Comparative Transcriptome Analysis Reveals Expression of Defense Pathways and Specific Protease Inhibitor Genes in Solanum lycopersicum in Response to Feeding by Tuta absoluta
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Solanum lycopersicum and Tuta absoluta
2.2. Sample Collection for Transcriptome Analysis
2.2.1. Plant Treatment with Tuta absoluta Feeding and Mechanical Damage
2.2.2. Collection of Different Developmental Stages of T. absoluta Larva
2.3. RNA Extraction and Transcriptome Sequencing
2.4. Transcriptome Data Analysis
2.5. Specific Gene Sequence Annotation
3. Results
3.1. Sequencing Data Statistics
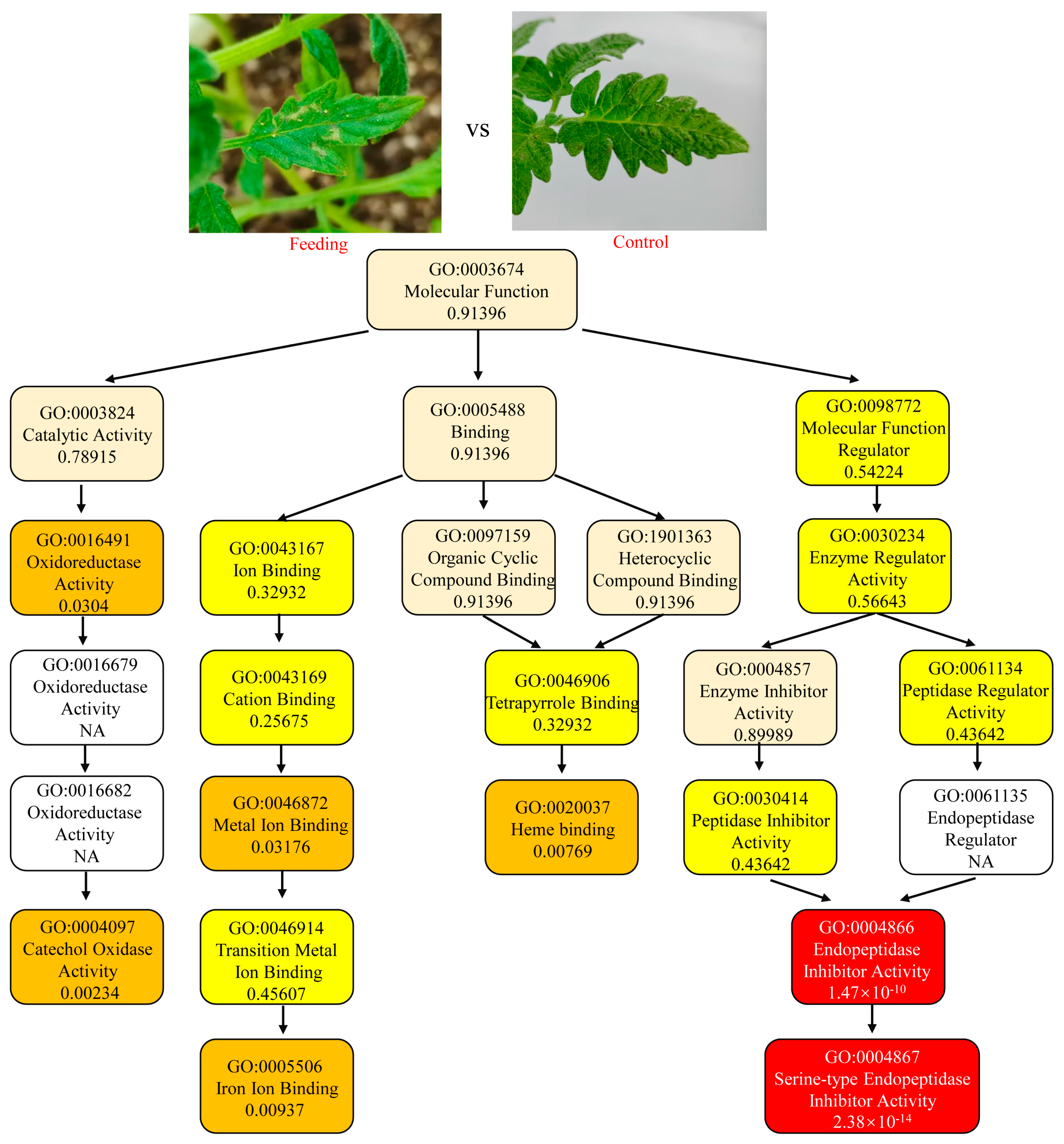
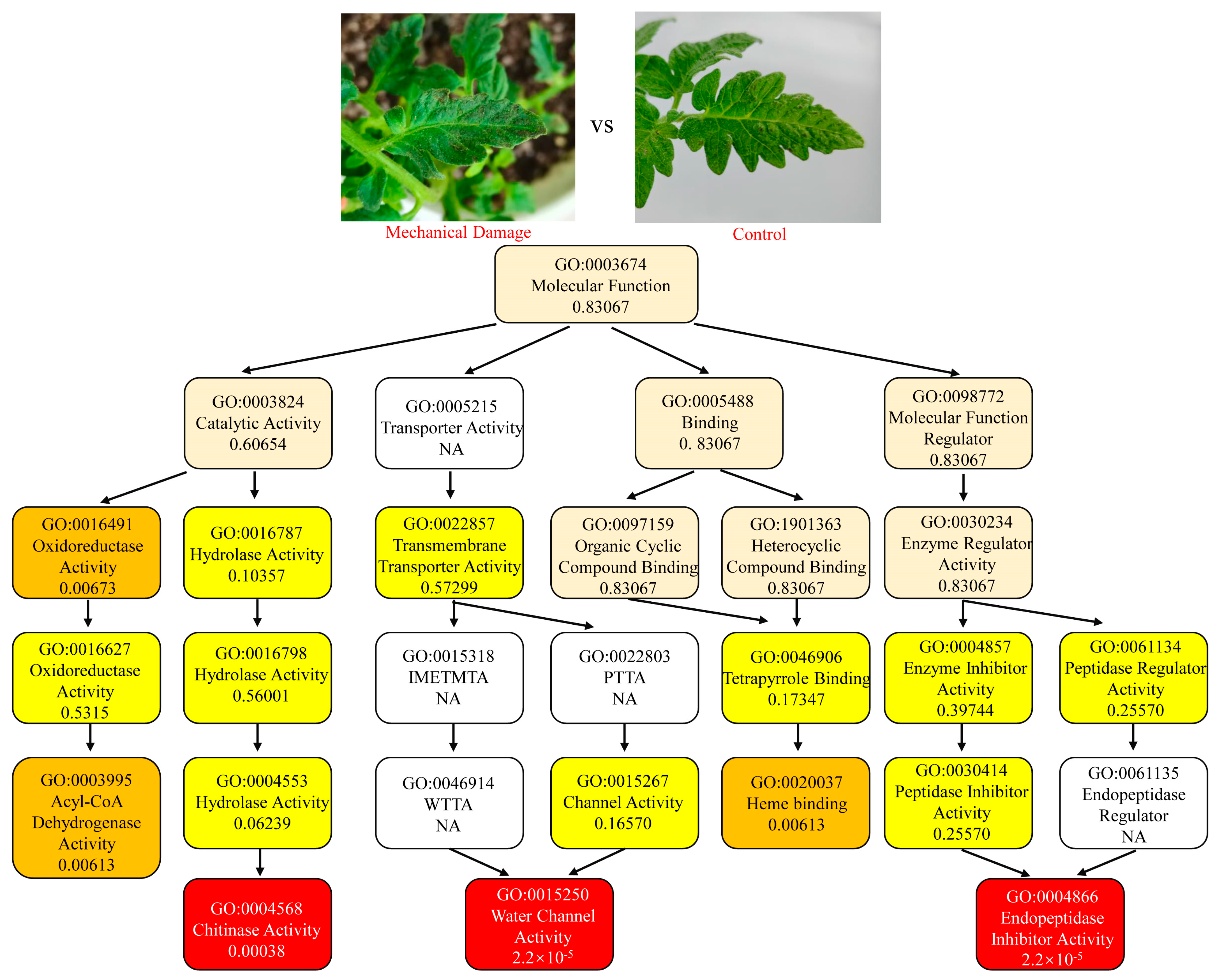
3.2. GO Enrichment of Response Genes to Mechanical and Feeding Damage
3.3. Bioinformatics and Expression Analysis of the Endopeptidase Inhibitor Genes of S. lycopersicum
3.3.1. Potato Inhibitor I Family
3.3.2. Potato Type II Proteinase Inhibitor Family
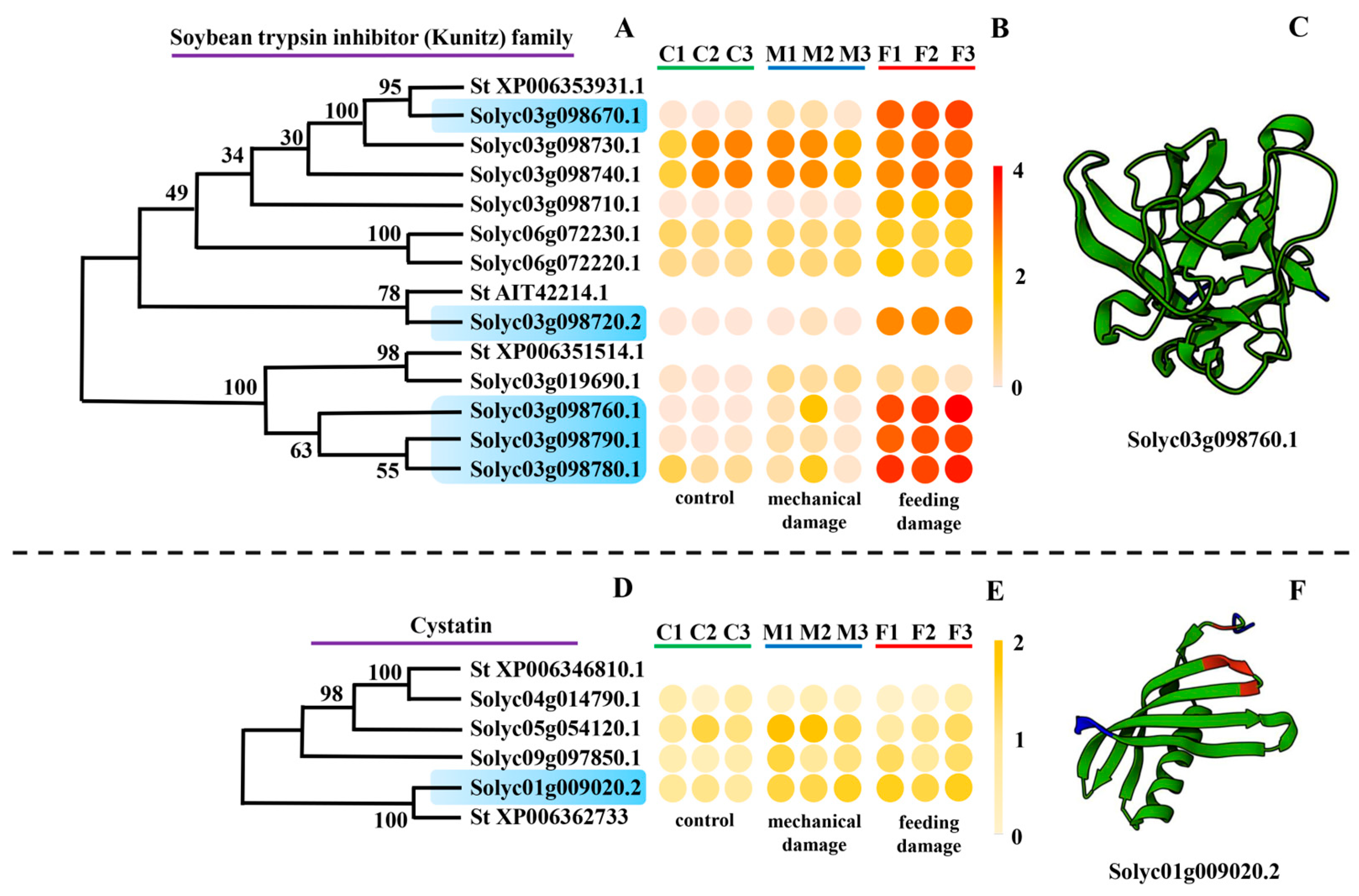
3.3.3. Soybean Trypsin Inhibitor (Kunitz) Family
3.3.4. Cystatin Family
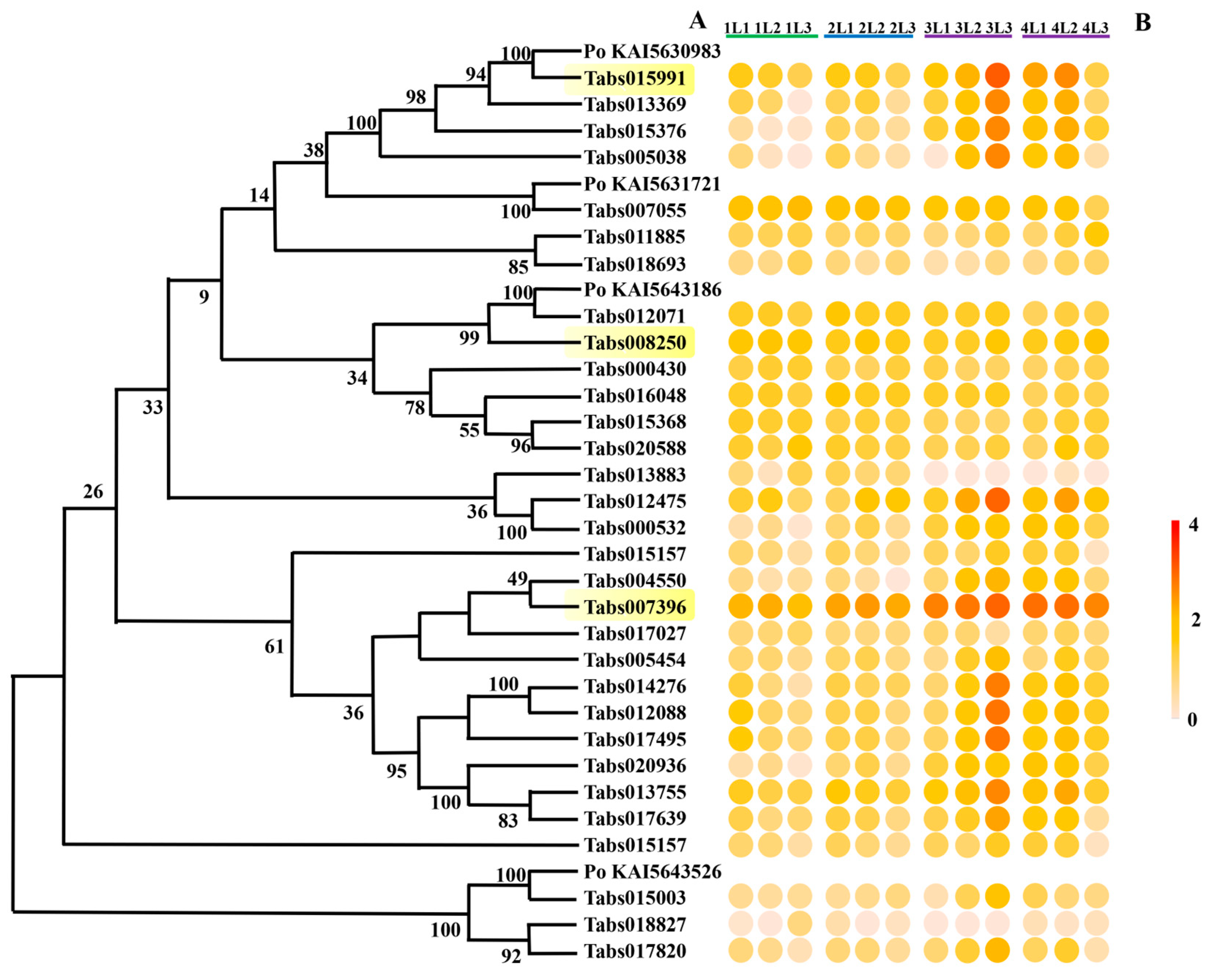
3.4. Bioinformatics and Expression Analysis of the Endopeptidase Genes of T. absoluta
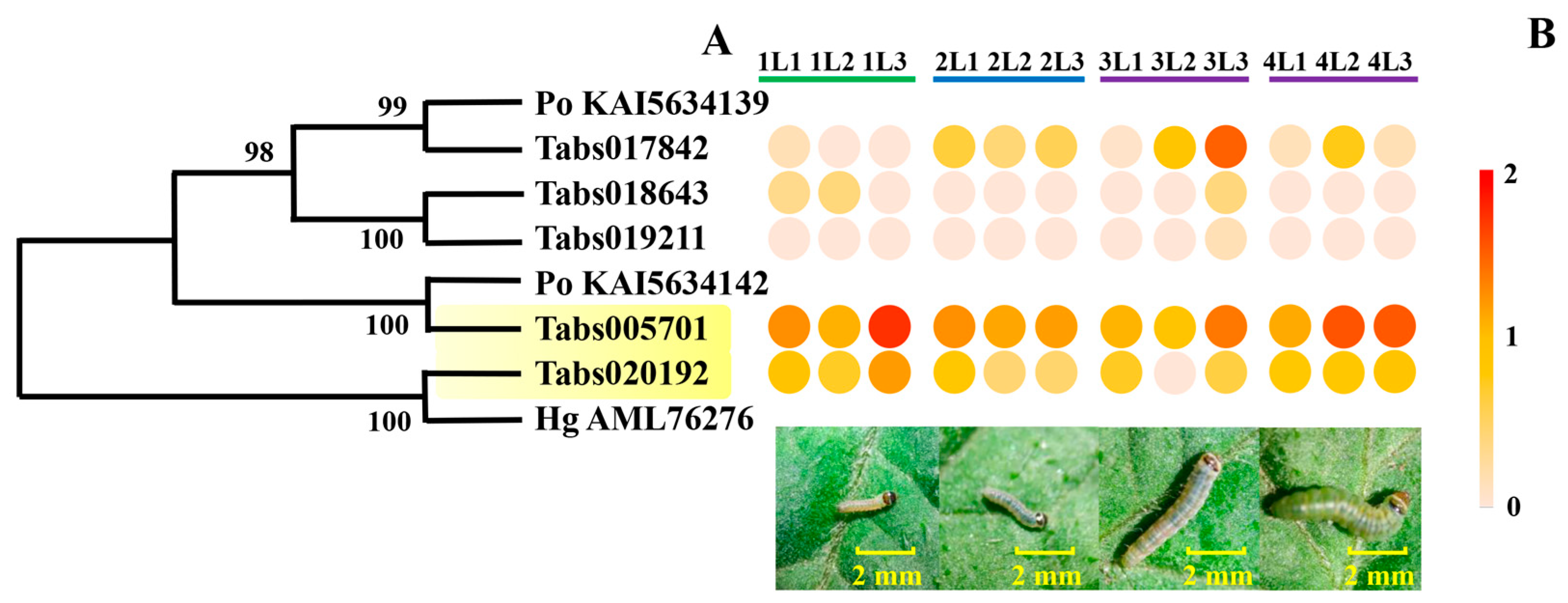
3.4.1. Expression of Genes Encoding Cysteine Proteases in the Developmental Stages of T. absoluta Larvae
3.4.2. Expression of Genes Encoding Serine Proteases in the Developmental Stages of T. absoluta Larvae
3.5. Prediction of Interactions Between Protease Inhibitors of S. lycopersicum and Proteases of T. absoluta
3.5.1. Interactions Between Serine Inhibitors of S. lycopersicum and Serine Protease
3.5.2. Interactions Between Serine Inhibitors of S. lycopersicum and Serine Protease Tabs007396 of T. absoluta
3.5.3. Interactions Between Cysteine Inhibitors of S. lycopersicum and Cysteine Protease Tabs005701 of T. absoluta
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Santana, P.A., Jr.; Kumar, L.; Da Silva, R.S.; Picanco, M.C. Global geographic distribution of Tuta absoluta as affected by climate change. J. Pest Sci. 2019, 92, 1373–1385. [Google Scholar] [CrossRef]
