Carbothermic Reduction of Ilmenite Concentrate with Sodium Carbonate Additive to Produce Iron Granules and High Titania Containing Slag
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
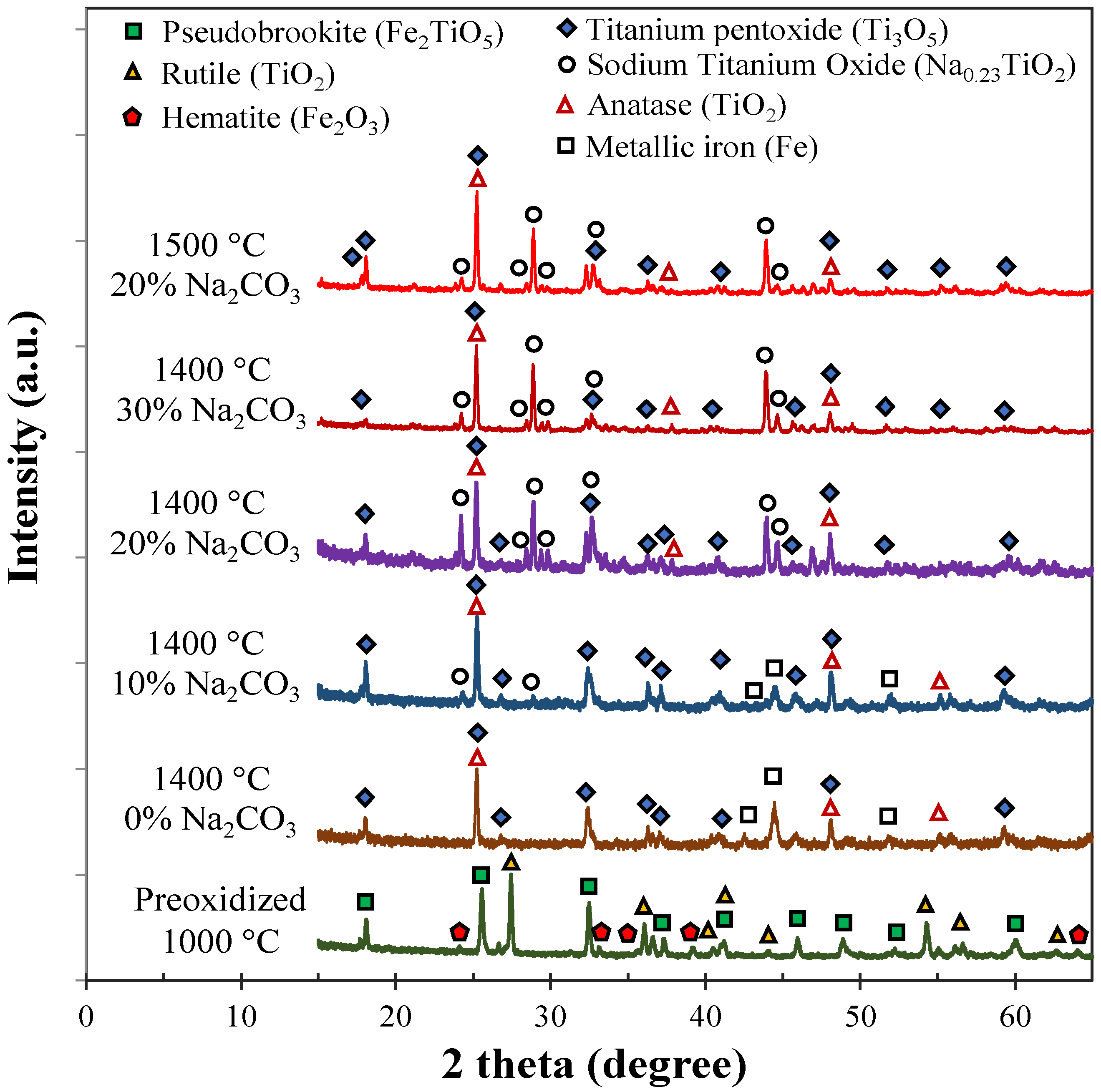
3.1. Pre-Oxidation of Ilmenite Concentrate
3.2. Effect of Final Temperature and Sodium Carbonate Addition
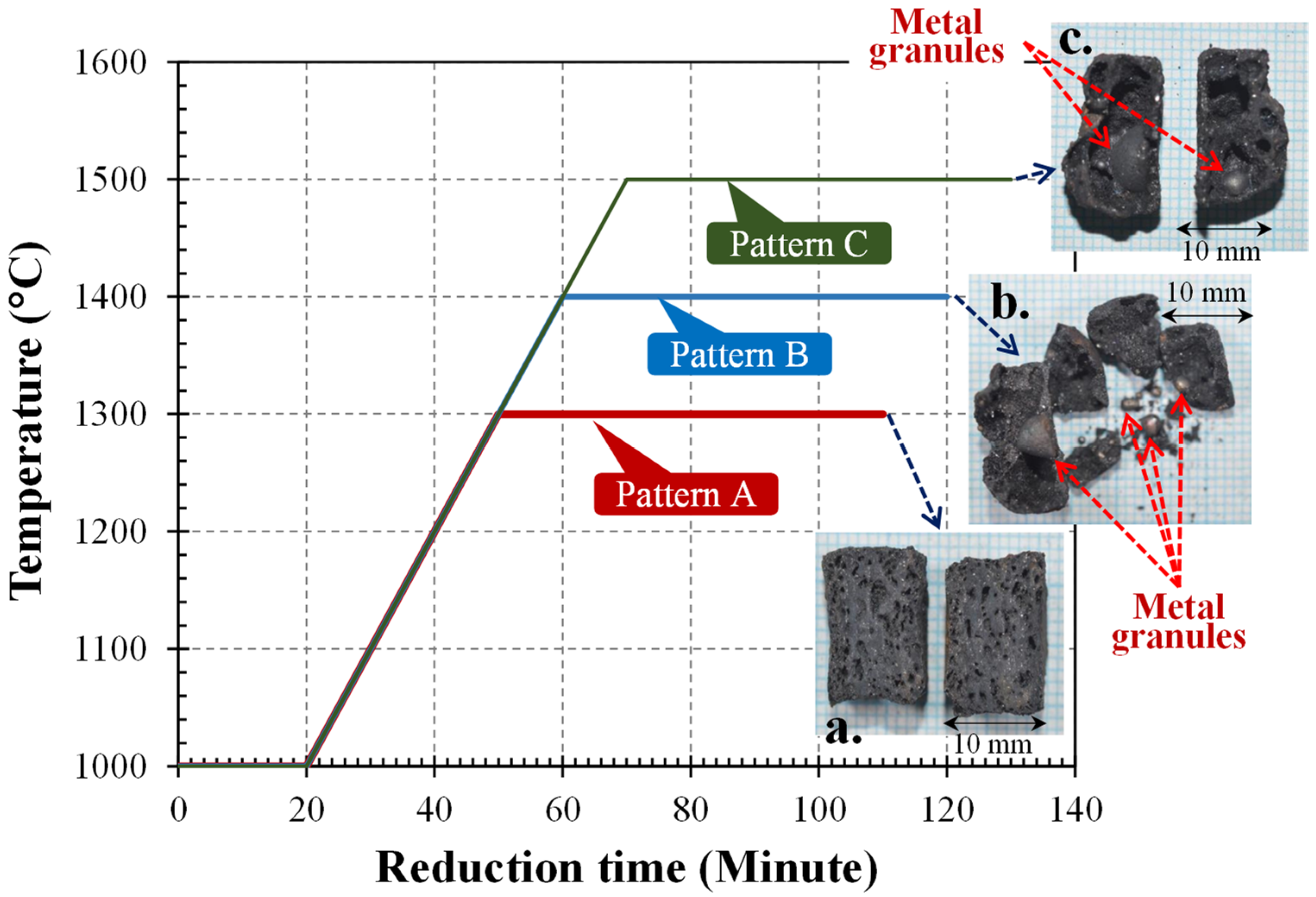
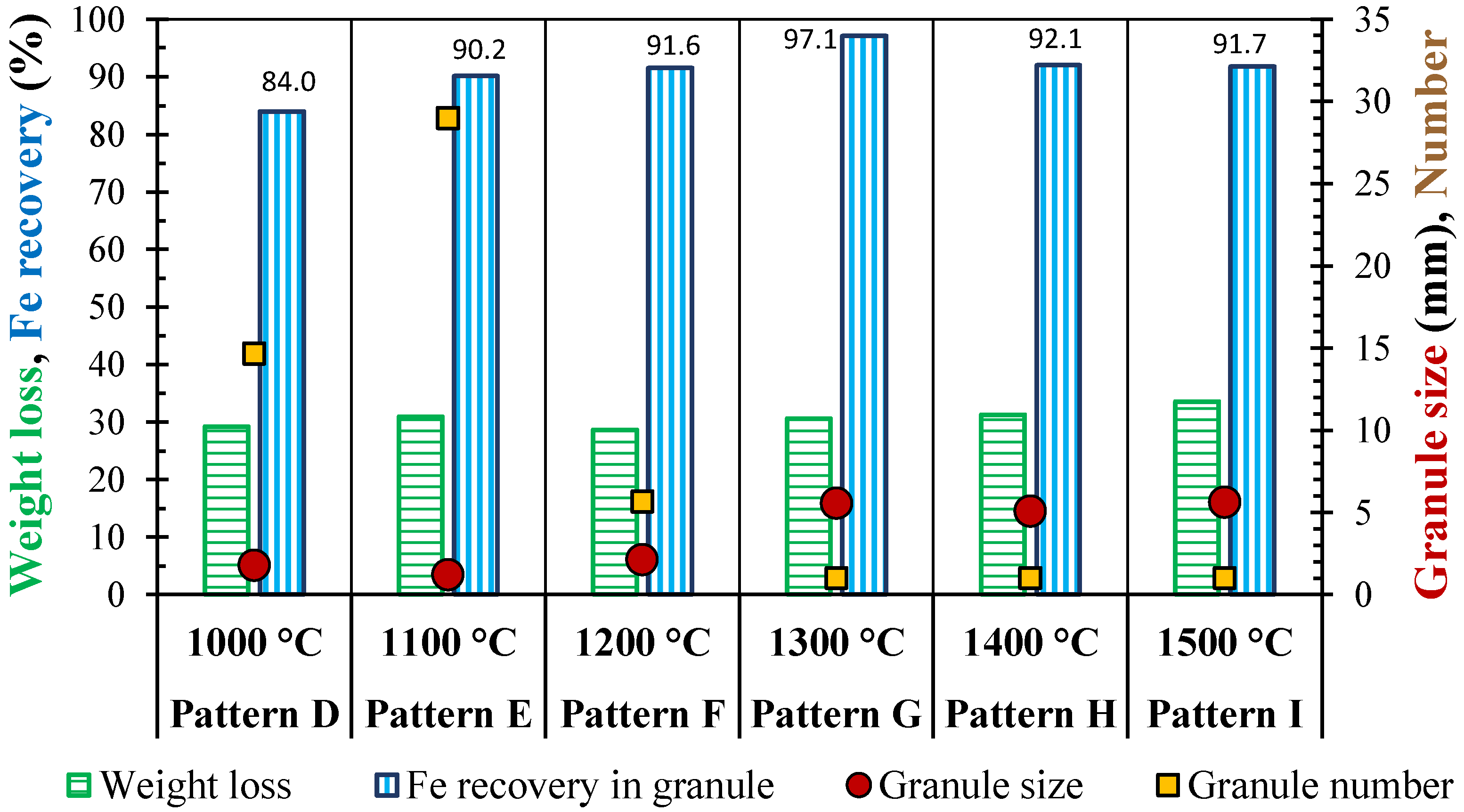
3.3. Effect of Initial Temperature
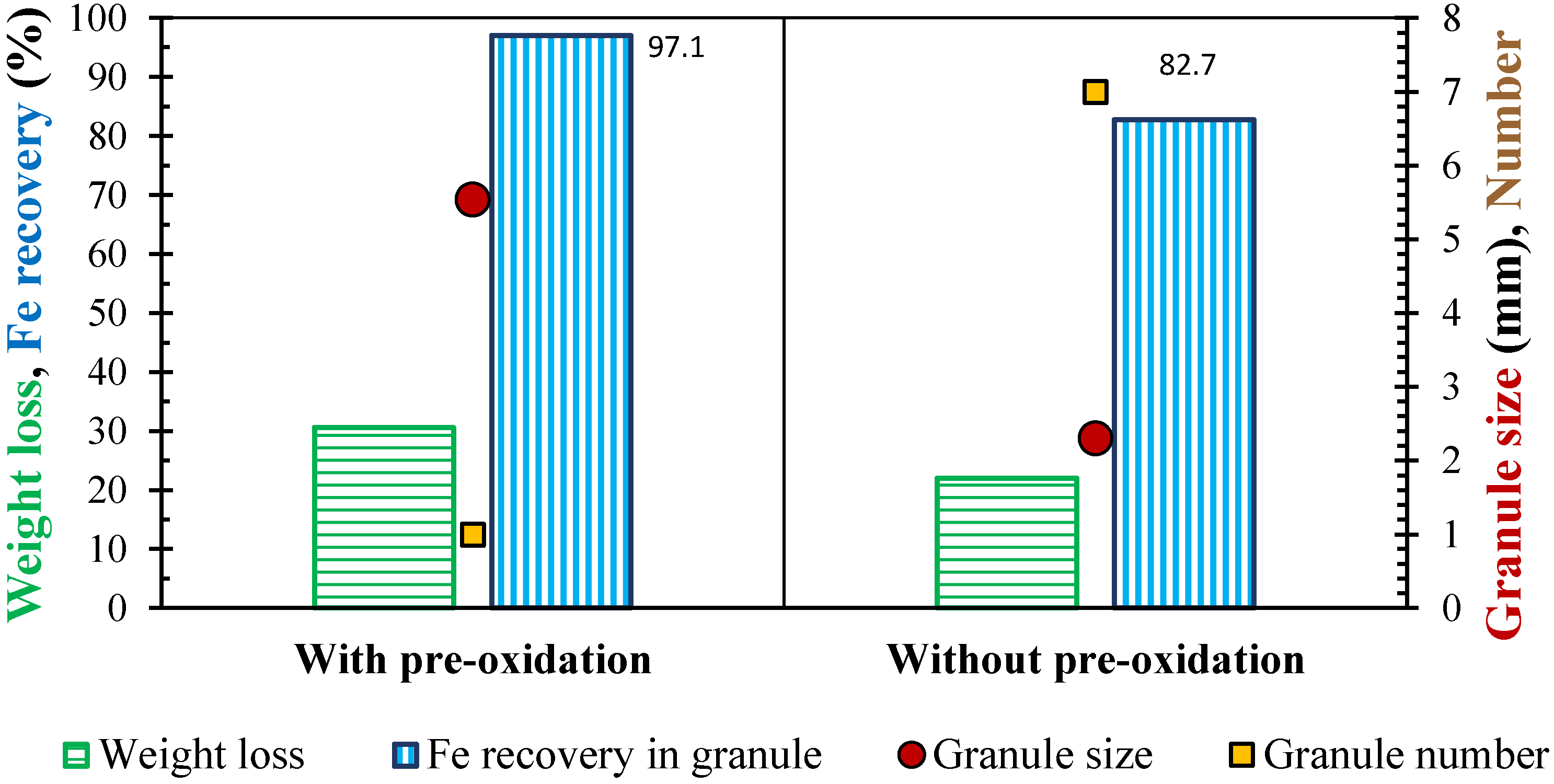
3.4. Effect of Pre-Oxidation
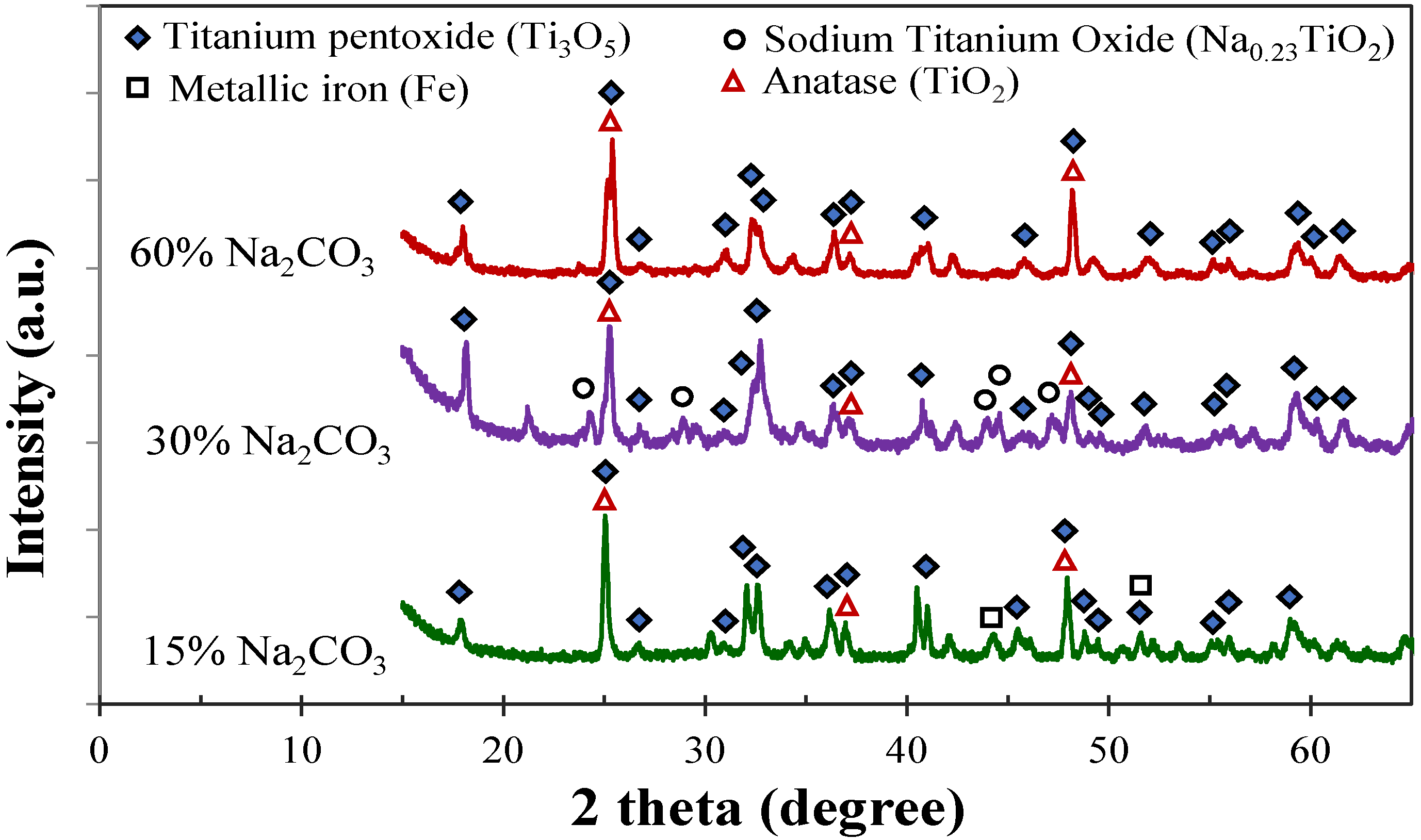
3.5. Effect of Sodium Carbonate Addition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Geological Survey. Titanium Statistics and Information. Available online: https://www.usgs.gov/centers/nmic/titanium-statistics-and-information (accessed on 13 July 2021).
- GVR. Titanium Dioxide Market Size, Share & Trends Analysis Report by Application (Paints & Coatings, Plastics, Pulp & Paper, Cosmetics), by Region (APAC, North America, Europe), and Segment Forecasts, 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/titanium-dioxide-industry (accessed on 13 July 2021).
