Endosymbiotic Bacteria Spiroplasma and Wolbachia in a Laboratory-Reared Insect Collection
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Insect Collection
2.2. DNA Extraction
2.3. PCR and Sequencing
2.4. Phylogenetic Analysis
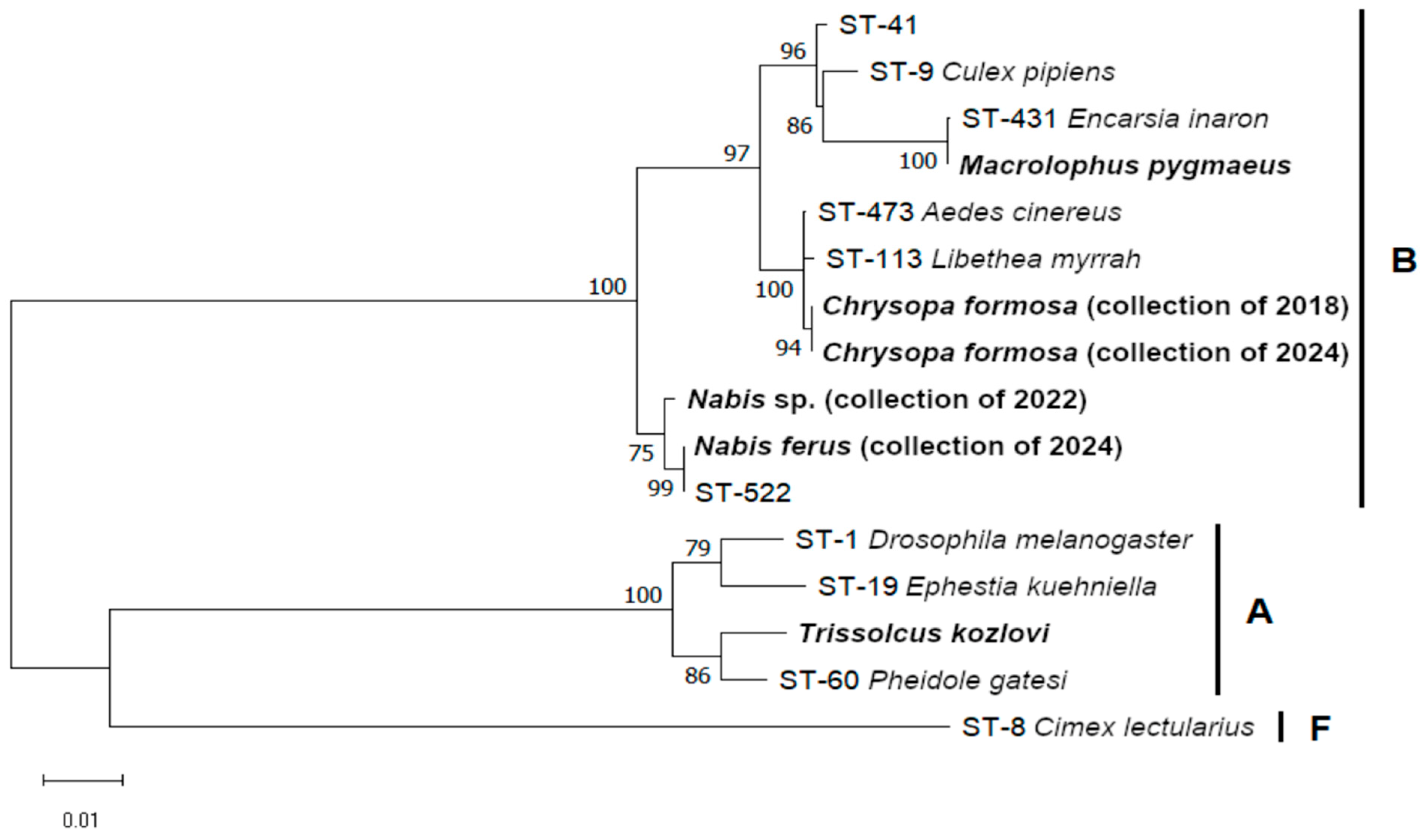
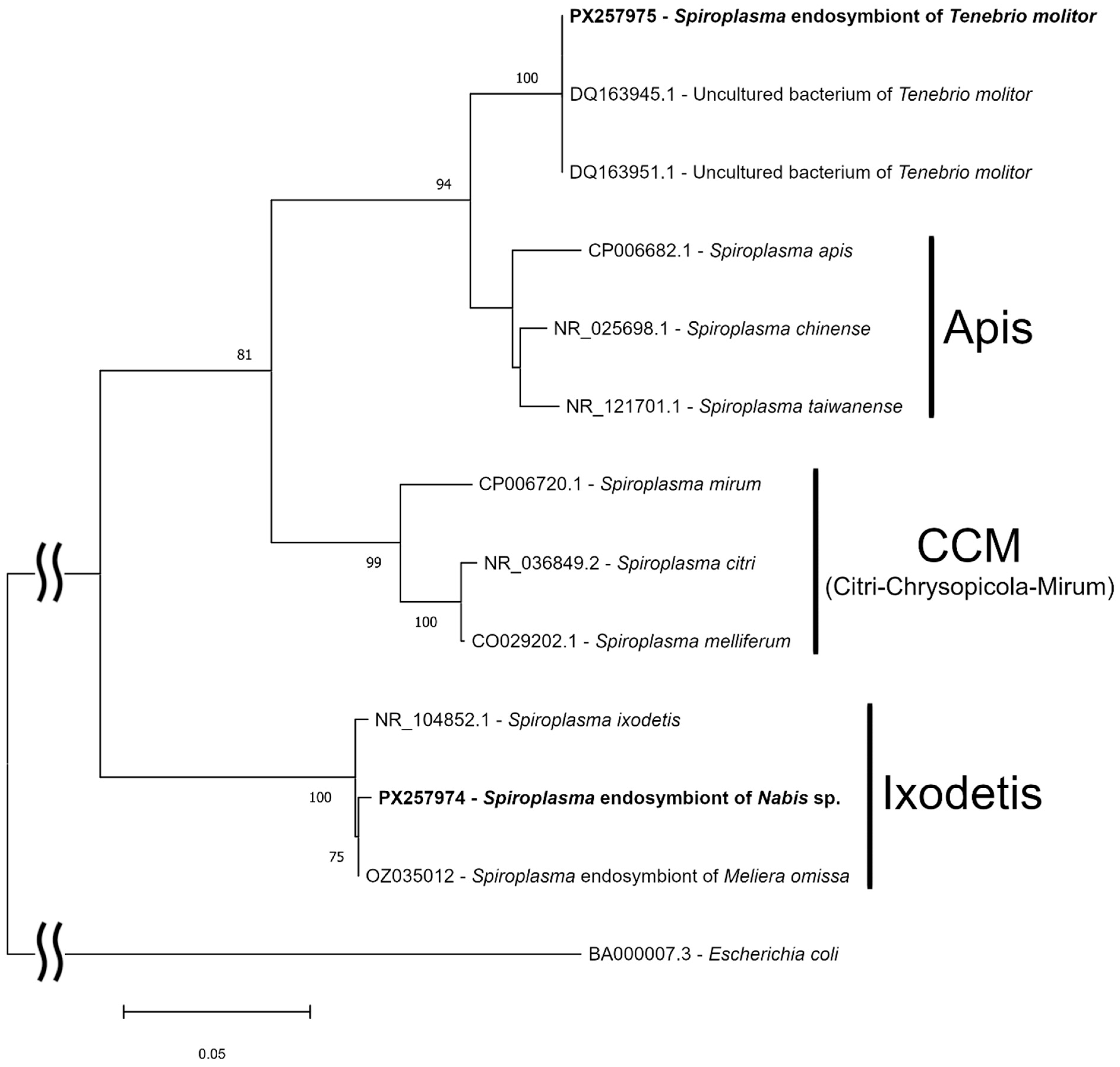
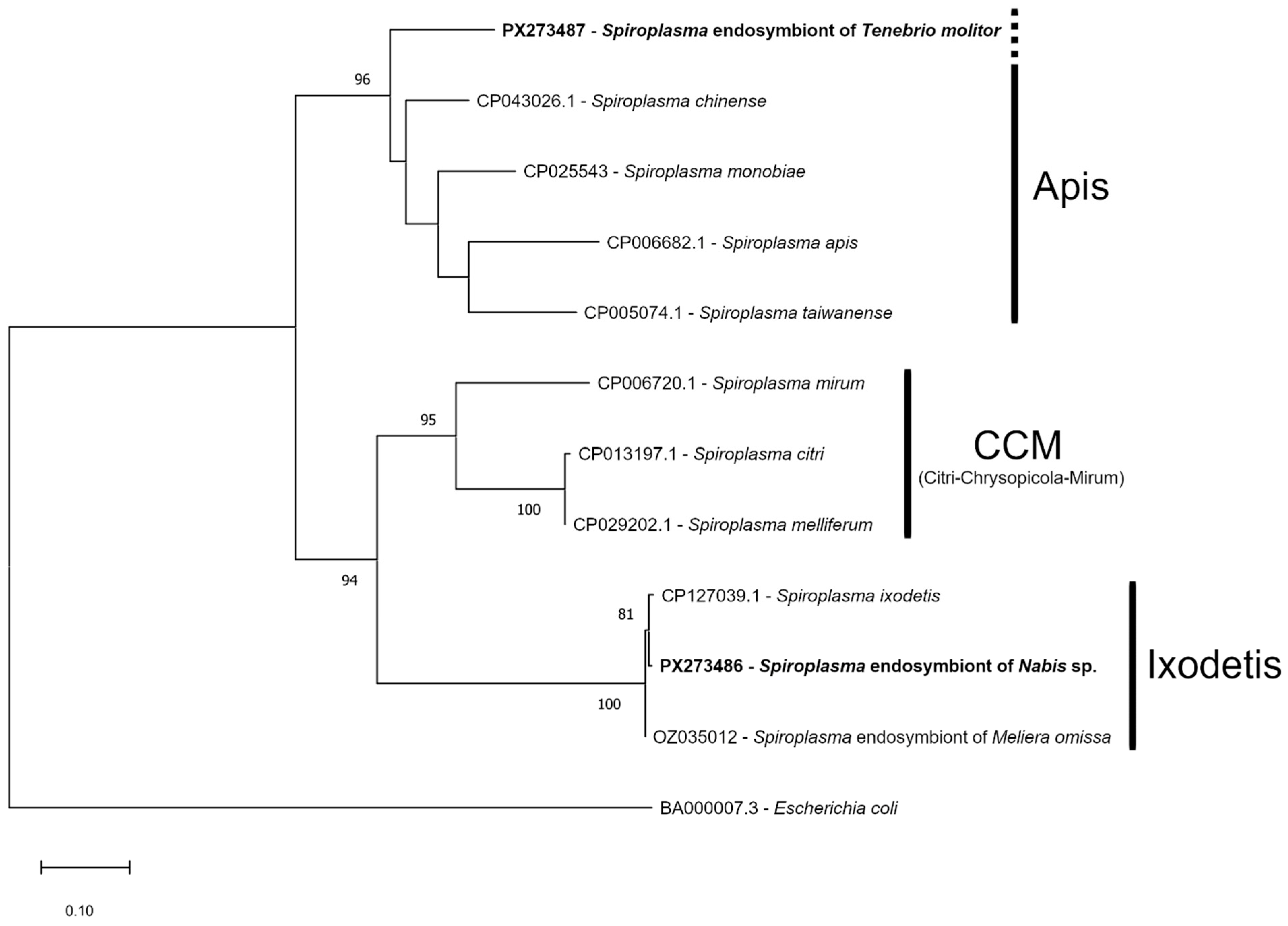
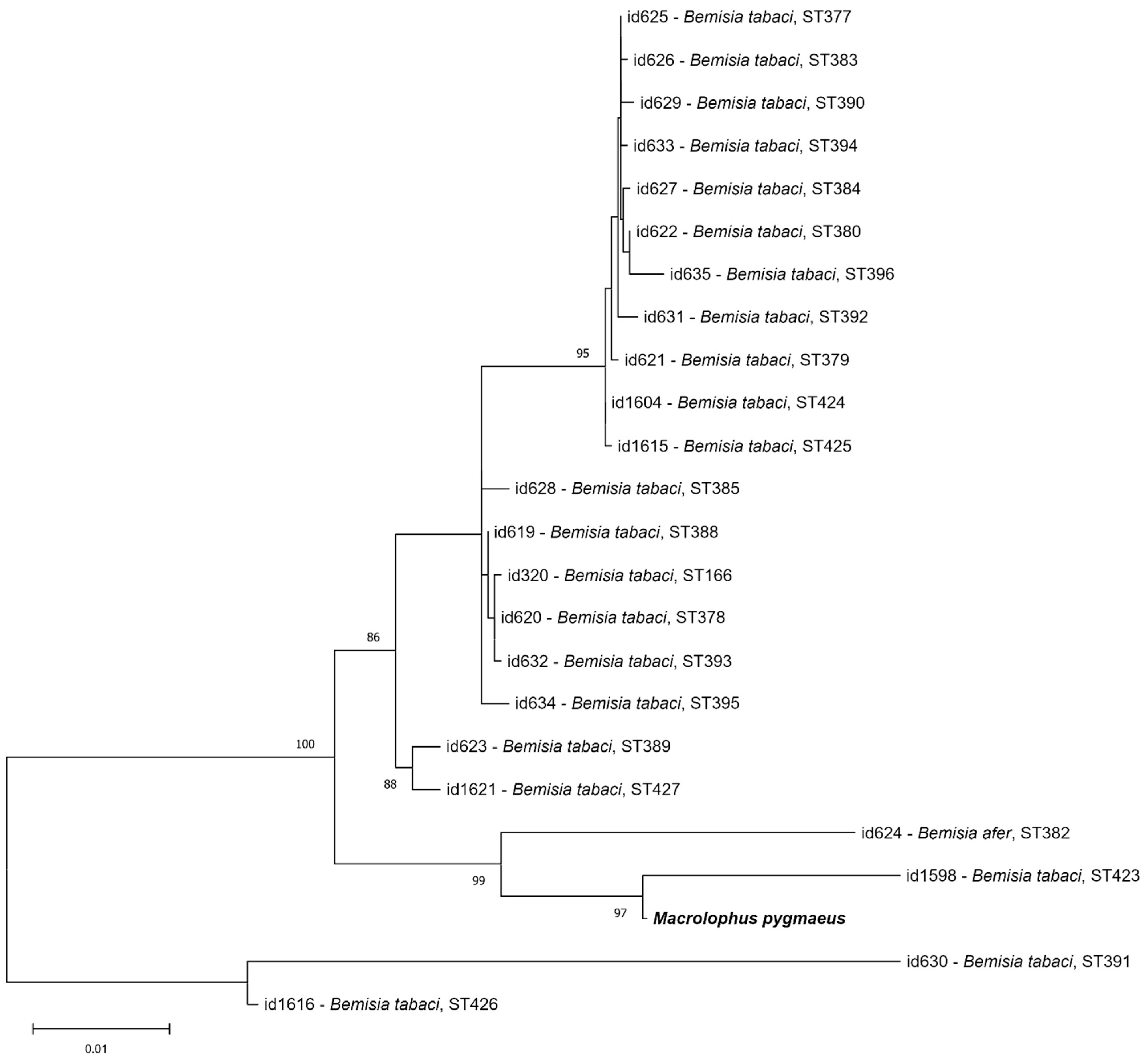
3. Results
4. Discussion
4.1. Barcoding Identification of the Symbiont-Harbored Species
4.2. Symbionts in Laboratory Stocks
4.3. Genetics of the Symbionts
4.4. How These Symbionts Can Be Used in Biocontrol
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Werren, J.H. Biology of Wolbachia. Annu. Rev. Entomol. 1997, 42, 587–609. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, M.J.; Perlman, S.J. The defensive Spiroplasma. Curr. Opin. Insect Sci. 2019, 32, 36–41. [Google Scholar] [CrossRef]
- Shishkina, O.D.; Gruntenko, N.E. Symbiosis of intracellular bacteria Wolbachia with insects: A hundred years of study summarized. Vavilov J. Genet. Breed. 2025, 29, 79. [Google Scholar] [CrossRef]
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How many species are infected with Wolbachia?—A statistical analysis of current data. FEMS Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef]
- Zug, R.; Hammerstein, P. Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 2012, 7, e38544. [Google Scholar] [CrossRef]
- Sazama, E.J.; Bosch, M.J.; Shouldis, C.S.; Ouellette, S.P.; Wesner, J.S. Incidence of Wolbachia in aquatic insects. Ecol. Evol. 2017, 7, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Fenn, K.; Blaxter, M. Wolbachia genomes: Revealing the biology of parasitism and mutualism. Trends Parasitol. 2006, 22, 60–65. [Google Scholar] [CrossRef]
- Haegeman, A.; Vanholme, B.; Jacob, J.; Vandekerckhove, T.T.; Claeys, M.; Borgonie, G.; Gheysen, G. An endosymbiotic bacterium in a plant-parasitic nematode: Member of a new Wolbachia supergroup. Int. J. Parasitol. 2009, 39, 1045–1054. [Google Scholar] [CrossRef]
- Kittayapong, P.; Jamnongluk, W.; Thipaksorn, A.; Milne, J.R.; Sindhusake, C. Wolbachia infection complexity among insects in the tropical rice-field community. Mol. Ecol. 2003, 12, 1049–1060. [Google Scholar] [CrossRef]
- Stahlhut, J.K.; Desjardins, C.A.; Clark, M.E.; Baldo, L.; Russell, J.A.; Werren, J.H.; Jaenike, J. The mushroom habitat as an ecological arena for global exchange of Wolbachia. Mol. Ecol. 2010, 19, 1940–1952. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.Z.; Li, S.J.; Xue, X.; Yin, X.J.; Ren, S.X.; Jiggins, F.M.; Greeff, J.M.; Qiu, B.L. The intracellular bacterium Wolbachia uses parasitoid wasps as phoretic vectors for efficient horizontal transmission. PLoS Pathog. 2015, 11, e1004672. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Windsor, D.; Guo, L.R. Distribution of Wolbachia among neotropical arthropods. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1995, 262, 197–204. [Google Scholar] [CrossRef]
- Bandi, C.; Anderson, T.J.; Genchi, C.; Blaxter, M.L. Phylogeny of Wolbachia in filarial nematodes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1998, 265, 2407–2413. [Google Scholar] [CrossRef] [PubMed]
- Lo, N.; Casiraghi, M.; Salati, E.; Bazzocchi, C.; Bandi, C. How many Wolbachia supergroups exist? Mol. Biol. Evol. 2002, 19, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Bordenstein, S.; Rosengaus, R.B. Discovery of a novel Wolbachia supergroup in Isoptera. Curr. Microbiol. 2005, 51, 393–398. [Google Scholar] [CrossRef]
- Ros, V.I.; Fleming, V.M.; Feil, E.J.; Breeuwer, J.A. How diverse is the genus Wolbachia? Multiple-gene sequencing reveals a putatively new Wolbachia supergroup recovered from spider mites (Acari: Tetranychidae). Appl. Environ. Microbiol. 2009, 75, 1036–1043. [Google Scholar] [CrossRef]
