Abstract
The heart rate slow component (scHR) is an intensity-dependent HR increment that emerges during constant exercises, partially dissociated from metabolism (O2). The scHR has been observed during constant-workload exercise in young and older adults. Unless this scHR is accounted for, exercise prescription using HR targets lead to an undesired reduction in metabolic intensity over time. Purpose: The purpose of this study is to characterize scHR across intensities, sex, and age to develop and validate a predictive equation able to maintain the desired metabolic stimulus over time in a constant aerobic exercise session. Methods: In our study, 66 individuals (35 females; 35 ± 13 yrs) performed the following: (i) a ramp-test for respiratory exercise threshold (GET and RCP) and maximal oxygen uptake (O2max) detection, and (ii) 6 × 9-minute constant exercises at different intensities. The scHR was calculated by linear fitting from the fifth minute of exercise (bpm⋅min−1). A multiple-linear equation was developed to predict the scHR based on individual and exercise variables. The validity of the equation was tested on an independent sample by a Pearson correlation and Bland–Altman analysis between the measured and estimated HR during constant exercises. Results: The scHR increases with intensity and is larger in males (p < 0.05). A multiple-linear equation predicts the scHR based on the relative exercise intensity to RCP, age, and sex (r2 = 0.54, SEE = 0.61 bpm⋅min−1). scHR (bpm⋅min−1) = −0.0514 + (0.0240 × relative exercise intensity to RCP) − (0.0172 × age) − (0.347 × Sex (males = 0 and females score = 1)). In the independent sample, we found an excellent correlation between the measured and estimated HR (r2 = 0.98, p < 0.001) with no bias (−0.01 b·min−1, z-score= −0.04) and a fair precision (±4.09 b·min−1). Conclusions: The dynamic of the scHR can be predicted in a heterogeneous sample accounting for the combined effects of relative intensity, sex, and age. The above equation provides the means to dynamically adapt HR targets over time, avoiding an undesired reduction in the absolute and relative training load. This strategy would allow the maintenance of the desired metabolic stimulus (O2) throughout an exercise session in a heterogeneous population.
1. Introduction
Aerobic physical activity induces specific metabolic stimuli and adaptations able to improve the cardiometabolic fitness in a given individual in a dose–response manner [1,2]. As such, aerobic exercise is a fundamental ingredient of any training intervention for health promotion [3]. The exercise prescription dose is typically quantified by these four elements: frequency, intensity, time, and type of exercise (according to the FITT scheme) [4]. Frequency, time, and type are relatively easy to determine, manipulate, and monitor, while intensity remains the most complex and elusive term of an exercise prescription dose [5,6].
Exercise intensity can be expressed in “absolute” or “relative” terms [7]. Absolute intensity refers to the energy required to perform a specific activity, and, for aerobic exercise, this can be measured through a metabolic equivalent as the oxygen uptake [5]. On the contrary, relative intensity refers to the stress imposed on the body’s homeostasis during exercise and is typically expressed as a percentage of anchor measurements, such as the maximal or reserve oxygen uptake (%O2max and %O2R) [5,8]. The implementation of either the absolute or relative exercise intensity outside of a laboratory environment typically entails the transition of the metabolic equivalent into an external load such as speed, watt, or pace that elicits the desired metabolic intensity (e.g., O2 or %O2max) [9]. Whenever this approach is impossible or impractical, the heart rate (HR) is commonly used as an easy-to-measure and inexpensive intensity index to prescribe and monitor the exercise intensity in both clinical and sports settings [10]. The prescription of exercise intensity using HR targets relies on the existence and constancy over time of a linear relationship between the HR and oxygen uptake (as either O2 or %O2max) [11,12]. Accordingly, guidelines for exercise intensity prescription typically include HR targets (%HRmax or %HRreserve) intended to generate specific metabolic stimuli and, in turn, specific training adaptations [3].
However, recent studies have raised our awareness of a problem that has been underappreciated: during prolonged constant-work exercise, a time-dependent mismatch emerges between HR and oxygen consumption as a result of a slow rise in HR, partially independent of metabolism (i.e., heart rate slow component) [13,14,15,16]. This phenomenon is present from the moderate to the severe domains, with an amplitude that appears larger with increasing intensity [13,14,15]. On the one hand, the failure of the heart rate to attain a steady state response hinders the accurate association of a univocal %HRmax or HR target to any exercise intensity [6,13]. On the other hand, when a constant HR target is maintained over time, the workload will be progressively reduced throughout the exercise session; this, in turn, will lead to an undesired reduction in the metabolic stimulus that was intended to be constant [6,15,16,17]. Therefore, the practical impact of ignoring the HR slow component when prescribing exercise (anchored to an %HRmax or HR target) may be minimal or severe depending on its amplitude, which can differ across intensities and in different populations, fitness levels, or diseases [13,14,16,17].
The heart rate slow component (scHR) has been repeatedly described in male populations (i.e., healthy adults [14,17], and adolescents suffering from obesity [15]), and in a group of postmenopausal women [13]. Interestingly, in postmenopausal women, the scHR was found to be one-third smaller compared to what was reported for adult males [13,14]. In our former study [13], we hypothesized that the observed smaller dynamic of HR could be related to (i) a higher potential for HR excursion (i.e., a larger HR reserve) in younger individuals compared to older ones, and/or (ii) a higher absolute heat production in younger and fitter males compared to older females that may have affected the core temperature over time.