- Zhang, W.; Zhu, Z.; Cheng, C.Y. A literature review of titanium metallurgical processes. Hydrometallurgy 2011, 108, 177–188. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present applications of titanium dioxide for photocatalytic removal of pollutants from water: A review. J. Environ. Manag. 2020, 270, 110906. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, S.; Zhou, S.; Feng, H.; Liu, Y.; Jia, G. Review of health safety aspects of titanium dioxide nanoparticles in food application. NanoImpact 2020, 18, 100224. [Google Scholar] [CrossRef]
- Chatterjee, S.; Webre, W.A.; Patra, S.; Rout, B.; Glass, G.A.; D’Souza, F.; Chatterjee, S. Achievement of superior efficiency of TiO2 nanorod-nanoparticle composite photoanode in dye sensitized solar cell. J. Alloy. Compd. 2020, 826, 154188. [Google Scholar] [CrossRef]
- Lv, W.; Bai, C.; Lv, X.; Hu, K.; Lv, X.; Xiang, J.; Song, B. Carbothermic reduction of ilmenite concentrate in semi-molten state by adding sodium sulfate. Powder Technol. 2018, 340, 354–361. [Google Scholar] [CrossRef]
- El-Guindy, M.I.; Davenport, W.G. Kinetics and mechanism of ilmenite reduction with graphite. Metall. Trans. 1970, 1, 1729–1734. [Google Scholar] [CrossRef]
- Gupta, S.K.; Rajakumar, V.; Grieveson, P. Kinetics of reduction of ilmenite with graphite at 1000 to 1100 °C. Metall. Trans. B 1987, 18B, 713–718. [Google Scholar] [CrossRef]
- El-Tawil, S.Z.; Morsi, I.M.; Francis, A.A. Kinetics of solid-state reduction of ilmenite ore. Can. Metall. Q. 1993, 32, 281–288. [Google Scholar] [CrossRef]
- Coley, K.S.; Terry, B.S.; Grieveson, P. Simultaneous reduction and carburization of ilmenite. Metall. Trans. B 1995, 26B, 485–494. [Google Scholar] [CrossRef]
- Chen, Y.; Hwang, T.; Williams, J.S. Ball milling induced low-temperature carbothermic reduction of ilmenite. Mater. Lett. 1996, 28, 55–58. [Google Scholar] [CrossRef]
