Abstract
Noble metal Pt catalyst has been identified as excellent electrocatalysts for the ammonia oxidation reaction (AOR). However, Pt’s scarcity, expensiveness, and toxicity hinder its large-scale commercial application. Herein, we report a facile and surfactant-free electrochemical synthesis method for the production of PtIr nanocubes. The PtIr nanocubes were in situ synthesized on carbon paper, and no organic additives were used at any stage in the synthesis of the catalyst. The formation of PtIr nanocubes was attributed to the synergy of the electro-adsorption/desorption of O-containing species and the preferential adsorption of hydrogen adatoms on PtIr(100) with a lower surface free energy. The obtained PtIr nanocubes exhibit an outstanding specific activity (SA) value of 1.34 mA cm−2, which is 1.5 and 3.8 times higher than Pt nanocubes (0.90 mA cm−2) and PtIr nanospheres (0.35 mA cm−2), respectively. The enhanced SA of the PtIr nanocubes can be ascribed to the synergic effects of multiple factors, including the (100) sites of the PtIr nanocubes, the dehydrogenation ability of Ir with respect to ammonia molecules, the electronic effects, and the clean surface of the catalyst due to the use of a “green” synthesis method. This work provides an effective strategy for the “green” synthesis of high-efficiency Pt-based metal catalysts with controllable shapes.
1. Introduction
Ammonia oxidation reactions (AORs) have drawn increasing attention due to their various applications in the field of energy and the environment [1], including with respect to direct ammonia fuel cells [2], hydrogen production [3], the decontamination of NH3 wastewater [4], and the development of direct electrochemical NH3 sensors [5]. Pt-based noble metal catalysts have been considered as the optimal electrocatalysts for the oxidation of ammonia [6]. Nonetheless, Pt’s scarcity, high cost, and toxicity hamper its large-scale commercial application [7]. Therefore, it is still a challenge to develop effective Pt-based catalysts with high activity, a low Pt loading, and anti-poisoning capacity for AOR.
To date, several strategies have been proposed for the development of high-performance Pt-based electrocatalysts. It is generally accepted that the catalytic performance of Pt catalysts strongly depends on their shape [8]. The control of shape to expose more active sites is an effective strategy to enhance Pt’s catalytic activity. In particular, the AOR is sensitive to the structure of Pt; for example, it occurs almost exclusively on Pt (100) [9]. Both experimentally and theoretically, previous studies have demonstrated that Nads intermediates on Pt (100) are rarely formed and readily removed owing to the lower adsorption energy of the poisonous product Nads on Pt (100) compared to Pt (111) and Pt (110) facets [10]. Hence, great efforts have been devoted to the synthesis of cubic Pt nanoparticle catalysts. For instance, Liu et al. [11] prepared cubic Pt particles with a preferential (100) orientation using an electrochemical method. The cubic Pt particles exhibited enhanced specific activity (SA) for the AOR, which was 3.6 times higher than that for spherical Pt particles. Peng et al. [12] synthesized a carbon-supported cubic Pt nanoparticle catalyst with clean surfaces using a mixture of H2 and CO as a reducing agent; the resulting catalyst exhibited greatly enhanced catalytic activity and excellent durability with respect to the electro-oxidation of ammonia. Tang et al. [13] developed well-defined Pt nanoparticles with a cuboid shape via L-Lysine-mediated synthesis; the Pt nanocubes demonstrated remarkably improved activity and stability towards the AOR. Feliu et al. [14] reported the synthesis of cubic Pt nanoparticles with a preferential orientation (100) using a microemulsion method. The cubic-like Pt nanoparticles with a high fraction of (100) sites exhibited the highest catalytic activity among the studied materials, which was more than three times higher than that of polycrystalline Pt nanoparticles. The above studies indicate that the controllable shape of Pt has a great influence on the improvement of its catalytic activity for ammonia electro-oxidation.
In addition, alloying Pt was regarded as one of the most effective strategies for enhancing the activity of Pt and reducing its usage [8]. Currently, various Pt-based bimetallic catalysts, such as PtPb [15], PtAu [16], PtRu [17], PtCu [18], PtNi [19], PtCo [20], PtPd [21], PtFe [22], and PtIr [23], have been synthesized. Among them, Ir is regarded as the most effective alloying element for the use of Pt toward ammonia oxidation owing to its good chemical stability and catalytic properties for the electro-oxidation of ammonia (despite its scarcity and high price) [24]. For instance, Baranova et al. [25] synthesized a series of PtIr bimetallic nanoparticles using the modified polyol method. It was found that the PtIr binary electrocatalysts exhibited improved catalytic activity and good stability towards ammonia electro-oxidation compared to monometallic Pt. Zhong et al. [26] reported cubic Pt-Ir nanoparticles enclosed by (100) facets that were synthesized using a solvothermal method. The SA of the cubic Pt-Ir nanoparticles was much higher than that of Pt-Ir nanoparticles with a polycrystalline structure and pure Pt nanocubes with respect to the AOR. Kim et al. [24] developed PtIr nanostructure alloys with a spiky surface using a template method. The obtained PtIr–Am catalyst showed significant performance enhancement with respect to the AOR compared to commercial Pt black. The enhanced catalytic performance of PtIr alloys toward the AOR can be explained by the synergetic interaction between the two metals or the electronic effect generated between Pt and Ir in the alloy [27]. Therefore, it remains a valuable research concept that the electrocatalytic activity of PtIr nanoparticles can be remarkably enhanced by combining the alloying of Pt and its morphology control.
At present, various synthetic methods are used for the controlled-shape preparation of Pt-based catalysts, such as wet chemical methods [28], hydrothermal methods [29], microemulsions [30], etc. However, most of these synthetic methods require the introduction of organic additives or capping agents and require additional steps to remove the residual capping agents [31]. The incomplete removal of organic additives can affect the performance of electrocatalysts [32]. In addition, during the assembly of a conventional electrode, the catalyst powder slurry must be transferred to the conductive substrate, and a conductive agent and binder must be introduced, which will also affect catalytic performance [11]. Electrodeposition is a facile and effective strategy for preparing metal nanoparticles due to its versatility, the high purity of the deposits, the fact that it is an easy-to-control procedure, and the low cost of its implementation [33]. The electrodeposition method can be used to directly prepare a nanoparticle catalyst on the surface of a conductive substrate and does not require the introduction of organic additives. Therefore, it is of great significance to develop electrodeposited PtIr nanoparticles with a clean surface to improve the catalytic performance of the AOR. To the best of our knowledge, there have been no reports regarding the synthesis of cubic PrIr bimetallic nanoparticles via the electrodeposition method towards AOR. The effects of electrodeposition parameters on the cubic shape of PtIr nanoparticles and the corresponding electrocatalytic activity towards the AOR have remained unclear [34].
In this work, we report our findings concerning PtIr nanocubes that were directly grown on a carbon paper surface using a facile and surfactant-free electrochemical method. No organic additives were used at any point in the entire catalyst synthesis process. The effects of key synthetic parameters (EL) of the periodic square-wave potential (PSWP) on the morphological evolution of the PtIr nanoparticles were systematically investigated. Ammonia electro-oxidation, acting as the model reaction, was performed to assess the electrocatalytic properties of the obtained PtIr nanoparticles. The obtained PtIr nanocubes exhibit a superior SA value of 1.34 mA cm−2, constituting a level that is 1.5 times and 3.8 times higher than Pt nanocubes (0.90 mA cm−2) and PtIr nanospheres (0.35 mA cm−2). Through a comparative analysis, the effects of the shape and composition of the PtIr nanocubes on their SA were revealed.
2. Experimental Section
2.1. Reagents and Materials
Chloroplatinic acid hexahydrate (H2PtCl6·6H2O, 99.95%) was obtained from Adamas Reagent Co., Ltd., Shanghai, China. Iridium trichloride trihydrate (IrCl3·3H2O, 98%) was acquired from Shanghai Macklin Biochemical Co. Ltd., Shanghai, China. H2SO4, (NH4)2SO4, and KOH were obtained from Jiangtian Chemical Technology Co., Ltd., Tianjin, China. Anhydrous ethanol (AR, 99%) was purchased from Tianjin Yuanli Chemical Technology Co., Ltd., Tianjin, China. Carbon paper (TGP-H-060) was obtained from Toray (Toray Industries, Tokyo, Japan). Deionized water (18.2 MΩ cm) for the preparation of all solutions was obtained using a Milli-Q water purification system.
2.2. Synthesis of Pt Nanocubes and PtIr Nanoparticles
Firstly, prior to the electrochemical synthesis, the carbon paper was cleaned via ultrasound in anhydrous ethanol and deionized water (separately) for 10 min. Pt nanocubes and PtIr nanoparticles were prepared on the cleaned carbon paper via pulsed electrodeposition in 5 mM H2PtCl6 + 5 mM IrCl3 + 0.5 M H2SO4 solution. Electrochemical synthesis was conducted in a classical three-electrode system at room temperature. A sheet of carbon paper (with an exposed area of 1 cm2) was used as the working electrode, while Pt foil (1 cm2) and a saturated calomel electrode (SCE) served as the counter electrode and reference electrode, respectively. All potentials in solution tests were compared with SCE. A series of PtIr nanoparticles was finally synthesized using an electrochemical PSWP method. The upper potential limit (EU), the upper potential pulse duration tU, and the lower potential pulse duration tL were 0.5 V (SCE), 1 s, and 0.01 s, respectively. The lower potential limit (EL) ranged from −0.7 to −0.6, −0.5, and −0.4 V(SCE). The total duration of electrochemical PSWP synthesis was about 34 min. For comparison, Pt nanocubes were prepared on carbon paper via pulsed electrodeposition in 5 mM H2PtCl6 + 0.5 M H2SO4 solution. The preparation parameters of EU, EL, tU, and tL were set to 0.5 V, −0.6 V, 1 s, and 0.01 s, respectively. The total duration of electrochemical synthesis was about 17 min. The other synthesis conditions are similar to those of PtIr nanoparticles.
2.3. Materials’ Characterization
The morphology, structure, and composition of the obtained PtIr nanoparticle catalysts were analyzed using a field-emission scanning electron microscope (SEM, JSM-7800F, Hitachi, Tokyo, Japan) and a transmission electron microscope (TEM, JEM-F2100, JELO Ltd., Tokyo Japan) equipped with energy-dispersive X-ray spectroscopy (EDS). The phases of the PtIr nanoparticles were characterized via X-ray diffraction (XRD, Bruker D8 Advanced, Billerica, MA, USA, with Cu Kα radiation, λ = 1.54056 Å). The chemical states and composition of the elements in the PtIr nanoparticle catalysts were characterized via X-ray photoelectron spectroscopy (XPS, ThermoFischer, ESCALAB 250Xi, Kratos Analytical Ltd, UK) with a monochromatized Al Kα source (hν = 1486.6 eV).
2.4. Electrochemical Tests
All electrochemical tests were conducted using a classical three-electrode system in a CHI760E potentiostat (Shanghai Chenhua Instrument Co., Ltd., Shanghai, China.). The obtained PtIr nanoparticles/carbon paper electrode served as the working electrode. A sheet of Pt foil and SCE were used as the counter electrode and the reference electrode, respectively. Cyclic voltammetry (CV) tests of PtIr nanoparticles were carried out in N2-saturated aqueous solution of 0.5 M H2SO4 at 50 mV s−1. The electrocatalytic activity and durability of the obtained nanoparticle catalysts towards AOR was evaluated using CV at 10 mV s−1 and chronoamperometry (CA) measurements in N2-saturated aqueous solution of 1 M KOH + 0.05 M (NH4)2SO4. All experiments were performed at 25 ± 1 °C.
3. Results and Discussion
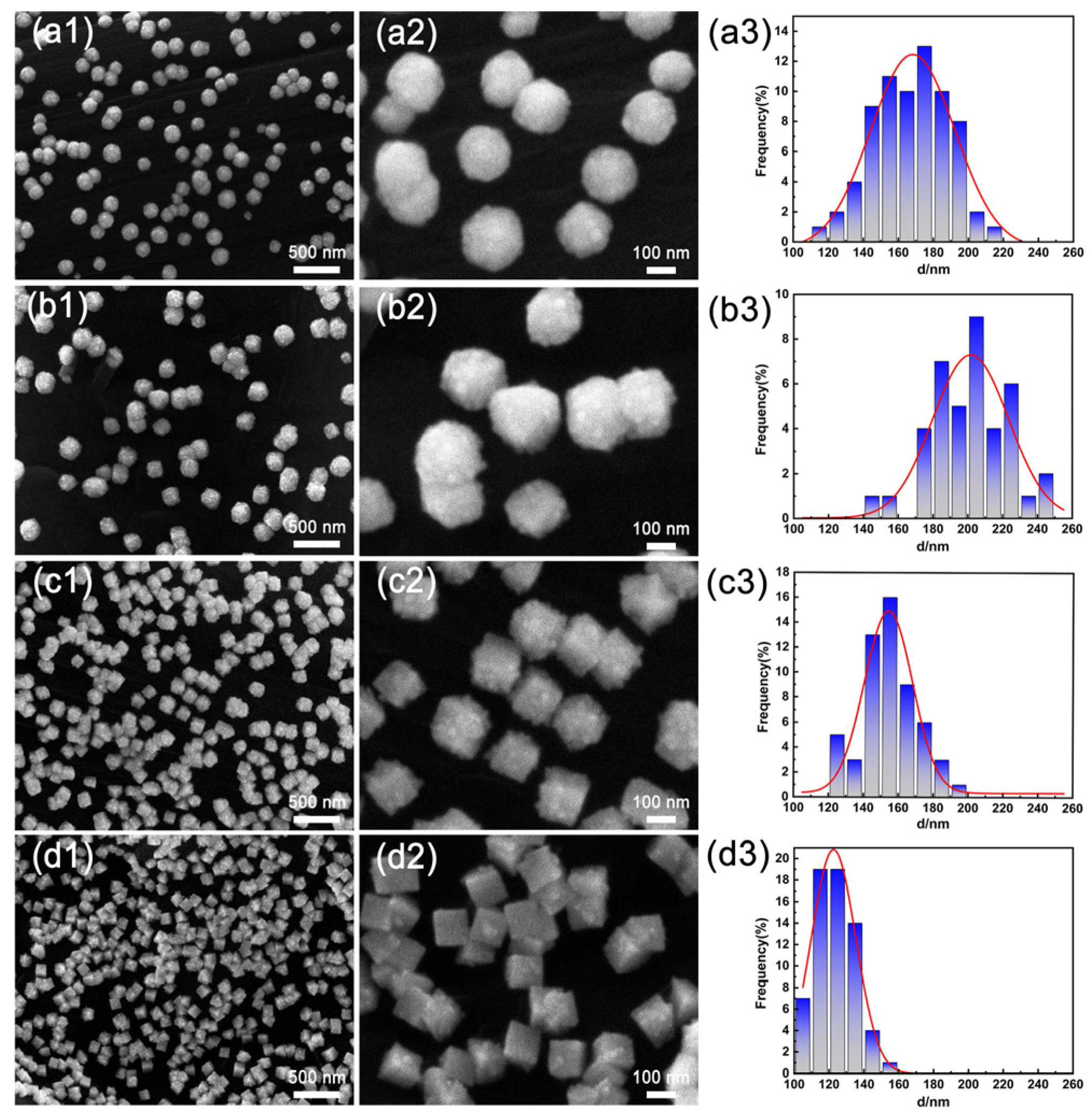
Figure 1 shows SEM images and the corresponding particle size distribution histograms for the PtIr nanoparticles with various morphologies prepared using PSPW. It can be seen that all the obtained PtIr nanoparticles exhibit good dispersibility and that the nanoparticle sizes are relatively uniform. It is worth noting that the lower potential limit of EL has a significant effect on the morphology of PtIr nanoparticles. When the EL is –0.4 V(SCE), the PtIr nanoparticles exhibit a smooth, spherical morphology (Figure 1(a1,a2)) and their average particle size is approximately 170 nm (Figure 1(a3)). When the EL decreases to –0.5 V(vs. SCE), the PtIr nanoparticles present a spherical morphology with a relatively rough surface (Figure 1(b1,b2)) and an average particle size of about 205 nm (Figure 1(b3)). As the EL further reduces to –0.6 V(SCE), the PtIr nanoparticles show a spiky cubic shape (Figure 1(c1,c2)) and a small average particle size of around 155 nm (Figure 1(c3)). When the lower limit potential EL is as low as –0.7 V(SCE), the obtained PtIr nanoparticles exhibit a cubic shape (Figure 1(d1,d2)). A smaller average size of about 122 nm for the PtIr nanocubes was observed (Figure 1(d3)). Obviously, with the decrease in the lower potential limit, the morphology of the PtIr nanoparticles evolves from smooth nanospheres to nanospheres with a rough surface, spiky nanocubes, and nanocubes. This suggests that the morphology of PtIr nanoparticles strongly depends on the lower potential limit of EL.
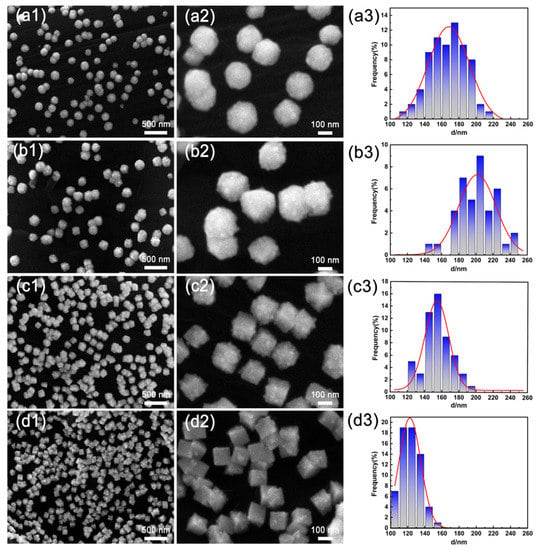
Figure 1.
SEM images of the surface morphology and the corresponding particle size distribution histograms of PtIr nanoparticles prepared via PSPW at the lower potential limit of (a1–a3) −0.4, (b1–b3) −0.5, (c1–c3) −0.6, and (d1–d3) −0.7 V (vs. SCE).
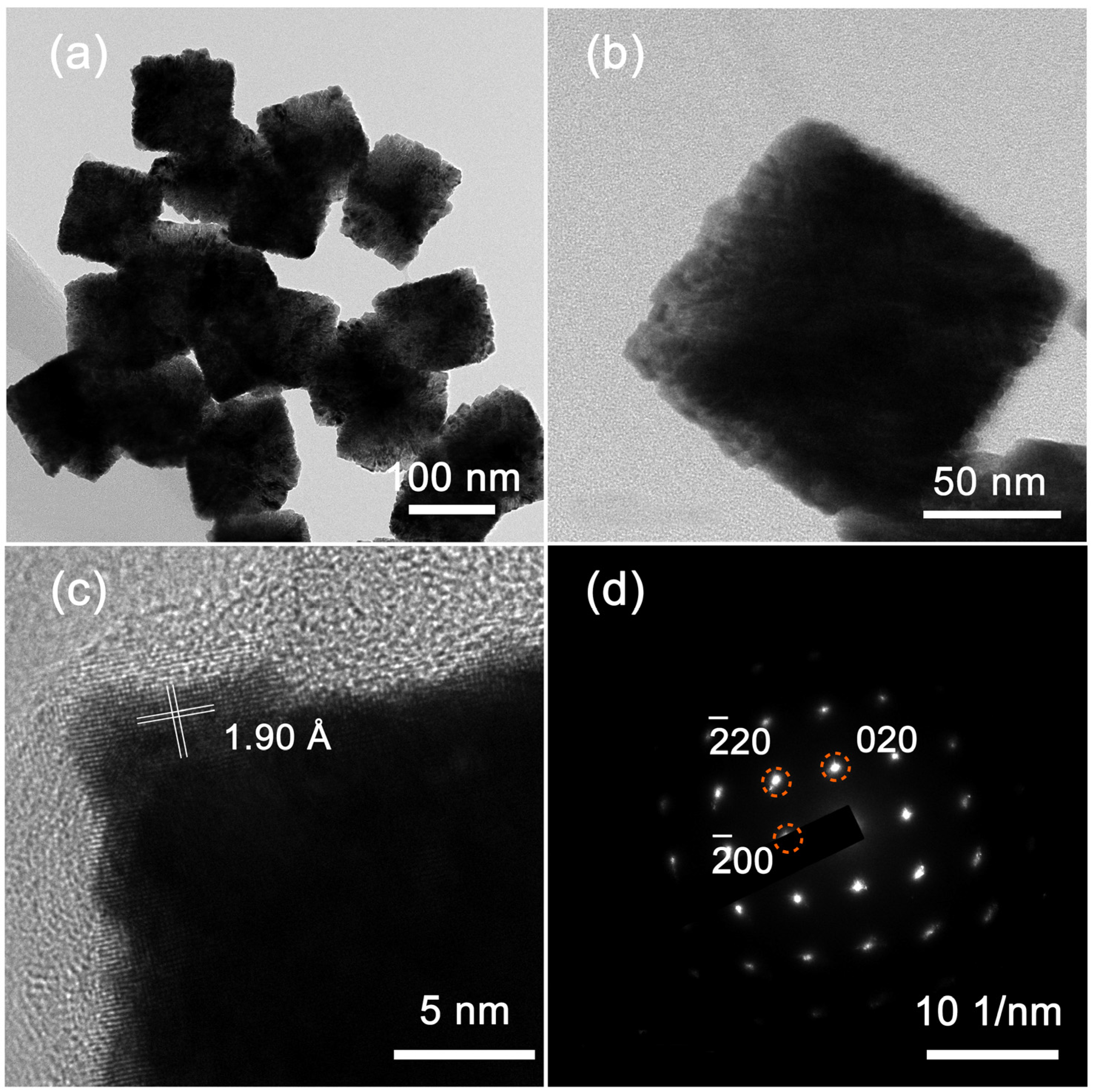
Figure 2a displays TEM images of the obtained PtIr nanocubes. The obtained PtIr nanoparticles possess a uniform cubic morphology. A single PtIr nanocube observed from the magnified TEM images (Figure 2b) shows a well-defined cubic profile. These results are consistent with the results of the SEM images (Figure 1(d1,d2)). Figure 2c shows a high-resolution TEM (HRTEM) image of PtIr nanocube. The clear lattice fringes indicate the high degree of crystallinity of the PtIr nanocubes. The measured interplanar spacing of the continuous lattice fringes was 1.90 Å, corresponding to the (200) crystal facet of face-centered cubic (FCC) PtIr. The corresponding selected area electron diffraction (SAED) pattern was observed along the [001] zone’s axis and displays a set of bright diffraction spots with four-fold rotational symmetry (Figure 2d), indicating the single-crystalline feature of the PtIr nanocubes with dominant Pt (100) facets.
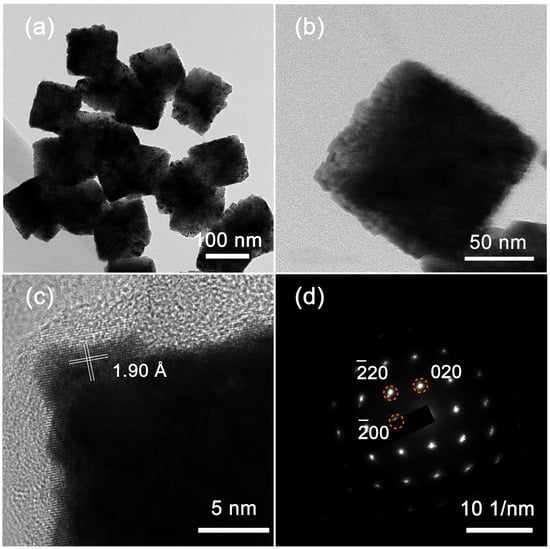
Figure 2.
(a) TEM image and (b) a magnified TEM image of PtIr nanocubes; (c) HRTEM image of PtIr nanocubes and (d) the corresponding SAED pattern.
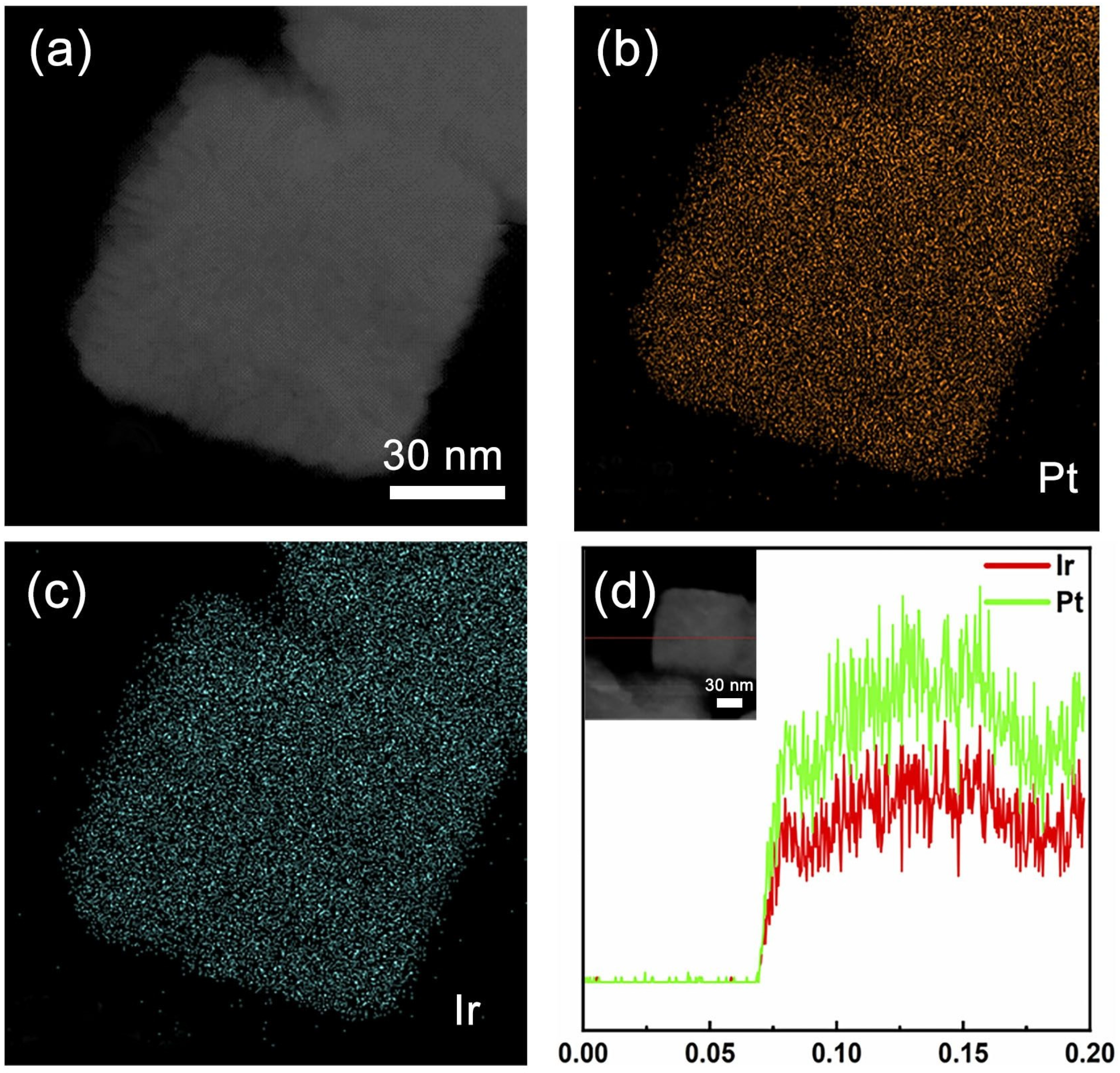
To study the elemental distribution of the PtIr nanocubes, high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) analysis was conducted. As shown in Figure 3a–c, the EDS mapping images reveal that Pt and Ir fractions were evenly distributed throughout the PtIr nanocubes. A line-profile analysis was conducted to determine the structure and elemental distribution of a single PtIr nanocube (Figure 3d), which further confirmed the uniform distribution of Pt and Ir throughout the nanocubes.
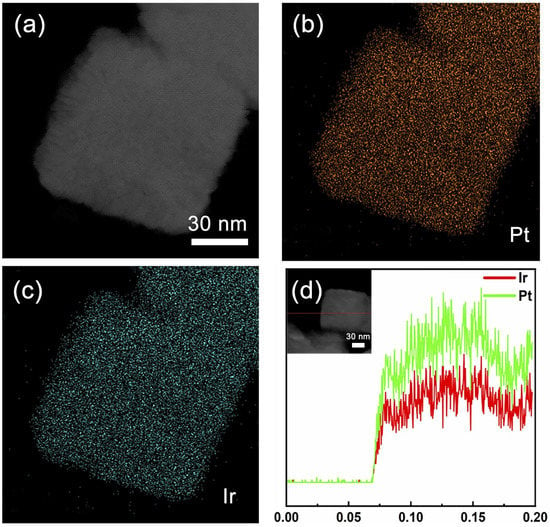
Figure 3.