Regarding the possible aging effect, a study conducted on obese adolescents (15) found an scHR slightly lower compared to what was reported for adult males [14] (respectively, in the moderate and heavy domain: ≈0.4 and ≈2.4 vs. ≈0.9 and ≈2.9 bpm⋅min−1). Nevertheless, this discrepancy might not have been derived from the age differences but from the lower catecholamine response affecting individuals suffering from obesity that, in turn, might have blunted the HR dynamics [15].
In addition, 3 weeks of moderate aerobic training has been shown to attenuate the scHR [15]; the findings appear to suggest an effect of the cardiorespiratory fitness level on the scHR. As such, the aging O2max decline (about ~10% every 10 years [18]) might, therefore, reduce the scHR in older individuals compared to young ones. However, nowadays, no studies have tested these hypotheses.
Lastly, in our former study, we also proposed that HR targets could be adjusted over time to ensure a constant metabolic intensity; with this aim, we provided a population-specific equation to predict the scHR in postmenopausal women [13]. However, a model with external validity needs to be developed in order to grant an accurate prediction of scHR at a given intensity, accounting for the possible role of sex, age, and fitness level in this dynamic.
To this aim, we performed a two-step study: (i) the development of a comprehensive model for scHR prediction across different intensities, sexes, ages, and cardiorespiratory fitness, and (ii) testing the validity of the developed model using an independent sample of individuals. We hypothesized that the intensity, sex, age, and cardiorespiratory fitness level would predictably affect the HR slow component, allowing for the HR estimation over time in the independent sample. If confirmed, the developed mathematical model would allow for adjusting the prescribed HR targets over time by accounting for the individually estimated slow component of HR and, in turn, maintaining the desired metabolic stimulus during prolonged sessions across individuals.
2. Materials and Methods
2.1. Participants
A total of one hundred and one healthy subjects were recruited by advertisement within the local community and agreed to participate in this two-step study.
The size required for each step was determined based on the power analysis reported above in the statistics analysis section. In addition, females and males were equally subdivided into three age groups (young <36, middle-aged between >36 and <55, and elderly >55 years [19]). As a result, sixty-five individuals (23 young, 12 females; 22 middle-aged, 12 females; and 20 elderly, 10 females) participated in Step 1, development of the prediction equation, while thirty-six (12 young, 6 females; 12 middle age, 6 females; and 12 elderly 6 females) participated in Step 2, validation of the prediction equation (see the respective results sections forparticipants’ characteristics). Inclusion criteria were individuals of both sexes and age > 18 years. Exclusion criteria were smoking and any medical condition or therapy that could influence the physiological responses during testing. Moreover, they were fully informed about the study procedures and the potential risks and discomfort associated with the exercise testing before agreeing to sign a written informed consent form. The study was approved by the Ethics Committee of the University of Verona (CARP) and conducted in conformity with the Declaration of Helsinki (no. 16-2019).
2.2. Study Design
After medical clearance, the subjects’ main anthropometric measurements were collected (body mass (digital scale, Seca877, Seca, Leicester, UK) and height (vertical stadiometer, Seca, Leicester, UK)) [20].
During Visit 1, all participants performed a ramp incremental test until volitional exhaustion on an electromagnetically braked cycle ergometer (Sport Excalibur, Lode, Groningen, The Netherlands) for the determination of gas exchange threshold (GET), respiratory compensation point (RCP), and maximal parameters (O2peak and HRpeak).
On the successive appointments, subjects performed the following constant work rate exercises: Step 1, during which participants performed six 9-minute constant-work-rate exercises: two below GET (i.e., moderate domain), two between GET and RCP (i.e., heavy domain), and two above RCP (i.e., severe domain); and Step 2, during which participants performed three constant-work-rate exercises lasting 15 min or until exhaustion: one in each domain (i.e., from the moderate to the severe domain). The constant-work-rate order was randomized and counterbalanced. Participants were instructed to avoid caffeine consumption and physical activity at least 8 h and 24 h before each visit, respectively [21]. All visits were separated by at least 48 h and completed within 30 days. Tests were conducted at the same time of the day (±2 h) in an environmentally controlled laboratory (22–25 °C, 55–65% relative humidity). The cycloergometer position was set at the first visit to the lab and recorded for successive tests. To minimize the variability of glycogen oxidation, participants consumed a standardized meal (i.e., 500 cc of water and 2 g⋅kg−1 of low-glycaemic-index carbohydrates) two hours before attending the laboratory [22].
2.3. Ramp Incremental Protocol
The ramp incremental test consisted of 6 min baseline cycling at 50–100, followed by 4 min baseline cycling at 20–40 W to calculate the mean response time (MRT) (see data analysis); thereafter, power output increases by 10–30 W every minute until volitional exhaustion [23]. The warm-up load and ramp increment were customized depending on the estimated fitness level of each subject to reach the individual’s exhaustion between 8 and 12 min, as described in detail elsewhere [24]. Participants were asked to pick a self-selected cadence between 70–90 rpm and to maintain it for all tests. Breath-by-breath pulmonary gas exchange and HR were continuously measured using a metabolic cart (Quark B2, Cosmed, Rome, Italy). Moreover, for Step 1 only, the rating of perceived effort was collected using a Borg 6–20 scale 20 min from the end of the ramp incremental test [25].
2.4. Constant-Work Protocol
All the constant-work-rate exercises were preceded by a 3 min freewheeling cycling warm-up followed by an instantaneous increase in power output.
