Cellular Uptake and Tissue Retention of Microplastics in Black Soldier Fly Larvae
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Husbandry and Selection
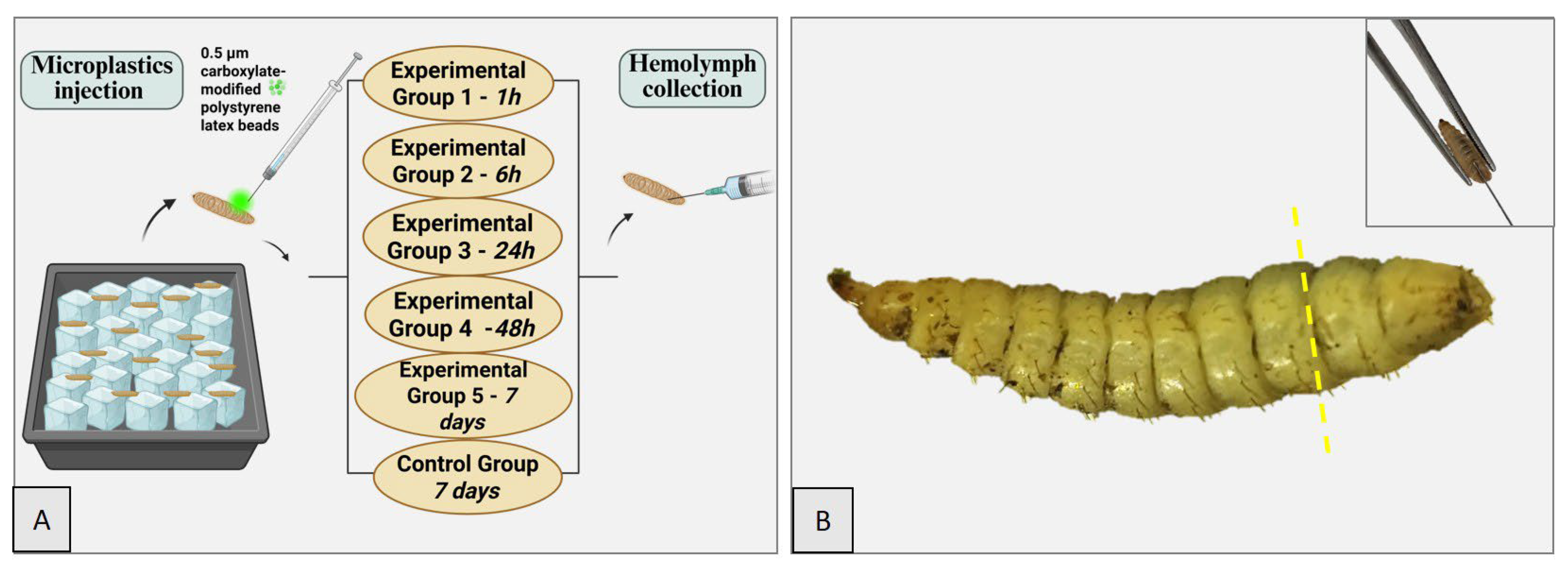
2.2. Experimental Design
2.3. Histopathological Analysis
2.4. Confocal Scanning Laser Microscopy
2.5. Microplastic Assessment
3. Results
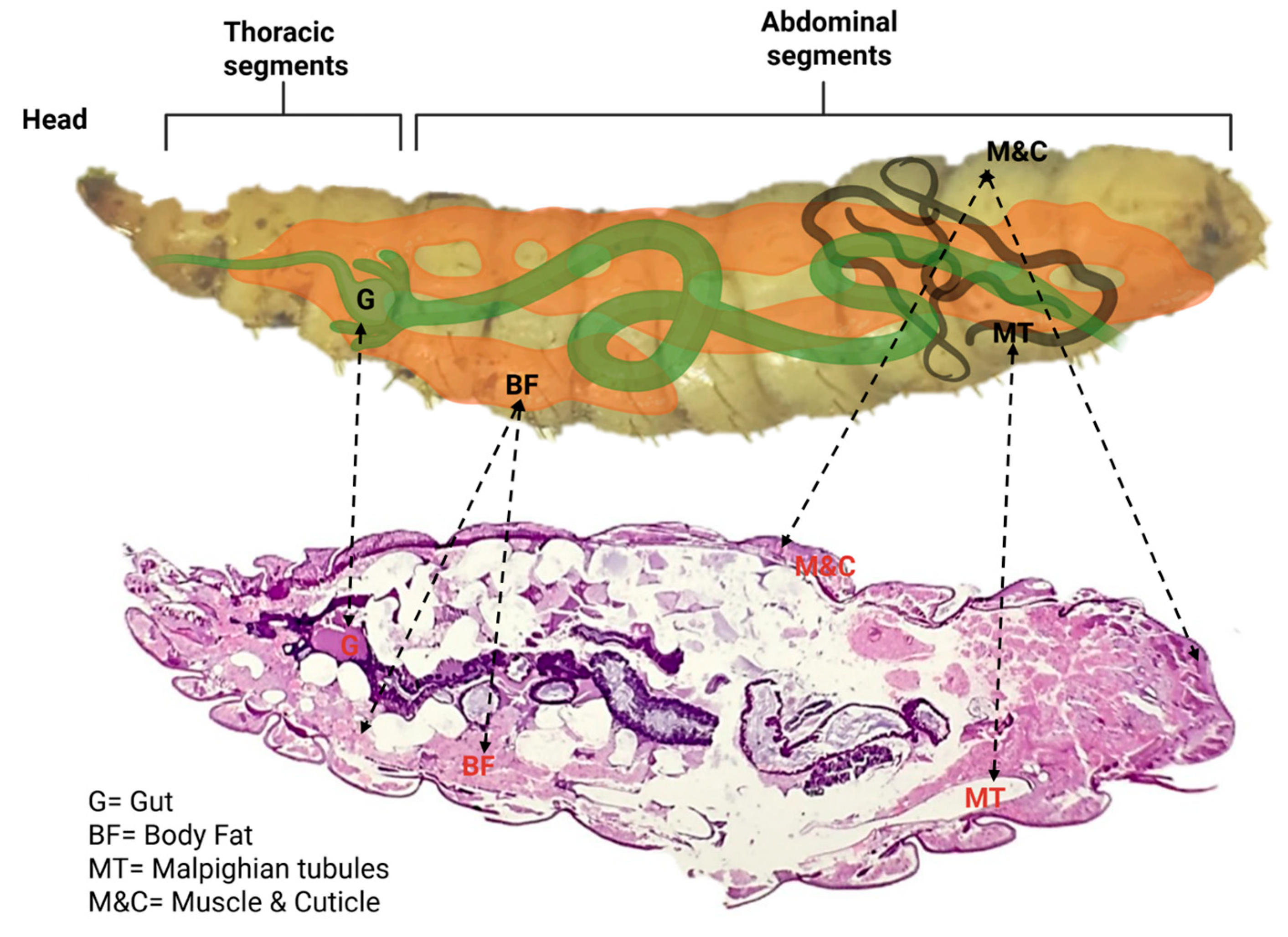
3.1. Normal Anatomy and Histology of BSFL
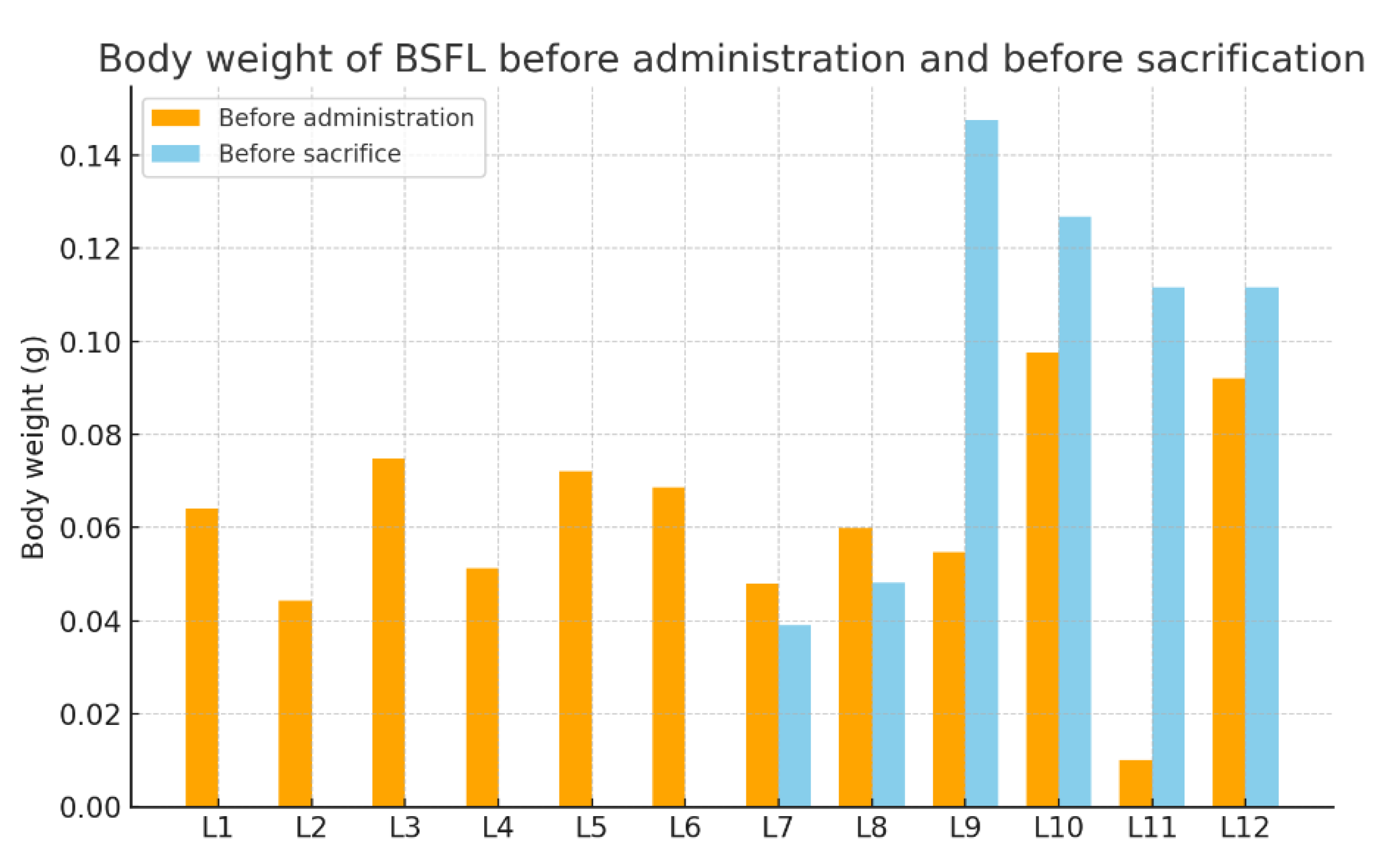
3.2. Weight Variation Before and After Administration
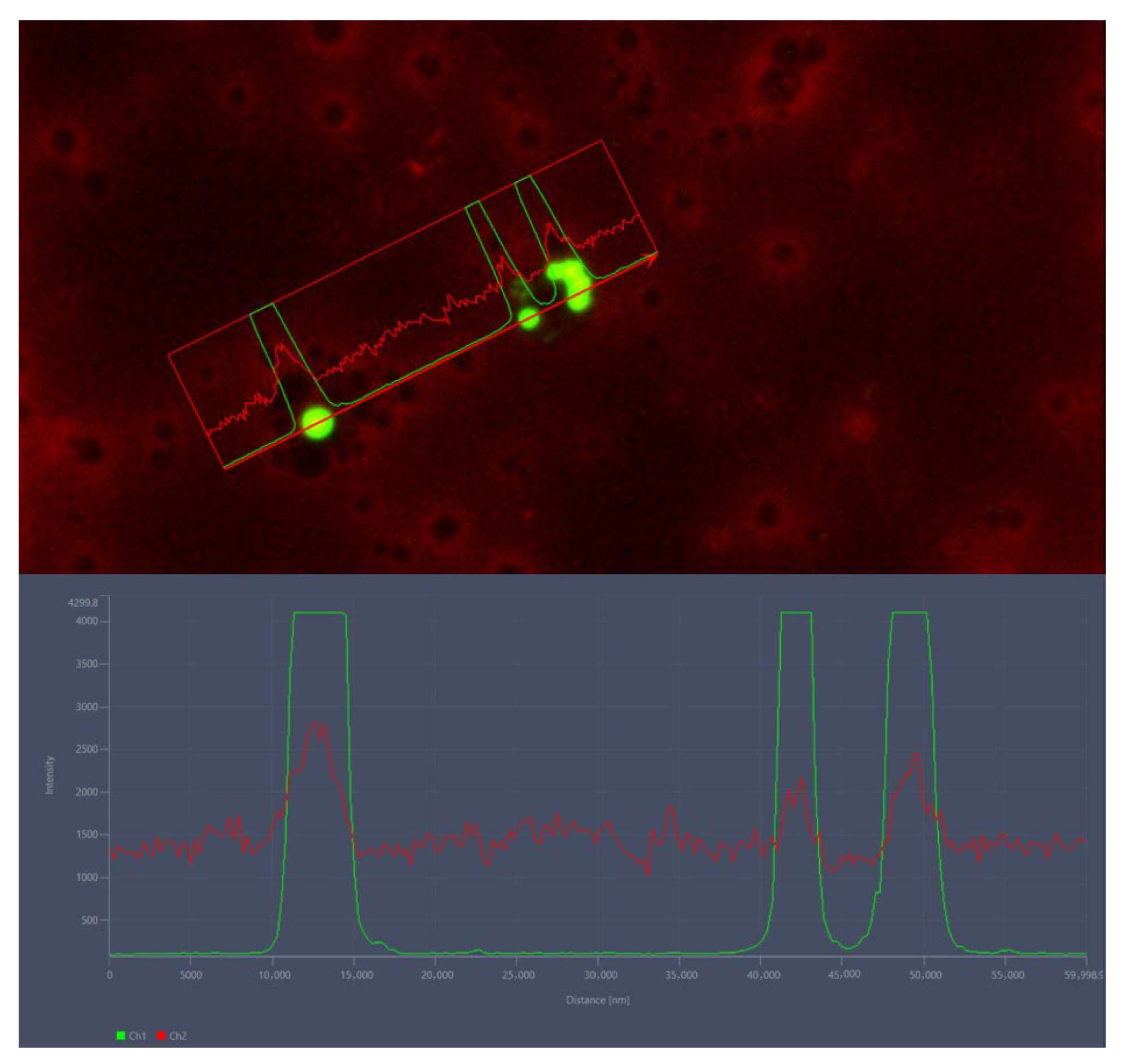
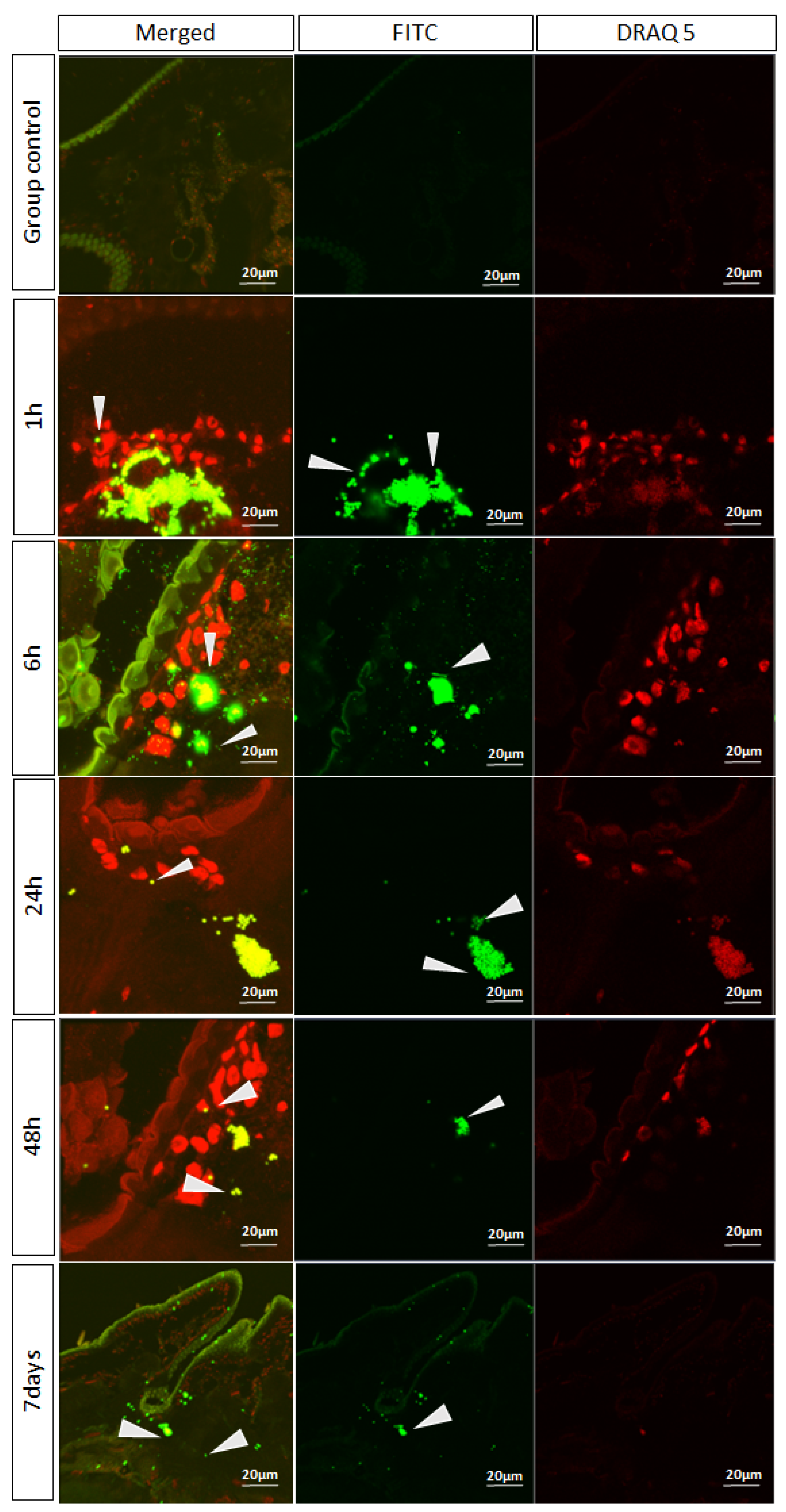
3.3. Microplastic Confocal Scanning Laser Microscopy Analysis