- Tropea Garzia, G.; Siscaro, G.; Biondi, A.; Zappala, L. Tuta absoluta, a South American pest of tomato now in the EPPO region: Biology, distribution and damage. EPPO Bulletin. 2012, 42, 205–210. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Shen, Y.; Gao, H.; Zhang, G.; Liu, W.; Jiang, H.; and Zhang, Y. Life table parameters of the tomato leaf miner Tuta absoluta (Lepidoptera: Gelechiidae) on five tomato cultivars in China. Insects 2024, 15, 208. [Google Scholar] [CrossRef]
- Loyani, L. Segmentation-based quantification of Tuta absoluta’s damage on tomato plants. Smart Agric. Technol. 2024, 7, 100415. [Google Scholar] [CrossRef]
- Gatehouse, J.A. Plant resistance towards insect herbivores: A dynamic interaction. New Phytol. 2002, 156, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Setotaw, Y.B.; Li, J.; Qi, J.; Ma, C.; Zhang, M.; Huang, C.; Wang, L.; Wu, J. Salicylic acid positively regulates maize defenses against lepidopteran insects. Plant Divers. 2024, 46, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Han, W.H.; Wang, J.X.; Zhang, F.B.; Ji, S.X.; Zhong, Y.W.; Liu, S.S.; Wang, X.W. Differential induction of JA/SA determines plant defense against successive leaf-chewing and phloem-feeding insects. J. Pest Sci. 2024. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, X.; Liu, Y.; Chai, L.; Liu, D.; Chen, Z.; Lue, S. Plant Chemical Defenses against Insect Herbivores-Using the Wild Tobacco as a Model. Phyton-int. J. Exp. Bot. 2024, 93, 641–659. [Google Scholar] [CrossRef]
- Kortbeek, R.W.J.; Galland, M.D.; Muras, A.; van der Kloet, F.M.; Andre, B.; Heilijgers, M.; van Hijum, S.A.F.T.; Haring, M.A.; Schuurink, R.C.; Bleeker, P.M. Natural variation in wild tomato trichomes; selecting metabolites that contribute to insect resistance using a random forest approach. BMC Plant Biol. 2021, 21, 315. [Google Scholar] [CrossRef] [PubMed]