For Step 1, the exercise intensity of the six trials was chosen as follows:
- (i)
- Moderate trials: 33% (M1) and 66% (M2) of the difference between rest O2 and O2 at GET;
- (ii)
- Heavy trials: 33% (H1) and 66% (H2) of the difference between O2 at GET and RCP;
- (iii)
- Severe trials: 33% (S1) and 66% (S2) of the difference between O2 at RCP and O2max.
For Step 2, the exercise intensity was chosen as follows:
- (i)
- Moderate trials: 50% of the difference between rest O2 and O2 at GET;
- (ii)
- Heavy trials: 50% of the difference between O2 at GET and RCP;
- (iii)
- Severe trials: 50% of the difference between O2 at RCP and O2max.
To identify the power output that elicits the above O2 targets, the individual O2/power output relationship derived from the incremental exercise was corrected for the O2 mean response time and slow component by applying the mathematical model recently proposed by Caen et al. [9].
Breath-by-breath pulmonary gas exchange, ventilation, and HR were continuously measured using the same method of the ramp incremental protocol. Moreover, the perceived effort ratings (RPEs) were collected using a Borg 6–20 scale at the fifth minute during each constant-work-rate exercise [25].
2.5. Data Analysis
Breath-by-breath gas exchange variables and HR were treated as follows: aberrant data points (that lay 3 standard deviations away from the local mean) were removed [26]. This was accomplished using a linear least-squares regression method whereby the baseline (fitting window: approximately −180 to 0 s) and steady-state period (fitting window: approximately 180– end trial) were fitted. The 99% prediction bands were used to identify any data points that lay three SD from the local mean. Care was taken not to delete data in the early portion of the transition (i.e., <180 s); thereafter, data were linearly interpolated at 1 s, and then mediated at 5 s intervals [27]. GET and RCP were determined from the ramp incremental test by three experts independently using the standard technique [28]. O2max (absolute and relative to the body weight), the maximal respiratory exchange ratio (RERmax), and HRmax were determined as, respectively, the average O2 and RER of the last 30 s and the highest HR achieved before exhaustion during the ramp incremental protocol. The steady-state O2, measured during the 50–100W bouts prior to the ramp incremental test, was used to correct the O2/power output relationship for the mean response time [29]. To correct the ramp-identified power output above the gas exchange threshold, an additional correction to account for the O2 slow component was applied [9]. The power outputs associated with the target O2 were obtained using the mathematical model developed by Caen et al. [9].
In each constant work rate, we calculated the following:
For Step 1: (i) oxygen pulse (O2/HR) at the fifth minute and ninth minute of exercise; and (ii) scHR, as the slope of the HR/time linear fitting from the fifth minute to the end of the exercise and expressed in both absolute units (bpm⋅min−1) and relative to the O2 at the fifth minute (scHR/5minO2);
For Step 2: the mean HR value at the fifth minute and last minute of exercise.
2.6. Statistical Analysis
All data are presented as mean ± SD. After assumption verification (i.e., normality, and homogeneity of variance), the within-subject coefficient of variation and a two-way repeated-measure ANOVA (trial × intensity) were used to evaluate HR data repeatability measured during each freewheeling.
A mixed RM-ANOVA was performed to compare the values at the fifth minute of power output, O2, HR, and %RCP, as well as the value of RPE, across exercise intensities, with sex as between-subjects factor.
To verify the presence and the amplitude of scHR and its relationship with O2, O2/HR between the 5th and the 9th minute was compared across intensities by a two-way RM-ANOVA (time × intensity). Moreover, scHR and scHR/5minO2 were compared by a mixed RM-ANOVA across intensities with sex as a between-subjects factor. A post hoc analysis was performed using the Holm–Sidak method.
To develop a multi-linear model for the prediction of the individual scHR, we proceeded as follows: (i) the sex parameter was classified as male = score 0 and female = score 1; (ii) relative exercise intensity to RCP (%RCP) was calculated based on the mean individual O2 measured at the 5th minute for each trial; and (iii) a forward multiple-linear regression was initially run including these variables: age, sex, %RCP, HRmax, absolute O2 at the 5th minute, and relative O2max to body weight. This analysis identified non-significant (p > 0.05) and cross-correlated predictors (i.e., correlation coefficient > 0.70, and variance inflation factor > 5) that were discarded from the model. Subsequently, a forward multiple regression was rerun until significant, non-cross-correlated predictors were identified, and the best prediction model was found.
To test the validity of the developed equation in Step 2, we proceed as follows: (i) verify the presence of an scHR by comparing the HR measure at the 5th minute with the last minute of exercise by a paired t-test; (ii) estimate the HR at the end of the exercise with the following formula:
where scHR was estimated using the previously developed equation derived from Step 1, and time is in minutes from the start of the exercise; and, (iii) lastly, compare the measured and the estimated HR at the end of all exercises by a mixed RM-ANOVA (method × sex), Pearson correlation, and Bland–Altman analysis.
HR@end = HR@5min + (scHR⋅(time − 5))
A power analysis was conducted a priori (G*Power 3.1). To develop a valid prediction model, the sample size required for Step 1 was 60 individuals. Moreover, based on the standard deviations of the primary outcomes (scHR for Step 1 and within-subject variability of HR for Step 2) detected in previous studies [13,14,15], a minimum of 18 subjects were required to identify significant differences with an α error of 0.05 and a statistical power (1 − β) of 0.80. All statistical analyses were performed using SigmaPlot (version 14.0). Statistical significance was accepted when p < 0.05.