- Welham, N.J.; Williams, J.S. Carbothermic reduction of ilmenite (FeTiO3) and rutile (TiO2). Metall. Trans. B 1999, 30B, 1075–1081. [Google Scholar] [CrossRef]
- Kucukkaragoz, C.S.; Eric, R.H. Solid state reduction of a natural ilmenite. Miner. Eng. 2006, 19, 334–337. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, Z. Reductive kinetics of the reaction between a natural ilmenite and carbon. Int. J. Miner. Process. 2006, 81, 133–140. [Google Scholar] [CrossRef]
- Francis, A.A.; El-Midany, A.A. An assessment of the carbothermic reduction of ilmenite ore by statistical design. J. Mater. Process. Technol. 2008, 199, 279–286. [Google Scholar] [CrossRef]
- Dewan, M.A.R.; Zhang, G.; Ostrovski, O. Carbothermal reduction of a primary ilmenite concentrate in different gas atmospheres. Metall. Trans. B 2010, 41B, 182–192. [Google Scholar] [CrossRef] [Green Version]
- Merk, R.; Pickles, C.A. Reduction of ilmenite by carbon monoxide. Can. Metall. Q. 1988, 27, 179–185. [Google Scholar] [CrossRef]
- Gupta, S.K.; Rajakumar, V.; Grieveson, P. The role of preheating in the kinetics of reduction of ilmenite with carbon. Can. Metall. Q. 1990, 29, 43–49. [Google Scholar] [CrossRef]
- Sun, K.; Takahashi, R.; Yagi, J.I. Nonisothermal behavior of the oxidation of natural ilmenite pellet. ISIJ Int. 1992, 32, 953–961. [Google Scholar] [CrossRef] [Green Version]
- Vijay, P.L.; Venugopalan, R.; Sathiyamoorthy, D. Preoxidation and hydrogen reduction of ilmenite in a fluidized bed reactor. Metall. Trans. B 1996, 27B, 731–738. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Q.; Xie, Z.; Lei, C.; Li, H. Morphological changes of Panzhihua ilmenite during oxidation treatment. Metall. Trans. B 2013, 44B, 897–905. [Google Scholar] [CrossRef]
- Xu, M.; Guo, M.; Zhang, J.; Wan, T.; Kong, L.J. Beneficiation of titanium oxides from ilmenite by self-reduction of coal bearing pellets. Iron. Steel Res. Int. 2006, 13, 6–9. [Google Scholar] [CrossRef]
