Substrate Impact on the Structure and Electrocatalyst Properties of Molybdenum Disulfide for HER from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrothermal Synthesis of MoS2 Species
2.3. Ti Surface Pretreatment, Anodizing, and Oxidation
2.4. Characterization Tests
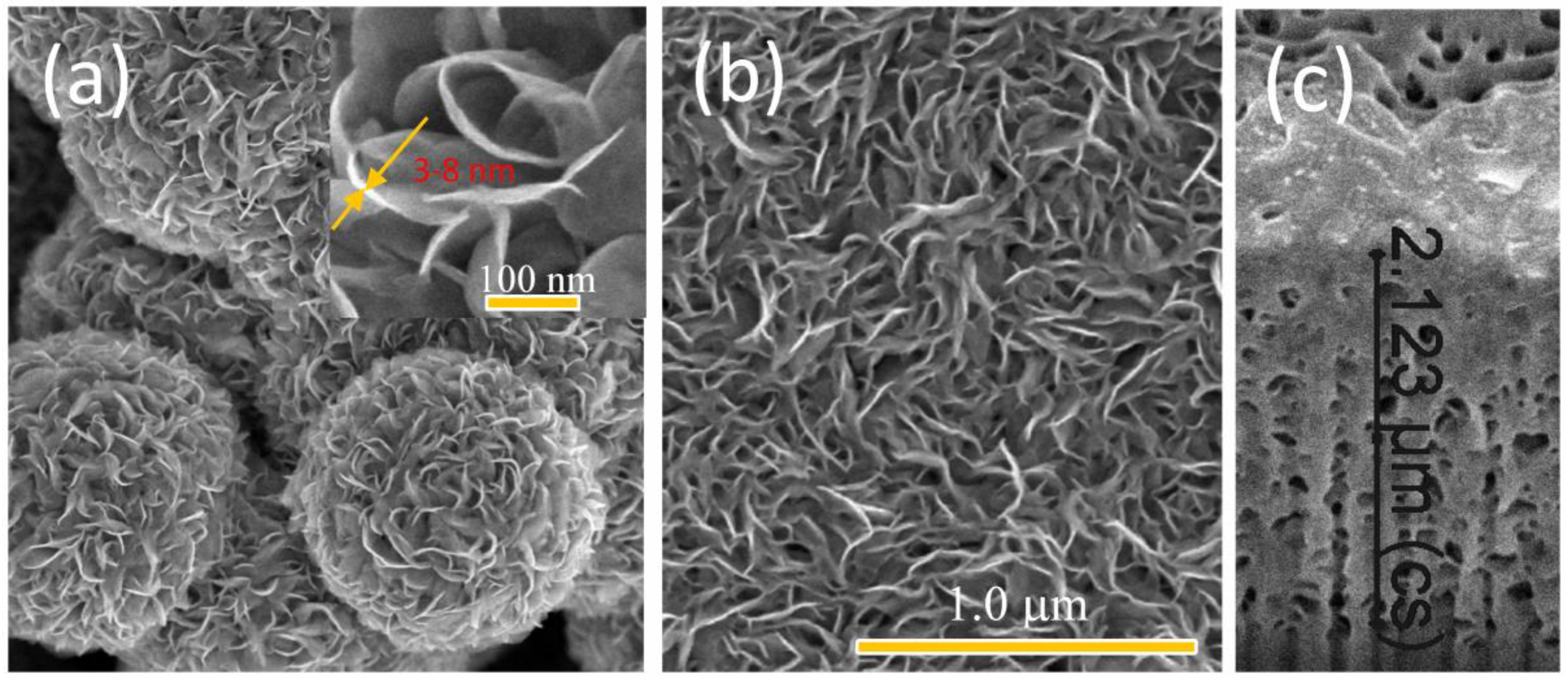
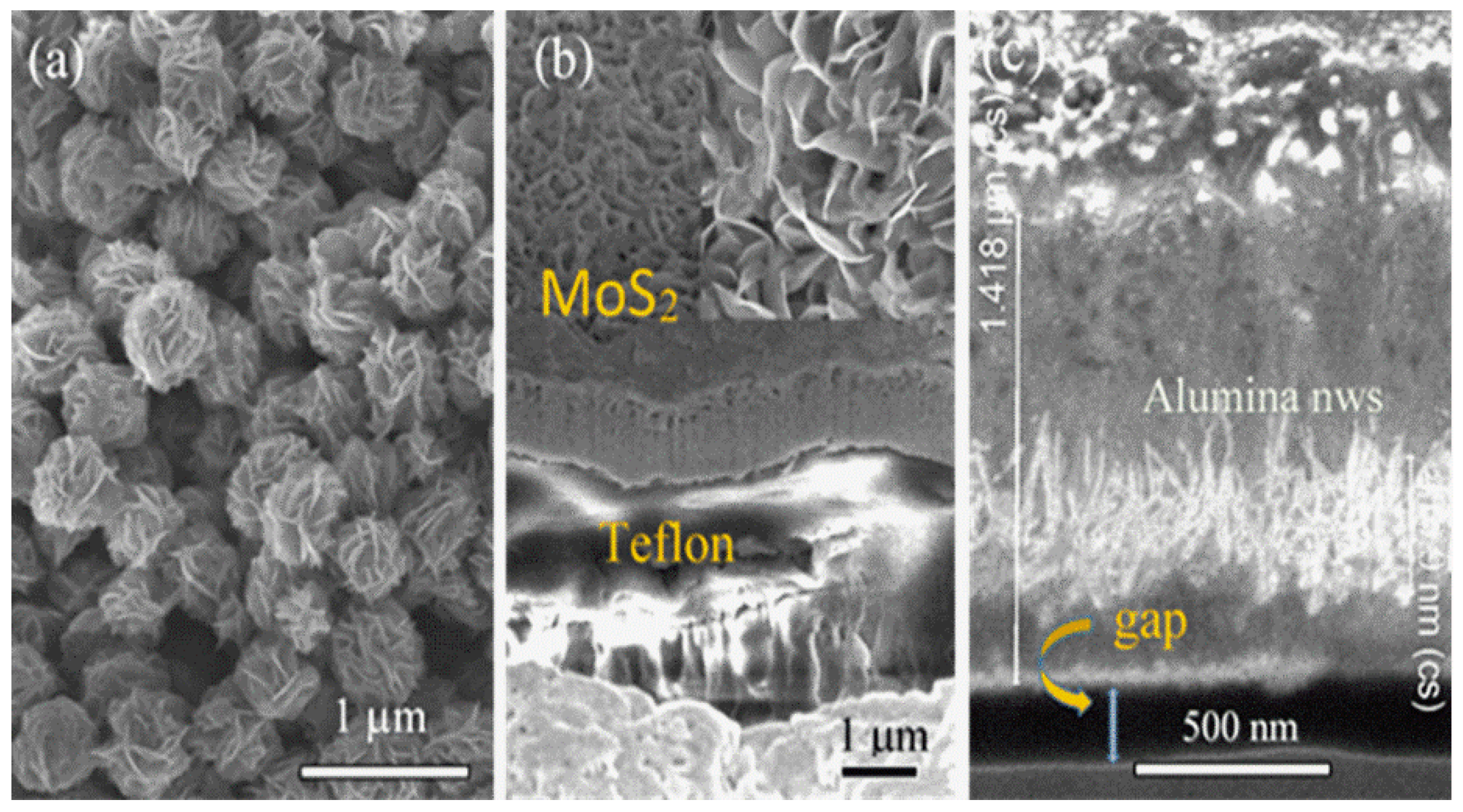
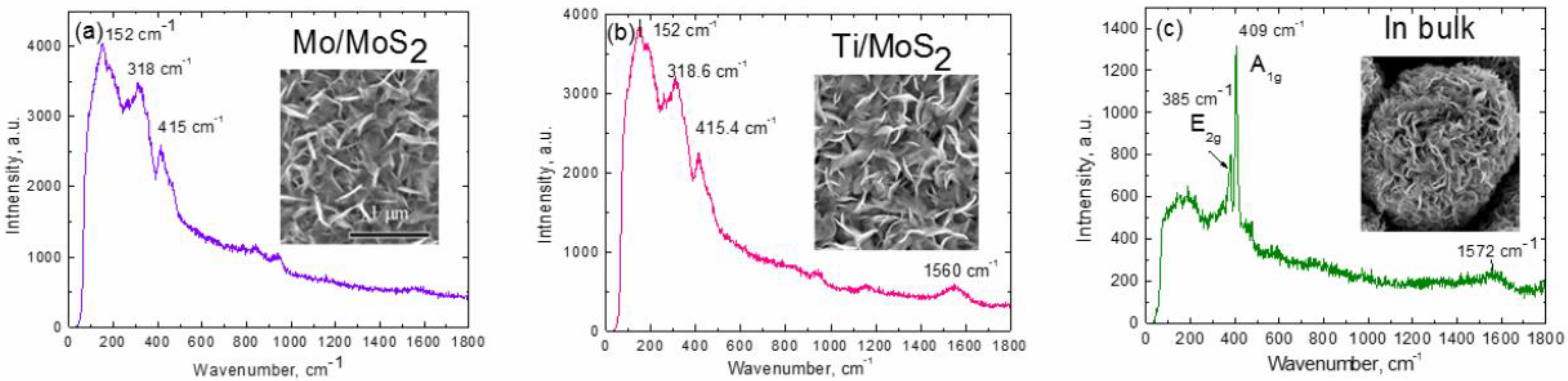
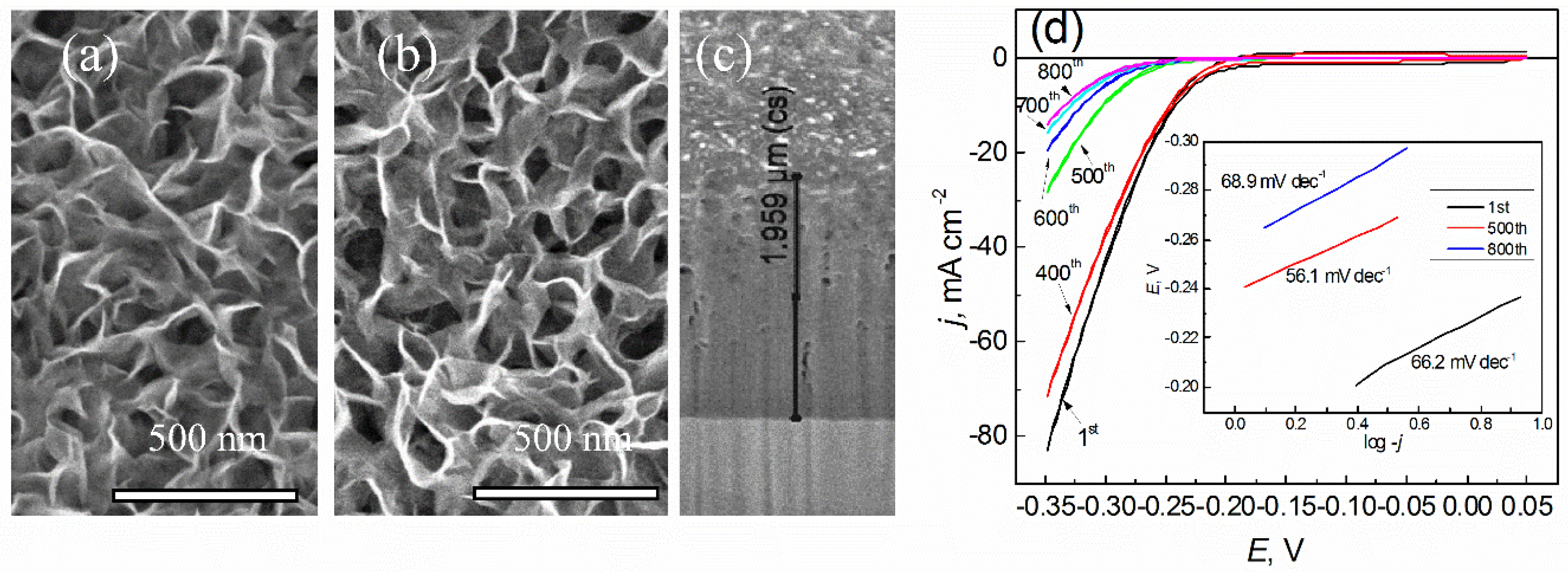
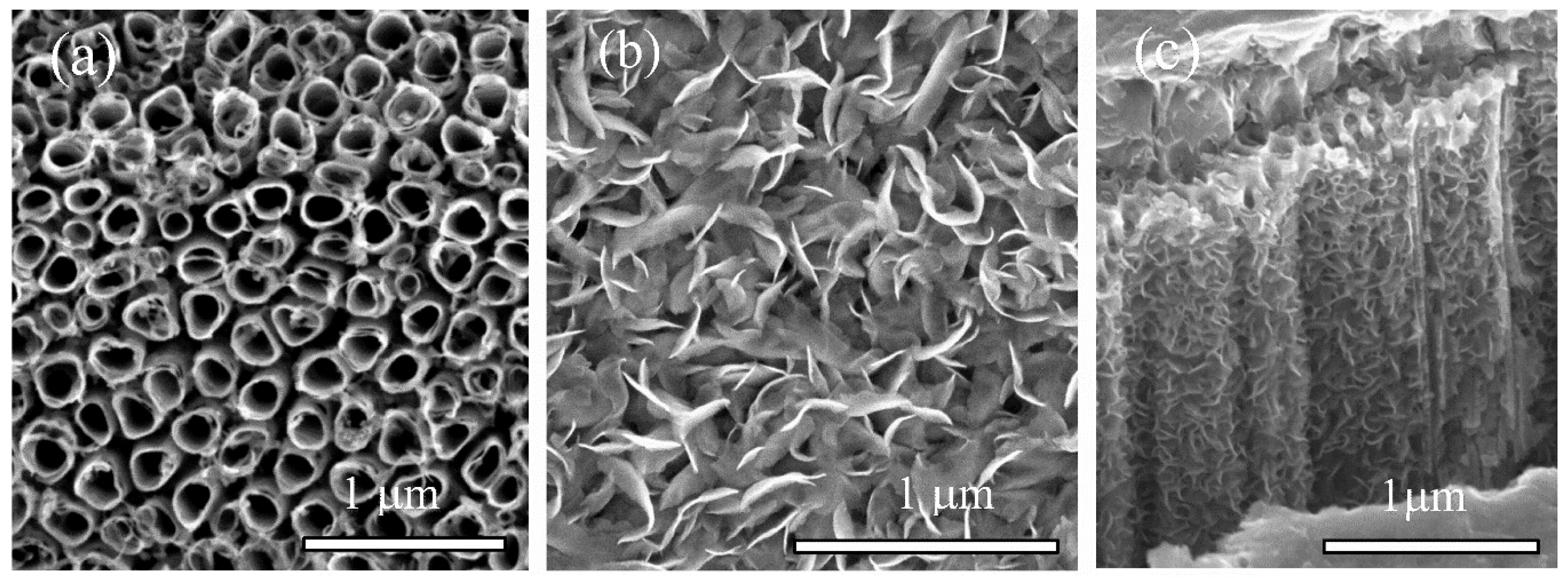
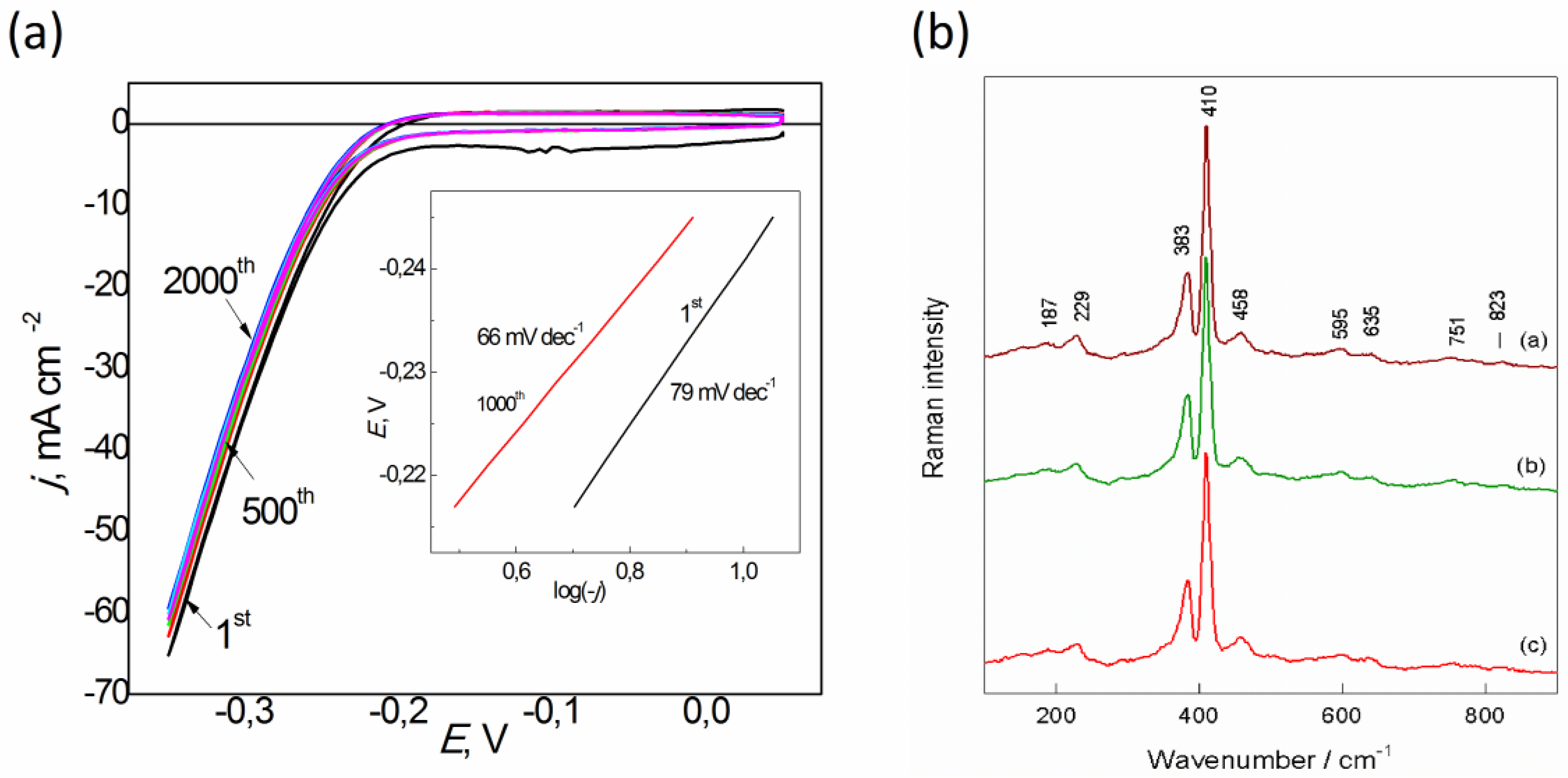
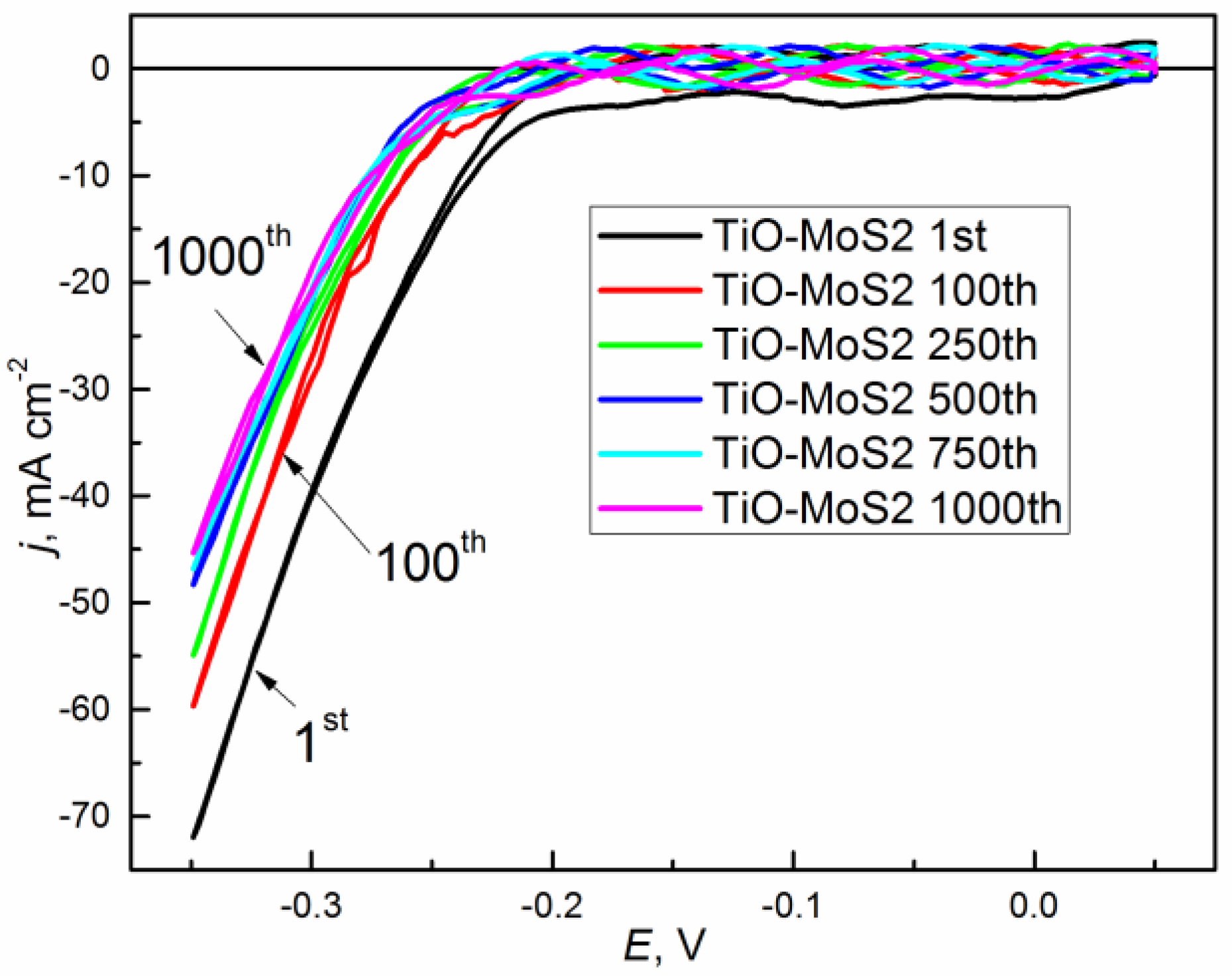
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, L.; Zhou, W.; Hou, D.; Zhai, K.; Li, G.; Tang, Z.; Li, L.; Chen, S. Porous metallic MoO2-supported MoS2 nanosheets for enhanced electrocatalytic activity in the hydrogen evolution reaction. Nanoscale 2015, 7, 5203–5208. [Google Scholar] [CrossRef]
- Kong, D.; Wang, H.; Cha, J.J.; Pasta, M.; Koski, J.; Yao, J.; Cui, Y. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett. 2013, 13, 1341–1347. [Google Scholar] [CrossRef]
- Tang, C.; Sun, A.; Xu, Y.; Wang, D. High specific surface area Mo2C nanoparticles as an efficient electrocatalyst for hydrogen evolution. J. Power Sources 2015, 296, 18–22. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, W.; Li, G.; Tang, Z.; Li, L.; Chen, S. MoS2 nanosheet-coated CoS2 nanowire arrays on carbon cloth as three-dimensional electrodes for efficient electrocatalytic hydrogen evolution. J. Mater. Chem. A 2015, 3, 22886–22891. [Google Scholar]
- Liu, Y.-R.; Hu, W.-H.; Han, G.-Q.; Dong, B.; Chai, Y.-M.; Liu, Y.-Q.; Liu, C.-G. Facile synthesis of one-dimensional MoOx-based nanostructure for electrocatalytic hydrogen evolution. ECS Electrochem. Lett. 2015, 4, H5–H9. [Google Scholar] [CrossRef]