(a) STEM image of Pt–Ir nanocubes. Elemental mapping images of (b) Pt and (c) Ir of PtIr nanocubes. (d) A line scan image of EDS for PtIr nanocubes.
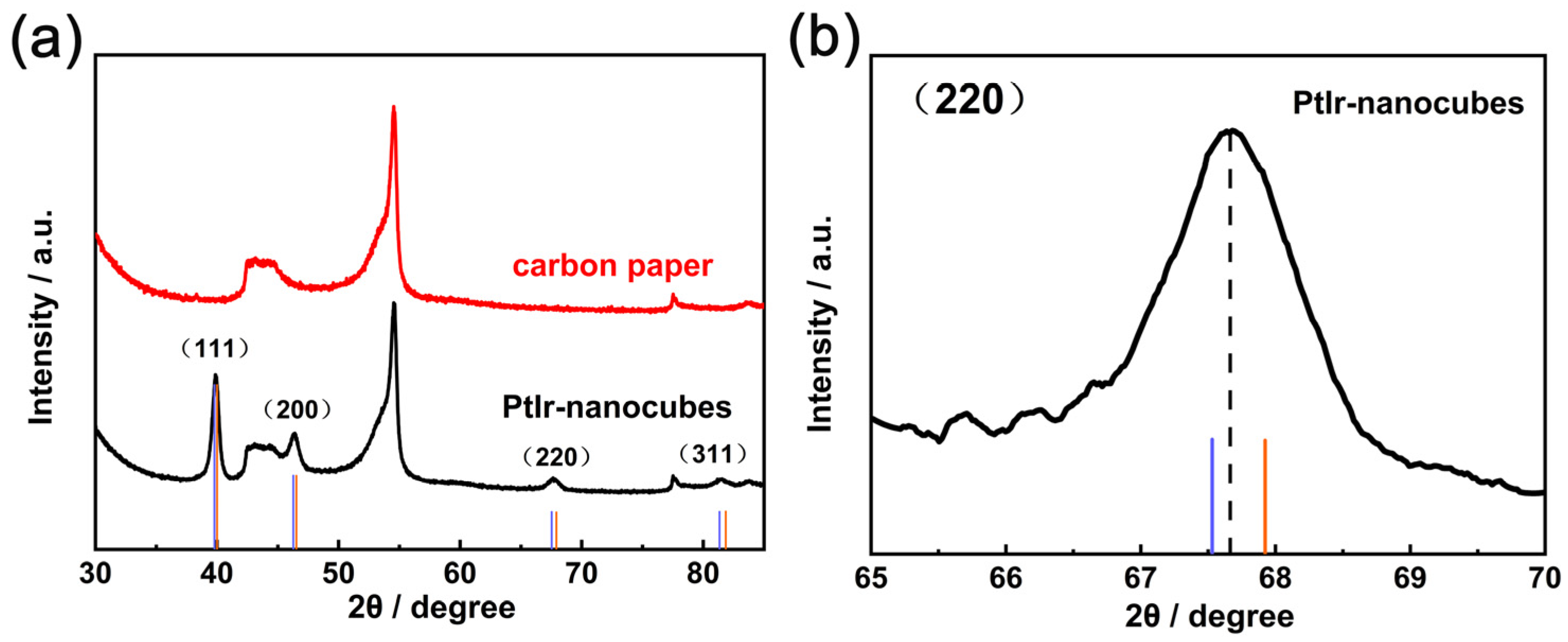
To further characterize their crystal structure, the XRD pattern of the PtIr nanocubes was measured. Figure 4a displays the characteristic diffraction peaks of the PtIr nanocubes at 39.3°, 46.4°, 67.7°, and 81.5°, which correspond to (111), (200), (220), and (311) of PtIr with a face-centered cubic (FFC) structure, respectively. The above results are in agreement with those of pure Pt (JCPDS 04-0802) since the lattice parameters of both Pt and Ir are very close and all possess an FCC structure. Notably, the enlarged diffraction peaks of the PtIr nanocubes in Figure 4b are shifted to the position between Pt and Ir (JCPDS 06–0598), indicating the alloying of Pt with Ir.
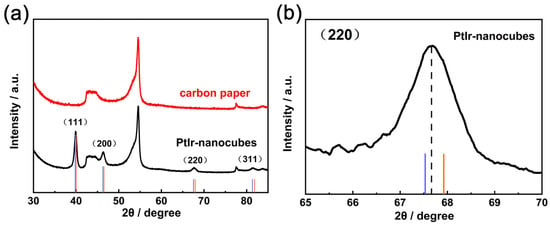
Figure 4.
(a) XRD spectra of PtIr nanocubes and (b) the corresponding partial enlargement. The standard values of Pt (blue line) and Ir (orange line).
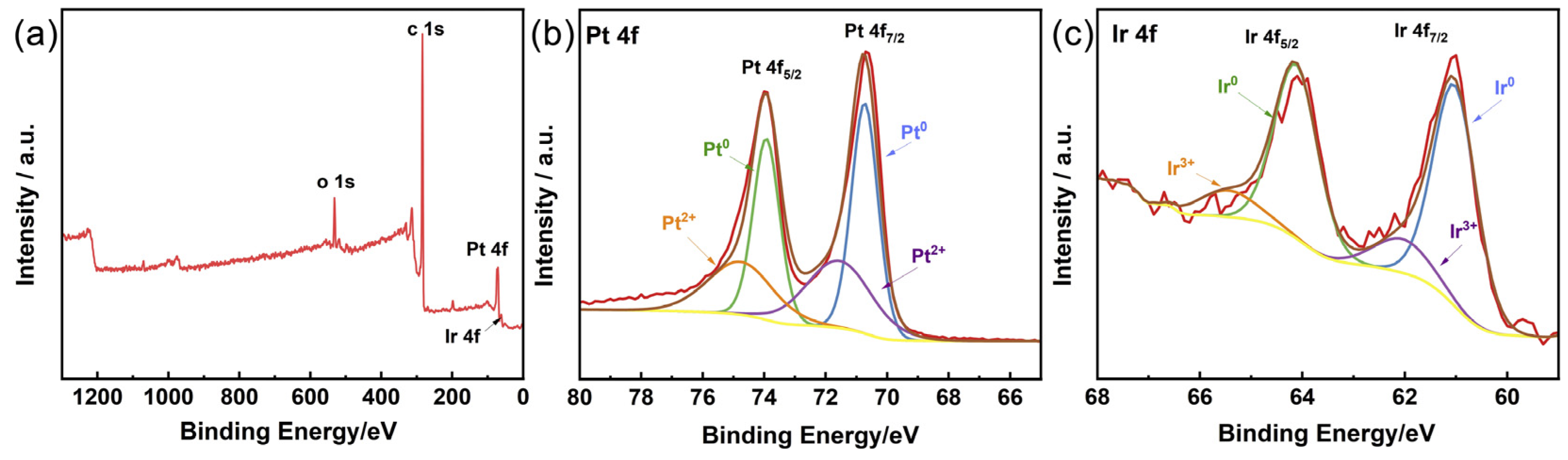
The chemical states of Pt and Ir in the PtIr nanocubes were determined using XPS. Figure 5a shows the XPS full spectrum of the PtIr nanocubes. The two peaks appearing at 70.6 and 74.0 eV correspond to Pt 4f7/2 and Pt 4f5/2, respectively (Figure 5b). The Pt 4f spectra can be deconvoluted into two sets of doublet peaks. The deconvoluted peaks at 70.6 and 74.0 eV correspond to Pt0, while the other peaks at 71.6 and 74.9 eV were all attributed to Pt2+. Regarding the Ir XPS spectrum, distinct peaks at 61.1 and 64.2 eV for Ir 4f7/2 and Ir 4f5/2 were observed, respectively (Figure 5c). Two sets of deconvoluted doublet peaks in the Ir 4f spectra correspond to the metallic Ir0 (at 61.1 eV for Ir 4f7/2 and at 64.2 eV for Ir 4f5/2) and Ir3+ (at 62.2 eV for Ir 4f7/2 and at 65.5 eV for Ir 4f5/2). Notably, compared with pure Pt (71.2 and 74.5 eV) and pure Ir (60.9 and 63.9 eV) [35], the binding energies of Pt 4f7/2 and Pt 4f5/2 display a slight negative shift, while a slight positive shift was observed for Ir 4f7/2 and Ir 4f5/2. The changes in binding energy can be ascribed to the strong electronic interaction between Pt and Ir [36], further suggesting the formation of a PtIr alloy.
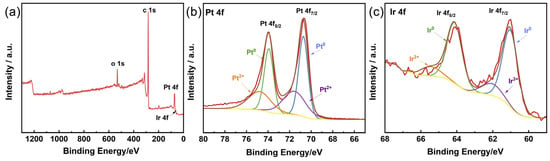
Figure 5.
(a) XPS full spectrum of PtIr nanocubes and the corresponding XPS spectra of (b) Pt 4f and (c) Ir 4f.
