Abstract
In the present paper, the leaching of copper from printed circuit boards (PCBs) using sulfuric acid with Cu2+ and O2 is proposed. The effects of various process parameters such as agitation speed, temperature, the type and the flow rate of gas, initial Cu2+ concentration, and pulp density were investigated to examine the dissolution behavior of Cu from PCBs in 1 mol/L sulfuric acid. The kinetic studies were performed using the obtained leaching data. The leaching rate of Cu from PCBs was found to be higher on addition of Cu2+ and O2 to the leachant in comparison with the addition of O2 or both Cu2+ and N2 in the leachant. The leaching efficiency of Cu was found to be increased with increasing agitation speed, temperature, O2 flow rate, and initial Cu2+ concentration and decreasing pulp density. The 96% of Cu leaching efficiency was obtained under the following conditions: sulfuric acid concentration, 1 mol/L; temperature, 90 °C; agitation speed, 600 rpm; pulp density, 1%; initial Cu2+ concentration, 10,000 mg/L; and O2 flow rate, 1000 cc/min. The leaching data and analyses indicate that the Cu leaching from PCBs followed the reaction-controlled model satisfactorily and determined that the activation energy was found to be 23.8 kJ/mol. Therefore, these results indicate that the sulfuric acid solution with Cu2+ and O2 as a mild leach medium without strong oxidants such as HNO3, H2O2, and Fe3+ is valid for Cu leaching from PCBs.
1. Introduction
Printed circuit boards (PCBs) are core parts of electric and electronic equipment (EEE) [1,2,3,4,5]. The market of PCBs in Korea grew to 13 trillion Korean won by 2016, and 60% of waste PCBs was recycled, while the rest was exported as used PCBs [6]. Waste PCBs have hazardous pollutants as well as valuable resources, which were recovered by smelters in Korea. However, only three smelters are in operation in Korea, and there is an increasing concern about hazardous gas emission from smelters.
Hydrometallurgical recycling processes have been recognized as alternative methods; generally, the processes consist of pretreatment (liberation), leaching, purification, and electrowinning. Some wastes need pretreatment such as grinding for liberation, which could facilitate contact between leaching reagent and metal components [1]. Even recently, a number of pretreatments and leaching processes for waste PCBs recycling have been proposed, such as the following examples. The pretreatment processes such as soaking in NaOH solution [7] and microwave pyrolysis [8] and mineral processing processes [9] such as flotation [10] were investigated to enhance the dissolution efficiency of valuable metals from PCBs. Leaching processes of PCBs have been examined using hydrogen peroxide (H2O2) [11,12,13,14], ammonia solution [10,14,15,16], HNO3 [16,17,18], halogen [16,17,19,20], glycine [12,13], and ionic liquid [21]. Bacterial leaching processes of PCBs have also been reported as environment-benign processes, where Phanerochaete chrysosporium [22], Acidithiobacillus ferrooxidans [23], Leptospirillum ferriphilum or its mixed culture with Acidithiobacillus ferrooxidans [24,25,26], and Frankia sp. [27] were used for Cu leaching. The fact that many processes are still being investigated and tried as above means that bio-hydrometallurgical recycling processes cannot be commercialized easily, despite more than 30 years of research.
The Korean government has also supported the development of hydrometallurgical recycling processes of waste PCBs for small-sized businesses for the last two decades [28]. Several pilot plants of waste PCBs recycling were operated to investigate the feasibility of the pre-treatment followed by hydrometallurgical recycling processes, but they did not reach the demo-plant or commercialization stage for two reasons. Firstly, precious metals, i.e., gold and silver were lost during extensive grinding for liberation and, secondly, capital and environmental costs increased by using nitric acid or hydrogen peroxide as leaching reagents for copper (Cu) dissolution. The amount of copper contained in waste PCBs found to be 5–20% [1,29,30], and Cu should be removed or recovered before the precious metal recovery process because Cu as an impurity could interfere with the recovery of precious metal such as gold [5,31]. Generally, strong oxidants such as HNO3, H2O2, and Fe3+ are required to dissolve copper (Cu) from waste PCBs because the standard electrode potential of copper is +0.34 V (Cu2+ + 2e = Cu) [32], which indicates Cu metal will not be dissolved in sulfuric acid solution.
Conventional oxidants have disadvantages such as NOx gas emission due to HNO3 leaching [33,34,35,36], increase in reagent consumption due to instability of H2O2 [37,38], and requirement of additional separation process due to the use of Fe3+ [38,39,40]. These drawbacks could increase the capital and environmental costs as mentioned above. Therefore, the development of mild leaching condition and minimization in reagent consumption and wastewater generation should be fulfilled for successful hydrometallurgical recycling processes. The use of sulfuric acid-based leaching medium could reduce the capital cost because sulfuric acid is less corrosive than nitric and hydrochloric acids, and sulfuric acid is favorable for purification and electrowinning processes.