- Green, E.S.; Zangerl, A.R.; Berenbaum, M.R. Effects of phytic acid and xanthotoxin on growth and detoxification in caterpillars. J. Chem. Ecol. 2001, 27, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Nardi, C.; Rech, C.; Ribeiro, L.K.; de Lima Filho, R.B.; de Oliveira, J.R.F.; Bento, J.M.S.; de Resende, J.T.V. Tomato plants selected to high levels of zingiberene influence herbivory and fecundity of Diabrotica speciosa. Arthropod-Plant Interact. 2024, 18, 905–916. [Google Scholar] [CrossRef]
- Mason, C.J.; Peiffer, M.; Hoover, K.; Felton, G. Tomato chemical defenses intensify corn earworm (Helicoverpa zea) mortality from opportunistic bacterial pathogens. J. Chem. Ecol. 2023, 49, 313–324. [Google Scholar] [CrossRef]
- Weber, N.C.; Sant’Ana, J.; Redaelli, L.R.; de Assis, L.S. Chemotaxis of Tuta absoluta to tomato plants exposed to methyl jasmonate and conspecific injuries. J. Appl. Entomol. 2024, 148, 508–517. [Google Scholar] [CrossRef]
- Campolo, O.; Cherif, A.; Ricupero, M.; Siscaro, G.; Grissa-Lebdi, K.; Russo, A.; Cucci, L.M.; Di Pietro, P.; Satriano, C.; Desneux, N.; et al. Citrus peel essential oil nanoformulations to control the tomato borer, Tuta absoluta: Chemical properties and biological activity. Sci Rep. 2017, 7, 10900. [Google Scholar] [CrossRef]
- Moreno, S.C.; Carvalho, G.A.; Picanco, M.C.; Morais, E.G.F.; Pereira, R.M. Bioactivity of compounds from Acmella oleracea against Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) and selectivity to two non-target species. Pest Manag. Sci. 2012, 68, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Ramos, V.d.S.; Cabrera, O.G.; Camargo, E.L.O.; Ambrosio, A.B.; Vidal, R.O.; da Silva, D.S.; Guimaraes, L.C.; Marangoni, S.; Parra, J.R.P.; Pereira, G.A.G.; et al. Molecular cloning and insecticidal effect of Inga laurina trypsin inhibitor on Diatraea saccharalis and Heliothis virescens. Comp. Biochem. Phys. C 2012, 156, 148–158. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Sato, S.; Tabata, S.; Hirakawa, H.; Asamizu, E.; Shirasawa, K.; Isobe, S.; Kaneko, T.; Nakamura, Y.; Shibata, D. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 2012, 485, 635–641. [Google Scholar]