- McKone, J.R.; Marinescu, S.C.; Brunschwig, B.S.; Winkler, J.R.; Gray, H.B. Earth-abundant hydrogen evolution electrocatalysts. Chem. Sci. 2014, 5, 865–878. [Google Scholar] [CrossRef]
- Xiao, P.; Yan, Y.; Ge, X.; Liu, Z.; Wang, J.Y.; Wang, X. Investigation of molybdenum carbide nano-rod as an efficient and durable electrocatalyst for hydrogen evolution in acidic and alkaline media. Appl. Catal. B Environ. 2014, 154–155, 232–237. [Google Scholar] [CrossRef]
- McEnaney, J.M.; Crompton, J.C.; Callejas, J.F.; Popczun, E.J.; Biacchi, A.J.; Lewis, N.S.; Schaak, R.E. Amorphous molybdenum phosphide nanoparticles for electrocatalytic hydrogen evolution. Chem. Mater. 2014, 26, 4826–4831. [Google Scholar] [CrossRef]
- Kong, D.; Cha, J.J.; Wang, H.; Lee, H.R.; Cui, Y. First-row transition metal dichalcogenide catalysts forhydrogen evolution reaction. Energy Environ. Sci. 2013, 6, 3553–3558. [Google Scholar] [CrossRef]
- Wu, Z.; Fang, B.; Wang, Z.; Wang, C.; Liu, Z.; Liu, F.; Wang, W.; Alfantazi, A.; Wang, D.; Wilkinson, D.P. MoS2 nanosheets: A designed structure with high active site density for the hydrogen evolution reaction. ACS Catal. 2013, 3, 2101–2107. [Google Scholar] [CrossRef]
- Bian, X.; Zhu, J.; Liao, L.; Scanlon, M.D.; Ge, P.; Ji, C.; Girault, H.H.; Liu, B. Nanocomposite of MoS2 on ordered mesoporous carbon nanospheres: A highly active catalyst for electrochemical hydrogen evolution. Electrochem. Commun. 2012, 22, 128–132. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jorgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS2 Nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Jagminas, A.; Niaura, G.; Žalnėravičius, R.; Trusovas, R.; Račiukaitis, G.; Jasulaitienė, V. Laser light-induced transformation of molybdenum disulfide-based nanoplatelet arrays. Sci. Rep. 2016, 6, 37514. [Google Scholar] [CrossRef] [PubMed]
- Bonde, J.; Moses, P.G.; Jaramillo, T.F.; Norskov, J.K.; Chorkendorff, J. Hydrogen evolution on nano-particulate transition metal sulfides. Faraday Discuss. 2008, 140, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, T.F.; Bonde, J.; Zhang, J.; Ooi, B.-L.; Andersson, K.; Ulstrup, J.; Chorkendorff, I. Hydrogen Evolution on Supported Incomplete Cubane-type [Mo3S4]4+ Electrocatalysts. J. Phys. Chem. C 2008, 112, 17492–17498. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 Nanoparticles Grown on Graphene: An Advanced Catalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Robinson, J.T.; Diankov, G.; Dai, H. Nanocrystal Growth on Graphene with Various Degrees of Oxidation. J. Am. Chem. Soc. 2010, 132, 3270–3271. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Wang, H.J.; Casalongue, H.S.; Chen, Z.; Dai, H.J. TiO2 Nanocrystals Grown on Graphene as Advanced Photocatalytic Hybrid Materials. Nano Res. 2010, 3, 701–705. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Bar, S.; Zhang, Z.; Fei, H.; Wu, Z. Phase engineering of a multiphasic 1T/2H MoS2 catalyst for highly efficient hydrogen evolution. J. Mater. Chem. A 2017, 5, 2681–2688. [Google Scholar] [CrossRef]
- Lu, X.J.; She, G.W.; Zhou, S.X.; Li, Y.M. Highly efficient electrocatalytic hydrogen production by nickel promoted molybdenum sulfide microspheres catalysts. RSC Adv. 2013, 3, 21231–21236. [Google Scholar]