In our previous study [40], although a new copper dissolution process in sulfuric acid solution using Cu2+ and O2 was proposed, the leaching tests were carried out to dissolve Cu from reagent-grade Cu metal powder. Therefore, in the present study, the leaching tests of PCBs were performed in sulfuric acid solution with Cu2+ and O2 to investigate the effects of agitation speed, temperature, the type and the flow rate of gas, initial Cu2+ concentration, and pulp density for Cu leaching from PCBs in 1 mol/L sulfuric acid solution and, further, activation energy was calculated based on the leaching data and kinetic study.
2. Materials and Methods
All waste PCBs were collected from spent hard disk drives of the same model, which were obtained from a recycling company. As shown in Figure 1, the electronic components on the waste PCBs were removed by dissolving solders [1,41], which was achieved by HCl leaching with Sn4+. The bare waste PCBs were cut with a cutting mill (SM100, Retsch GmbH & Co., Haan, Germany) equipped with a 5 mm sieve, and the product was screened with a 1 mm sieve. The 1 mm oversized product was ground further with a mixer mill (MM400, Retsch GmbH & Co., Haan, Germany). Therefore, all waste PCBs samples with less than 1 mm were used in the leaching tests. The waste PCBs samples contain 31.7% of Cu, which was determined by 2-hour aqua-regia digestion (over 100 °C, less than 1 mm PCBs, and 5 g PCBs/100 mL aqua regia) before the leaching tests. All reagents used in this study were of reagent grade.
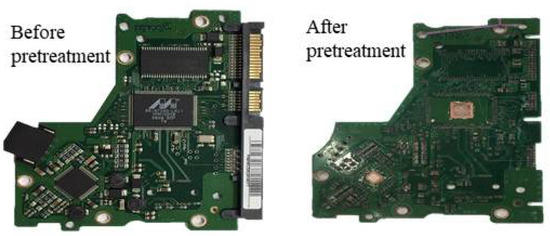
Figure 1.
Waste PCBs sample before and after the pretreatment with HCl and Sn4+.
The leaching tests of the ground waste PCBs were performed in a 500 mL three-necked Pyrex glass reactor using a heating mantle to maintain the temperature. The reactor was fitted with a reflux condenser and an agitator. The reflux condenser was inserted into one port to avoid solution loss at high temperatures. In a typical run, 200 mL of 1 mol/L sulfuric acid solution with 0, 3000, 5000, 7000, or 10,000 mg/L Cu2+ was poured into the reactor with an agitation speed of 200–800 rpm. After the temperature of solution reached the thermal equilibrium (30–90 °C), 200–1000 cc/min of oxygen, air, or nitrogen gas was introduced into the reactor and 2 g of the ground waste PCBs was added to the reactor in all the experiments. During the leaching tests, 3 mL of the solution was withdrawn periodically at a desired time interval (15−120 min) by a syringe. The sample was filtered using a 0.45 µm membrane filter, and the obtained filtrate was diluted with 5% HNO3 solution. The leach residue was digested with aqua regia and the solution was diluted with distilled water. The concentration of Cu was measured using optima 8300 ICP-OES (inductively coupled plasma optical emission spectrometer, PerkinElmer Inc., Waltham, MA, USA). The leaching efficiency of Cu was calculated using the following equation.
where Msolution (g) and Mresidue (g) represent the mass of Cu in the leach solution and residue, respectively, and Msolution does not contain the initial Cu2+, which was added before the leaching tests.
The leaching efficiency of Cu (%) = Msolution/(Msolution + Mresidue) × 100,
3. Results and Discussion
Generally, it has been found that PCBs contain various metals, but the metals, except Au and Cu, are contained in electronic components such as IC chips, capacitors, and resistors, while Au and Cu are distributed in both electronic components and bare PCBs. In the recycling of bare PCBs, Cu could act as an impurity in the hydrometallurgical Au recovery process using cyanide, and Cu must be removed before the Au recovery from PCBs. Therefore, in this study, the electronic components were removed by HCl leaching with Sn4+, and then the leaching behaviors of Cu were investigated as follows.
The leaching tests of Cu from PCBs were carried out to investigate the effect of Cu2+ and O2 addition on the improvement of Cu leaching. Figure 2 shows the leaching behaviors of Cu from PCBs in 1 mol/L sulfuric acid solution with or without 10,000 mg/L Cu2+ at a temperature of 90 °C and agitation speed of 600 rpm with 1% pulp density and 1000 cc/min N2 or O2 introduction. The fastest leaching efficiency of Cu was obtained by adding both O2 and Cu2+, and when only O2 was introduced in the solution, the leaching efficiency was higher than that with Cu2+ and N2 introduction. In the previous study [40], the mechanism of Cu leaching with Cu2+ and O2 was explained as follows. The added cupric ion (Cu2+) oxidizes Cu metal into cuprous ion (Cu+), and then the added O2 oxidizes Cu+ ion further into Cu2+. The reactions are expressed in Equations (2) and (3).