3.4. Hemolymph Analysis
3.5. Histological Analysis
3.6. Confocal Scanning Laser Microscopy Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSFL | Black Soldier Fly Larvae |
| CSLM | Confocal Scanning Laser Microscopy |
| H&E | Hematoxylin and Eosin (histological staining method) |
| FITC | Fluorescein Isothiocyanate (fluorescent dye, used to label microplastics) |
| ROI | Region of Interest (in microscopy/image analysis) |
| OCT | Optimal Cutting Temperature (compound for cryosection embedding) |
References
- Wang, Y.-S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef]
- Heussler, C.D.; Dittmann, I.L.; Egger, B.; Robra, S.; Klammsteiner, T. A Comparative Study of Effects of Biodegradable and Non-Biodegradable Microplastics on the Growth and Development of Black Soldier Fly Larvae (Hermetia illucens). Waste Biomass Valoriz. 2024, 15, 2313–2322. [Google Scholar] [CrossRef]
- Nsiah-Gyambibi, R.; Antwi, B.Y.; Animpong, M.A.B.; Aniagyei, A.; Kwakye, R.; Osei-Owusu, J.; Bayitse, R. Valorization of Wheat-Bran Substrate with Microplastic Inclusions Using Black Soldier Fly (Hermetia illucens) Bio-Processing and the Effect on Frass and Larval Development. Waste Biomass Valoriz. 2025, 1–8. [Google Scholar] [CrossRef]