3. Results
Step 1. Participants’ characteristics and maximal parameters derived from the ramp incremental test are reported in Table 1. The average body mass index and cardiorespiratory variables were indicative of a normal weight and active population [4]. The two-way ANOVA on the HR during freewheeling shows no significant effect among the six trials (p = 0.11). The mean within-subject coefficient of variation was 2.7 ± 2.6%.

Table 1.
Anagraphic, anthropometric, and cardiorespiratory variables of the Step 1 subjects.
Participants’ HR and O2 responses during the constant-workload exercises are shown in Figure 1, while variables at the fifth minute are reported in Table 2. A mixed RM-ANOVA on power output, O2, HR, %RCP, and RPE found, as expected, a significant effect of the relative exercise intensity (for all variables p < 0.001), while a significant main effect regarding sex was found for the power output and O2 only (power output and O2: p < 0.01; HR: p = 0.11; %RCP: p = 0.39; RPE: p = 0.28).
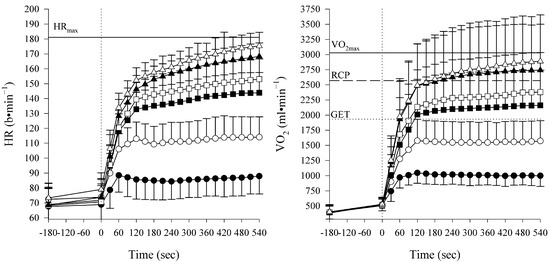
Figure 1.
Data are expressed as mean ± SD of the heart rate (HR) and oxygen uptake (O2) during different constant-workload intensities (moderate: M1 ● and M2 ○; heavy: H1 ■ and H2 □; and severe trials: S1 ▲; S2 ∆). As identified from the ramp test, mean HR and O2 peak values (HRmax and O2max) are displayed as horizontal lines, respectively, in the left and right panels. Gas exchange threshold (GET) and respiratory compensation point (RCP) were plotted as, respectively, dotted line and dashed line.

Table 2.
Variables measured during the constant workload exercises.
A two-way RM-ANOVA on O2/HR found a significant main effect for both time and intensity (respectively, p < 0.05 and p < 0.01). A post hoc analysis for time shows a lower O2/HR at the ninth minute compared to the fifth minute in all the intensities from the moderate to the heavy domain (mean difference ±SD: M1 −0.17 ± 0.63, M2 −0.32 ±0.68, H1 −0.45 ±0.60, and H2 −0.60 ± 0.57 mL⋅b−1). In contrast, no difference was found in both intensities in the severe domain (mean difference: S1 −0.13 ± 0.74, and S2 0.14 ± 0.72 mL⋅b−1).
The scHR in absolute values and relative to the fifth minute of O2 are shown in Figure 2. A mixed RM-ANOVA on scHR found a significant main effect of relative exercise intensity and sex (respectively, p < 0.001 and p < 0.05) and no interaction (p = 0.48). Moreover, when the scHR was expressed relative to the 5minO2, a significant main effect of intensity was confirmed (p < 0.001), while the effect of sex disappeared (p = 0.24) with no interaction (p = 0.18). The post hoc analysis for sex within each intensity is shown in Figure 2. The post hoc analysis for intensity within each domain (i.e., S2 vs. S1, H2 vs. H1, and M2 vs. M1) showed no difference for either scHR or scHR/5minO2 (p > 0.05 for all comparisons).
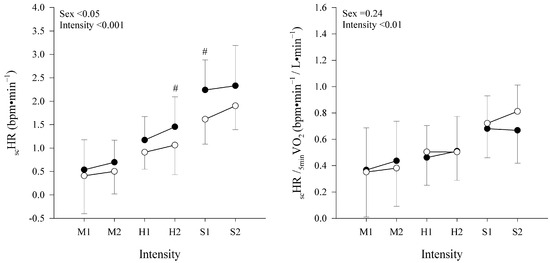
Figure 2.
Absolute HR slow component (scHR) and relative to the O2 at the 5th minute (scHR/5minO2) are displayed as mean ± SD in, respectively, left and right panel. Data are shown at different constant-workload intensities (moderate: M1 and M2;heavy: H1 and H2;and severe trials: S1, and S2) in males (●) and females (○). Main effects of sex and intensity are displayed for both variables. # indicates a significant difference from females at the same exercise intensity.
The iterative application of the forward multiple-linear regression to estimate the scHR indicated that all the analyzed factors were significant predictors of the scHR (p < 0.05). However, the O2 at the fifth minute, HRmax, and relative O2max to body weight were cross-correlated with %RCP and/or age (r2 > 0.70 and variance inflation factor >10) and discarded from the analysis as their removals did not significantly reduce the explanatory power of the model (i.e., p and r2). Thus, the most relevant and not cross-correlated predictor of the scHR was %RCP along with sex and age (p < 0.01). Then, the following predicting equation for the scHR was found:
r2 = 0.53; standard error of estimate = 0.61
scHR (bpm⋅min−1) = −0.0514 + (0.0240 ⋅ intensity expressed in %RCP) − (0.0172 ⋅ age) − (0.347 ⋅ Sex (males = score 0 and females = score 1))
Step 2. To investigate the external validity of the present equations, we tested the model on an independent sample (Table 3 for individual features).

Table 3.
Anagraphic, anthropometric, and cardiorespiratory variables of the Step 2 subjects.
