Abstract
It is worth it to explore the extraction performance for vanadium by the imidazole ionic liquids. The extraction of vanadium (V) was studied using [Omim]Cl, [Omim]Br, and [Omim][BF4] as extractants. The effects of various diluents, equilibrium time, extraction temperature, and anion species were investigated. The structure-activity relationship of vanadium and ILs was discussed by calculating the lattice energy of ILs based on the Glasser theory and the volume of anions. The results show that n-pentanol is the optimum diluent. Under the extraction conditions of an equilibrium time of 60 s and extraction temperature of 25 °C, the extraction rates of V (V) by [Omim]Cl, [Omim]Br, and [Omim][BF4] reached 97.93%, 96.59%, and 87.01%, respectively. Furthermore, based on the Glasser theory, the lattice energy of ionic liquids decreased in the order [Omim]Cl > [Omim]Br > [Omim]BF4. The volume of the anions increased in the order Cl− < Br− < BF4− < HVO42−. The extraction rate of V (V) depended on the size of the anions and the strength of the interaction between the anion and imidazolium cation. The results of counterevidence experiments verified the larger the anion volume, the easier it is to combine with cation in the organic phase, and the lattice energy of extracted compound is lower. The statistical analysis showed that the effect of the equilibrium time and temperature were not significant in the model, and the anions species showed a significant effect on the extraction efficiency of V (V).
1. Introduction
Vanadium (V) is an important rare metal element which has been known as “alloy vitamins”, and has been extensively applied in the fields of ferrous metallurgy, aerospace industry, chemical industry, and medicine due to its excellent physicochemical properties [1,2,3,4]. At present, more than 80% of vanadium products in the world originated from vanadium-titanium magnetite [5]. Since vanadium-titanium magnetite is a complex mineral with multiple elements such as iron, vanadium, titanium, and chromium, the separation and extraction of vanadium products are quite difficult. The main separation and abstraction technology including chemical precipitation [6,7], the crystallization method [8], ion exchange [9,10], the electrochemical method [11], and solvent extraction [12,13,14] were used to recover vanadium from the aqueous phase. Solvent extraction is widely used for its advantages, such as high separating efficiency, high purity product, and simple process.
The organic phosphoric acid extractants, such as Di-(2-ethylhexyl) phosphoric acid (HDEHP) and 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester (HEHEHP), have been widely used to recover the VO2+ from lower initial pH aqueous solutions. However, the equipment requires good corrosion resistance under highly acidic conditions [15,16]. Moreover, the compositions of vanadium in the aqueous phase are very complicated. As reported previously, the vanadium exists in the negative charged forms of HlVmOnl+5m−2n, VxOy5x−2y at the pH range of 4–13 [17]. The neutral condition is friendly to the environment and has lower requirements on equipment. However, the acidic extractants such as HDEHP and HEHEHP are unsuitable for the extraction of vanadium due to the complex variability of the forms of V (V) in the neutral condition. Therefore, it is necessary to explore the new extraction system for vanadium [18,19].
Ionic liquids (ILs), room temperature molten salts composed of organic cations, and organic or inorganic anions have been called “designable solvents” [20,21]. As a new type of green organic solvent, ionic liquids have gradually attracted people’s attention and are widely used in many fields such as organic synthesis, biocatalysis, material preparation, electrochemistry, and separation technology [22,23,24,25,26]. The extraction of vanadium by ionic liquids has been reported by several researchers [27,28,29]. At present, imidazole and pyridine ionic liquids are mainly studied and widely used. Hu et al. studied the selective extraction of vanadium from chromium by hydrophobic imidazole ionic liquids, and the mechanism of V (V) extraction by [C8mim][PF6] was proven to be an anion exchange between HV10O273− and PF6− [30].
The anion vanadium complex ions in a neutral condition can combine with organic cations in ionic liquids to form a liquid ionic extracted compound which can dissolve in the organic phase. The liquid ionic extracted compound can move to the organic phase by phase transfer and achieve the purpose of separating vanadium according to its polarity and hydrophobic property. Therefore, the separation and extraction of vanadium is affected by the strong interactions between the vanadium composition, the extractant structure, and the diluents. The Glasser theory of ionic liquids [31,32] could characterize the relationship between the structure and properties of ionic liquids and calculate the lattice energy of ionic liquids. The method is simple and has high accuracy.