- Chen, Z.B.; Cummins, D.; Reinecke, B.N.; Clark, E.; Sunkara, M.K.; Jaramillo, T.F. Core-shell MoO3-MoS2 nanowires for hydrogen evolution: A functional design for electrocatalytic materials. Nano Lett. 2011, 11, 4168–4175. [Google Scholar] [CrossRef] [PubMed]
- Aditya, T.; Nayak, A.K.; Pradhan, D.; Pal, A.; Pal, T. Fabrication of MoS2 decorated reduced graphene oxide sheets from solid Mo-precursor for electrolytic hydrogen evolution reaction. Electrochimica Acta 2019, 313, 341–351. [Google Scholar] [CrossRef]
- Yan, Y.; Ge, X.M.; Liu, Z.L.; Wang, J.Y.; Lee, J.M.; Wang, X. Facile synthesis of low crystalline MoS2 nanosheet-coated CNTs for enhanced hydrogen evolution reaction. Nanoscale 2013, 5, 7768–7771. [Google Scholar] [CrossRef]
- Liao, L.; Zhu, J.; Bian, X.; Zhu, L.; Scanlon, M.D.; Girault, H.H.; Liu, B. MoS2 formed on mesoporous graphene as a highly active catalyst for hydrogen evolution. Adv. Func. Mater. 2013, 23, 5326–5333. [Google Scholar] [CrossRef]
- Park, S.; Chun, D.; Ko, D.; Sung, Y.; Piao, Y. Three-dimensional carbon foam/N-doped graphene@MoS2 hybrid nanostructures as effective electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 12720–12725. [Google Scholar] [CrossRef]
- Geng, X.; Sun, W.; Wu, W.; Chen, B.; Hilo, A.A.; Benamara, M.; Zhu, H.; Watanabe, F.; Cui, J.; Chen, T.P. Pure and stable metallic phase molybdenum disulphide nanosheets for hydrogen evolution reaction. Nat. Commun. 2016, 7, 10672. [Google Scholar] [CrossRef]