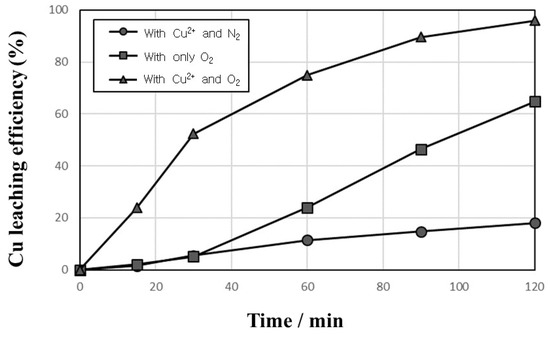
Figure 2.
Leaching behaviors of Cu in 1 mol/L sulfuric acid solution with or without 10,000 mg/L Cu2+ at 90 °C and 600 rpm with 1% pulp density and 1000 cc/min N2 or O2 introduction.
Equations (2) and (3) could be rearranged into Equation (4):
As shown in Figure 2, when Cu2+ and N2 were added to sulfuric acid solution, the dissolution efficiency of Cu increased gradually to only 18%. This dissolution would result from partly oxidized surface of Cu during grinding, and an oxidized part of Cu could be dissolved in sulfuric acid solution [30,40]. When only Cu2+ ions are added without O2, even though Cu+ ions are formed, as shown in Equation (2), because Cu+ ions are unstable in sulfuric acid solution, the Cu+ ions are changed into Cu2+ or Cu as follows [30]:
If no any other oxidant is added in the solution, when one of the two Cu+ oxidizes to Cu2+ by losing an electron, the remaining Cu+ must reduce to elemental copper (Cu0) by receiving the electron. When only O2 is added without Cu2+, the leaching efficiency of Cu increases gradually, and then increases rapidly after 30 min. Although Cu2+ was not added, because Cu ions were dissolved out in the beginning of leaching, the Cu leaching was achieved by O2 addition with a small amount of Cu2+, which was dissolved from PCBs.
As discussed above, Cu+ ions are extremely unstable in sulfuric acid solution and it is impossible to detect Cu+ as an intermediate in Equations (1) and (2). Therefore, three conditions—Cu2+/O2 addition, only O2 addition, and Cu2+/N2 addition—were compared and the synergetic effect of simultaneous addition of Cu2+ and O2 on the improvement of Cu leaching was confirmed as shown in Figure 2.
Figure 3 shows the effect of agitation speed on the leaching efficiency of Cu to examine the effect of liquid film boundary diffusion surrounding the solid particles on the dissolution efficiency in 1 mol/L sulfuric acid solution at 90 °C with 10,000 mg/L Cu2+, 1% pulp density, and 1000 cc/min O2 introduction. The dissolution efficiency increased gradually with leaching time, and the higher leaching efficiency was obtained at a higher agitation speed. Similar dissolution behaviors of Cu were observed in the leaching test with 600 rpm and 800 rpm. Therefore, in all subsequent leaching tests, a working agitation speed of 600 rpm was selected to provide effective particle suspension in the solution. The effect of temperature (30–90 °C) was investigated in 1 mol/L sulfuric acid solution at 600 rpm with 10,000 mg/L Cu2+, 1% pulp density, and 1000 cc/min O2 introduction, and the result is presented in Figure 4. The elevated temperatures yielded higher dissolution rates of Cu from PCBs, and the leaching efficiency of Cu at 90 °C increased gradually to 96.0% within 120 min.
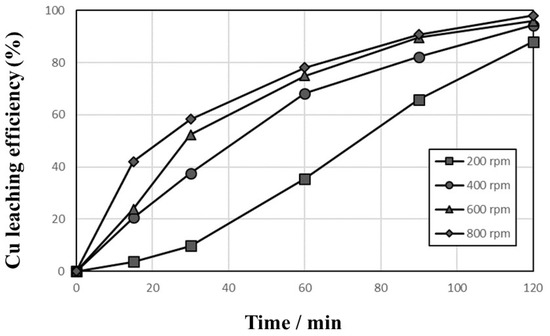
Figure 3.
Effects of agitation speed on the leaching behaviors of Cu in 1 mol/L sulfuric acid solution at 90 °C with 10,000 mg/L Cu2+, 1% pulp density, and 1000 cc/min O2 introduction.
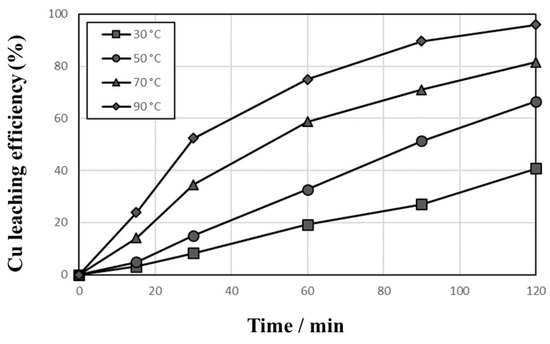
Figure 4.
Effects of temperature on the leaching behaviors of Cu in 1 mol/L sulfuric acid solution at 600 rpm with 10,000 mg/L Cu2+, 1% pulp density, and 1000 cc/min O2 introduction.