According to the previous studies, the imidazole ionic liquids have an excellent extraction performance for vanadium and the extraction mechanism is anion exchange [33]. However, there is still a certain gap in the extraction efficiency. In order to obtain a better understanding of the factors that determine the extraction rate of vanadium, four different ILs with the same cation [Omim]+ but different anions have been used to investigate the extraction behaviors for vanadium in this paper. The effects of the main factors on the extraction process such as various diluents, equilibrium time, extraction temperature, and anion species were investigated. The structure-activity relationship of vanadium and ILs was determined by calculating the lattice energy of ILs based on the Glasser theory, combined with the volume of anions and the extraction rate of vanadium.
2. Materials and Methods
2.1. Reagents and Instruments
All of the reagents used were of analytical grade. First, 1-octyl-3-methylimidazolium chloride ([Omim]Cl), 1-octyl-3-methylimidazolium bromide ([Omim]Br), 1-octyl-3-methylimidazolium tetrafluoroborate ([Omim][BF4]), and 1-octyl-3-methylimidazolium hexafluorophosphate ([Omim][PF6]) were bought from Linzhou Keneng Material Technology Co. Ltd. (Linzhou, China). Sodium vanadate (NaVO3), sodium hydroxide (NaOH), sulphuric acid (H2SO4), and diluents such as n-pentanol, n-hexanol, n-octanol, and n-dodecane were supplied by Sinopharm Chemical Reagent Co. Ltd. (Shenyang, China). The needed organic phases were prepared by dissolving the ILs in the corresponding diluents. A standard stock solution of 1.75 g·L−1 V (V) was prepared by dissolving NaVO3 in deionized water. The pH of the aqueous solution was adjusted with the appropriate amount of 0.1 mol·L−1 NaOH and 0.1 mol·L−1 H2SO4 unless otherwise stated.
The following instruments were used: PXSJ-216 pH meter (Shanghai Leici Instrument Co., Ltd., Shanghai, China), SHA-C thermostat water bath cauldron oscillator (Gongyi Yuhua Instrument Co., Ltd., Gongyi, China), TG-60 high speed centrifuge (Gongyi Yuhua Instrument Co., Ltd., Gongyi, China), and DF-101S magnetic stirrer (Gongyi Yuhua Instrument Co., Ltd., Gongyi, China).
2.2. Experimental Procedure
All the extraction experiments were performed by contacting 20 mL of organic phase and 20 mL of aqueous phase in a conical flask in the thermostat water bath cauldron oscillator under the conditions of corresponding time, temperature, and rotating at 250 r·min−1 of the oscillator. After the mixture was centrifuged at 4000 r·min−1 for 5 min, the concentration of V (V) in the lower aqueous phase was measured by ammonium ferrous sulfate titration [34] and the amount of metal ion extracted into the organic phase was determined by material balance. Each group of data was measured three times in parallel, and the average value was given. The extraction efficiency (E) was calculated according to the following Equation (1):
where ρ0 and ρ1 are the initial and equilibrium concentrations of V (V) in the aqueous phase, respectively, g·L−1.
2.3. Uncertainty and Statistical Analysis
The main uncertainty sources for the experiment considered in this work are: (i) Type-A uncertainty (uA) arising from different measurements performed under repeatability conditions; and (ii) Type-B uncertainty (uB) inherent to the measurement instrument resolution. The standard uncertainty u could be calculated according to the standard JJF1035-2005 [35]. An ANOVA analysis of these results was performed using Spss software (Statistical Package the Social Sciences, Spss 24.0, IBM Corporation, America).
3. Results and Discussion
3.1. Effect of Various Diluents on the Extraction of V (V)
The diluents play an important role in the extraction system. The proper diluent is not only to reduce the viscosity of the organic phase and improve the extraction and separation effect, but also to enhance the efficiency and economic indicators of the extraction process [36,37]. Different diluents such as n-pentanol, n-hexanol, n-octanol, and n-dodecane were used to carry out the extraction process of V (V) using [Omim][BF4] as the extractant. The extraction rate of V (V) has been shown in Figure 1. Table 1 shows the related parameters of diluents.
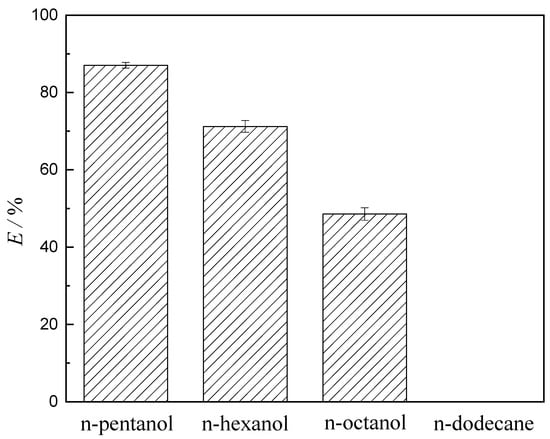
Figure 1.