- Liu, Q.; Fang, Q.; Chu, W.; Wan, Y.; Li, X.; Xu, W.; Habib, M.; Tao, S. Electron-doped 1T-MoS2 via interface engineering for enhanced electrocatalytic hydrogen evolution. Chem. Mater. 2017, 29, 4738–4744. [Google Scholar] [CrossRef]
- Hu, J.J.; Zabinski, J.S.; Sanders, J.H.; Bultman, J.E.; Vacvodin, A.A. Pulsed Laser Syntheses of Layer-Structured WS2 Nanomaterials in Water. J. Phys. Chem. B 2006, 110, 8914–8916. [Google Scholar] [CrossRef]
- Zelenski, C.M.; Dorhout, R.K. Template Synthesis of Near-Monodisperse1 Microscale Nanofibers and Nanotubules of MoS2. J. Am. Chem. Soc. 1998, 120, 734–742. [Google Scholar] [CrossRef]
- Feldman, Y.; Wassermann, E.; Srolovitz, D.J.; Tenne, R. High-Rate, Gas-Phase Growth of MoS2 Nested Inorganic Fullerenes and Nanotubes. Science 1995, 267, 222–225. [Google Scholar] [CrossRef]
- Ellmer, K.; Mientus, R.; Seeger, S.; Weiβ, V. Highly (001)-textured WS2−x films prepared by reactive radio frequency magnetron sputtering. Phys. Status Solidi A 2004, 201, R97–R100. [Google Scholar] [CrossRef]
- Wu, Z.; Tang, C.; Zhou, P.; Liu, Z.; Xu, Y.; Wang, D.; Fang, B. Enhanced hydrogen evolution catalysis from osmotically swollen ammoniated MoS2. J. Mater. Chem. A 2015, 3, 13050–13056. [Google Scholar] [CrossRef]
- Ma, L.; Chen, W.X.; Li, H.; Xu, Z.D. Synthesis and characterization of MoS2 nanostructures with different morphologies via an ionic liquid-assisted hydrothermal route. Mater. Chem. Phys. 2009, 116, 400–405. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, H.; Zheng, Y.; Hu, Y.; MacLennan, A. Understanding the effect of synthesis temperature on the crystallization and activity of nano-MoS2 catalyst. Appl. Catal. B Environ. 2015, 165, 537–546. [Google Scholar] [CrossRef]