As discussed above, the leaching of Cu was improved by adding Cu2+ and O2, where the O2 used in the leaching reaction indicates dissolved oxygen. Generally, the concentration of dissolved oxygen in water decreases with increasing temperature, and the oxygen was introduced continuously into the reactor during the leaching tests. The effect of the O2 gas flow rate was investigated in 1 mol/L sulfuric acid solution at 90 °C and 600 rpm with 10,000 mg/L Cu2+, 1% pulp density, and 200–1000 cc/min O2 introduction. The results presented in Figure 5 indicate that the leaching efficiencies of Cu increased gradually up to 95% in all flow rates, but the tests with less than 400 cc/min showed a slower leaching rate. As oxygen plays a role of oxidant for the oxidation of cuprous ion (Cu+) to cupric ion (Cu2+), as shown in Equation (4), it is important to provide sufficient oxygen into the leaching system. Therefore, 1000 cc/min flow rate of oxygen was selected in further leaching tests.
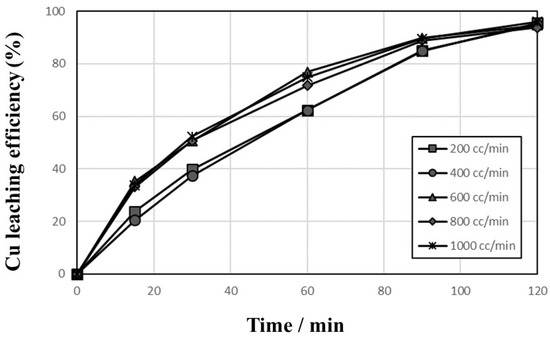
Figure 5.
Effects of O2 gas flow rate on the leaching behaviors of Cu in 1 mol/L sulfuric acid solution at 90 °C and 600 rpm with 10,000 mg/L Cu2+, 1% pulp density, and 200–1000 cc/min O2 introduction.
Leaching experiments were carried out to investigate the effect of initial cupric ion concentration as an oxidant for the dissolution of Cu metal in 1 mol/L sulfuric acid solution, temp. 90 °C, agitation speed 600 rpm, Cu2+ concentration 0–10,000 mg/L, pulp density 1%, and flow rate of O2 in solution1000 cc/min. Equation (4) represents the chemical reaction of Cu leaching, where Cu2+ ion acts as an oxidant for the dissolution of Cu metal. Figure 6 indicates that the leaching efficiencies of Cu increased gradually with time and the Cu2+ concentration; therefore, the use of Cu2+ is favorable for Cu metal leaching in sulfuric acid solution. When no Cu2+ was added to the leaching system (0 mg/L initial Cu2+ in Figure 6), the leaching efficiency of Cu increased up to 64.7% and was comparatively slower. As discussed above, partly oxidized Cu could be dissolved in sulfuric acid solution and the dissolved Cu ions could act as an oxidant for Cu metal leaching.
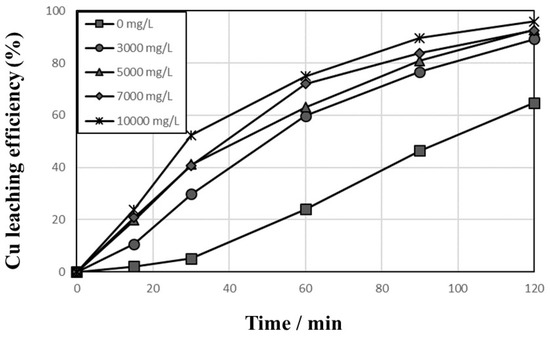
Figure 6.
Effects of initial Cu2+ concentration on the leaching behaviors of Cu in 1 mol/L sulfuric acid solution at 90 °C and 600 rpm with 0–10,000 mg/L Cu2+, 1% pulp density, and 1000 cc/min O2 introduction.
In Figure 7, the effects of pulp density on the leaching behaviors of Cu were investigated in 1 mol/L sulfuric acid solution at a temp. of 90 °C and agitation rate of 600 rpm with Cu2+ concentration 10,000 mg/L, pulp density 1–5%, and flow rate of O2 addition 1000 cc/min. The leaching efficiency of Cu increased rapidly with the decreasing pulp density. The leaching efficiency with 1% pulp density increased slower in the beginning of leaching than that with 3% pulp density, but they show similar leaching behaviors after 60 min. The leaching efficiency with 5% pulp density was retarded. If all Cu metals in the PCBs samples dissolved, the Cu concentrations of sample were found to be 3170 mg/L, 9510 mg/L, and 15,850 mg/L at 1%, 3%, and 5% pulp density, respectively. In Equation (4), 1 mol of Cu2+ should be required to oxidize 1 mol of Cu metal (the stoichiometric mole ratio is 1). Because the initial Cu2+ concentration was 10,000 mg/L, in the case of 5% pulp density, the initial Cu2+ concentration was not enough to oxidize all the Cu metal from PCBs samples, but the dissolved Cu could be changed into Cu2+ ion, as shown in Equation (4), and the leaching efficiency with 5% increased to 85.8% in 120 min, even though it was slow.