- Augustinos, A.A.; Santos-Garcia, D.; Dionyssopoulou, E.; Moreira, M.; Papapanagiotou, A.; Scarvelakis, M.; Doudoumis, V.; Ramos, S.; Aguiar, A.F.; Borges, P.A.V.; et al. Detection and characterization of Wolbachia infections in natural populations of aphids: Is the hidden diversity fully unraveled? PLoS ONE 2011, 6, e28695. [Google Scholar] [CrossRef]
- Wang, Z.; Su, X.M.; Wen, J.; Jiang, L.Y.; Qiao, G.X. Widespread infection and diverse infection patterns of Wolbachia in Chinese aphids. Insect Sci. 2014, 21, 313–325. [Google Scholar] [CrossRef]
- Glowska, E.; Dragun-Damian, A.; Dabert, M.; Gerth, M. New Wolbachia supergroups detected in quill mites (Acari: Syringophilidae). Infect. Genet. Evol. 2015, 30, 140–146. [Google Scholar] [CrossRef]
- Lefoulon, E.; Clark, T.; Borveto, F.; Perriat-Sanguinet, M.; Moulia, C.; Slatko, B.E.; Gavotte, L. Pseudoscorpion Wolbachia symbionts: Diversity and evidence for a new supergroup S. BMC Microbiol. 2020, 20, 188. [Google Scholar] [CrossRef]
- Sharma, A.K.; Som, A. Assigning new supergroups V and W to the Wolbachia diversity. Bioinformation 2023, 19, 336. [Google Scholar] [CrossRef]
- Duron, O.; Bouchon, D.; Boutin, S.; Bellamy, L.; Zhou, L.; Engelstädter, J.; Hurst, G.D. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 2008, 6, 27. [Google Scholar] [CrossRef]
- Cisak, E.; Wójcik-Fatla, A.; Zajac, V.; Sawczyn, A.; Sroka, J.; Dutkiewicz, J. Spiroplasma—An emerging arthropod-borne pathogen? Ann. Agric. Environ. Med. 2015, 22, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Gasparich, G.E.; Whitcomb, R.F.; Dodge, D.; French, F.E.; Glass, J.; Williamson, D.L. The genus Spiroplasma and its non-helical descendants: Phylogenetic classification, correlation with phenotype and roots of the Mycoplasma mycoides clade. Int. J. Syst. Evol. Microbiol. 2004, 54, 893–918. [Google Scholar] [CrossRef] [PubMed]
- Bolanos, L.M.; Servin-Garciduenas, L.E.; Martinez-Romero, E. Arthropod–Spiroplasma relationship in the genomic era. FEMS Microbiol. Ecol. 2015, 91, 1–8. [Google Scholar] [CrossRef]
- Binetruy, F.; Bailly, X.; Chevillon, C.; Martin, O.Y.; Bernasconi, M.V.; Duron, O. Phylogenetics of the Spiroplasma ixodetis endosymbiont reveals past transfers between ticks and other arthropods. Ticks Tick-Borne Dis. 2019, 10, 575–584. [Google Scholar] [CrossRef]
- Poulson, D.F.; Sakaguchi, B. Nature of “sex-ratio” agent in Drosophila. Science 1961, 133, 1489–1490. [Google Scholar] [CrossRef] [PubMed]
- Hurst, G.D.D.; von der Schulenburg, J.G.; Majerus, T.M.O.; Bertrand, D.; Zakharov, I.A.; Baungaard, J.; Völkl, W.; Stouthamer, R.; Majerus, M.E.N. Invasion of one insect species, Adalia bipunctata, by two different male-killing bacteria. Insect Mol. Biol. 1999, 8, 133–139. [Google Scholar] [CrossRef]
- Williamson, D.L.; Sakaguchi, B.; Hackett, K.J.; Whitcomb, R.F.; Tully, J.G.; Carle, P.; Bové, J.M.; Adams, J.R.; Konai, M.; Henegar, R.B. Spiroplasma poulsonii sp. nov., a new species associated with male-lethality in Drosophila willistoni, a neotropical species of fruit fly. Int. J. Syst. Evol. Microbiol. 1999, 49, 611–618. [Google Scholar] [CrossRef]
- Tinsley, M.C.; Majerus, M.E.N. A new male-killing parasitism: Spiroplasma bacteria infect the ladybird beetle Anisosticta novemdecimpunctata (Coleoptera: Coccinellidae). Parasitology 2006, 132, 757–765. [Google Scholar] [CrossRef]
- Jiggins; Hurst; Dolman; Majerus. High-prevalence male-killing Wolbachia in the butterfly Acraea encedana. J. Evol. Biol. 2000, 13, 495–501. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Tabata, J.; Hattori, Y.; Sakamoto, H.; Yukuhiro, F.; Fujii, T.; Kugimiya, S.; Mochizuki, A.; Ishikawa, Y.; Kageyama, D. Male killing and incomplete inheritance of a novel Spiroplasma in the moth Ostrinia zaguliaevi. Microb. Ecol. 2011, 61, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Pollmann, M.; Moore, L.D.; Krimmer, E.; D’Alvise, P.; Hasselmann, M.; Perlman, S.J.; Ballinger, M.J.; Steidle, J.L.M.; Gottlieb, Y. Highly transmissible cytoplasmic incompatibility by the extracellular insect symbiont Spiroplasma. iScience 2022, 25, 104335. [Google Scholar] [CrossRef]
- Starr, D.J.; Cline, T.W. A host–parasite interaction rescues Drosophila oogenesis defects. Nature 2002, 418, 76–79. [Google Scholar] [CrossRef]
- Brownlie, J.C.; Cass, B.N.; Riegler, M.; Witsenburg, J.J.; Iturbe-Ormaetxe, I.; McGraw, E.A.; O’Neill, S.L. Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog. 2009, 5, e1000368. [Google Scholar] [CrossRef]
- Ikeya, T.; Broughton, S.; Alic, N.; Grandison, R.; Partridge, L. The endosymbiont Wolbachia increases insulin/IGF-like signalling in Drosophila. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2009, 276, 3799–3807. [Google Scholar] [CrossRef]
- Zug, R.; Hammerstein, P. Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biol. Rev. 2015, 90, 89–111. [Google Scholar] [CrossRef]
- Jaenike, J.; Stahlhut, J.K.; Boelio, L.M.; Unckless, R.L. Association between Wolbachia and Spiroplasma within Drosophila neotestacea: An emerging symbiotic mutualism? Mol. Ecol. 2010, 19, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Vilchez, I.; Mateos, M. Spiroplasma bacteria enhance survival of Drosophila hydei attacked by the parasitic wasp Leptopilina heterotoma. PLoS ONE 2010, 5, e12149. [Google Scholar] [CrossRef]
- Higashi, C.H.; Patel, V.; Kamalaker, B.; Inaganti, R.; Bressan, A.; Russell, J.A.; Oliver, K.M. Another tool in the toolbox: Aphid-specific Wolbachia protect against fungal pathogens. Environ. Microbiol. 2024, 26, e70005. [Google Scholar] [CrossRef]
- Perlmutter, J.I.; Atadurdyyeva, A.; Schedl, M.E.; Unckless, R.L. Wolbachia enhances the survival of Drosophila infected with fungal pathogens. BMC Biol. 2025, 23, 42. [Google Scholar] [CrossRef] [PubMed]
- Mouches, C.; Bové, J.M.; Albisetti, J. Pathogenicity of Spiroplasma apis and other spiroplasmas for honey-bees in Southwestern France. Ann. L’Institut Pasteur/Microbiol. 1984, 135, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, B.; Schroeder, J.; Reischl, U. First evidence of an endogenous Spiroplasma sp. infection in humans manifesting as unilateral cataract associated with anterior uveitis in a premature baby. Graefes Arch. Clin. Exp. Ophthalmol. 2002, 240, 348–353. [Google Scholar] [CrossRef]
- Santa Olga Cacciola, A.B.; Pane, A.; Furneri, P.M. Spiroplasma spp.: A plant, arthropod, animal and human pathogen. In Citrus Pathology; Gill, H., Garg, H., Eds.; InTech: London, UK, 2017; pp. 31–51. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Yang, K.; Zhu, Y.X.; Hong, X.Y. Symbiont-conferred reproduction and fitness benefits can favour their host occurrence. Ecol. Evol. 2018, 8, 1626–1633. [Google Scholar] [CrossRef]
- Xie, K.; Lu, Y.J.; Yang, K.; Huo, S.M.; Hong, X.Y. Co-infection of Wolbachia and Spiroplasma in spider mite Tetranychus truncatus increases male fitness. Insect Sci. 2020, 27, 921–937. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Song, Z.R.; Song, Y.L.; Hong, X.Y. Double infection of Wolbachia and Spiroplasma alters induced plant defense and spider mite fecundity. Pest Manag. Sci. 2020, 76, 3273–3281. [Google Scholar] [CrossRef]
- Tarbinsky, S.P.; Plavilshchikov, N.N. (Eds.) Opredelitel Nasekomykh Evropeiskoi Chasti SSSR [Key to the Insects of the European Part of the USSR]; OGIZ-Selkhozgiz: Moscow, Russia, 1948. (In Russian) [Google Scholar]
- Bei-Bienko, G.Y. (Ed.) Zhestkokrylye i verokrylye [Coleoptera and Strepsiptera]. In Opredelitel Nasekomykh Evropeiskoi Chasti SSSR [Key to the Insects of the European Part of the USSR]; Nauka: Moscow, Russia, 1965; Volume 2, pp. 1–668. (In Russian) [Google Scholar]