- Dhas, N.A.; Suslikk, K.S. Sonochemical Preparation of Hollow Nanospheres and Hollow Nanocrystals. J. Am. Chem. Soc. 2005, 127, 2368–2369. [Google Scholar] [CrossRef]
- Merki, D.; Vrubel, H.; Rovelli, L.; Fierro, S.; Hu, X. Fe, Co, and Ni ions promote the catalytic activity of amorphous molybdenum sulfide films for hydrogen evolution. Chem. Sci. 2012, 3, 2515–2525. [Google Scholar] [CrossRef]
- Jagminas, A.; Ramanavicius, S.; Jasulaitienė, V.; Šimėnas, M. Hydrothermal synthesis and characterization of nanostructured titanium monoxide films. RSC Adv. 2019, 9, 40727–40735. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, D.; Sun, A. Preparation of MoS2 nanoflakes by a novel mechanical activation method. J. Cryst. Growth 2010, 312, 340–343. [Google Scholar] [CrossRef]
- Xiong, X.Q.; Luo, W.; Hu, X.L.; Chen, C.J.; Qie, L.; Hou, D.F.; Huang, Y.H. Flexible Membranes of MoS2/C Nanofibers by Electrospinning as Binder-Free Anodes for High-Performance Sodium-Ion Batteries. Sci. Rep. 2015, 5, 9254. [Google Scholar]
- Kerr, T.A.; Wu, H.; Nazar, L.F. Concurrent polymerization and insertion of aniline in molybdenum trioxide: Formation and properties of a [Poly(aniline)]0.24MoO3 nanocomposite. Chem. Mater. 1996, 8, 2005–2015. [Google Scholar] [CrossRef]
- Kanatzidis, G.; Bissessur, R.; deGroot, D.C.; Schindler, J.L.; Kannewurf, C.R. New intercalation compounds of conjugated polymers. Encapsulation of polyaniline in MoS2. Chem. Mater. 1993, 5, 595–596. [Google Scholar] [CrossRef]
- Seguin, L.; Figlarz, M.; Cavagnat, R.; Lassegues, J.-C. Infrared and Raman spectra of MoO3 molybdenum trioxides and MoO3 xH2O molybdenum trioxide hydrates. Spectrochim. Acta Part A 1995, 51, 1323–1344. [Google Scholar] [CrossRef]