Effect of various diluents on the extraction of V (V) (experimental conditions: concentration of V (V) in the aqueous phase 1.75 g·L−1, concentration of extractant 0.2 mol·L−1, aqueous phase pH 8.051, extraction temperature 25 °C, and equilibrium time 60 s).

Table 1.
The related parameters of diluents.
As shown in Figure 1, the maximum extraction rate of V (V) was observed when n-pentanol was a diluent. The extraction rate of V (V) with different diluents decreased in the order n-pentanol > n-hexanol > n-octanol > n-dodecane. When n-dodecane was applied as diluent, there was almost no effect on the extraction of V (V) using [Omim][BF4] as the extractant. The phenomenon can be explained by the theory of “similarity and intermiscibility” [38]. The n-pentanol, n-hexanol, n-octanol, and [Omim][BF4] are polar organic solvents. Conversely, the n-dodecane belongs to a non-polar organic solvent. Therefore, [Omim][BF4] is more easily dissolved in n-pentanol, n-hexanol, and n-octanol and hardly dissolved in n-dodecane. From Table 1, comparing the four kinds of diluents of experiment, it was found that the extraction rate was enhanced with increasing the dielectric constant of diluents. Jackson [39] reported that the diluents reduce the interactions between the oppositely charged ions through their high relative dielectric constant and facilitate the formation of an extracted compound; thus, the reaction is more likely to occur [40]. This conclusion fits well with our result presented here. The n-pentanol was the most appropriate diluent in this paper. Furthermore, the dielectric constant of diluent was related to the polarity. The polarity was found to increase with the increasing dielectric constant of the diluent. When the polarity of the diluent increased, the ILs were more soluble in the diluent. Higher polarity of the diluent brings about higher extraction efficiency [41,42]. The polarizability among the four diluents had the order of n-pentanol > n-hexanol > n-octanol > n-dodecane. The Minimum Polarizability Principle [43] states that “the natural evolution of any system is toward a state of minimum polarizability”. It is further proved that n-pentanol was the most appropriate diluent, and the result is confirmed by the conclusions above. Hence, n-pentanol was used as a diluent throughout the study.
[Omim]Cl, [Omim]Br, [Omim][BF4], and [Omim][PF6] were selected as the extractants in this paper for the experiment. The water and oil repellence of [Omim][PF6] was found during the experiment; therefore, [Omim][PF6] was not suitable for the study.
3.2. Effect of Equilibrium Time on the Extraction of V (V)
The effects of equilibrium time on the extraction of V (V) were investigated at a varying time interval from 5 to 120 s using n-pentanol as the diluent, and the results are listed in Figure 2. It is clearly observed from Figure 2 that the V (V) extraction efficiency sharply increased with an increase of shaking time. The extraction rate of V (V) by [Omim]Cl reached 97.93% at 40 s and after that the curve began to level off. The extraction rates of V (V) by [Omim]Br and [Omim][BF4] reached 96.59% and 87.01% at 60 s, respectively. After shaking for 60 s, the increase trends were no longer obvious. Hence, the equilibrium time on the extraction of V (V) was fixed at 60 s throughout the study.
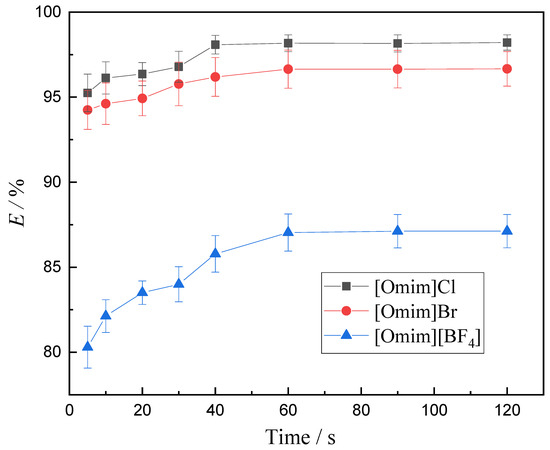
Figure 2.
Effect of equilibrium time on the extraction of V (V) (experimental conditions: concentration of V (V) in the aqueous phase 1.75 g·L−1, concentration of extractant 0.2 mol·L−1, aqueous phase pH 8.051, and extraction temperature 25 °C; relative standard uncertainty: confidence probability p = 95%, urA = 0.72%, urB = 0.35%, ur = 0.8%).
3.3. Effect of Temperature on the Extraction of V (V)
The temperature of the extraction process could affect the movement rate of particles and the viscosity of the organic phase, which could also affect the structure of extracted compound. Therefore, the suitable temperature of the extraction process has great significance in achieving the ideal extraction rate. The effect of temperature on V (V) extraction was investigated with a varying temperature interval from 25 to 45 °C, and the results are depicted in Figure 3.