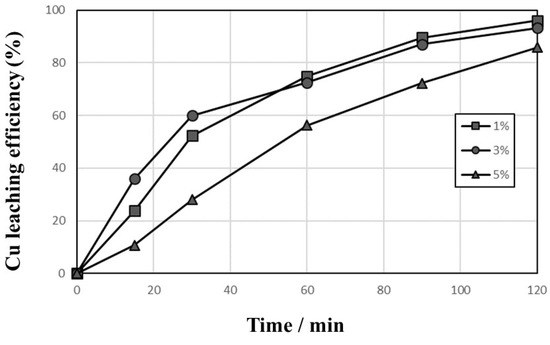
Figure 7.
Effects of pulp density on the leaching behaviors of Cu in 1 mol/L sulfuric acid solution at 90 °C and 600 rpm with 10,000 mg/L Cu2+, 1–5% pulp density, and 1000 cc/min O2 introduction.
Studies on the leaching kinetics of Cu from PCBs were reported previously and investigations were carried out using HCl solution with Cl2 [42], NH4OH solution with H2O2 [14], HNO3 solution [43], and H2SO4 solution with Fe3+ [44]. Activation energy values were calculated using shrinking core model [14,42,43,44] and were determined to be 20.7–24.5 kJ/mol in HCl solution with Cl2 [42], 47.39 kJ/mol in NH4OH solution with H2O2 [14], 23.35 kJ/mol in HNO3 solution [43], and 14.87 kJ/mol in H2SO4 solution with Fe3+ [44], respectively. The shrinking core model can be presented by [45]
where Equation (6) represents the shrinking core model basically based on the surface chemical reaction (reaction-controlled model), while Equation (7) is based on the diffusion reaction (diffusion-controlled model). The value for x in the equations represents the fraction reacted and is obtained from the leaching efficiencies, while kr and kd are rate constants.
The data in Figure 4 were fitted to the models as shown in Figure 8 (reaction-controlled model) and Figure 9 (diffusion-controlled model). The correlation coefficients (R2) are 0.9768 to 0.9879 in Figure 8 and 0.8019 to 0.9774 in Figure 9, respectively, which indicate that the reaction-controlled model is more suitable to this leaching behavior than the diffusion-controlled model. Generally, PCB has a multi-layered structure composed of copper foil and resin. However, as shown in Figure 10, because the copper in PCBs was exposed during extensive milling with cutting and mixer mills, the reaction-controlled model is suitable for this PCBs’ leaching. Rate constants (k) for different temperature were calculated by plotting Figure 8, and the Arrhenius plots using the rate constants over T−1 are presented in Figure 11, where the activation energy is found to be 23.8 kJ/mol. The activation energy is similar to the data reported in conventional studies [14,42,43,44], which indicate that the leachate containing sulfuric acid with Cu2+ is effective for Cu leaching.
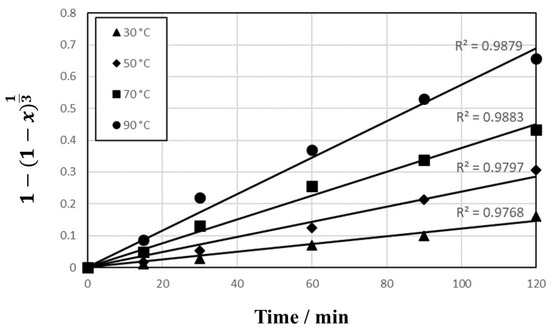
Figure 8.
Plot of 1 − (1 − x)1/3 vs. T as a function of time for Cu leaching at temperature 30–90 °C in 1 mol/L sulfuric acid solution.
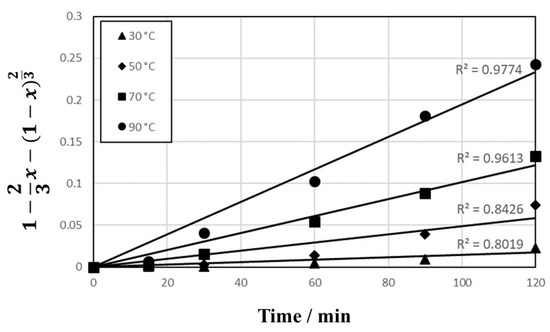
Figure 9.
Plot of 1 − 2/3x − (1 − x)2/3 vs. T as a function of time for Cu leaching at temperature 30–90 °C in 1 mol/L sulfuric acid solution.
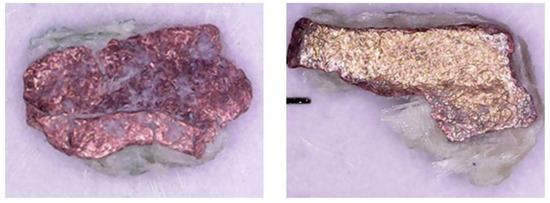
Figure 10.