A paired t-test showed significant increments in the HR from the fifth minute to the end (12 ± 3 min) of the exercise (HR mean of the three intensities at, respectively, the fifth minute, 129 ± 21 b⋅min−1, and the end of the exercise, 136 ± 24 b⋅min−1, p < 0.05).
A mixed RM-ANOVA showed higher end-exercise HR in females compared to males (p < 0.001); however, it showed no difference between the measured and estimated HR (p = 0.86) and no interaction (sex × method, p = 0.82) (respectively, the mean of the three intensities for the estimated and directly measured HR for females is 146 ± 27 and 145 ± 26 b⋅min−1; and males, 126 ± 19 and 127 ± 18 b⋅min−1). Figure 3 displays the correlation and Bland–Altman analysis performed between the measured and estimated end-exercises’ HR, indicating an excellent correlation and correspondence with the small, non-significant bias of −0.01 ± 4.09 b⋅min−1 (LoA: lower = −7.84 b⋅min−1 and upper = 7.81 b⋅min−1; z-score = −0.04) that was within the day-to-day variability detected in the present study for HR values: 2.7 ± 2.6%.
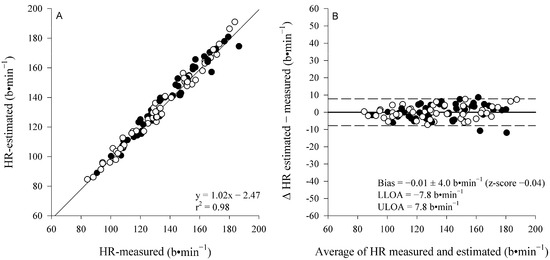
Figure 3.
Pearson correlation and Bland–Altman analysis (panel A and B, respectively) between the measured and estimated HR at the end of each constant-work-rate exercise. Males (●) and females (○). Since no interaction effects (sex × method) were detected, cumulative coefficient of determination (r2), bias, z-score, and precision are shown.
4. Discussion
The purpose of this study was to develop a comprehensive prediction model for the scHR across exercise intensities in both sexes and different ages and to test the validity of the developed model using an independent sample of individuals.
The study confirmed the presence of an scHR in moderate, heavy, and severe domains, which proportionally increases with relative intensity. Moreover, the study demonstrated, for the first time, a significant effect of sex and age on the amplitude of the scHR. Finally, the study developed a comprehensive equation that, by including %RCP, sex, and age, accurately predicted the scHR in an independent sample of males and females of different ages across the three domains.
The individual and anthropometric characteristics of the subjects enrolled in the study were in line with what we expected from the existing literature for healthy, active individuals [3].
In agreement with previous findings on either male-only [14,15] or female-only [13] populations, our data confirmed the presence of the scHR from the moderate to the severe domain and confirmed its relationship with the relative exercise intensity [13,14,15,16].
However, in male individuals, previous studies described a higher scHR compared to our male sample (i.e., mean, respectively, of ≈0.9, ≈2.9, and ≈6.7 bpm⋅min−1 versus ≈0.55, ≈1.35, and ≈2.2 bpm⋅min−1, for the moderate, heavy, and severe domain) [14]. On the contrary, in females, the scHR in the present study was approximately double the size of the values reported previously in postmenopausal women (i.e., mean, respectively, of ≈0.22, ≈0.99, and ≈1.8 bpm⋅min−1 versus ≈0.21, ≈0.31, and ≈0.99 bpm⋅min−1 for the moderate, heavy, and severe domains) [13].
As suggested by our predictive equation, these discrepancies may result from the different exercise intensities performed and/or ages. Indeed, in the current study, exercise intensities were anchored to the individual rest O2, gas exercise thresholds, and O2max, compared to previous studies in which different approaches were adopted, i.e., %GET, %ΔGET/O2peak [14], or %O2max [13]. These differences may have led to unmatched exercise intensities among studies and, in turn, to different scHR amplitude.
To our knowledge, this is the first study directly comparing the scHR between age-matched adults of both sexes across several exercise intensities from the moderate to severe domain. Our data show an scHR reduced by aging (about 10% in our elderly versus young individuals) and one-third lower in females compared to males.
A possible explanation for the sex and age differences in the amplitude of the scHR may partially derive from the metabolic heat production on the one hand and heat dissipation capacity on the other hand, both of which will affect the core temperature and, possibly, the scHR over time [30,31,32]. The metabolic heat production for a given relative exercise intensity will be higher in individuals with a higher absolute O2max (e.g., for trained vs. untrained, young vs. old, male vs. female, and heavier vs. lighter individuals) [30,32]. Indeed, males and females differ anthropometrically, with the first being generally heavier and taller, as well as displaying higher O2max values [33]. In the present study, males were, on average, ≈17% heavier and ≈7% taller compared to female participants and, consequently, presented a ≈30% higher O2max and ≈25% higher absolute O2 values at the fifth minute for each of the matched relative exercise intensity. Indeed, the O2 at the fifth minute was found to be a significant predictor of the scHR (even if discarded from our predictive equation due to its cross-correlation with the relative exercise intensity to RCP), so much so that, when the individual scHR were normalized for the O2 at the fifth minute, the sex difference disappeared (see Figure 2), suggesting an effect of the absolute oxygen uptake on the observed sex differences in the scHR.
In addition, the ability to eliminate heat (dissipation) through vasodilation and sweating is affected by body dimensions (mainly related to the ratio between the body surface area and mass), sex, age, and aerobic fitness (that affect the threshold and sensitivity of the sweating mechanism) [30]. In particular, while the larger surface/mass ratio in women may represent an advantage for heat dissipation, the lower sweating rate vs. men may represent a disadvantage [30]. Unfortunately, no measures of body temperature, sweating rate, or peripheral blood flow were taken in our study; therefore, we have no means to quantify the overall effect of sex and body geometry on this variable. Notably, in a previous study, in which exercise intensities between sexes were matched for metabolic heat production, the anecdotal HR increments during exercise were similar in males vs. females (from the 30th to 90th minute of about 0.71 and 0.61 bpm⋅min−1, respectively) [30]. However, the authors reported HR values only above 30 min of exercise, when different factors (e.g., decreasing stroke volume and dehydration) started affecting HR dynamics in a well-known phenomenon called cardiovascular drift [34]. Thus, further studies need to examine the effect of heat production and dissipation on the HR slow component during the early phase of exercise (i.e., <15 min).
Similarly, the progressive decrease in the scHR amplitude with aging can be partially due to the lower absolute O2 observed in older people compared to young individuals. A decrease in O2max with aging is a well-documented phenomenon quantified by about ~10% every 10 years [18]. The aging O2max decline may, therefore, be partially related to the lower scHR that characterizes older individuals compared to young ones. In addition, reductions in the intrinsic maximal heart rate and in the chronotropic responsiveness to β-adrenergic stimulation with aging [35] reduce the HR reserve and might lower the potential for the HR excursion during exercise.
To summarize, we think it is fair to hypothesize that the metabolic heat production (i.e., absolute O2), the temperature regulation capacity, and the potential for a maximal HR excursion may play a role in the discrepancy observed between sexes and ages. However, further investigation is needed to fully understand the physiological underpinnings of the scHR dynamics as well as to examine the HR dynamics over more prolonged exercise (i.e., >15 min of exercise) to better elucidate the interplay roles between the HR slow component and cardiovascular drift phenomenon.
Moreover, it remains to be determined if the menstrual cycle phase may affect the scHR component’s dynamic, possibly explaining the differences between young and postmenopausal women. However, a previous study exploring the menstrual cycle’s effect on cardiovascular drift excludes such a possibility. In fact, the cycle phase affected the absolute HR at a given workload, yet not its time-dependent slow dynamic [36].
In conclusion, whenever exercise is anchored to HR targets, the presence of an scHR that is partially dissociated from O2 will cause an undesired, time- and intensity-dependent reduction in work rate (approximately 6 and 14% over a moderate and heavy intensity 30 min session, respectively) during the training session [13,14,15]. Notably, at intensities closest to the exercise intensity boundaries (e.g., H1 in the present study), a time-dependent work rate decline could also cause an undesired switch to the lower-intensity exercise domain (i.e., from heavy-to-moderate or severe-to-heavy). Therefore, ignoring the scHR when fixed HR targets are used for exercise prescription may be responsible for a reduction in the overall energy expenditure and a down-shift in the intensity domain with important implications for the health and training outcomes [5,13,14,15]. The practical impact of ignoring the HR slow component when prescribing exercise (anchored to a %HRmax or HR target) may be minimal or severe depending on its amplitude, which can differ across intensities and in different populations, fitness levels, or diseases [13,14,16,17]. Notably, previous studies showed that the uncertainty in exercise prescription deriving from the scHR is not more severe in obese patients compared to healthy controls, as the scHR amplitude was similar in each domain [15]. On the contrary, this issue seems to be less relevant in cardiac patients’ β-Blockers, as the reduction in workload over time during exercise at a fixed HR was less pronounced than in healthy individuals [17]. Further studies should be conducted on different patient populations.
Lastly, it should be noted that, whenever a measure of external load is possible, this can be used as a proxy of metabolic intensity to prescribe exercise. This is typically the case in cycling or running, where wearable sensors offer the opportunity to monitor the external load, although at a cost [5,6]. However, in a variety of situations (e.g., activities where the metabolic cost is affected by the terrain conditions, inclination, and skills of the individual), HR offers a reliable, time-resolved, and accessible index of metabolic intensity. Among the advantages of this approach is the immediate transferability of the metabolic intensity from a cardiorespiratory test to any health/rehabilitation center, gym, and external environment, and across different ergometers [10,11,12,37,38]. The decision to use either an external load (e.g., speed or power output) or a proxy of metabolic intensity (e.g., HR) ultimately depends upon the type and context of the activity, as well as the affordability of the instruments.
5. Practical Implications and Limitations
The adjustment of the HR target over time is made possible by our predictive equation with the following steps: (i) from a previous cardiopulmonary test, detect the desired metabolic intensity and the associated HR target; and, (ii) during the exercise training beyond the fifth minute, dynamically update the HR target based on the rate of increment (scHR, i.e., bpm⋅min−1) estimated with our equation (i.e., based on the training intensity in %RCP, sex, and age of the individual). This procedure would grant that the desired stimulus is maintained throughout the exercise session in a given individual.
It should be noted that the developed predictive equation does not take into account factors that may potentially affect HR kinetics, such as fatigue, overtraining, nutrition, hydration, and environmental conditions such as temperature and humidity, which were controlled for in our study to the best of our abilities. In addition, the sample tested in the present study was representative of moderately active to active individuals. Thus, to confirm or refuse the absence of the fitness level’s effect on the scHR’s dynamic, further studies are needed on a more heterogeneous population (e.g., sedentary individuals vs. elite athletes). Lastly, the assumption of a linear nature of the scHR kinetics and, therefore, the validity of our predictive equation needs to be confirmed over longer exercise sessions.