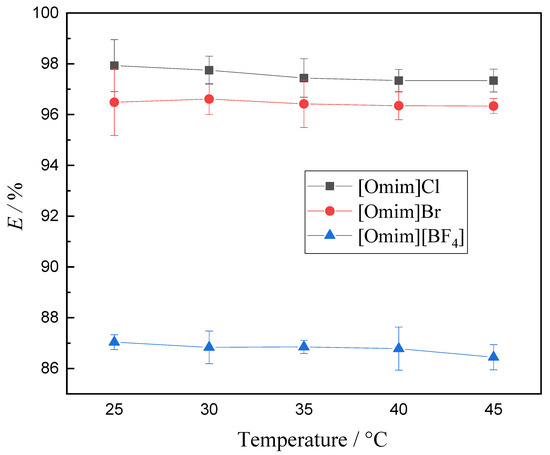
Figure 3.
Effect of temperature on the extraction of V (V) (experimental conditions: concentration of V (V) in the aqueous phase 1.75 g·L−1, concentration of extractant 0.2 mol·L−1, aqueous phase pH 8.051, equilibrium time 60 s; relative standard uncertainty: confidence probability p = 95%, urA = 0.66%, urB = 0.35%, ur = 0.75%).
As shown in Figure 3, all of the V (V) extraction rates had a slightly decrease. This finding indicates that a high extraction temperature could affect the viscosity of the ionic liquids, and cause the loss of ionic liquids, leading to the decrease in the extraction efficiency of V (V). Meanwhile, the production energy consumption increased with an increasing temperature. Hence, 25 °C was the optimum temperature in this study.
3.4. Effect of Anions Species on the Extraction of V (V)
For relatively simple salts, such as type MX, lattice energies can be calculated with the following Equation (2) based on the Glasser theory:
where U is the lattice energy, kJ·mol−1; Vm is the volume of ionic compound, nm3.
For the salts of formula MX with a charge ratio of 1:1, I = 1, α = 117.3 kJ·mol−1·nm, and β = 51.9 kJ·mol−1. The calculation results for the lattice energy of ionic liquids are listed in Table 2.

Table 2.
The lattice energy of ionic liquids.
Figure 4 shows the extraction rates of V (V) using [Omim]Cl, [Omim]Br, and [Omim][BF4] as extractants under the same experimental conditions.
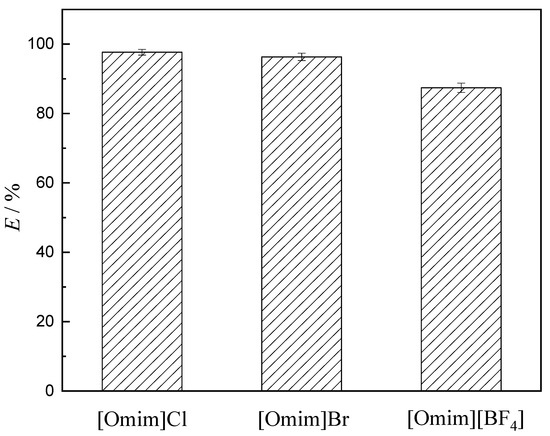
Figure 4.
The extraction of V (V) by using the three ionic liquids (experimental conditions: concentration of V (V) in the aqueous phase 1.75 g·L−1, concentration of extractant 0.2 mol·L−1, aqueous phase pH 8.051, extraction temperature 25 °C, and equilibrium time 60 s).
From Table 2, the lattice energy of ionic liquids followed this trend: [Omim]Cl > [Omim]Br > [Omim]BF4. As shown in Figure 4, the extraction rates of V (V) followed the same trend: [Omim]Cl > [Omim]Br > [Omim]BF4. This finding indicates that the extraction rates of V (V) were found to increase with the increasing lattice energy of ionic liquids.
According to the concentration of V (V), and the previous studies [17,33], it was found that V (V) mainly existed in the form of HVO42− in this study. Figure 5 shows the ball-stick models of Cl−, Br−, BF4−, and HVO42− at the same proportion which were drawn by ChemBioOffice software (14.0, CambridgeSoft, America).
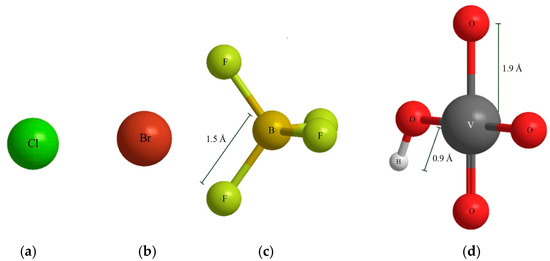
Figure 5.