Photos of ground PCBs used in the leaching tests.
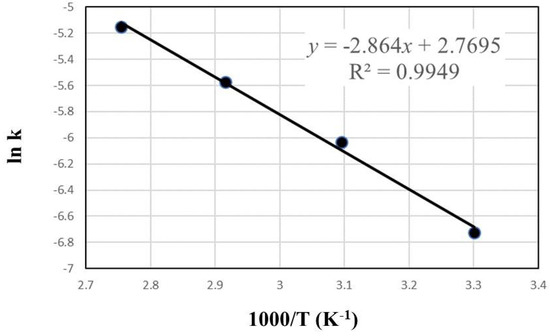
Figure 11.
Arrhenius plot for Cu leaching in 1 mol/L sulfuric acid solution.
4. Conclusions
Mild leaching conditions using Cu2+ and O2 in sulfuric acid solution were investigated to leach Cu from PCBs. When Cu2+ and O2 were added to the leach solution, the leaching efficiency of Cu increased faster than that of the leaching test performed without Cu2+ and O2. On the other hand, the simultaneous use of Cu2+ and O2 improved Cu leaching from PCBs. The leaching efficiency of Cu increased with the increasing agitation speed, temperature, gas flow rate of O2, and initial Cu2+ concentration and decreasing pulp density. The leaching efficiency of Cu increased to 96% in 1 mol/L sulfuric acid solution at temperature 90 °C, agitation speed 600 rpm, pulp density 1%, Cu2+ concentration 10,000 mg/L, and O2 introduction flow rate 1000 cc/min. The kinetic data indicate that the leaching behavior of Cu followed the reaction-controlled model well rather than the diffusion-controlled model, and the activation energy was calculated to be 23.8 kJ/mol, which is similar to the data reported in the conventional studies. Therefore, these results suggest that the sulfuric acid solution with Cu2+ and O2 addition will be a suitable leaching condition to leach Cu from PCBs.
Author Contributions
Methodology, Y.P. and K.Y.; writing—original draft preparation, Y.P. and K.Y.; data curation, Y.P., Y.E., and K.Y.; writing—review and editing, K.Y. and M.K.J.; providing ideas, Y.P. and K.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Technology Innovation Program (or Industrial Strategic Technology Development Program—Development of Material Component Technology) (20011176, Development of Advanced Technology in Hydrometallurgy for High Added Value of Resources Recovery) Funded by the Ministry of Trade, Industry, and Energy (MOTIE, Korea).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jung, M.; Yoo, K.; Alorro, R.D. Dismantling of electric and electronic components from waste printed circuit boards by hydrochloric acid leaching with stannic ions. Mater. Trans. 2017, 58, 1076–1080. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.Y.; Kim, M.S.; Lee, J.C.; Jha, M.K.; Yoo, K.; Jeong, J. Effect of cuprous ions on Cu leaching in the recycling of waste PCBs, using electro-generated chlorine in hydrochloric acid solution. Miner. Eng. 2008, 21, 121–128. [Google Scholar] [CrossRef]
- Yoo, J.M.; Jeong, J.; Yoo, K.; Lee, J.C.; Kim, W. Enrichment of the metallic components from waste printed circuit boards by a mechanical separation process using a stamp mill. Waste Manag. 2009, 29, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Na, H.; Yoo, K.; Jha, M.K.; Tabelin, C.B. The separation of aluminum and stainless-steel scraps using vibrating mixed-size ball bed. Metals 2020, 10, 868. [Google Scholar] [CrossRef]
- Bae, M.; Lee, H.; Kim, S.; Yoo, K. Destruction of cyanide and removal of copper from waste printed circuit boards leach solution using electro-generated hypochlorite followed by magnetite adsorption. Metals 2019, 9, 963. [Google Scholar] [CrossRef] [Green Version]
- An, H.; Kang, L.; Lee, C.G. Analysis of Commercial Recycling Technology and Research Trend of Printed Circuit Boards in Korea. Resour. Recycl. 2017, 26, 9–18. [Google Scholar] [CrossRef]
- Moyo, T.; Chirume, B.H.; Petersen, J. Assessing alternative pre-treatment methods to promote metal recovery in the leaching of printed circuit boards. Resour. Conserv. Recycl. 2020, 152, 104545. [Google Scholar] [CrossRef]
- Huang, Y.F.; Pan, M.W.; Lo, S.L. Hydrometallurgical metal recovery from waste printed circuit boards pretreated by microwave pyrolysis. Resour. Conserv. Recycl. 2020, 163, 105090. [Google Scholar] [CrossRef]
- Jeon, S.; Ito, M.; Tabelin, C.B.; Pongsumrankul, R.; Tanaka, S.; Kitajima, N.; Saito, A.; Park, I.; Hiroyoshi, N. A physical separation scheme to improve ammonium thiosulfate leaching of gold by separation of base metals in crushed mobile phones. Miner. Eng. 2019, 138, 168–177. [Google Scholar] [CrossRef]