- Lv, W.; Lv, X.; Xiang, J.; Zhang, Y.; Li, S.; Bai, C.; Song, B.; Han, K. A novel process to prepare high-titanium slag by carbothermic reduction of pre-oxidized ilmenite concentrate with the addition of Na2SO4. Int. J. Miner. Process. 2017, 167, 68–78. [Google Scholar] [CrossRef]
- Lv, W.; Lv, X.; Xiang, J.; Wang, J.; Lv, X.; Bai, C.; Song, B. Effect of pre-oxidation on the carbothermic reduction of ilmenite concentrate powder. Int. J. Miner. Process. 2017, 169, 176–184. [Google Scholar] [CrossRef]
- El-Tawil, S.Z.; Morsi, I.M.; Yehia, A.; Francis, A.A. Alkali reductive roasting of ilmenite ore. Can. Metall. Q. 1996, 35, 31–37. [Google Scholar] [CrossRef]
- Zulhan, Z.; Lo, F. Iron nugget formation from iron sand/coal composite pellets under isothermal-temperature gradient profiles. Ironmak. Steelmak. 2000, 48, 1022–1029. [Google Scholar] [CrossRef]
- Glasser, F.P.; Marr, J. Phase Relations in the System Na2O-TiO2-SiO2. J. Am. Ceram. Soc. 1979, 62, 42–47. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, G.; Liu, M.; Li, Z. Removal of residual element tin in the ferrous metallurgy process: A review. Metals 2019, 9, 834. [Google Scholar] [CrossRef] [Green Version]