6. Conclusions
The prediction equation for scHR developed and validated in the current study provides the means to dynamically adapt HR targets over time, avoiding an undesired reduction in absolute and relative training load. This strategy would allow the maintenance of the desired metabolic stimulus throughout an exercise session in a heterogeneous population.
Author Contributions
Conceptualization, J.B. and S.P.; formal analysis, M.T.; investigation, M.T., A.L.C., M.L. and S.P.; supervision, J.B. and S.P.; writing—original draft, M.T.; writing—review and editing, A.L.C., J.B. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
Funding from the Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Italy, supported this study. The authors express their gratitude to the participants who made this data collection possible.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the ethics committee of the University of Verona (CARU) (no. 16-2019, approval date: 8 August 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data supporting the findings of this study are not publicly available due to privacy/ethical restrictions. The data are available from the corresponding author upon reasonable request.
Conflicts of Interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Abbreviations
The following abbreviations are used in this manuscript:
| GET | Gas exchange threshold |
| HR | Heart rate |
| HRpeak | Peak heart rate |
| RM-ANOVA | Repeated measures analysis of variance |
| RCP | Respiratory compensation point |
| O2peak | Peak oxygen uptake |
| O2R | Reserve oxygen uptake |
| scHR | Heart rate slow component |
References
- McLaughlin, M.; Jacobs, I. Exercise is medicine, but does it interfere with medicine? Exerc. Sport Sci. Rev. 2017, 45, 127–135. [Google Scholar] [CrossRef]
- Gronwald, T.; Törpel, A.; Herold, F.; Budde, H. Perspective of Dose and Response for Individualized Physical Exercise and Training Prescription. J. Funct. Morphol. Kinesiol. 2020, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Riebe, D.; Ehrman, J.; Liguori, G.; Magal, M. ACSM’s Guidelines for Exercise Testing and Pescription, 10th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2018. [Google Scholar]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Jamnick, N.A.; Pettitt, R.W.; Granata, C.; Pyne, D.B.; Bishop, D.J. An Examination and Critique of Current Methods to Determine Exercise Intensity; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Iannetta, D.; Inglis, E.C.; Mattu, A.T.; Fontana, F.Y.; Pogliaghi, S.; Keir, D.A.; Murias, J.M. A Critical Evaluation of Current Methods for Exercise Prescription in Women and Men. Med. Sci. Sports Exerc. 2020, 52, 466–473. [Google Scholar] [CrossRef]
- Herold, F.; Müller, P.; Gronwald, T.; Müller, N.G. Dose–Response Matters!—A Perspective on the Exercise Prescription in Exercise–Cognition Research. Front. Psychol. 2019, 10, 2338. [Google Scholar] [CrossRef] [PubMed]
- Vanhatalo, A.; Black, M.I.; DiMenna, F.J.; Blackwell, J.R.; Schmidt, J.F.; Thompson, C.; Wylie, L.J.; Mohr, M.; Bangsbo, J.; Krustrup, P.; et al. The mechanistic bases of the power-time relationship: Muscle metabolic responses and relationships to muscle fibre type. J. Physiol. 2016, 594, 4407–4423. [Google Scholar] [CrossRef]
- Caen, K.; Boone, J.; Bourgois, J.G.; Colosio, A.L.; Pogliaghi, S. Translating Ramp O2 into Constant Power Output: A Novel Strategy that Minds the Gap. Med. Sci. Sports Exerc. 2020, 52, 2020–2028. [Google Scholar] [CrossRef]
- Achten, J.; Jeukendrup, A.E. Heart Rate Monitoring. Sports Med. 2003, 33, 517–538. [Google Scholar] [CrossRef]
- Colosio, A.L.; Pedrinolla, A.; Da Lozzo, G.; Pogliaghi, S. Heart Rate-Index Estimates Oxygen Uptake, Energy Expenditure and Aerobic Fitness in Rugby Players. J. Sports Sci. Med. 2018, 17, 633–639. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30479532 (accessed on 11 January 2021).
- Colosio, A.L.; Lievens, M.; Pogliaghi, S.; Bourgois, J.G.; Boone, J. Heart rate-index estimates aerobic metabolism in professional soccer players. J. Sci. Med. Sport 2020, 23, 1208–1214. [Google Scholar] [CrossRef]
- Teso, M.; Colosio, A.L.; Pogliaghi, S. An Intensity-dependent Slow Component of HR Interferes with Accurate Exercise Implementation in Postmenopausal Women. Med. Sci. Sports Exerc. 2022, 54, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Zuccarelli, L.; Porcelli, S.; Rasica, L.; Marzorati, M.; Grassi, B. Comparison between Slow Components of HR and O2 Kinetics: Functional Significance. Med. Sci. Sports Exerc. 2018, 50, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Zuccarelli, L.; Sartorio, A.; De Micheli, R.; Tringali, G.; Grassi, B. Obese Patients Decrease Work Rate in Order to Keep a Constant Target Heart Rate. Med. Sci. Sports Exerc. 2021, 53, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, G.; Zuccarelli, L.; Manferdelli, G.; Manfredini, V.; Marzorati, M.; Pilotto, A.; Porcelli, S.; Rasica, L.; Šimunič, B.; Pišot, R.; et al. Decrease in work rate in order to keep a constant heart rate: Biomarker of exercise intolerance following a 10-day bed rest. J. Appl. Physiol. 2022, 132, 1569–1579. [Google Scholar] [CrossRef]
- Baldassarre, G.; Azzini, V.; Zuccarelli, L.; Degano, C.; Graniero, F.; Plett, G.; Floreani, M.; Lazzer, S.; Mos, L.; Grassi, B. In Cardiac Patients β-Blockers Attenuate the Decrease in Work Rate during Exercise at a Constant Submaximal Heart Rate. Med. Sci. Sports Exerc. 2023, 55, 1995–2001. [Google Scholar] [CrossRef]
- Kim, C.H.; Wheatley, C.M.; Behnia, M.; Johnson, B.D. The effect of aging on relationships between lean body mass and VO2max in rowers. PLoS ONE 2016, 11, e0160275. [Google Scholar] [CrossRef]
- Petry, N.M. A comparison of young, middle-aged, and older adult treatment-seeking pathological gamblers. Gerontologist 2002, 42, 92–99. [Google Scholar] [CrossRef]
- Ferrari, L.; Teso, M.; Colosio, A.L.; Pogliaghi, S. Performance and Anthropometrics of Classic Powerlifters: Which characteristics Matter? J. Strength. Cond. Res. 2020, in press. [CrossRef]
- de Roia, G.; Pogliaghi, S.; Adami, A.; Papadopoulou, C.; Capelli, C. Effects of priming exercise on the speed of adjustment of muscle oxidative metabolism at the onset of moderate-intensity step transitions in older adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R1158–R1166. [Google Scholar] [CrossRef]
- Pogliaghi, S.; Teso, M.; Ferrari, L.; Boone, J.; Murias, J.M.; Colosio, A.L. Easy Prediction of the Maximal Lactate Steady-State in Young and Older Men and Women. J. Sports Sci. Med. 2023, 22, 68–74. [Google Scholar] [CrossRef]
- Stuer, L.; Teso, M.; Colosio, A.L.; Loi, M.; Mucci, P.; Pogliaghi, S.; Boone, J.; Caen, K. The impact of skinfold thickness and exercise intensity on the reliability of NIRS in the vastus lateralis. Eur. J. Appl. Physiol. 2024, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Wilkerson, D.P.; Jones, A.M. Validity of criteria for establishing maximal O2 uptake during ramp exercise tests. Eur. J. Appl. Physiol. 2008, 102, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A.V. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Keir, D.A.; Murias, J.M.; Paterson, D.H.; Kowalchuk, J.M. Breath-by-breath pulmonary O2 uptake kinetics: Effect of data processing on confidence in estimating model parameters. Exp. Physiol. 2014, 99, 1511–1522. [Google Scholar] [CrossRef]
- Colosio, A.L.; Teso, M.; Pogliaghi, S. Prolonged static stretching causes acute, nonmetabolic fatigue and impairs exercise tolerance during severe-intensity cycling. Appl. Physiol. Nutr. Metab. 2020, 45, 902–910. [Google Scholar] [CrossRef]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. 1986, 121, 2020–2027. [Google Scholar] [CrossRef]
- Iannetta, D.; Murias, J.M.; Keir, D.A. A Simple Method to Quantify the VO2 Mean Response Time of Ramp-Incremental Exercise. Med. Sci. Sports Exerc. 2019, 51, 1080–1086. [Google Scholar] [CrossRef]
- Gagnon, D.; Kenny, G.P. Does sex have an independent effect on thermoeffector responses during exercise in the heat? J. Physiol. 2012, 590, 5963–5973. [Google Scholar] [CrossRef]
- Rowell, L.B. Human Cardiovascular Adjustments to Exercise and Thermal Stress. Physiol. Rev. 1974, 54, 75–159. [Google Scholar] [CrossRef]
- Yanovich, R.; Ketko, I.; Charkoudian, N. Sex differences in human thermoregulation: Relevance for 2020 and beyond. Am. Physiol. Soc. 2020, 35, 177–184. [Google Scholar] [CrossRef]
- Santisteban, K.J.; Lovering, A.T.; Halliwill, J.R.; Minson, C.T. Sex Differences in VO2max and the Impact on Endurance-Exercise Performance. Int. J. Environ. Res. Public Health 2022, 19, 4946. [Google Scholar] [CrossRef] [PubMed]
- Coyle, E.F.; González-Alonso, J. Cardiovascular Drift during Prolonged Exercise: New Perspectives. Exerc. Sport Sci. Rev. 2001, 29, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Christou, D.D.; Seals, D.R. Decreased maximal heart rate with aging is related to reduced β-adrenergic responsiveness but is largely explained by a reduction in intrinsic heart rate. J. Appl. Physiol. 2008, 105, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.; Earley, R.L.; Burnash, S.G.; Wingo, J.E. Menstrual cycle effects on cardiovascular drift and maximal oxygen uptake during exercise heat stress. Eur. J. Appl. Physiol. 2021, 121, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Weltman, A.; Snead, D.; Seip, R.; Schurrer, R.; Weltman, J.; Rutt, R.; Rogol, A. Percentages of maximal heart rate, heart rate reserve, and VO2peak for determining endurance training intensity in sedentary women. Int. J. Sports Med. 1989, 10, 212–216. [Google Scholar] [CrossRef]
- Pettitt, R.W.; Symons, J.D.; Taylor, J.E.; Eisenman, P.A.; White, A.T. Adjustment for gas exchange threshold enhances precision of heart rate-derived VO2 estimates during heavy exercise. Appl. Physiol. Nutr. Metab. 2008, 33, 68–74. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).