- Lewald, K.M.; Tabuloc, C.A.; Godfrey, K.E.; Arno, J.; Perini, C.R.; Guedes, J.C.; Chiu, J.C. Genome Assembly and Population Sequencing Reveal Three Populations and Signatures of Insecticide Resistance of Tuta absoluta in Latin America. Genome Biol. Evol. 2023, 15. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.H.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Canene-Adams, K.; Campbell, J.K.; Zaripheh, S.; Jeffery, E.H.; Erdman, J.W. The tomato as a functional food. J. Nutr. 2005, 135, 1226–1230. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics 2006, 22, 195–201. [Google Scholar] [CrossRef]
- Yan, Y.; Tao, H.; He, J.; Huang, S.-Y. The HDOCK server for integrated protein-protein docking. Nat. Protoc. 2020, 15, 1829–1852. [Google Scholar] [CrossRef]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodova, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koca, J.; Rose, A.S. Mol* Viewer: Modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, F.E.; Smith, P.; Peiffer, M.; Helms, A.; Tooker, J.; Felton, G.W. Phytohormones in Fall Armyworm Saliva Modulate Defense Responses in Plants. J. Chem. Ecol. 2019, 45, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Basu, S.; Rivera-Vega, L.J.; Acevedo, F.E.; Louis, J.; Felton, G.W.; Luthe, D.S. Lessons from the Far End: Caterpillar FRASS-Induced Defenses in Maize, Rice, Cabbage, and Tomato. J. Chem. Ecol. 2016, 42, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Larbat, R.; Adamowicz, S.; Robin, C.; Han, P.; Desneux, N.; Le Bot, J. Interrelated responses of tomato plants and the leaf miner Tuta absoluta to nitrogen supply. Plant Biol. 2016, 18, 495–504. [Google Scholar] [CrossRef]
- D’Esposito, D.; Manzo, D.; Ricciardi, A.; Garonna, A.P.; De Natale, A.; Frusciante, L.; Pennacchio, F.; Ercolano, M.R. Tomato transcriptomic response to Tuta absoluta infestation. BMC Plant Biol. 2021, 21, 1–14. [Google Scholar] [CrossRef]