- Dam, N.L.; van der Borg, G.; Hosseini, A.; van Raamsdonk, L.W.D.; Zheng, R.; Schmitt, E.; Hedemann, J.; Ruis, S.; van Bemmel, G.; Meijer, N. Determination of Microplastics in Reared Black Soldier Fly Larvae (Hermetia illucens) Using Polarised Light Optical Microscopy. J. Insects Food Feed 2024, 11, 1289–1303. [Google Scholar] [CrossRef]
- Lievens, S.; Vervoort, E.; Bruno, D.; Van der Donck, T.; Tettamanti, G.; Seo, J.W.; Poma, G.; Covaci, A.; De Smet, J.; Van Der Borght, M. Ingestion and Excretion Dynamics of Microplastics by Black Soldier Fly Larvae and Correlation with Mouth Opening Size. Sci. Rep. 2023, 13, 4341. [Google Scholar] [CrossRef] [PubMed]
- Planche, C.; Lievens, S.; Van der Donck, T.; Sicard, J.; Van Der Borght, M. Exposure of Black Soldier Fly Larvae to Microplastics of Various Sizes and Shapes: Ingestion and Egestion Dynamics and Kinetics. Waste Manag. 2025, 203, 114852. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Bonelli, M.; Bruno, D.; Sequino, G.; Montali, A.; Reguzzoni, M.; Pasolli, E.; Savy, D.; Cangemi, S.; Cozzolino, V. Plastics Shape the Black Soldier Fly Larvae Gut Microbiome and Select for Biodegrading Functions. Microbiome 2023, 11, 205. [Google Scholar] [CrossRef]
- Gold, M.; Lievens, S.; De Smet, J.; Peguero, D.A.; