics of Sulfuric Acid Leaching of Low-Grade Zinc Silicate Ore. Hydrometallurgy 2000, 55, 247–254. [Google Scholar] [CrossRef]
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N.; Wikstroè, C.; Wold, S. Design of Experiments, Principles and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001; Volume 15, pp. 38–39. ISBN 9197373001. [Google Scholar]
- Mohapatra, D.; Park, K.H. Selective Recovery of Mo, Co and Al from Spent Co/Mo/γ -Al 2O3 Catalyst: Effect of Calcination Temperature. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 2007, 42, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Mbedzi, N.; Ibana, D.; Dyer, L.; Browner, R. The Effect of Oxidant Addition on Ferrous Iron Removal from Multi-Element Acidic Sulphate Solutions. AIP Conf. Proc. 2017, 1805. [Google Scholar] [CrossRef]
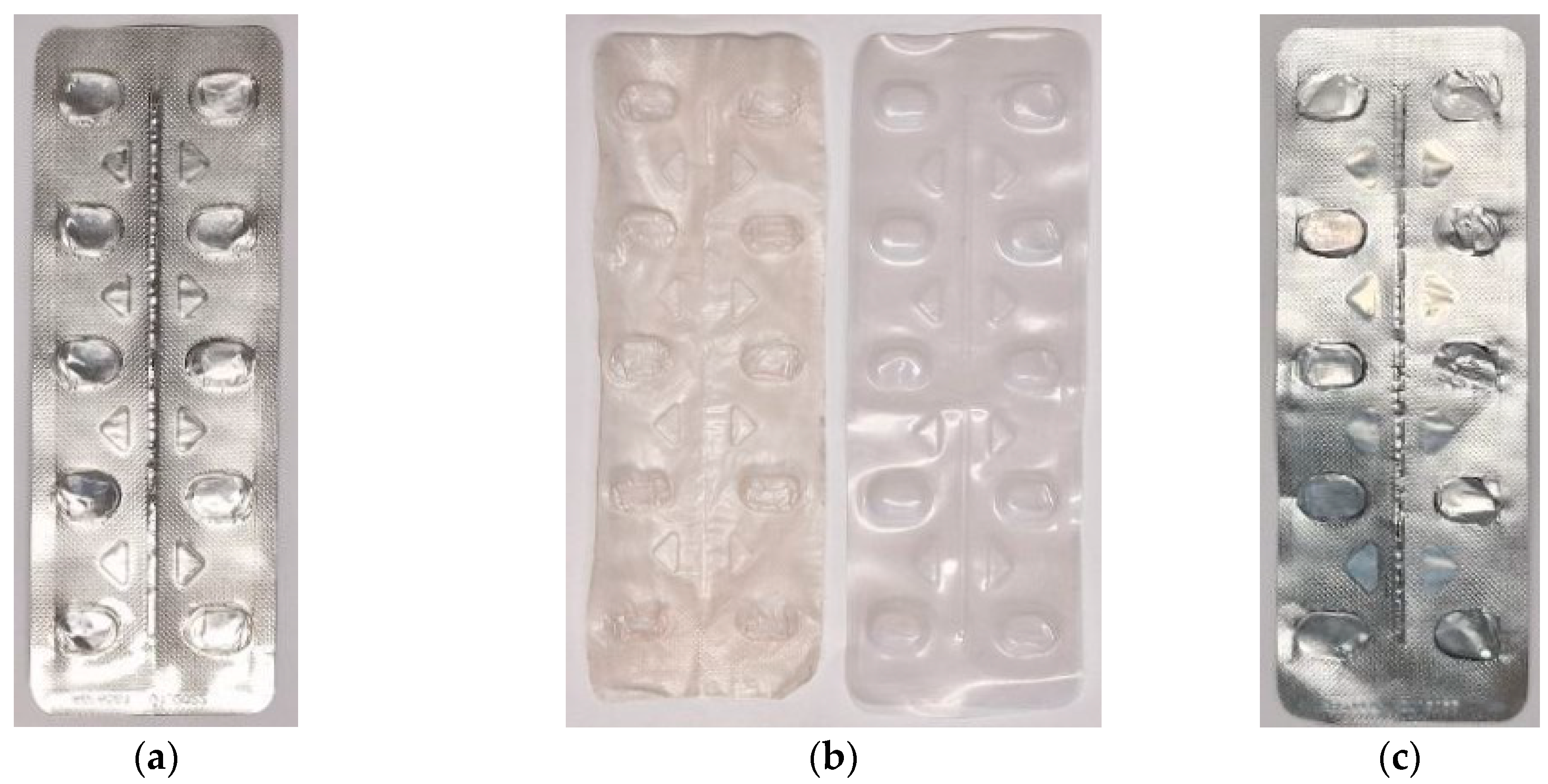




| O | Al | Cl | Fe | Total | |
|---|---|---|---|---|---|
| Aluminium Film | 1.03 | 95.57 | 1.93 | 0.47 | 100 |
| Experimental Set | Experiment Code | Temperature (°C) | H2SO4 (M) | S:L Ratio (g/L) | Time (h) |
| PE | PE-1 | 80 | 0.25 | 4.0 | 24 |
| PE-2 | 80 | 0.50 | 4.0 | 24 | |
| PE-3 | 80 | 0.75 | 4.0 | 24 | |
| PE-4 | 80 | 1.00 | 4.0 | 24 | |
| Experimental Set | Experiment Code | Temperature (°C) | H2O2 (vol.%) | S:L ratio (g/L) | Time (h) |
| T1 | A1 * | 40 | 0 | 4.5 | 24 |
| A2 * | 60 | 0 | 4.5 | 24 | |
| A3 *# | 80 | 0 | 4.5 | 24 | |
| A4 * | 40 | 1.25 | 4.5 | 24 | |
| A5 * | 60 | 1.25 | 4.5 | 24 | |
| A6 *# | 80 | 1.25 | 4.5 | 24 | |
| A7 * | 40 | 2.5 | 4.5 | 24 | |
| A8 * | 60 | 2.5 | 4.5 | 24 | |
| A9 *# | 80 | 2.5 | 4.5 | 24 | |
| T2 | A10 | 60 | 1.25 | 4.5 | 24 |
| A11 | 60 | 1.25 | 4.5 | 24 | |
| A12 | 60 | 1.25 | 4.5 | 24 | |
| T3 | A13 | 50 | 0 | 4.5 | 5 |
| A14 | 70 | 0 | 4.5 | 5 | |
| A15 | 50 | 1.25 | 4.5 | 5 | |
| A16 | 70 | 1.25 | 4.5 | 5 | |
| A17 | 40 | 0.625 | 4.5 | 5 | |