- Roumani, M.; Le Bot, J.; Boisbrun, M.; Magot, F.; Pere, A.; Robin, C.; Hilliou, F.; Larbat, R. Transcriptomics and Metabolomics Analyses Reveal High Induction of the Phenolamide Pathway in Tomato Plants Attacked by the Leafminer Tuta absoluta. Metabolites 2022, 12, 484. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.A.; Balls, A.K. An inhibitor of chymotrypsin from Solanum tuberosum and its behavior toward trypsin. Proc. Natl. Acad. Sci. USA 1962, 48, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- Margossian, L.J.; Federman, A.D.; Giovannoni, J.J.; Fischer, R.L. Ethylene-regulated expression of a tomato fruit ripening gene encoding a proteinase inhibitor I with a glutamic residue at the reactive site. Proc. Natl. Acad. Sci. USA 1988, 85, 8012–8016. [Google Scholar] [CrossRef]
- Wang, H.Y.; Huang, Y.C.; Chen, S.F.; Yeh, K.W. Molecular cloning, characterization and gene expression of a water deficiency and chilling induced proteinase inhibitor I gene family from sweet potato (Ipomoea batatas Lam.) leaves. Plant Sci. 2003, 165, 191–203. [Google Scholar] [CrossRef]
- Bateman, K.S.; James, M.N.G. Plant protein proteinase inhibitors: Structure and mechanism of inhibition. Curr. Protein Pept. Sci. 2011, 12, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Yadav, R.; Sanyal, I. Evaluating the pesticidal impact of plant protease inhibitors: Lethal weaponry in the co-evolutionary battle. Pest Manag. Sci. 2022, 78, 855–868. [Google Scholar] [CrossRef]
- Rizzo, E.; Sherman, T.; Manosalva, P.; Gomez, S.K. Assessment of local and systemic changes in plant gene expression and aphid responses during potato interactions with arbuscular mycorrhizal fungi and potato aphids. Plants 2020, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Schoenherr, A.P.; Rizzo, E.; Jackson, N.; Manosalva, P.; Gomez, S.K. Mycorrhiza-induced resistance in potato involves priming of defense responses against cabbage looper (Noctuidae: Lepidoptera). Environ. Entomol. 2019, 48, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Mahajan, N.; Tamhane, V.A.; Kulkarni, M.J.; Baldwin, I.T.; Gupta, V.S.; Giri, A.P. Stress inducible proteinase inhibitor diversity in Capsicum annuum. BMC Plant Biol. 2012, 12, 217. [Google Scholar] [CrossRef]