- Zhu, X.N.; Nie, C.C.; Ni, Y.; Zhang, T.; Li, B.; Wang, D.Z.; Qu, S.; Qiao, F.; Lyu, X.; Qiu, J.; et al. Advanced utilization of copper in waste printed circuit boards: Synthesis of nano-copper assisted by physical enrichment. J. Hazard. Mater. 2020, 401, 123294. [Google Scholar] [CrossRef]
- Dávila-Pulido, G.I.; Salinas-Rodríguez, A.; Carrillo-Pedroza, F.R.; González-Ibarra, A.A.; Méndez-Nonell, J.; Garza-García, M. Leaching kinetics of electronic waste for the recovery of copper: Rate-controlling step and rate process in a multisize particle system. Int. J. Chem. Kinet. 2020, 53, 379–389. [Google Scholar] [CrossRef]
- Han, Y.; Yi, X.; Wang, R.; Huang, J.; Chen, M.; Sun, Z.; Sun, S.; Shu, J. Copper extraction from waste printed circuit boards by glycine. Sep. Purif. Technol. 2020, 253, 117463. [Google Scholar] [CrossRef]
- Li, H.; Oraby, E.; Eksteen, J. Extraction of copper and the co-leaching behaviour of other metals from waste printed circuit boards using alkaline glycine solutions. Resour. Conserv. Recy. 2020, 154, 104624. [Google Scholar] [CrossRef]
- Oluokun, O.O.; Otunniyi, I.O. Kinetic analysis of Cu and Zn dissolution from printed circuit board physical processing dust under oxidative ammonia leaching. Hydrometallurgy 2020, 193, 105320. [Google Scholar] [CrossRef]
- El-Nasr, R.S.; Abdelbasir, S.M.; Kamel, A.H.; Hassan, S.S. Environmentally friendly synthesis of copper nanoparticles from waste printed circuit boards. Sep. Purif. Technol. 2020, 230, 115860. [Google Scholar] [CrossRef]
- Tan, Q.; Liu, L.; Yu, M.; Li, J. An innovative method of recycling metals in printed circuit board (PCB) using solutions from PCB production. J. Hazard. Mater. 2020, 390, 121892. [Google Scholar] [CrossRef]
- Barnwal, A.; Dhawan, N. Recycling of discarded mobile printed circuit boards for extraction of gold and copper. Sustain. Mater. Technol. 2020, 25, e00164. [Google Scholar] [CrossRef]
- Tanısalı, E.; Özer, M.; Burat, F. Precious Metals Recovery from Waste Printed Circuit Boards by Gravity Separation and Leaching. Min. Proc. Ext. Met. Rev. 2021, 42, 1–14. [Google Scholar] [CrossRef]
- Cui, H.; Anderson, C. Hydrometallurgical Treatment of Waste Printed Circuit Boards: Bromine Leaching. Metals 2020, 10, 462. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.M.; Costa, F.O.; Gomes, R.F.; Rodrigues, M.L.M.; da Silva, G.A.; Leão, V.A. Multivariate study of a novel hydrometallurgical route employing chloride/hypochlorite for leaching silver from printed circuit boards. Chem. Eng. Res. Des. 2020, 163, 115–124. [Google Scholar] [CrossRef]
- He, J.; Yang, J.; Tariq, S.M.; Duan, C.; Zhao, Y. Comparative investigation on copper leaching efficiency from waste mobile phones using various types of ionic liquids. J. Clean. Prod. 2020, 256, 120368. [Google Scholar] [CrossRef]
- Liu, Q.; Bai, J.F.; Gu, W.H.; Peng, S.J.; Wang, L.C.; Wang, J.W.; Li, H.X. Leaching of copper from waste printed circuit boards using Phanerochaetechrysosporium fungi. Hydrometallurgy 2020, 196, 105427. [Google Scholar] [CrossRef]
- Van Yken, J.; Cheng, K.Y.; Boxall, N.J.; Nikoloski, A.N.; Moheimani, N.; Valix, M.; Sahajwalla, V.; Kaksonen, A.H. Potential of metals leaching from printed circuit boards with biological and chemical lixiviants. Hydrometallurgy 2020, 196, 105433. [Google Scholar] [CrossRef]
- Erust, C.; Akcil, A.; Tuncuk, A.; Panda, S. Intensified acidophilic bioleaching of multi-metals from waste printed circuit boards (WPCBs) of spent mobile phones. J. Chem. Technol. Biotechnol. 2020, 95, 2272–2285. [Google Scholar] [CrossRef]
- Sodha, A.B.; Tipre, D.R.; Dave, S.R. Optimisation of biohydrometallurgical batch reactor process for copper extraction and recovery from non-pulverized waste printed circuit boards. Hydrometallurgy 2020, 191, 105170. [Google Scholar] [CrossRef]
- Tong, L.; Zhao, Q.; Kamali, A.R.; Sand, W.; Yang, H. Effect of Graphite on Copper Bioleaching from Waste Printed Circuit Boards. Minerals 2020, 10, 79. [Google Scholar] [CrossRef] [Green Version]