The ball-stick models of anions. (a) Cl−; (b) Br−; (c) BF4−; (d) HVO42−.
It is observed from Figure 5 that the bond lengths of B-F and V-O were 1.5 Å and 1.9 Å, respectively. The V-O bond length was longer than B-F obviously. The radius of HVO42− was evaluated through the bond-additivity using a V-O bond length of 190 pm, V (V) ionic radius of 54 pm, and oxygen ion radius of 140 pm. The volume of HVO42− was calculated by ChemBioOffice2014 software. The radius and volume of anions are shown in Table 3.

Table 3.
The radius and volume of anions.
As can be seen from Table 3, the volume of the anions increased in the order Cl− < Br− < BF4− < HVO42−. The largest lattice energy of [Omim]Cl was observed for the smallest anion, Cl−, and the smallest lattice energy of [Omim][BF4] was observed for the larger anion, BF4−. The extraction rates of V (V) using ILs decreased with the increase in the volume of the anion. This is owing to the fact that more cations can be accommodated around with the larger anion [45]. The study indicates that the complexation ability of HVO42− with [Omim]+ was stronger than other anions (Cl−, Br−, BF4−) during the extraction process. Therefore, HVO42− can carry out an ion exchange with the Cl−, Br−, and BF4− due to the larger volume compared with that of Cl−, Br−, and BF4−. Furthermore, due to the smallest volume of Cl−, it preferred to carry out ion exchange with HVO42−; thus, the ability of Cl− ions in enhancing the extraction of HVO42− ions was the strongest.
According to the cavity effect [12], the excellent extraction efficiency is attributed to the formation of the larger volume of the extracted compound in the organic phase. The volume of extracted compound [Omim]2[HVO4] must be larger than [Omim]Cl, [Omim]Br, and [Omim][BF4] due to the largest volume of HVO42− among the four anions, leading to the smallest lattice energy compared with [Omim]Cl, [Omim]Br, and [Omim][BF4]. As mentioned above, the lower interactions between the oppositely charged ions would lead to the stable extracted compound in the organic phase, and then the extraction efficiency is improved. Due to the largest lattice energy gap between [Omim]Cl and [Omim]2[HVO4], the ion exchange is more complete during the extraction process; thus, the best extraction efficiency of V (V) can be obtained. Obviously, the differences of extraction efficiency of HVO42− by ILs could be well understood from the combined consideration of the lattice energy and the volume of the anions.
The differences in extraction efficiency of [Omim]Cl, [Omim]Br, and [Omim][BF4] can also be explained by the modified Born energy. As reported, the modified Born energy of ion increases as the ionic radius decreases in water or organic solvent [46]. Therefore, the smaller anions (i.e., Cl−) in the low-dielectric organic phase will be solvated more preferentially by the higher-dielectric water solvent through an ion-dipole interaction. It is easier for Cl− and Br− to dissociate into the aqueous phase and exchange with HVO42− ions, leading to the higher extraction efficiency.
In order to further verify the above conclusions, the counterevidence experiments were conducted in this paper. The standard stock solution of 0.2 mol·L−1 NH4Cl and NH4Br were prepared by dissolving NH4Cl and NH4Br in deionized water, respectively. The extraction of anions was studied using 0.2 mol·L−1 [Omim]Cl, [Omim]Br, and [Omim][BF4] as extractants, and n-pentanol as the diluent. The shaking time was 60 s, and the extraction temperature was 25 °C. The concentration of anions in the lower aqueous phase was measured by the Inductive Coupling Plasma Emission Spectrograph (ICP), and the experimental results are shown in Table 4.

Table 4.
Experimental results of the counterevidence experiments.
As shown in Table 4, when NH4Br was extracted by [Omim]Cl, the concentration of Cl− in the aqueous phase was 0.200 mol·L−1. Almost all the Cl− in the organic phase transferred into aqueous phase. When NH4Br and NH4Cl were extracted by [Omim][BF4], the concentrations of BF4− in the aqueous phase were 0.078 mol·L−1 and 0.062 mol·L−1, respectively. It indicates that most of the BF4− was still in the organic phase, and hardly carried out the ion exchange with Cl− and Br− in the aqueous phase. Therefore, the experimental results show that the larger the anion volume, the easier it is to combine with cation in the organic phase, and the lattice energy of the extracted compound is lower.
3.5. Statistical Analysis
A statistical analysis of the experimental data is presented in Table 5. An analysis of variance (ANOVA) measures the significance of the parameters. The significances of the equilibrium time, temperature, and anions species were determined by the Fisher’s F-test and p value. The p values were used as a tool to check the significance of each model. The larger the magnitudes of the F-value, the smaller the p-value; the more significant was the corresponding models. A confidence level of 95% was used, and p-values < 0.05, between 0.05 and 0.1, and more than 0.1 indicated a significant effect, almost meaningful effect, and an insignificant effect, respectively [47]. From the presented results in Table 5, it can be concluded that the effect of the equilibrium time and temperature are not significant in the model; however, the anions species show a significant effect on the extraction efficiency of V (V).

Table 5.
ANOVA results of the experimental data.
4. Conclusions
In this paper, the octyl imidazole ionic liquids such as [Omim]Cl, [Omim]Br, and [Omim][BF4] can be used as extractants for the extraction of V (V). The results show that n-pentanol is the most appropriate diluent. The extraction rates of V (V) by [Omim]Cl, [Omim]Br, and [Omim][BF4] reach 97.93%, 96.59%, and 87.01% under an equilibrium time of 60 s and extraction temperature of 25 °C. The structure-activity relationship of vanadium and ILs was discussed by calculating the lattice energy of Ils based on the Glasser theory and the volume of anions. The extraction rate of V (V) depends on the size of the anions and the strength of interaction between the anion and imidazolium cation. The results of the counterevidence experiments also verify the larger the anion volume, the easier it is to combine with cation in the organic phase, and the lattice energy of the extracted compound is lower. The statistical analysis shows that the effect of the equilibrium time and temperature are not significant in the model; however, the anions species show a significant effect on the extraction efficiency of V (V).
Author Contributions
Conceptualization, J.H.; methodology, J.H.; validation, W.T.; investigation, J.H. and W.T.; resources, J.H. and W.T.; data curation, G.D.; writing—original draft preparation, J.H. and G.D.; writing—review and editing, J.H.; supervision, J.H.; project administration, J.H.; funding acquisition, J.H. and W.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the key program of the National Natural Science Foundation of China (Grant No. 51904192, 51804071), Liaoning Doctoral Research Start-up Fund Project of Liaoning Provincial Department of Science and Technology (Grant No. 2020-BS-155), Basic Scientific Research Project of Liaoning Provincial Department of Education (General Project) (Grant No. LJKZ0248), and Scientific Research Support Program for Introducing High-Level Talents of Shenyang Ligong University (Grant No. 1050002000611).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, G.; Luo, D.; Deng, C.; Lv, L.; Liang, B.; Li, C. Simultaneous extraction of vanadium and titanium from vanadium slag using ammonium sulfate roasting-leaching process. J. Alloys Compd. 2018, 742, 504–511. [Google Scholar] [CrossRef]
- Tang, Z.; Zhou, Z.; Jin, J.; Sun, Y.; Han, Y.; Zhang, Y. Vanadium extraction from stone coal using a novel two-stage roasting technology. Fuel 2022, 321, 124031. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, F.; Zhu, Z.; Chen, D.; Zhao, H.; Liu, Y.; Zhen, Y.; Qi, T.; Zheng, S.; Wang, M.; et al. A novel process to prepare high-purity vanadyl sulfate electrolyte from leach liquor of sodium-roasted vanadium slag. Hydrometallurgy 2022, 208, 105805. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, H.; Hu, G.; Qi, T.; Yu, H.; Zhang, G.; Wang, L.; Wang, W. An extraction process to recover vanadium from low-grade vanadium-bearing titanomagnetite. J. Hazard. Mater. 2015, 294, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Rajab, V.R. Vanadium market in the world. Steel World 2007, 13, 19–22. [Google Scholar]