- Leley, A.S. (Ed.) Opredelitel Nasekomykh Dalnego Vostoka SSSR. Tom II. Ravnokrylye i Poluzhestkokrylye [Key to the Insects of the Soviet Far East. Vol. II. Homoptera and Heteroptera]; Nauka: Leningrad, Russia, 1988. (In Russian) [Google Scholar]
- Vinokurov, N.N.; Kanyukova, E.V. Heteropteran Insects (Heteroptera) of Siberia; Nauka, Siberian Publishing Firm of the Russian Academy of Sciences: Novosibirsk, Russia, 1995. (In Russian) [Google Scholar]
- Andreeva, I.V.; Shatalova, E.I.; Ulyanova, E.G. Sposob Razvedeniya Kapustnoi Moli Plutella xylostella L. [A Method for Breeding the Diamondback Moth Plutella xylostella L.]. Russian Patent RU 2,735,251 C1, 29 October 2020. [Google Scholar]
- Andreeva, I.V.; Khodakova, A.V.; Shatalova, E.I. Sposob Razvedeniya Laboratornoi Kultury Entomofaga Trissolcus kozlovi Rjachovsky [A Method for Breeding a Laboratory Culture of the Entomophagous Trissolcus kozlovi Rjachovsky]. Russian Patent RU 2,839,960 C1, 14 May 2025. [Google Scholar]
- Shatalova, E.I.; Agricolyanskaya, N.I.; Andreeva, I.V.; Ulyanova, E.G.; Khodakova, A.V. Sposob Razvedeniya Klopa Podizusa Podisus maculiventris Say [A Method for Breeding the Spined Soldier Bug Podisus maculiventris Say]. Russian Patent RU 2,795,991 C1, 16 May 2023. [Google Scholar]
- Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Baldo, L.; Dunning Hotopp, J.C.; Jolley, K.A.; Bordenstein, S.R.; Biber, S.A.; Choudhury, R.R.; Hayashi, C.; Maiden, M.C.J.; Tettelin, H.; Werren, J.H. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 2006, 72, 7098–7110. [Google Scholar] [CrossRef]
- Ilinsky, Y. Coevolution of Drosophila melanogaster mtDNA and Wolbachia genotypes. PLoS ONE 2013, 8, e54373. [Google Scholar] [CrossRef] [PubMed]
- Sanada-Morimura, S.; Matsumura, M.; Noda, H. Male killing caused by a Spiroplasma symbiont in the small brown planthopper, Laodelphax striatellus. J. Hered. 2013, 104, 821–829. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.K.; Stabb, E.V. Culture-independent characterization of the microbiota of the ant lion Myrmeleon mobilis (Neuroptera: Myrmeleontidae). Appl. Environ. Microbiol. 2005, 71, 8784–8794. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cascales, J.I.; Cenis, J.L.; Sanchez, J.A. Differentiation of Macrolophus pygmaeus (Rambur 1839) and Macrolophus melanotoma (Costa 1853) (Heteroptera: Miridae) based on molecular data. Bull. OILB/SROP 2006, 29, 223–227. [Google Scholar]
- Castañé, C.; Agustí, N.; Arnó, J.; Gabarra, R.; Riudavets, J.; Comas, J.; Alomar, O. Taxonomic identification of Macrolophus pygmaeus and Macrolophus melanotoma based on morphometry and molecular markers. Bull. Entomol. Res. 2013, 103, 204–215. [Google Scholar] [CrossRef]
- Pasquer, F.; Pfunder, M.; Frey, B.; Frey, J.E. Microarray-based genetic identification of beneficial organisms as a new tool for quality control of laboratory cultures. Biocontrol. Sci. Technol. 2009, 19, 809–833. [Google Scholar] [CrossRef]
- Jung, J.; Heo, A.; Park, Y.W.; Kim, Y.J.; Koh, H.; Park, W. Gut microbiota of Tenebrio molitor and their response to environmental change. J. Microbiol. Biotechnol. 2014, 24, 888–897. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y. Investigation of gut-associated bacteria in Tenebrio molitor (Coleoptera: Tenebrionidae) larvae using culture-dependent and DGGE methods. Ann. Entomol. Soc. Am. 2015, 108, 941–949. [Google Scholar] [CrossRef]
- Machtelinckx, T.; Van Leeuwen, T.; Vanholme, B.; Gehesquière, B.; Dermauw, W.; Vandekerkhove, B.; Gheysen, G.; De Clercq, P. Wolbachia induces strong cytoplasmic incompatibility in the predatory bug Macrolophus pygmaeus. Insect Mol. Biol. 2009, 18, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Fukatsu, T. Diversity of Wolbachia endosymbionts in heteropteran bugs. Appl. Environ. Microbiol. 2003, 69, 6082–6090. [Google Scholar] [CrossRef]
- Li, G.; Sun, J.; Meng, Y.; Yang, C.; Chen, Z.; Wu, Y.; Tian, L.; Song, F.; Cai, W.; Zhang, X.; et al. The impact of environmental habitats and diets on the gut microbiota diversity of true bugs (Hemiptera: Heteroptera). Biology 2022, 11, 1039. [Google Scholar] [CrossRef] [PubMed]
- Guz, N.; Kocak, E.; Emre Akpinar, A.; Oktay Gurkan, M.; Neset Kilincer, A. Wolbachia infection in Trissolcus species (Hymenoptera: Scelionidae). Eur. J. Entomol. 2012, 109, 169–174. [Google Scholar] [CrossRef]
- Zakharov, I.A.; Goriacheva, I.I.; Shaĭkevich, E.V.; Schulenburg, J.H.; Majerus, E.N. Wolbachia—A new bacteria causing sex ratio bias in the two-spot lady-bird Adalia bipunctata L. Genetika 2000, 36, 482–486. [Google Scholar]
- Sokolova, M.I.; Zinkevich, N.S.; Zakharov, I.A. Bacteria in ovarioles of females from maleless families of ladybird beetles Adalia bipunctata L. (Coleoptera: Coccinellidae) naturally infected with Rickettsia, Wolbachia, and Spiroplasma. J. Invertebr. Pathol. 2002, 79, 72–79. [Google Scholar] [CrossRef]
- Ahmed, M.Z.; Ren, S.X.; Mandour, N.S.; Greeff, J.M.; Qiu, B.L. Prevalence of Wolbachia supergroups A and B in Bemisia tabaci (Hemiptera: Aleyrodidae) and some of its natural enemies. J. Econ. Entomol. 2010, 103, 1848–1859. [Google Scholar] [CrossRef]
- Shaikevich, E.V.; Romanov, D.A.; Zakharov, I.A. The diversity of Wolbachia and its effects on host reproduction in a single Adalia bipunctata (Coleoptera: Coccinellidae) population. Symbiosis 2021, 85, 249–257. [Google Scholar] [CrossRef]
- Romanov, D.A.; Zakharov, I.A. Distribution of symbiotic bacteria Spiroplasma, Rickettsia, Wolbachia in populations of Adalia bipunctata (Linnaeus, 1758). Symbiosis 2023, 91, 1–13. [Google Scholar] [CrossRef]
- Archer, J.; Hurst, G.D.; Hornett, E.A. Male-killer symbiont screening reveals novel associations in Adalia ladybirds. Access Microbiol. 2023, 5, 000585-v3. [Google Scholar] [CrossRef] [PubMed]
- Goryacheva, I.I.; Blekhman, A.V.; Andrianov, B.V.; Gorelova, T.V.; Zakharov, I.A. Genotypic diversity of Wolbachia pipientis in native and invasive Harmonia axyridis Pall., 1773 (Coleoptera, Coccinellidae) populations. Russ. J. Genet. 2015, 51, 731–736. [Google Scholar] [CrossRef]
- Goryacheva, I.; Blekhman, A.; Andrianov, B.; Romanov, D.; Zakharov, I. Spiroplasma infection in Harmonia axyridis-Diversity and multiple infection. PLoS ONE 2018, 13, e0198190. [Google Scholar] [CrossRef]
- Awad, M.; Piálková, R.; Haelewaters, D.; Nedvěd, O. Infection patterns of Harmonia axyridis (Coleoptera: Coccinellidae) by ectoparasitic microfungi and endosymbiotic bacteria. J. Invertebr. Pathol. 2023, 197, 107887. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.; Poncelet, N.; He, K.; Francis, F.; Wang, Z. Detection and geographic distribution of seven facultative endosymbionts in two Rhopalosiphum aphid species. MicrobiologyOpen 2019, 8, e00817. [Google Scholar] [CrossRef]
- Majeed, M.Z.; Sayed, S.; Bo, Z.; Raza, A.; Ma, C.S. Bacterial symbionts confer thermal tolerance to cereal aphids Rhopalosiphum padi and Sitobion avenae. Insects 2022, 13, 231. [Google Scholar] [CrossRef]
- Zytynska, S.E.; Meyer, S.T.; Sturm, S.; Ullmann, W.; Mehrparvar, M.; Weisser, W.W. Secondary bacterial symbiont community in aphids responds to plant diversity. Oecologia 2016, 180, 735–747. [Google Scholar] [CrossRef]
- Romanov, D.A.; Zakharov, I.A.; Shaikevich, E.V. Wolbachia, Spiroplasma, and Rickettsia symbiotic bacteria in aphids (Aphidoidea). Vavilov J. Genet. Breed. 2020, 24, 673. [Google Scholar] [CrossRef] [PubMed]
- Owashi, Y.; Minami, T.; Kikuchi, T.; Yoshida, A.; Nakano, R.; Kageyama, D.; Adachi-Hagimori, T. Microbiome of zoophytophagous biological control agent Nesidiocoris tenuis. Microb. Ecol. 2023, 86, 2923–2933. [Google Scholar] [CrossRef]
- Shibata, T.; Shimoda, M.; Kobayashi, T.; Arai, H.; Owashi, Y.; Uehara, T. High-quality genome of the zoophytophagous stink bug, Nesidiocoris tenuis, informs their food habit adaptation. G3 2024, 14, jkad289. [Google Scholar] [CrossRef]
- Fernandez-Cassi, X.; Söderqvist, K.; Bakeeva, A.; Vaga, M.; Dicksved, J.; Vagsholm, I.; Jansson, A.; Boqvist, S. Microbial communities and food safety aspects of crickets (Acheta domesticus) reared under controlled conditions. J. Insects Food Feed 2020, 6, 429–440. [Google Scholar] [CrossRef]
- Weinert, L.A.; Tinsley, M.C.; Temperley, M.; Jiggins, F.M. Are we underestimating the diversity and incidence of insect bacterial symbionts? A case study in ladybird beetles. Biol. Lett. 2007, 3, 678–681. [Google Scholar] [CrossRef]
- Škaljac, M.; Žanić, K.; Hrnčić, S.; Radonjić, S.; Perović, T.; Ghanim, M. Diversity and localization of bacterial symbionts in three whitefly species (Hemiptera: Aleyrodidae) from the east coast of the Adriatic Sea. Bull. Entomol. Res. 2013, 103, 48–59. [Google Scholar] [CrossRef]
- Skaljac, M.; Kanakala, S.; Zanic, K.; Puizina, J.; Lepen Pleic, I.; Ghanim, M. Diversity and phylogenetic analyses of bacterial symbionts in three whitefly species from Southeast Europe. Insects 2017, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Kapantaidaki, D.E.; Ovčarenko, I.; Fytrou, N.; Knott, K.E.; Bourtzis, K.; Tsagkarakou, A. Low levels of mitochondrial DNA and symbiont diversity in the worldwide agricultural pest, the greenhouse whitefly Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). J. Hered. 2015, 106, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Tat, Z.; Koçak, E. Composition of Secondary Endosymbiont Bacteria in Two Whitefly Species (Hemiptera: Aleyrodidae). Akdeniz Univ. Ziraat Fak. Derg. 2024, 19, 39–46. [Google Scholar] [CrossRef]
- Jeyaprakash, A.; Hoy, M.A. Long PCR improves Wolbachia DNA amplification: Wsp sequences found in 76% of sixty-three arthropod species. Insect Mol. Biol. 2000, 9, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.M.; Cook, J.M. Effects of a sex-ratio distorting endosymbiont on mtDNA variation in a global insect pest. BMC Evol. Biol. 2009, 9, 49. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, T.; He, A.; Zhang, L.; Li, J.; Li, T.; Miao, X.; You, M.; You, S. Diversity of Wolbachia infection and its influence on mitochondrial DNA variation in the diamondback moth, Plutella xylostella. Mol. Phylogenet. Evol. 2023, 182, 107751. [Google Scholar] [CrossRef]
- Jiang, X.F.; Wang, L.; Zhang, L.; Luo, L.Z. Molecular detection of Wolbachia in three species of vegetable aphids collected from Beijing suburb. Plant Prot. 2009, 35, 63–65. [Google Scholar]
- Eddoubaji, Y.; Aldeia, C.; Campos-Madueno, E.I.; Moser, A.I.; Kundlacz, C.; Perreten, V.; Hilty, M.; Endimiani, A. A new in vivo model of intestinal colonization using Zophobas morio larvae: Testing hyperepidemic ESBL-and carbapenemase-producing Escherichia coli clones. Front. Microbiol. 2024, 15, 1381051. [Google Scholar] [CrossRef]
- Bailly-Bechet, M.; Martins-Simões, P.; Szöllősi, G.J.; Mialdea, G.; Sagot, M.F.; Charlat, S. How long does Wolbachia remain on board? Mol. Biol. Evol. 2017, 34, 1183–1193. [Google Scholar] [CrossRef]
- Łukasik, P.; van Asch, M.; Guo, H.; Ferrari, J.; Charles, J.; Godfray, H. Unrelated facultative endosymbionts protect aphids against a fungal pathogen. Ecol. Lett. 2013, 16, 214–218. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.H.C.; Hrček, J.; Parker, B.J.; Mathé-Hubert, H.; Kaech, H.; Paine, C.; Godfray, H.C.J. Multiple phenotypes conferred by a single insect symbiont are independent. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2020, 287, 20200562. [Google Scholar] [CrossRef]
- Majerus, T.M.O.; von der Schulenburg, J.H.; Majerus, M.E.N.; Hurst, G.D.D. Molecular identification of a male-killing agent in the ladybird Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). Insect Mol. Biol. 1999, 8, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Majerus, M.E.; Hinrich, J.; Schulenburg, G.V.D.; Zakharov, I.A. Multiple causes of male-killing in a single sample of the two-spot ladybird, Adalia bipunctata (Coleoptera: Coccinellidae) from Moscow. Heredity 2000, 84, 605–609. [Google Scholar] [CrossRef]
- Takamatsu, T.; Arai, H.; Abe, N.; Nakai, M.; Kunimi, Y.; Inoue, M.N. Coexistence of two male-killers and their impact on the development of oriental tea tortrix Homona magnanima. Microb. Ecol. 2021, 81, 193–202. [Google Scholar] [CrossRef]
- Nai, Y.S.; Su, P.Y.; Hsu, Y.H.; Chiang, C.H.; Kim, J.S.; Chen, Y.W.; Wang, C.H. A new spiroplasma isolate from the field cricket (Gryllus bimaculatus) in Taiwan. J. Invertebr. Pathol. 2014, 120, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Hercus, M.; Dagher, H. Population dynamics of the Wolbachia infection causing cytoplasmic incompatibility in Drosophila melanogaster. Genetics 1998, 148, 221–231. [Google Scholar] [CrossRef]
- Arthofer, W.; Riegler, M.; Avtzis, D.N.; Stauffer, C. Evidence for low-titre infections in insect symbiosis: Wolbachia in the bark beetle Pityogenes chalcographus (Coleoptera, Scolytinae). Environ. Microbiol. 2009, 11, 1923–1933. [Google Scholar] [CrossRef]
- Bykov, R.; Kerchev, I.; Demenkova, M.; Ryabinin, A.; Ilinsky, Y. Sex-specific Wolbachia infection patterns in populations of Polygraphus proximus Blandford (Coleoptera; Curculionidae: Scolytinae). Insects 2020, 11, 547. [Google Scholar] [CrossRef]
- Schilthuizen, M.O.; Stouthamer, R. Horizontal transmission of parthenogenesis–inducing microbes in Trichogramma wasps. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1997, 264, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Huigens, M.E.; De Almeida, R.P.; Boons, P.A.H.; Luck, R.F.; Stouthamer, R. Natural interspecific and intraspecific horizontal transfer of parthenogenesis–inducing Wolbachia in Trichogramma wasps. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2004, 271, 509–515. [Google Scholar] [CrossRef]
- Nikoh, N.; Hosokawa, T.; Moriyama, M.; Oshima, K.; Hattori, M.; Fukatsu, T. Evolutionary origin of insect–Wolbachia nutritional mutualism. PNAS Nexus 2014, 111, 10257–10262. [Google Scholar] [CrossRef]
- Łukasik, P.; Guo, H.; Van Asch, M.; Ferrari, J.; Godfray, H.C.J. Protection against a fungal pathogen conferred by the aphid facultative endosymbionts Rickettsia and Spiroplasma is expressed in multiple host genotypes and species and is not influenced by co-infection with another symbiont. J. Evol. Biol. 2013, 26, 2654–2661. [Google Scholar] [CrossRef]
- Garcia-Arraez, M.G.; Masson, F.; Escobar, J.C.P.; Lemaitre, B. Functional analysis of RIP toxins from the Drosophila endosymbiont Spiroplasma poulsonii. BMC Microbiol. 2019, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- Jaenike, J.; Unckless, R.; Cockburn, S.N.; Boelio, L.M.; Perlman, S.J. Adaptation via symbiosis: Recent spread of a Drosophila defensive symbiont. Science 2010, 329, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Ebbert, M.A.; Nault, L.R. Improved overwintering ability in Dalbulus maidis (Homoptera: Cicadellidae) vectors infected with Spiroplasma kunkelii (Mycoplasmatales: Spiroplasmataceae). Environ. Entomol. 1994, 23, 634–644. [Google Scholar] [CrossRef]
| Order | Species | Purpose of Culture | Origin (Year) | Nutrient Substrate | Wolbachia | Spiroplasma |
|---|---|---|---|---|---|---|
| Orthoptera | Acheta domesticus | IF | PS, Msk, (2022) | Vegetables | 0 | 0 |
| Coleoptera | Adalia bipunctata | BC | SFSCA, Nsk (2024) | Aphids | 0 | 0 |
| Hemiptera | Aphis fabae | test, IF | SFSCA, Nsk (2017) | Bean | 0 | 0 |
| Hemiptera | Arma custos | BC | SFSCA, Nsk (2023) | Tenebrio molitor | 0 | 0* |
| Hemiptera | Brevicoryne brassicae | test, IF | SFSCA, Nsk (2023) | Cabbage | 0 | 0 |
| Coleoptera | Bruchus rufimanus | test | PS, Msk (2024) | Mash | 0 | 0 |