- Marappa, N.; Ramachandran, L.; Dharumadurai, D.; Nooruddin, T. Recovery of gold and other precious metal resources from environmental polluted E-waste printed circuit board by bioleaching Frankia. Int. J. Environ. Res. 2020, 14, 165–176. [Google Scholar] [CrossRef]
- Cho, B.G.; Cho, Y.J.; Lee, J.C.; Yoo, K. Korea’s metal resources recycling research project–valuable recycling. Geosyst. Eng. 2019, 22, 48–58. [Google Scholar] [CrossRef]
- Lim, Y.; Kwon, O.H.; Lee, J.; Yoo, K. The ammonia leaching of alloy produced from waste printed circuit boards smelting process. Geosyst. Eng. 2013, 16, 216–224. [Google Scholar] [CrossRef]
- Park, I.; Yoo, K.; Alorro, R.D.; Kim, M.S.; Kim, S.K. Leaching of copper from cuprous oxide in aerated sulfuric acid. Mater. Trans. 2017, 58, 1500–1504. [Google Scholar] [CrossRef] [Green Version]
- Bae, M.; Lee, H.; Yoo, K.; Kim, S. Copper (I) selective chemisorption on magnetite (Fe3O4) over gold (I) ions in chloride solution with cyanide. Hydrometallurgy 2021, 201, 105560. [Google Scholar] [CrossRef]
- Brett, C.M.A.; Brett, A.M.O. Electrochemistry; Oxford University Press Inc.: New York, NY, USA, 1993; pp. 416–419. [Google Scholar]
- Jeon, S.; Park, I.; Yoo, K.; Ryu, H. The effects of temperature and agitation speed on the leaching behaviors of tin and bismuth from spent lead free solder in nitric acid leach solution. Geosyst. Eng. 2015, 18, 213–218. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.C.; Lee, K.S.; Yoo, K.; Alorro, R.D. Separation of tin, silver and copper from waste Pb-free solder using hydrochloric acid leaching with hydrogen peroxide. Mater. Trans. 2014, 55, 1885–1889. [Google Scholar] [CrossRef] [Green Version]
- Yoo, K.; Lee, J.C.; Lee, K.S.; Kim, B.S.; Kim, M.S.; Kim, S.K. Recovery of Sn, Ag and Cu from waste Pb-free solder using nitric acid leaching. Mater. Trans. 2012, 53, 2175–2180. [Google Scholar] [CrossRef] [Green Version]
- Yoo, K.; Lee, K.; Jha, M.K.; Lee, J.C.; Cho, K. Preparation of nano-sized tin oxide powder from waste Pb-free solder by direct nitric acid leaching. J. Nanosci. Nanotechnol. 2016, 16, 11238–11241. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, J.C.; Yoo, K. Leaching of tin from waste Pb-free solder in hydrochloric acid solution with stannic chloride. Hydrometallurgy 2016, 165, 143–147. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoo, K.; Jha, M.K.; Lee, J.C. Separation of Sn from waste Pb-free Sn–Ag–Cu solder in hydrochloric acid solution with ferric chloride. Hydrometallurgy 2015, 157, 184–187. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Yoo, K.; Jha, M.K.; Park, J.; Choi, U.; Choe, H.; Lee, J.C. Removal of heavy metals from tailing in citrate solution with ferric chloride. Mater. Trans. 2018, 59, 1665–1668. [Google Scholar] [CrossRef]
- Yoo, K.; Park, Y.; Choi, S.; Park, I. Improvement of Copper Metal Leaching in Sulfuric Acid Solution by Simultaneous Use of Oxygen and Cupric Ions. Metals 2020, 10, 721. [Google Scholar] [CrossRef]
- Park, Y.; Yoo, K. Dismantling of Components from Waste Printed Circuit Boards Using Stannic Chloride Solution. Resour. Recy. 2021, 30, 24–30. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, M.S.; Lee, J.C.; Jeong, J.; Pandey, B.D. Leaching kinetics of copper from waste printed circuit boards by electro-generated chlorine in HCl solution. Hydrometallurgy 2011, 107, 124–132. [Google Scholar] [CrossRef]
- Dutta, D.; Panda, R.; Kumari, A.; Goel, S.; Jha, M.K. Sustainable recycling process for metals recovery from used printed circuit boards (PCBs). Sustain. Mater. Technol. 2018, 17, e00066. [Google Scholar] [CrossRef]
- Laubertová, M.; Derin, B.; Trpčevská, J.; Šándorová, K.; Sminčáková, E. Metals Recovery: Study of the Kinetic Aspects of Copper Acidic Leaching Waste Printed Circuit Boards from Discarded Mobile Phones. ActaMontanisticaSlovaca 2019, 24, 223–233. [Google Scholar]
- Lee, S.H.; Kwon, O.; Yoo, K.; Alorro, R.D. Removal of Zn from contaminated sediment by FeCl3 in HCl solution. Metals 2015, 5, 1812–1820. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).