- Saikhedkar, N.S.; Joshi, R.S.; Bhoite, A.S.; Mohandasan, R.; Yadav, A.K.; Fernandes, M.; Kulkarni, K.A.; Giri, A.P. Tripeptides derived from reactive centre loop of potato type II protease inhibitors preferentially inhibit midgut proteases of Helicoverpa armigera. Insect Biochem. Molec. 2018, 95, 17–25. [Google Scholar] [CrossRef]
- Vorster, J.; Westhuizen, W.v.d.; Plessis, G.d.; Marais, D.; Sparvoli, F.; Cominelli, E.; Camilli, E.; Ferrari, M.; Le Donne, C.; Marconi, S.; et al. In order to lower the antinutritional activity of serine protease inhibitors, we need to understand their role in seed development. Front. Plant Sci. 2023, 14, 1252223. [Google Scholar] [CrossRef]
- Srinivasan, A.; Giri, A.P.; Harsulkar, A.M.; Gatehouse, J.A.; Gupta, V.S. A Kunitz trypsin inhibitor from chickpea (Cicer arietinum L.) that exerts anti-metabolic effect on podborer (Helicoverpa armigera) larvae. Plant Mol. Biol. 2005, 57, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Vilela Oliva, M.L.; Ferreira, R.d.S.; Ferreira, J.G.; Andrade de Paula, C.A.; Salas, C.E.; Sampaio, M.U. Structural and Functional Properties of Kunitz Proteinase Inhibitors from Leguminosae: A Mini Review. Curr. Protein Pept. Sci. 2011, 12, 348–357. [Google Scholar] [CrossRef]
- Guimaraes, L.C.; Ramalho de Oliveira, C.F.; Marangoni, S.; Lourenco de Oliveira, D.G.; Rodrigues Macedo, M.L. Purification and characterization of a Kunitz inhibitor from Poincianella pyramidalis with insecticide activity against the Mediterranean flour moth. Pestic. Biochem. Phys. 2015, 118, 1–9. [Google Scholar] [CrossRef]
- Rehder, A.; Sorensen, J.C.; Markedal, K.E.; Sorensen, H.; Sorensen, S.; Petersen, I.L. Targeted inactivation of soybean proteinase inhibitors using zinc. Food Chem. 2021, 349, 129049. [Google Scholar] [CrossRef] [PubMed]