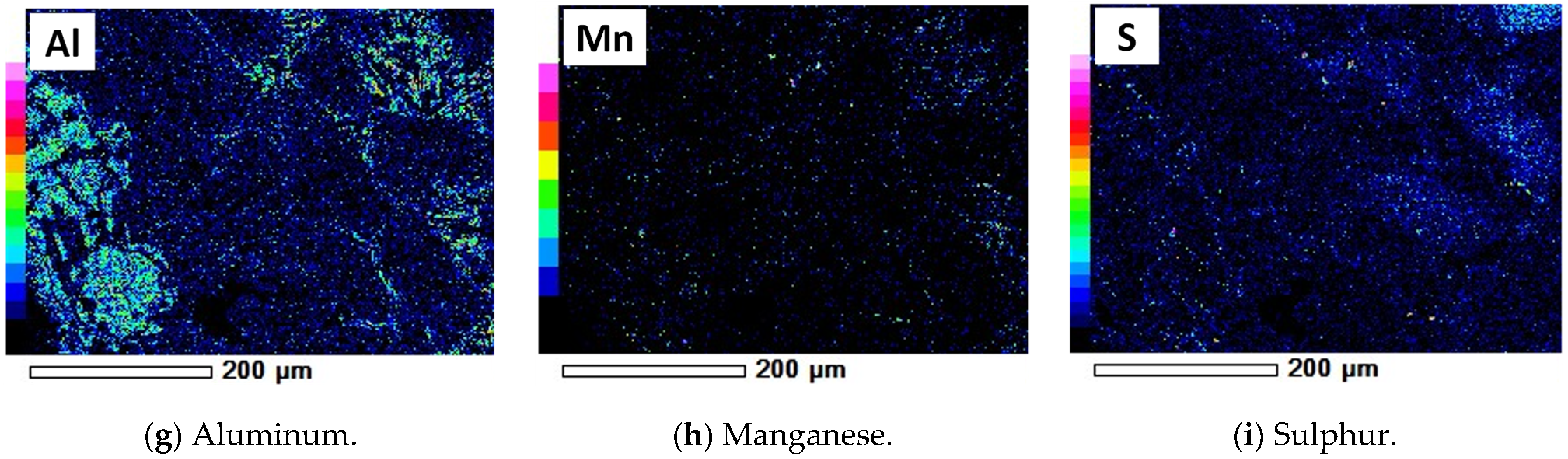
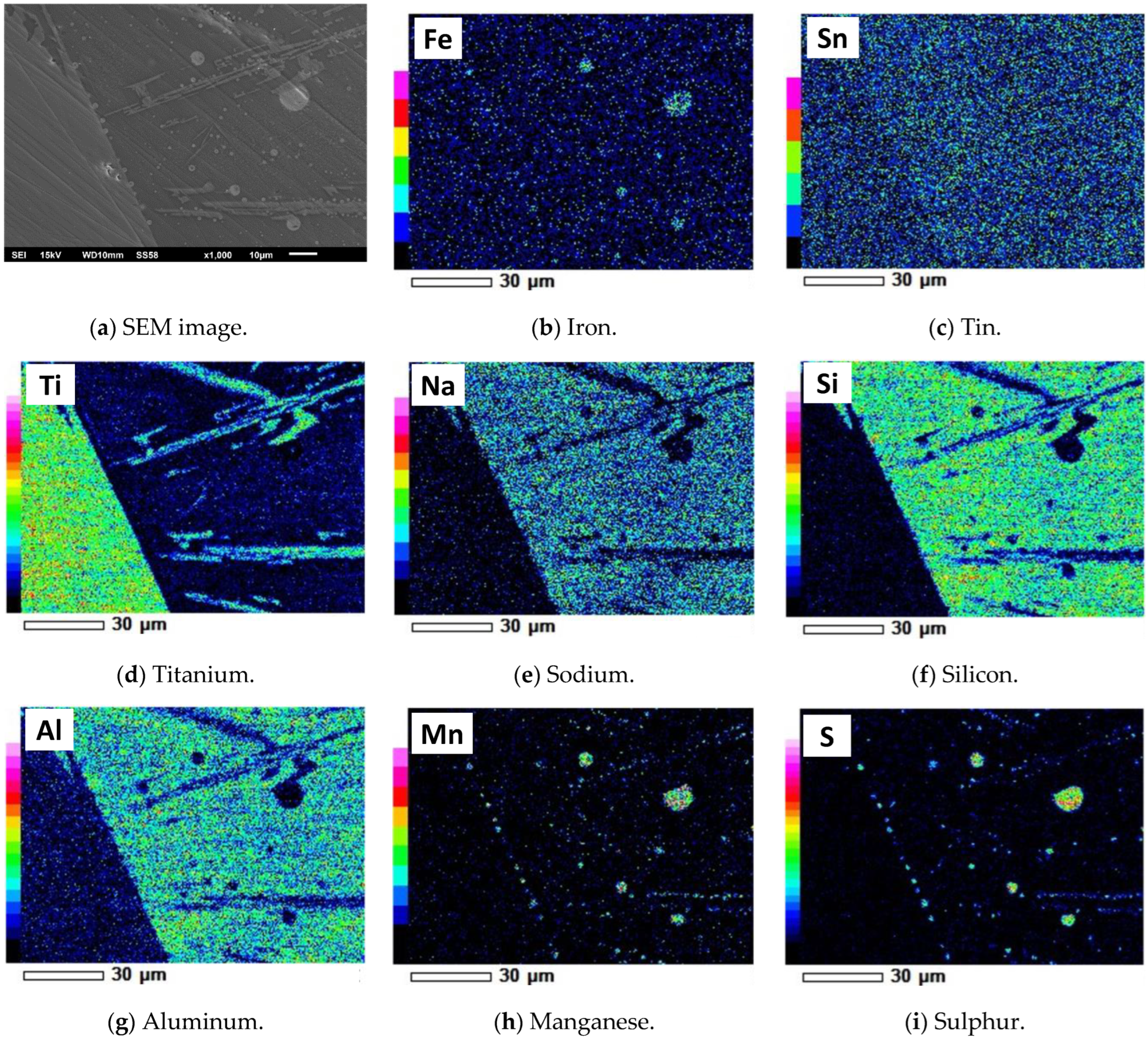

| Ti | Fe | Mn | Sn | S | Cl | Si | Al | Nb | Y | Ta | W | Pb | O and Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 33.23 | 21.21 | 2.03 | 1.88 | 0.98 | 0.67 | 0.65 | 0.64 | 0.62 | 0.32 | 0.29 | 0.23 | 0.17 | 37.08 |
| Fixed Carbon | Ash | Volatile Matter | Inherent Moisture |
|---|---|---|---|
| 44.63 | 12.20 | 40.00 | 3.17 |
| C | H | O | N | S |
|---|---|---|---|---|
| 65.77 | 5.34 | 14.21 | 1.19 | 1.29 |
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | MnO | K2O | Na2O | SO3 | Other |
|---|---|---|---|---|---|---|---|---|---|
| 47.03 | 24.88 | 14.29 | 3.76 | 1.15 | 0.22 | 0.52 | 0.25 | 3.81 | 2.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulhan, Z.; Dinillah, R.; Yulianton, T.; Santoso, I.; Hidayat, T. Carbothermic Reduction of Ilmenite Concentrate with Sodium Carbonate Additive to Produce Iron Granules and High Titania Containing Slag. Metals 2022, 12, 963. https://doi.org/10.3390/met12060963
Zulhan Z, Dinillah R, Yulianton T, Santoso I, Hidayat T. Carbothermic Reduction of Ilmenite Concentrate with Sodium Carbonate Additive to Produce Iron Granules and High Titania Containing Slag. Metals. 2022; 12(6):963. https://doi.org/10.3390/met12060963
Chicago/Turabian StyleZulhan, Zulfiadi, Rifda Dinillah, Toto Yulianton, Imam Santoso, and Taufiq Hidayat. 2022. "Carbothermic Reduction of Ilmenite Concentrate with Sodium Carbonate Additive to Produce Iron Granules and High Titania Containing Slag" Metals 12, no. 6: 963. https://doi.org/10.3390/met12060963
APA StyleZulhan, Z., Dinillah, R., Yulianton, T., Santoso, I., & Hidayat, T. (2022). Carbothermic Reduction of Ilmenite Concentrate with Sodium Carbonate Additive to Produce Iron Granules and High Titania Containing Slag. Metals, 12(6), 963. https://doi.org/10.3390/met12060963