Clearly, the above results confirmed that PtIr alloy nanocubes with (100) preferential facets were successfully synthesized using a facile electrochemical PSWP method without the addition of organic additives. During PSWP electrodeposition, the selective electro-dissolution of the metals and the selective electrodeposition of precursor metal ions take place in the anodic and cathodic half-cycles, respectively, accompanied by electro-adsorption/desorption of O-containing species (e.g., OH) and hydrogen adatoms [11,37]. These processes will contribute to the formation of PtIr alloy nanocubes. As shown in Figure 1(d1,d2), PtIr alloy nanocubes are formed at an EL of −0.7 V (vs SCE). A strong hydrogen evolution reaction takes place at this potential; thus, numerous hydrogen adatoms were easily electrosorbed on the exposed facets of the PtIr nanoparticles. It has been found that the occurrence of hydrogen adsorption will result in a lower surface free energy of Pt (100) than that of Pt (111). As a result, PtIr (100) also has a lower surface free energy than PtIr (111) when hydrogen adsorption has taken place, thereby promoting the formation of more PtIr (100) sites. As EL shifts positively from −0.7 to −0.4 V (vs SCE), the hydrogen evolution reaction gradually weakens, resulting in a decrease in the number of hydrogen adatoms adsorbed on the PtIr nanoparticles. As a result, the shape of the PtIr nanoparticles, which is associated with the arrangement of exposed surface atoms, gradually evolves from nanocubes to spiky nanocubes, nanospheres with a rough surface, and smooth nanospheres (Figure 1). This indicates that hydrogen adsorption plays a crucial role in the formation of the PtIr nanocubes with preferential (100) crystal facets. Notably, a previous study conducted by our group [11] showed that hydrogen adsorption alone cannot generate cubic-shaped Pt nanoparticles in the absence of the adsorption/desorption of OH species. Hence, the selective adsorption/desorption of OH species is necessary for the formation of the PtIr nanocubes. In this work, the upper potential limit of EU is 0.5 V (vs. SCE), which is in the range where the electrosorption of OH species occurs. The selective dissolution of the PtIr metal, resulting from the periodic electroadsorption/electrodesorption of OH species, will contribute to the rearrangement of the surfaces of Pt and Ir atoms. The coupling of the periodic adsorption/desorption of OH species and hydrogen adatoms promotes the development of (100) active sites and thus the formation of PtIr nanocubes with preferential (100) facets.
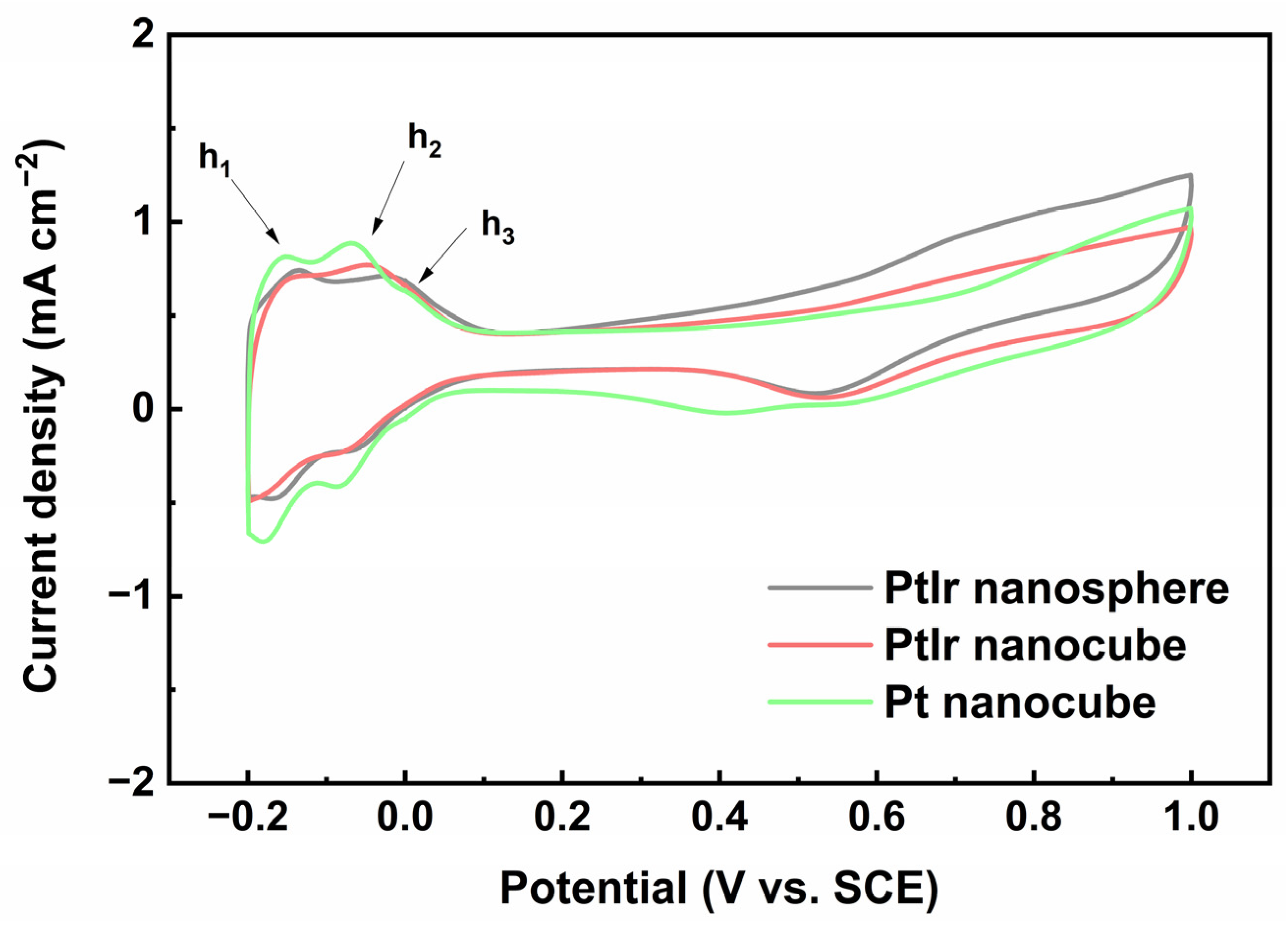
To investigate the electrochemical behavior of the obtained PtIr nanocubes, CV tests of the electrodes were conducted. Figure 6 shows the CV profiles of the obtained nanoparticles recorded in an N2-saturated 0.5 M H2SO4 solution. The obtained pure Pt nanocubes (Figure S1, Support Information) were used for comparative purposes. All the CV curves display typical potential regions: hydrogen adsorption/desorption (–0.20 V~0.08 V (vs. SCE)), an electric double layer (0.08 V~0.31 V (vs. SCE)), and the formation/reduction of Pt/Ir oxide (0.31 V~1.00 V (vs. SCE)). Notably, three main anodic peaks of h1, h2, and h3 are observed at about –0.15, –0.06, and 0.02 V (vs. SCE) for the Pt nanocubes and PtIr nanocubes in the forward scan, corresponding to the hydrogen desorption of (110) sites, (100) step sites, and (100) terrace sites, respectively [38]. By contrast, only h1 and h2 anodic peaks appear for the PtIr nanospheres. The anodic peak of h1 results from the desorption of weakly bonded hydrogen species, while the anodic peaks of h2 and h3 originate from strongly bonded hydrogen species [39]. Regarding the Pt nanocubes, the anodic peak of h2 is distinctly higher than that of h1, which is a typical feature of Pt with preferential (100) facets [11]. Moreover, the appearance of the h3 peak further proves its preferential Pt (100) crystal orientation. The PtIr nanocubes also display a significantly higher h2 peak than h1 as well as an h3 peak corresponding to (100) terrace sites, which is similar to Pt nanocubes. This indicates once again that the obtained PtIr nanocubes possess a preferential (100) crystal orientation. This is consistent with the HRTEM results (Figure 2c). Moreover, the CV profile of the PtIr nanospheres is similar to that of polycrystalline PtIr nanoparticles reported in the literature [26], indicating the typical polycrystalline feature of PtIr nanospheres. Since hydrogen desorption is very structure-sensitive to Pt, the CV profiles can be used to qualitatively estimate the number of active sites on the Pt surface. The electrochemically active surface area (ECSA) is estimated from the charge of the hydrogen desorption peak in the forward CV scan, assuming a charge density of 210 C cm−2 for one monolayer of hydrogen desorption on Pt [40].
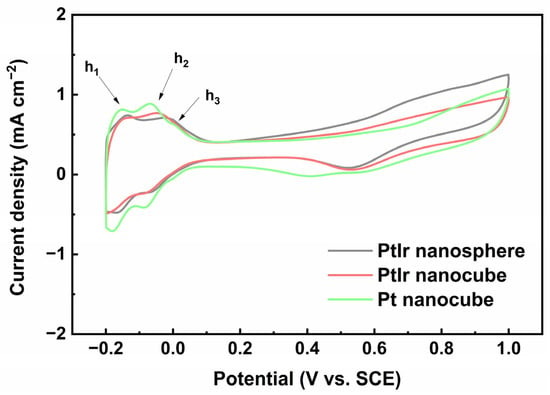
Figure 6.
CVs of PtIr nanosphere, PtIr nanocube, and Pt nanocube recorded in 0.5 M H2SO4 solution at 0.05 V s−1.