- da Silva Junior, N.R.; Vital, C.E.; Barros, R.d.A.; Faustino, V.A.; Monteiro, L.P.; Barros, E.; de Oliveira, E.E.; de Oliveira Ramos, H.J.; de Almeida Oliveira, M.G. Intestinal proteolytic profile changes during larval development of Anticarsia gemmatalis caterpillars. Arch. Insect Biochem. 2020, 103, e21631. [Google Scholar] [CrossRef] [PubMed]
- Kipgen, L.; Aggarwal, K.K. Gut protease profiles of different instars of Helicoverpa armigera (Lepidoptera: Noctuidae). Int. J. Trop. Insect Sci. 2014, 34, 172–178. [Google Scholar] [CrossRef]
- Li, X.Y.; Si, F.L.; Zhang, X.X.; Zhang, Y.J.; Chen, B. Characteristics of Trypsin genes and their roles in insecticide resistance based on omics and functional analyses in the malaria vector Anopheles sinensis. Pestic. Biochem. Phys. 2024, 201, 105883. [Google Scholar] [CrossRef]
- Amirhusin, B.; Shade, R.E.; Koiwa, H.; Hasegawa, P.M.; Bressan, R.A.; Murdock, L.L.; Zhu-Salzman, K. Protease inhibitors from several classes work synergistically against Callosobruchus maculatus. J. Insect. Physiol. 2007, 53, 734–740. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, W.; Reiser, G. Trypsin and trypsin-like proteases in the brain: Proteolysis and cellular functions. Cell. Mol. Life Sci. 2008, 65, 237–252. [Google Scholar] [CrossRef]
- de Almeida Barros, R.; Merino-Cabrera, Y.; Vital, C.E.; da Silva Junior, N.R.; de Oliveira, C.N.; Lessa Barbosa, S.; Marques Goncalves Assis, J.V.; Ramos, H.J.O.; de Almeida Oliveira, M.G. Small peptides inhibit gut trypsin-like proteases and impair Anticarsia gemmatalis (Lepidoptera: Noctuidae) survival and development. Pest Manag. Sci. 2021, 77, 1714–1723. [Google Scholar] [CrossRef] [PubMed]
- Turra, D.; Vitale, S.; Marra, R.; Woo, S.L.; Lorito, M. Heterologous expression of PKPI and Pin1 proteinase inhibitors enhances plant fitness and broad-spectrum resistance to biotic threats. Front. Plant Sci. 2020, 11, 461. [Google Scholar] [CrossRef] [PubMed]
- Tanpure, R.S.; Barbole, R.S.; Dawkar, V.V.; Waichal, Y.A.; Joshi, R.S.; Giri, A.P.; Gupta, V.S. Improved tolerance against Helicoverpa armigera in transgenic tomato over-expressing multi-domain proteinase inhibitor gene from Capsicum annuum. Physiol. Mol. Biol. Plants 2017, 23, 597–604. [Google Scholar] [CrossRef]