- Hu, P.; Zhang, Y.; Liu, T.; Huang, J.; Yuan, Y.; Yang, Y. Separation and recovery of iron impurity from a vanadium-bearing stone coal via an oxalic acid leaching-reduction precipitation process. Sep. Purif. Technol. 2017, 180, 99–106. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, Y.; Bao, S.; Huang, J. Precipitation of vanadium using ammonium salt in alkaline and acidic media and the effect of sodium and phosphorus. Hydrometallurgy 2018, 180, 113–120. [Google Scholar] [CrossRef]
- Zheng, S.; Du, H.; Wang, S.; Zhang, Y.; Chen, D.; Bai, R. Efficient and cleaner technology of vanadium extraction from vanadium slag by sub-molten salt method. Iron Steel Vanadium Titan. 2012, 33, 15–19. (In Chinese) [Google Scholar]
- Fan, Y.Y. Separation and Recovery of Vanadium (V) and Chromium (Ⅳ) from Vanadium-Containing Chromate Solution. Master’s Thesis, Central South University, Changsha, China, 2013. (In Chinese). [Google Scholar]
- Zhu, X.; Huo, G.; Ni, J.; Song, Q. Removal of tungsten and vanadium from molybdate solutions using ion exchange resin. Trans. Nonferrous Met. Soc. China 2017, 27, 2727–2732. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, S.; Wang, S.; Zhang, Y.; Du, H. The electrowinning of vanadium oxide from alkaline solution. Hydrometallurgy 2016, 165, 244–250. [Google Scholar] [CrossRef]
- Li, Y.X.; Liu, Y.Z.; Zhou, X.Z.; Zhou, X.M. Separation Chemistry and Technology; Chemical Industry Press: Beijing, China, 2017; pp. 19–119. (In Chinese) [Google Scholar]
- Ye, G.; Hu, Y.; Tong, X.; Lu, L. Extraction of vanadium from direct acid leaching solution of clay vanadium ore using solvent extraction with N235. Hydrometallurgy 2018, 177, 27–33. [Google Scholar] [CrossRef]
- Razavi, S.M.; Haghtalab, A.; Khanchi, A.R. Optimization of vanadium(V) extraction by 2-ethyl-1-hexanol and the study of extraction reaction mechanism. Miner. Eng. 2021, 170, 106984. [Google Scholar] [CrossRef]
- Li, X.; Wei, C.; Wu, J.; Li, C.; Li, M.; Deng, Z.; Xu, H. Thermodynamics and mechanism of vanadium(IV) extraction from sulphate medium with D2EHPA, EHEHPA and CYANEX 272 in kerosene. T. Nonferr. Metal. Soc. 2012, 22, 461–466. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.; Lv, G.; Zhang, G.; Liu, Y.; Zhang, W. Synergistic extraction of vanadium(IV) in sulfuric acid media using a mixture of D2EHPA and EHEHPA. Hydrometallurgy 2016, 166, 87–93. [Google Scholar] [CrossRef]
- Nayl, A.A.; Aly, H.F. Solvent extraction of V(V) and Cr(III) from acidic leach liquors of ilmenite using Aliquat 336. T. Nonferr. Metal. Soc. 2015, 25, 4183–4191. [Google Scholar] [CrossRef]
- Wen, J.; Liu, F.; Cao, H.; Ning, P.; Zhang, Y. Insights into the extraction of various vanadium species by primary amine. Hydrometallurgy 2017, 173, 57–62. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Y.; Huang, J.; Zheng, Q.; Liu, H. Extraction of vanadium(V) from a vanadium-bearing shale leachate through bifunctional coordination in Mextral 984H extraction system. Sep. Purif. Technol. 2022, 288, 120452. [Google Scholar] [CrossRef]
- Boruń, A. Conductance and ionic association of selected imidazolium ionic liquids in various solvents: A review. J. Mol. Liq. 2019, 276, 214–224. [Google Scholar] [CrossRef]
- Silva, D.M.; Ribeiro, T.; Branco, L.C.; Colaço, R.; Silva, A.G.; Saramago, B. Hydrophobic ionic liquids at liquid and solid interfaces. Tribol. Int. 2019, 129, 459–467. [Google Scholar] [CrossRef]
- Pei, Y.; Zhang, Y.; Ma, J.; Fan, M.; Zhang, S.; Wang, J. Ionic liquids for advanced materials. Mater. Today Nano 2022, 17, 100159. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H. Ionic liquids for electrochemical energy storage devices applications. J. Mater. Sci. Technol. 2019, 35, 674–696. [Google Scholar] [CrossRef]
- Egorova, K.S.; Ananikov, V.P. Fundamental importance of ionic interactions in the liquid phase: A review of recent studies of ionic liquids in biomedical and pharmaceutical applications. J. Mol. Liq. 2018, 272, 271–300. [Google Scholar] [CrossRef]
- Yin, J.J.; Sun, H.Y.; Zhu, Y.; Gao, Y. Research advance of synthesis of nitrogenous chiral ionic liquids and its application. J. Lanzhou Univ. Technol. 2008, 34, 66–72. (In Chinese) [Google Scholar]