| A18 | 60 | 0.625 | 4.5 | 5 | |
| A19 | 40 | 1.875 | 4.5 | 5 | |
| A20 | 60 | 1.875 | 4.5 | 5 | |
| T4 | A21 | 60 | 1.25 | 2.25 | 5 |
| A22 | 60 | 1.25 | 3.375 | 5 | |
| A23 | 60 | 1.25 | 5.625 | 5 | |
| A24 | 60 | 1.25 | 6.75 | 5 | |
| A25 | 80 | 1.25 | 2.25 | 5 | |
| A26 | 80 | 1.25 | 3.375 | 5 | |
| A27 | 80 | 1.25 | 5.625 | 5 | |
| A28 | 80 | 1.25 | 6.75 | 5 |
| A5 | A10 | A11 | A12 | |
|---|---|---|---|---|
| Slope: | 11.70 | 10.04 | 10.18 | 10.22 |
| Mean for the slopes: | 8.59 | |||
| Standard deviation for the slopes: | 0.78 | |||
| Coefficient of variation (CV): | 0.09 | |||
| Initial Lixiviant | PLS (A3 #) | PLS (A6 #) | PLS (A9 #) | |
|---|---|---|---|---|
| [H+] conc. (mol/L) | 0.265 | 0.159 | 0.060 | 0.053 |
| pH | 0.58 | 0.80 | 1.23 | 1.27 |
| Model | Q2 | R2 | R2 (adj.) | Reproducibility |
|---|---|---|---|---|
| Reaction rate constant (t = 5 h) | 0.471 | 0.918 | 0.871 | 0.997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shukla, S.; Chernyaev, A.; Halli, P.; Aromaa, J.; Lundström, M. Leaching of Waste Pharmaceutical Blister Package Aluminium in Sulphuric Acid Media. Metals 2023, 13, 1118. https://doi.org/10.3390/met13061118
Shukla S, Chernyaev A, Halli P, Aromaa J, Lundström M. Leaching of Waste Pharmaceutical Blister Package Aluminium in Sulphuric Acid Media. Metals. 2023; 13(6):1118. https://doi.org/10.3390/met13061118
Chicago/Turabian StyleShukla, Sugam, Alexander Chernyaev, Petteri Halli, Jari Aromaa, and Mari Lundström. 2023. "Leaching of Waste Pharmaceutical Blister Package Aluminium in Sulphuric Acid Media" Metals 13, no. 6: 1118. https://doi.org/10.3390/met13061118
APA StyleShukla, S., Chernyaev, A., Halli, P., Aromaa, J., & Lundström, M. (2023). Leaching of Waste Pharmaceutical Blister Package Aluminium in Sulphuric Acid Media. Metals, 13(6), 1118. https://doi.org/10.3390/met13061118