- Ye, M.; Liu, C.; Li, N.; Yuan, C.; Liu, M.; Xin, Z.; Lei, S.; Sun, X. A constitutive serine protease inhibitor suppresses herbivore performance in tea (Camellia sinensis). Hortic. Res. 2023, 10, uhad178. [Google Scholar] [CrossRef] [PubMed]
- Dunse, K.M.; Stevens, J.A.; Lay, F.T.; Gaspar, Y.M.; Heath, R.L.; Anderson, M.A. Coexpression of potato type I and II proteinase inhibitors gives cotton plants protection against insect damage in the field. Proc. Natl. Acad. Sci. USA 2010, 107, 15011–15015. [Google Scholar] [CrossRef]
- Zhu, C.; Yi, X.; Yang, M.; Liu, Y.; Yao, Y.; Zi, S.; Chen, B.; Xiao, G. Comparative transcriptome analysis of defense response of potato to Phthorimaea operculella infestation. Plants 2023, 12, 3092. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Pan, Y.; Liu, J.; Yang, W.; Shen, G. Comparative Transcriptome Analysis Reveals Expression of Defense Pathways and Specific Protease Inhibitor Genes in Solanum lycopersicum in Response to Feeding by Tuta absoluta. Insects 2025, 16, 166. https://doi.org/10.3390/insects16020166
Zhou Y, Pan Y, Liu J, Yang W, Shen G. Comparative Transcriptome Analysis Reveals Expression of Defense Pathways and Specific Protease Inhibitor Genes in Solanum lycopersicum in Response to Feeding by Tuta absoluta. Insects. 2025; 16(2):166. https://doi.org/10.3390/insects16020166
Chicago/Turabian StyleZhou, Yan, Yongyi Pan, Jia Liu, Wenjia Yang, and Guangmao Shen. 2025. "Comparative Transcriptome Analysis Reveals Expression of Defense Pathways and Specific Protease Inhibitor Genes in Solanum lycopersicum in Response to Feeding by Tuta absoluta" Insects 16, no. 2: 166. https://doi.org/10.3390/insects16020166
APA StyleZhou, Y., Pan, Y., Liu, J., Yang, W., & Shen, G. (2025). Comparative Transcriptome Analysis Reveals Expression of Defense Pathways and Specific Protease Inhibitor Genes in Solanum lycopersicum in Response to Feeding by Tuta absoluta. Insects, 16(2), 166. https://doi.org/10.3390/insects16020166