- Li, S.L. Qantitative Raman spectrum and reliable thickness identification for atomic layers on insulating substrates. ACS. Nano. 2012, 6, 7381–7388. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Zhang, Y.; Gao, T.; Zhang, Y.; Ma, D.; Liu, M.; Chen, Y.; Qiao, X.; Tan, P.H.; Kan, M. Epitaxial Monolayer MoS2 on Mica with Novel Photoluminescence. Nano Lett. 2013, 13, 3870–3877. [Google Scholar] [CrossRef]
- Das, S.; Chen, H.-Y.; Penumatch, A.V.; Appenzeller, J. High performance multilayer MoS2 transistors with scandium contacts. Nano. Lett. 2013, 13, 100–105. [Google Scholar] [CrossRef]
- He, Z.; Que, W. Molybdenum disulfide nanomaterials: Structure, properties, synthesis and recent progress on hydrogen evolution reaction. Appl. Mater. Today 2016, 3, 23–56. [Google Scholar] [CrossRef]
- Li, G.; Liu, Z.-Q.; Lu, J.; Wang, L.; Zhang, Z. Effect of calcination temperature on the morphology and surface properties of TiO2 nanotube array. Appl. Surf. Sci. 2009, 255, 7323–7328. [Google Scholar] [CrossRef]
- Paulose, M.; Varghese, O.K.; Mor, G.K.; Grimes, C.A.; Ong, K.G. Unprecedented ultra-high hydrogen gas sensitivity in undopted titania nanotubes. Nanotechnology 2005, 17, 398–402. [Google Scholar] [CrossRef]
- Chen, G.; Song, X.; Guan, L.; Chai, J.; Zhang, H.; Wang, S.; Pan, J.; Tao, J. Defect assisted coupling of a MoS2/TiO2 interface and tuning of its electronic structure. Nanotechnology 2016, 27, 355203. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Guo, H.; Chen, W.; Yuan, J.; Martinez, U.; Gupta, G.; Mohite, A.; Ajayan, P.M.; Lou, J. Unveiling Active Sites for the Hydrogen Evolution Reaction of Monolayer MoS2. Adv. Mater. 2017, 29, 1701955. [Google Scholar] [CrossRef] [PubMed]