- Luo, D.; Huang, J.; Zhang, Y.; Liu, H.; Hu, P. Efficient and environment-friendly vanadium (V) extraction from vanadium shale leachate using tri-n-octylmethylammonium chloride. Sep. Purif. Technol. 2020, 237, 116482. [Google Scholar] [CrossRef]
- Tran, T.T.; Liu, Y.; Lee, M.S. Recovery of pure molybdenum and vanadium compounds from spent petroleum catalysts by treatment with ionic liquid solution in the presence of oxidizing agent. Sep. Purif. Technol. 2021, 255, 117734. [Google Scholar] [CrossRef]
- Kurniawan, K.; Kim, S.; Lee, J. Chapter 19—Ionic liquids-assisted extraction of metals from electronic waste. Ionic Liquid-Based Technol. Environ. Sustain. 2022, 295–329. [Google Scholar] [CrossRef]
- Hu, Q.; Zhao, J.; Wang, F.; Huo, F.; Liu, H. Selective extraction of vanadium from chromium by pure [C8mim][PF6]: An anion exchange process. Sep. Purif. Technol. 2014, 131, 94–101. [Google Scholar] [CrossRef]
- Glasser, L. Lattice and phase transition thermodynamics of ionic liquids. Thermochim. Acta 2004, 421, 87–93. [Google Scholar] [CrossRef]
- Kaya, S. Relationships between lattice energies of inorganic ionic solids. Physica B 2018, 538, 25–28. [Google Scholar] [CrossRef]
- Wei, J.Y. Extraction and Separation of V (V) and Cr (VI) from Vanadium-Chromium Slags Acid Leaching Liquor Using Imidazole Ionic Liquids. Master’s Thesis, Northeastern University, Shenyang, China, 2018. (In Chinese). [Google Scholar]
- Wang, M.; Zhang, G.; Wang, X.; Zhang, J. Solvent extraction of vanadium from sulfuric acid solution. Rare Metals 2009, 28, 209–211. [Google Scholar] [CrossRef]
- Miranda, S.; Llera, J.C.; Miranda, E. Uncertainty on measurement of elastomeric isolators effective properties. Measurement 2021, 180, 109511. [Google Scholar] [CrossRef]
- Keshav, A.; Wasewar, K.L.; Chand, S. Extraction of propionic acid with tri-n-octyl amine in different diluents. Sep. Purif. Technol. 2008, 63, 179–183. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Zhang, G.Q.; Tang, R.R. Fundamentals and Practice of Solvent Extraction in Hydrometallurgy; Central South University Press: Changsha, China, 2014; pp. 124–125. (In Chinese) [Google Scholar]
- Liu, L.; Chen, D.L.; Ding, L.N.; Wan, L.P. The application of similar miscibility principle in the TCM chemical technology. Vocat. Technol. 2016, 15, 60–62. (In Chinese) [Google Scholar]
- Gilkerson, W.R.; Jackson, M.D. Ion-Solvent Interaction. Effects of added polar compounds on the conductances of several alkali metal salts in 2-Butanone at 25 °C. J. Am. Chem. Soc. 1979, 101, 4096–4100. [Google Scholar] [CrossRef]
- Sprakel, L.M.J.; Schuur, B. Solvent developments for liquid-liquid extraction of carboxylic acids in perspective. Sep. Purif. Technol. 2019, 211, 935–957. [Google Scholar] [CrossRef]
- Wang, S.Y.; Wang, Y. Synthesis of quaternary ammonium salt from chloropropene and dimethylamine. Taiyuan Sci. Technol. 1999, A06, 58, 60. (In Chinese) [Google Scholar]
- Beniwal, V.; Kumar, A. Understanding positive and negative deviations in polarity of ionic liquid mixtures by pseudo-solvent approach. Phys. Chem. Chem. Phys. 2016, 18, 23853–23863. [Google Scholar] [CrossRef]
- Pan, S.; Solà, M.; Chattaraj, P.K. On the validity of the maximum hardness principle and the minimum electrophilicity principle during chemical reactions. J. Phys. Chem. A 2013, 117, 1843–1852. [Google Scholar] [CrossRef]
- Sun, H.Y. Synthesis and Structure-Activity Relationship of Imidazolium Ionic Liquids. Master’s Thesis, Lanzhou University of Technology, Lanzhou, China, 2009. (In Chinese). [Google Scholar]
- Kolbeck, C.; Lehmann, J.; Lovelock, K.R.J.; Cremer, T.; Paape, N.; Wasserscheid, P.; Fröba, A.P.; Maier, F.; Steinrück, H.P. Density and Surface Tension of Ionic Liquids. J. Phys. Chem. B 2010, 114, 17025–17036. [Google Scholar] [CrossRef]
- Nakamura, I. Effects of dielectric inhomogeneity and electrostatic correlation on the solvation energy of ions in liquids. J. Phys. Chem. B 2018, 122, 6064–6071. [Google Scholar] [CrossRef] [PubMed]
- Katoozi, E.; Anari, Z. Statistical optimization of extraction column parameters for the zinc (II) solvent extraction by D2EHPA in a continuous mode. Miner. Eng. 2021, 174, 107255. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).