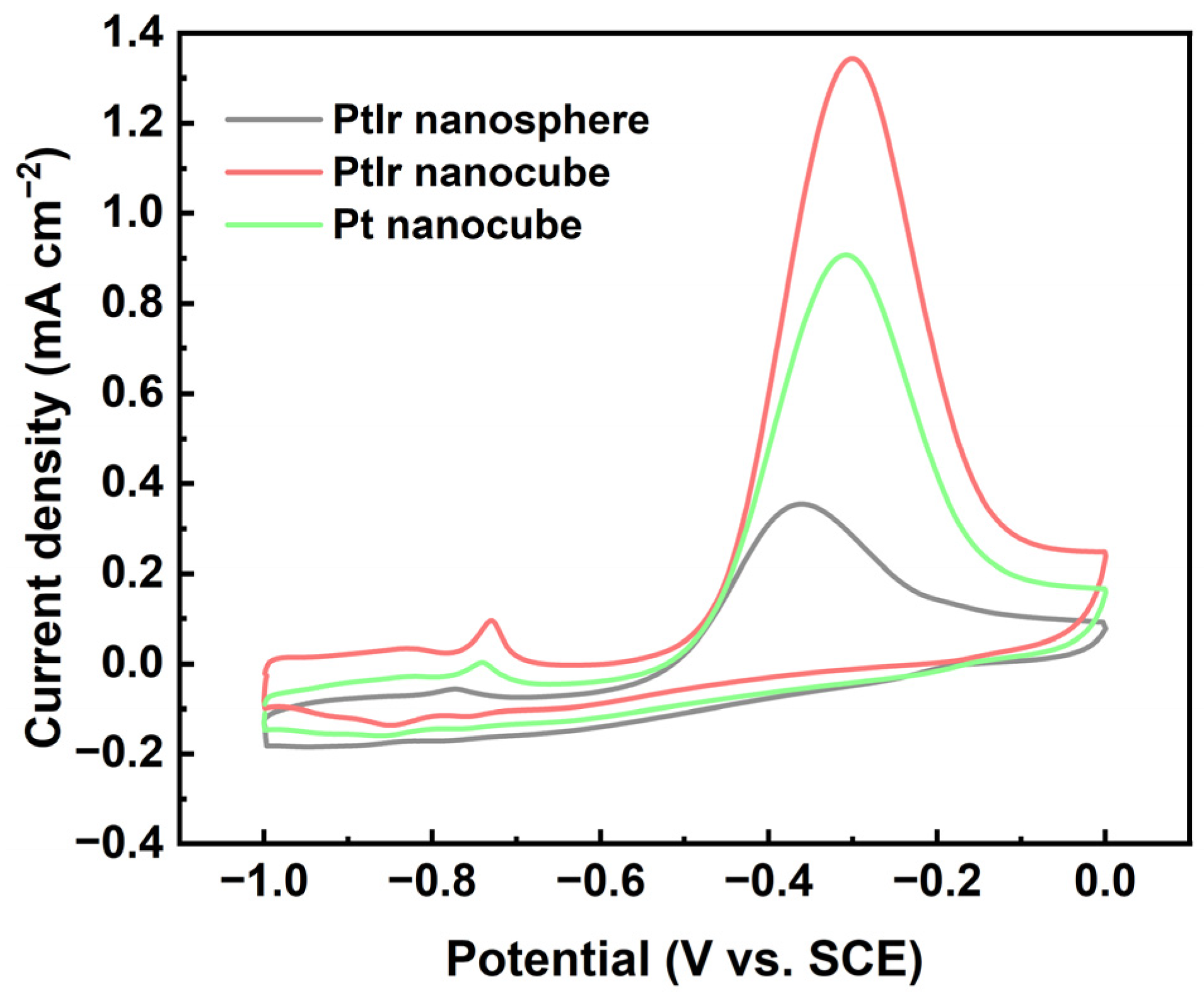
To evaluate the electrocatalytic properties of the PtIr nanoparticles, a typical AOR in alkaline media was initiated. Figure 7 shows the CVs of the obtained PtIr nanospheres, PtIr nanocubes, and Pt nanocubes with respect to the AOR. The current density values were normalized via ECSA to reveal the intrinsic activity (specific activity) of the obtained nanoparticles. Prominent anodic peaks appear at around −0.3 V (SCE), which are related to the ammonia oxidation of N2 and N adatoms. Clearly, the PtIr nanocubes exhibit an outstanding SA value of 1.34 mA cm−2, which is 3.8 times higher than that of the PtIr nanospheres (0.35 mA cm−2) and 1.5 times higher than that of the Pt nanocubes (0.90 mA cm−2), indicating the strong effects of the shape and composition of the Pt nanocubes on the SA towards AOR. SA is usually affected by many factors, including preferential crystal orientation, surface morphology, chemical composition, and preparation methods. The generally accepted mechanism of the AOR on Pt is that the adsorbed NH3 is dehydrogenated to the adsorbed NHx (x = 1, 2) intermediates and N adatoms [41]. Partially dehydrogenated NH2, as the active intermediate, can promote the formation of N2H4, which is further dehydrogenated into N2, but completely dehydrogenated N adatoms will induce the toxification of the Pt surface [42]. The corresponding reaction equations are given below [42]:
NH3 (aq) → NH3, ads
NH3, ads + OH− → NH2, ads+ H2O + e−
NH2, ads + OH− → NHads+ H2O + e−
NHx, ads + NHy, ads→ N2Hx + y, ads
N2Hx + y, ads + (x + y)OH− → N2 + (x + y)H2O + (x + y)e−
NHads + OH− → Nads+ H2O + e−
(x = 1 or 2 and y =1 or 2)
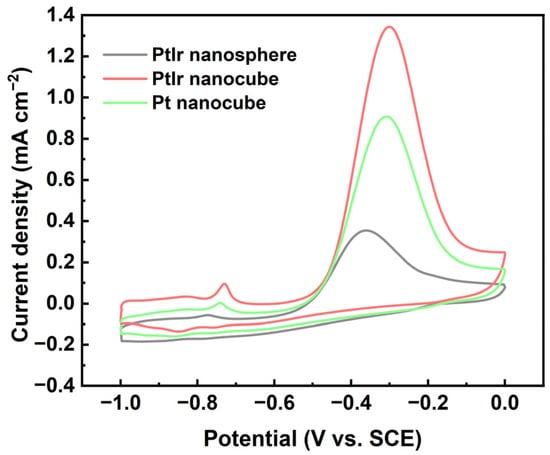
Figure 7.
CVs of PtIr nanosphere, PtIr nanocube, and Pt nanocube nanoparticles tested in 1 M KOH + 0.05 M (NH4)2SO4 aqueous solution at 10 mV s−1.
Both theoretically and experimentally, the studies have shown that ammonia electro-oxidation takes place almost exclusively on Pt (100) sites [43,44]. This is attributed to the fact that Pt (100) can stabilize the adsorbed active intermediate of NH2, thereby promoting the formation of N2H4 and then further dehydrogenation to N2 [45,46], whereas Pt (111) is easily toxified, which is related to the deeper dehydrogenation of NH3 on Pt (111) to strongly adsorbed N adatoms [45]. Therefore, the obtained Pt nanocubes and PtIr nanocubes all exhibited higher SA than the polycrystalline PtIr nanospheres in the present work. In addition, chemical composition also has a great influence on SA. A previous study [47] found that compared with pure Pt nanoparticles, the introduction of Ir into Pt can only enhance SA at a low oxidation potential, while an important loss of maximum activity was also observed. This loss of activity may be attributed to the reduction of Pt (100) sites via the introduction of Ir. This also explains the result in the present work in which the SA of the Pt nanocubes is much larger than that of the polycrystalline PtIr nanospheres (Figure 7). Notably, the PtIr nanocubes displayed higher SA than the Pt nanocubes in the present work. The enhanced SA of the PtIr nanocubes can be ascribed to the synergic effects of multiple factors, including the well-preserved (100) sites of the PtIr nanocubes (Figure 2c), the dehydrogenation ability of Ir toward ammonia molecules, the electronic effects of Pt and Ir, and the clean surfaces of the catalyst due to the use of a “green” synthesis method.
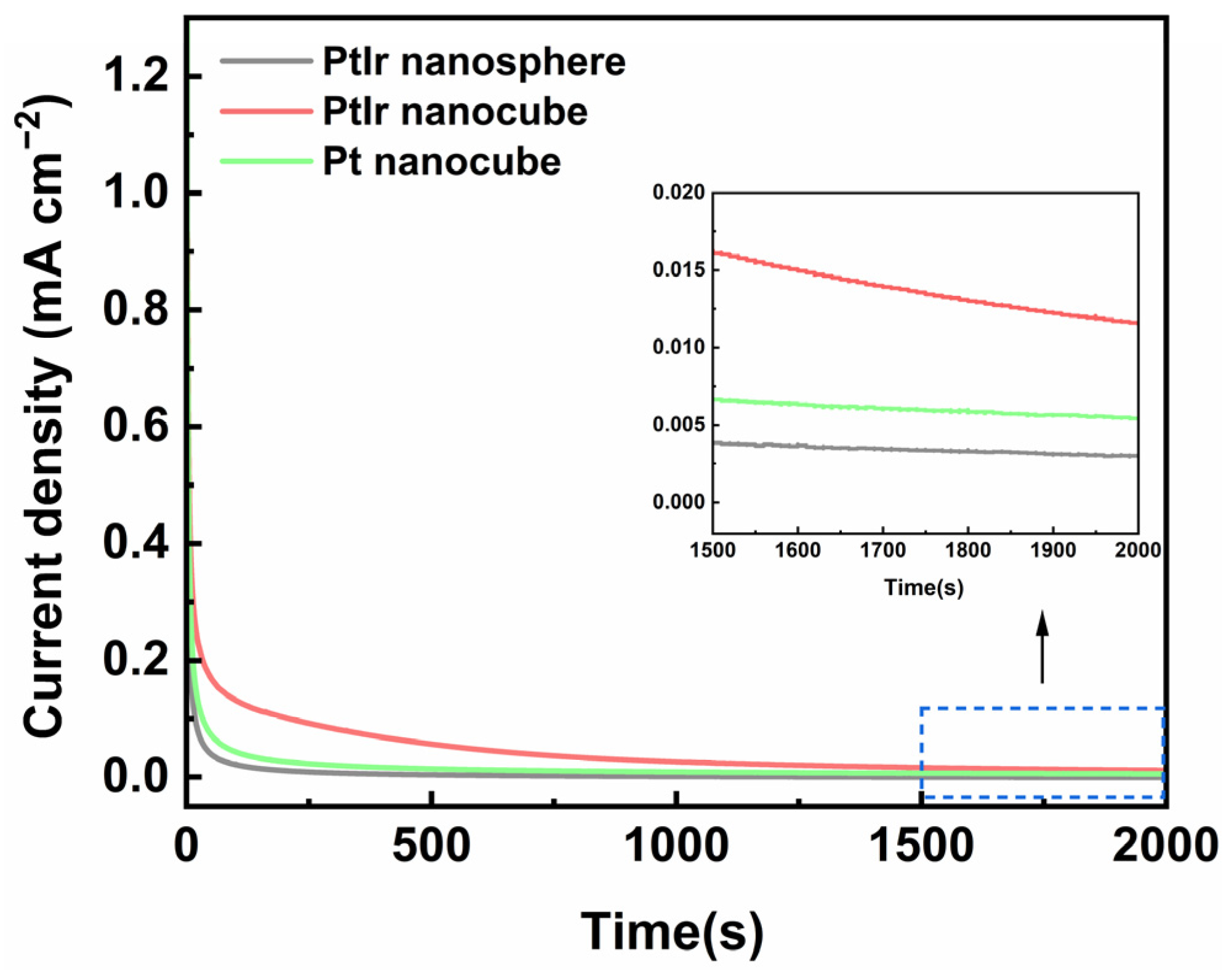
To assess the stability of the obtained PtIr nanocubes towards the AOR, a chronoamperometric test (CA) was performed at −0.36 V (vs. SCE) for 2000 s. Figure 8 shows a typical set of CA curves for these catalysts. The current densities of the obtained PtIr nanospheres, PtIr nanocubes, and Pt nanocubes all drop sharply at the initial stage, which is related to electric double-layer charging [48]. Afterwards, the current density decays slowly to a steady-state, which is associated with the consumption of the electroactive species near the electrode surface [48] and the toxification of N adatoms on the surface of the catalysts [49]. At 2000 s, the steady-state current densityvalues of the PtIr nanocubes are about 0.012 mA cm−2, which are 4 and 2.3 times higher than those of the PtIr nanospheres (0.003 mA cm−2) and Pt nanocubes (0.005 mA cm−2), respectively. This indicates that the obtained PtIr nanocube catalyst possesses high stability towards AOR in an alkaline medium. The (100) sites of the PtIr nanocubes can stabilize the adsorbed active intermediate of NH2, favoring the formation of the final product, N2 [45,46], and thus alleviating the deactivation of the Pt catalyst. On the one hand, introducing Ir into Pt will enhance the capacity of the NHx intermediates to recombine and thus be further oxidized into N2. Hence, the high stability towards the AOR for the PtIr nanocube catalyst can be ascribed to the synergy of (100) sites and the introduced Ir for the PtIr nanocubes [50].
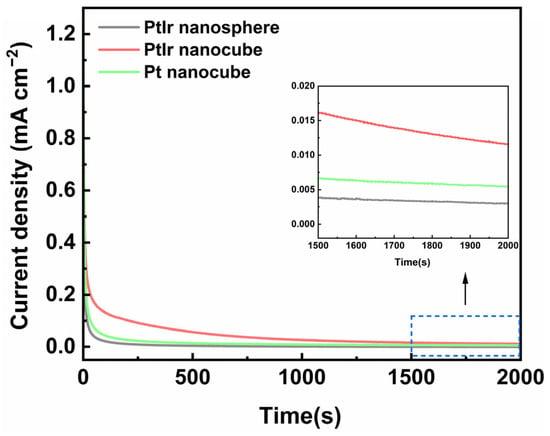
Figure 8.
CA curves of PtIr nanospheres, PtIr nanocubes, and Pt nanocubes tested in 1 M KOH + 0.05 M (NH4)2SO4 aqueous solution.
4. Conclusions
In conclusion, PtIr nanocube catalysts with preferential (100) facets were successfully in situ synthesized on a carbon paper surface via a facile and surfactant-free electrochemical PSWP method. No organic additives were used at any stage in the entire catalyst synthesis process. The formation of PtIr nanocubes is related to the synergy of the electro-adsorption/desorption of O-containing species and the preferential adsorption of hydrogen adatoms on PtIr (100) with a lower surface free energy. The obtained PtIr nanocubes exhibit an outstanding SA value of 1.34 mA cm−2 towards the AOR, which is 3.8 times higher than that of the PtIr nanospheres (0.35 mA cm−2) and 1.5 times higher than that of the Pt nanocubes (0.90 mA cm−2). The enhanced SA of the PtIr nanocubes can be ascribed to the synergic effects of multiple factors, including the well-preserved (100) sites of the PtIr nanocubes, the dehydrogenation ability of Ir toward ammonia molecules, the electronic effects of Pt and Ir, and the clean surfaces of the catalysts due to the use of a “green” synthesis method. In addition, the PtIr nanocube catalyst displays high stability towards AOR in an alkaline medium, which can be related to the synergy of (100) sites and the introduced Ir for the PtIr nanocubes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/met13050901/s1. Figure S1: SEM image of Pt nanocubes.
Author Contributions
Conceptualization, J.L. and Y.T.; methodology, Y.T., D.G. and J.Z.; investigation, L.H. and Y.T.; data curation, X.H., Z.C. and Y.T.; writing—original draft preparation, J.L., Y.T. and D.X.; writing—review and editing, J.L. and D.X.; supervision, J.L.; project administration, J.L. and D.X.; funding acquisition, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 52271224 and 51801134) and the Tianjin Natural Science Foundation (20JCQNJC01130).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Y.; Pillai, H.S.; Wang, T.; Hwang, S.; Zhao, Y.; Qiao, Z.; Mu, Q.; Karakalos, S.; Chen, M.; Yang, J.; et al. High-performance ammonia oxidation catalysts for anion-exchange membrane direct ammonia fuel cells. Energy Environ. Sci. 2021, 14, 1449–1460. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Z.; Esan, O.C.; Xu, X.; An, L. Performance Characteristics of a Direct Ammonia Fuel Cell with an Anion Exchange Membrane. Energy Fuels 2022, 36, 13203–13211. [Google Scholar] [CrossRef]
- Lucentini, I.; Garcia, X.; Vendrell, X.; Llorca, J. Review of the Decomposition of Ammonia to Generate Hydrogen. Ind. Eng. Chem. Res. 2021, 60, 18560–18611. [Google Scholar] [CrossRef]
- Mandal, P.; Yadav, M.K.; Gupta, A.K.; Dubey, B.K. Chlorine mediated indirect electro-oxidation of ammonia using non-active PbO2 anode: Influencing parameters and mechanism identification. Sep. Purif. Technol. 2020, 247, 116910. [Google Scholar] [CrossRef]
- de Mishima, B.A.L.; Lescano, D.; Holgado, T.M.; Mishima, H.T. Electrochemical oxidation of ammonia in alkaline solutions: Its application to an amperometric sensor. Electrochim. Acta 1998, 43, 395–404. [Google Scholar] [CrossRef]
- Kim, H.; Hong, S.; Kim, H.; Jun, Y.; Kim, S.Y.; Ahn, S.H. Recent progress in Pt-based electrocatalysts for ammonia oxidation reaction. Appl. Mater. Today 2022, 29, 101640. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.; Zhang, X.; Ye, J.; Xue, F.; Zhen, C.; Liao, X.; Li, H.; Li, P.; Liu, M.; et al. Quatermetallic Pt-based ultrathin nanowires intensified by Rh enable highly active and robust electrocatalysts for methanol oxidation. Nano Energy 2020, 71, 104623. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Zhang, X.; Rao, S.; Li, J.; Wang, W.; Sun, Z.; Yang, J. Surface Structure Engineering of PtPd Nanoparticles for Boosting Ammonia Oxidation Electrocatalysis. Acs Appl. Mater. Interfaces 2022, 14, 28816–28825. [Google Scholar] [CrossRef]
- Wallace, S.W.; McCrum, I.T.; Janik, M.J. Ammonia electro-oxidation mechanism on the platinum (100) surface. Catal. Today 2021, 371, 50–57. [Google Scholar] [CrossRef]
- Chan, Y.T.; Siddharth, K.; Shao, M. Investigation of cubic Pt alloys for ammonia oxidation reaction. Nano Res. 2020, 13, 1920–1927. [Google Scholar] [CrossRef]
- Liu, J.; Fan, X.; Liu, X.; Song, Z.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Synthesis of Cubic-Shaped Pt Particles with (100) Preferential Orientation by a Quick, One-Step and Clean Electrochemical Method. Acs Appl. Mater. Interfaces 2017, 9, 18856–18864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hwang, S.Y.; Peng, Z. Shape-enhanced ammonia electro-oxidation property of a cubic platinum nanocrystal catalyst prepared by surfactant-free synthesis. J. Mater. Chem. A 2013, 1, 14402–14408. [Google Scholar] [CrossRef]
- Fu, G.-T.; Liu, C.; Wu, R.; Chen, Y.; Zhu, X.-S.; Sun, D.-M.; Tang, Y.-W.; Lu, T.-H. l-Lysine mediated synthesis of platinum nanocuboids and their electrocatalytic activity towards ammonia oxidation. J. Mater. Chem. A 2014, 2, 17883–17888. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, R.A.; Vidal-Iglesias, F.J.; Solla-Gullón, J.; Cabrera, C.R.; Feliu, J.M. Synthesis and Electrocatalytic Properties of H2SO4-Induced (100) Pt Nanoparticles Prepared in Water-in-Oil Microemulsion. ChemPhysChem 2014, 15, 1997–2001. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Zhang, N.; Guo, S.; Zhang, X.; Li, J.; Yao, J.; Wu, T.; Lu, G.; Ma, J.-Y.; Su, D.; et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 2016, 354, 1410–1414. Available online: https://www.science.org/doi/10.1126/science.aah6133 (accessed on 18 April 2023). [CrossRef] [PubMed]
- Liu, C.-W.; Wei, Y.-C.; Wang, K.-W. Preparation and characterization of carbon-supported Pt-Au cathode catalysts for oxygen reduction reaction. J. Colloid Interface Sci. 2009, 336, 654–657. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Gasteiger, H.A.; Behm, R.J. Methanol electrooxidation on a colloidal PtRu-alloy fuel-cell catalyst. Electrochem. Commun. 1999, 1, 1–4. [Google Scholar] [CrossRef]
- Kugai, J.; Moriya, T.; Seino, S.; Nakagawa, T.; Ohkubo, Y.; Nitani, H.; Daimon, H.; Yamamoto, T.A. CeO2-supported Pt-Cu alloy nanoparticles synthesized by radiolytic process for highly selective CO oxidation. Int. J. Hydrogen Energy 2012, 37, 4787–4797. [Google Scholar] [CrossRef]
- Cui, C.; Gan, L.; Li, H.-H.; Yu, S.-H.; Heggen, M.; Strasser, P. Octahedral PtNi Nanoparticle Catalysts: Exceptional Oxygen Reduction Activity by Tuning the Alloy Particle Surface Composition. Nano Lett. 2012, 12, 5885–5889. [Google Scholar] [CrossRef]
- Xia, B.Y.; Wu, H.B.; Li, N.; Yan, Y.; Lou, X.W.; Wang, X. One-Pot Synthesis of Pt–Co Alloy Nanowire Assemblies with Tunable Composition and Enhanced Electrocatalytic Properties. Angew. Chem. Int. Ed. 2015, 54, 3797–3801. [Google Scholar] [CrossRef]