- Roland, U.; Braunscheig, T.; Roessner, F. On the nature of spilt-over hydrogen. J. Mol. Catal. A 1997, 127, 61–84. [Google Scholar] [CrossRef]
- Jagminas, A.; Kovger, J.; Selskis, A.; Reza, A. Effect of hydrogen doping on the loading of titania nanotube films with copper selenide species via alternating current deposition. J. Appl. Electrochem. 2015, 45, 1141–1151. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagminas, A.; Naujokaitis, A.; Gaigalas, P.; Ramanavičius, S.; Kurtinaitienė, M.; Trusovas, R. Substrate Impact on the Structure and Electrocatalyst Properties of Molybdenum Disulfide for HER from Water. Metals 2020, 10, 1251. https://doi.org/10.3390/met10091251
Jagminas A, Naujokaitis A, Gaigalas P, Ramanavičius S, Kurtinaitienė M, Trusovas R. Substrate Impact on the Structure and Electrocatalyst Properties of Molybdenum Disulfide for HER from Water. Metals. 2020; 10(9):1251. https://doi.org/10.3390/met10091251
Chicago/Turabian StyleJagminas, Arūnas, Arnas Naujokaitis, Paulius Gaigalas, Simonas Ramanavičius, Marija Kurtinaitienė, and Romualdas Trusovas. 2020. "Substrate Impact on the Structure and Electrocatalyst Properties of Molybdenum Disulfide for HER from Water" Metals 10, no. 9: 1251. https://doi.org/10.3390/met10091251
APA StyleJagminas, A., Naujokaitis, A., Gaigalas, P., Ramanavičius, S., Kurtinaitienė, M., & Trusovas, R. (2020). Substrate Impact on the Structure and Electrocatalyst Properties of Molybdenum Disulfide for HER from Water. Metals, 10(9), 1251. https://doi.org/10.3390/met10091251