| Neuroptera | Chrysopa formosa 1 | BC | SFSCA, Nsk (2018) | Aphids | 1 | 0 |
| Neuroptera | Chrysopa formosa 2 | BC | SFSCA, Nsk, (2024) | Aphids | 1 | 0 |
| Coleoptera | Coccinella septempunctata | BC | SFSCA, Nsk (2022) | Aphids | 0 | 0 |
| Orthoptera | Grillus bimaculatus | IF | PS, Msk (2022) | Vegetables | 0 | 0 |
| Coleoptera | Harmonia axyridis | BC | SFSCA, Nsk (2022) | Aphids | 0 | 0 |
| Coleoptera | Harmonia (Leis) dimidiata | BC | VIZR, SPb (2024) | Aphids | 0 | 0 |
| Coleoptera | Hippodamia variegata | BC | SFSCA, Nsk (2022) | Aphids | 0 | 0 |
| Lepidoptera | Lacanobia oleracea | test, IF | SFSCA, Nsk (2023) | Cabbage | 0 | 0 |
| Hemiptera | Macrolophus pygmaeus | BC | CS (2017) | T. vaporariorum | 1 | 0 |
| Hemiptera | Megoura viciae | test, IF | SFSCA, Nsk (2017) | Bean | 0 | 0 |
| Hemiptera | Nabis sp. | BC | SFSCA, Nsk (2023) | Aphids | 1 | 1 |
| Hemiptera | Nabis ferus | BC | SFSCA, Nsk (2024) | Aphids | 1 | 0 |
| Hemiptera | Nesidiocoris tenuis | BC | VIZR, SPb (2023) | T. vaporariorum | 0 | 0 |
| Hemiptera | Platymeris biguttatus | test | PS, T (2023) | T. molitor, Z. morio | 0 | 0 |
| Lepidoptera | Plutella xylostella | IF | SFSCA, Nsk (2019) | Rape | 0 | 0 |
| Hemiptera | Podisus maculiventris | BC | VIZR (2017) | T. molitor, Z. morio | 0 | 0* |
| Hemiptera | Psyttala horrida | test | PS, K (2024) | T. molitor, Z. morio | 0 | 0 |
| Hemiptera | Rhopalosiphum padi | test, IF | SFSCA, Nsk (2018) | Wheat | 0 | 0 |
| Hemiptera | Schizaphis graminum | test, IF | SFSCA, Nsk (2017) | Wheat | 0 | 0 |
| Coleoptera | Subcoccinella vigintiquatuorpunctata | test | SFSCA, Nsk (2023) | Alfalfa | 0 | 0 |
| Coleoptera | Tenebrio molitor | test, IF | PS, Nsk (2020) | Bran, vegetables | 0 | 1 |
| Hemiptera | Trialeurodes vaporariorum | test, IF | SFSCA, Nsk (2021) | Tobacco | 0 | 0 |
| Hymenoptera | Trissolcus kozlovi | BC | SFSCA, Nsk (2021) | P. maculiventris | 1 | 0 |
| Coleoptera | Zophobas morio | IF | PS, Nsk (2020) | Bran, vegetables | 0 | 0 |
| Morphological Identification (GenBank Acc. Num.) | BOLD (% Identity) | GenBank (% Identity) |
|---|---|---|
| Trissolcus kozlovi (PX259647) | Scelionidae (99.53) | T. kozlovi (99.12) |
| Tenebrio molitor (PX259651) | T. molitor (99.85) | T. molitor (99.85) |
| Nabis sp. (PX259649) | N. rugosus, N. pseudoferus, N. brevis (99.8), N. ericetorum (99.7), N. ferus (99.54) | N. rugosus, N. pseudoferus (99.7), N. ferus (99.54) |
| Nabis ferus (PX259650) | N. ferus (99.7) | N. ferus (99.54) |
| Chrysopa formosa 1 (PX259648) | C. formosa (100) | C. formosa (100) |
| Chrysopa formosa 2 (PX259652) | C. formosa (99.85) | C. formosa (99.7) |
| Macrolophus pygmaeus (PX259646) | M. pygmaeus, M. caliginosus (100) | M. pygmaeus, M. caliginosus (100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bykov, R.; Shatalova, E.; Andreeva, I.; Khodakova, A.; Ryabinin, A.; Demenkova, M.; Ilinsky, Y. Endosymbiotic Bacteria Spiroplasma and Wolbachia in a Laboratory-Reared Insect Collection. Insects 2025, 16, 1168. https://doi.org/10.3390/insects16111168
Bykov R, Shatalova E, Andreeva I, Khodakova A, Ryabinin A, Demenkova M, Ilinsky Y. Endosymbiotic Bacteria Spiroplasma and Wolbachia in a Laboratory-Reared Insect Collection. Insects. 2025; 16(11):1168. https://doi.org/10.3390/insects16111168
Chicago/Turabian StyleBykov, Roman, Elena Shatalova, Irina Andreeva, Alevtina Khodakova, Artem Ryabinin, Mary Demenkova, and Yury Ilinsky. 2025. "Endosymbiotic Bacteria Spiroplasma and Wolbachia in a Laboratory-Reared Insect Collection" Insects 16, no. 11: 1168. https://doi.org/10.3390/insects16111168
APA StyleBykov, R., Shatalova, E., Andreeva, I., Khodakova, A., Ryabinin, A., Demenkova, M., & Ilinsky, Y. (2025). Endosymbiotic Bacteria Spiroplasma and Wolbachia in a Laboratory-Reared Insect Collection. Insects, 16(11), 1168. https://doi.org/10.3390/insects16111168