- Hong, J.W.; Kang, S.W.; Choi, B.-S.; Kim, D.; Lee, S.B.; Han, S.W. Controlled Synthesis of Pd–Pt Alloy Hollow Nanostructures with Enhanced Catalytic Activities for Oxygen Reduction. ACS Nano 2012, 6, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-W.; Zhang, H.; Li, M. Potentiostatic Electrodeposition of Pt-Fe Alloy Catalyst and Application in PEMFC Cathode. J. Inorg. Mater. 2013, 28, 1217–1222. Available online: http://www.jim.org.cn/en/10.3724/sp.j.1077.2013.13118 (accessed on 18 April 2023). [CrossRef]
- Zhang, T.; Li, S.-C.; Zhu, W.; Zhang, Z.-P.; Gu, J.; Zhang, Y.-W. Shape-tunable Pt–Ir alloy nanocatalysts with high performance in oxygen electrode reactions. Nanoscale 2017, 9, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Won, D.; Nahm, K.S.; Kim, P. Facile Preparation of Hollow Pt- and PtIr-Nanostructures with Spiky Surface for the Electro-Oxidation of Ammonia. Catal. Lett. 2014, 144, 469–477. [Google Scholar] [CrossRef]
- Lomocso, T.L.; Baranova, E.A. Electrochemical oxidation of ammonia on carbon-supported bi-metallic PtM (M = Ir, Pd, SnOx) nanoparticles. Electrochim. Acta 2011, 56, 8551–8558. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, J.; Ni, Z.; Deng, Y.; Chen, B.; Hu, W. Shape-controlled synthesis of Pt-Ir nanocubes with preferential (100) orientation and their unusual enhanced electrocatalytic activities. Sci. China-Mater. 2014, 57, 13–25. [Google Scholar] [CrossRef]
- Allagui, A.; Oudah, M.; Tuaev, X.; Ntais, S.; Almomani, F.; Baranova, E.A. Ammonia electro-oxidation on alloyed PtIr nanoparticles of well-defined size. Int. J. Hydrogen Energy 2013, 38, 2455–2463. [Google Scholar] [CrossRef]
- Li, D.; Wang, L.; Zhang, P.; Chen, S.; Xu, J. Effects of the composition on the active carbon supported Pd-Pt bimetallic catalysts for HI decomposition in the iodine-sulfur cycle. Int. J. Hydrogen Energy 2013, 38, 6586–6592. [Google Scholar] [CrossRef]
- Hanifah, M.F.R.; Jaafar, J.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Yusof, N.; Salleh, W.N.W.; Aziz, F.; Ajid, A.Z.A. Advanced ternary RGO/bimetallic Pt-Pd alloy/CeO2 nanocomposite electrocatalyst by one-step hydrothermal-assisted formic acid reduction reaction for methanol electrooxidation. J. Environ. Chem. Eng. 2021, 9, 104991. [Google Scholar] [CrossRef]
- He, Q.; Mukerjee, S. Electrocatalysis of oxygen reduction on carbon-supported PtCo catalysts prepared by water-in-oil micro-emulsion. Electrochim. Acta 2010, 55, 1709–1719. [Google Scholar] [CrossRef]
- Barbosa, J.R.; Leon, M.N.; Fernandes, C.M.; Antoniassi, R.M.; Alves, O.C.; Ponzio, E.A.; Silva, J.C.M. PtSnO2/C and Pt/C with preferential (100) orientation: High active electrocatalysts for ammonia electro-oxidation reaction. Appl. Catal. B-Environ. 2020, 264, 118458. [Google Scholar] [CrossRef]
- Chin, T.-K.; Liao, M.-W.; Perng, T.-P. Enabling higher electrochemical activity of Pt nanoparticles uniformly coated on cubic titanium oxynitride by vertical forced-flow atomic layer deposition. J. Power Sources 2019, 434, 226716. [Google Scholar] [CrossRef]
- Le Vot, S.; Roue, L.; Belanger, D. Electrodeposition of iridium onto glassy carbon and platinum electrodes. Electrochim. Acta 2012, 59, 49–56. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, G.; Yu, M.; Xu, J.; Qiao, S.; Cheng, X.; Yang, F. High Mass and Specific Activity for Ammonia Electro-oxidation through Optimization of Dispersion Degree and Particle Size of Pt-Ir Nanoparticles over N-Doped Reductive Graphene Oxide. Chemistryselect 2018, 3, 3433–3443. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer, Physical Electronics Division: Eden Prairie, MN, USA, 1992; pp. 178–181. [Google Scholar]
- Chen, W.; Chen, S. Iridium-platinum alloy nanoparticles: Composition-dependent electrocatalytic activity for formic acid oxidation. J. Mater. Chem. 2011, 21, 9169–9178. [Google Scholar] [CrossRef]
- Dai, H.; Dong, K.; Zhang, T.; Pu, H.; Wang, Y.; Deng, Y. Electrodeposition of shaped PtIr alloy nanocrystals with high–index facets for the electro–catalytic oxidation of alcohols. Appl. Surf. Sci. 2023, 609, 155225. [Google Scholar] [CrossRef]
- Ponrouch, A.; Garbarino, S.; Bertin, E.; Andrei, C.; Botton, G.A.; Guay, D. Highly Porous and Preferentially Oriented {100} Platinum Nanowires and Thin Films. Adv. Funct. Mater. 2012, 22, 4172–4181. [Google Scholar] [CrossRef]
- Shibata, S.; Sumino, M.P. The electrochemical Peltier heat for the adsorption and desorption of hydrogen on a platinized platinum electrode in sulfuric acid solution. J. Electroanalytical. Chem. Interfacial Electrochem. 1985, 193, 135–143. [Google Scholar] [CrossRef]
- Antolini, E.; Giorgi, L.; Pozio, A.; Passalacqua, E. Influence of Nafion loading in the catalyst layer of gas-diffusion electrodes for PEFC. J. Power Sources 1999, 77, 136–142. [Google Scholar] [CrossRef]
- Liu, J.; Hu, W.; Zhong, C.; Cheng, Y.F. Surfactant-free electrochemical synthesis of hierarchical platinum particle electrocatalysts for oxidation of ammonia. J. Power Sources 2013, 223, 165–174. [Google Scholar] [CrossRef]
- Gerischer, H.; Mauerer, A. Untersuchungen Zur anodischen Oxidation von Ammoniak an Platin-Elektroden. J. Electroanal. Chem. Interfacial Electrochem. 1970, 25, 421–433. [Google Scholar] [CrossRef]
- Le Vot, S.; Roué, L.; Bélanger, D. Study of the electrochemical oxidation of ammonia on platinum in alkaline solution: Effect of electrodeposition potential on the activity of platinum. J. Electroanal. Chem. 2013, 691, 18–27. [Google Scholar] [CrossRef]
- Novell-Leruth, G.; Valcárcel, A.; Clotet, A.; Ricart, J.M.; Pérez-Ramírez, J. DFT Characterization of Adsorbed NHx Species on Pt(100) and Pt(111) Surfaces. J. Phys. Chem. B 2005, 109, 18061–18069. [Google Scholar] [CrossRef] [PubMed]
- Rosca, V.; Koper, M.T.M. Electrocatalytic oxidation of ammonia on Pt(111) and Pt(100) surfaces. Phys. Chem. Chem. Phys. 2006, 8, 2513–2524. [Google Scholar] [CrossRef]
- Rosca, V.; Koper, M.T.M. Electrocatalytic oxidation of hydrazine on platinum electrodes in alkaline solutions. Electrochim. Acta 2008, 53, 5199–5205. [Google Scholar] [CrossRef]
- Vidal-Iglesias, F.J.; Solla-Gullón, J.; Montiel, V.; Feliu, J.M.; Aldaz, A. Screening of electrocatalysts for direct ammonia fuel cell: Ammonia oxidation on PtMe (Me: Ir, Rh, Pd, Ru) and preferentially oriented Pt(100) nanoparticles. J. Power Sources 2007, 171, 448–456. [Google Scholar] [CrossRef]
- Liu, J.; Chen, B.; Kou, Y.; Liu, Z.; Chen, X.; Li, Y.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Pt-Decorated highly porous flower-like Ni particles with high mass activity for ammonia electro-oxidation. J. Mater. Chem. A 2016, 4, 11060–11068. [Google Scholar] [CrossRef]
- de Vooys, A.C.A.; Koper, M.T.M.; van Santen, R.A.; van Veen, J.A.R. The role of adsorbates in the electrochemical oxidation of ammonia on noble and transition metal electrodes. J. Electroanal. Chem. 2001, 506, 127–137. [Google Scholar] [CrossRef]
- Le Vot, S.; Roué, L.; Bélanger, D. Synthesis of Pt–Ir catalysts by coelectrodeposition: Application to ammonia electrooxidation in alkaline media. J. Power Sources 2013, 223, 221–231. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).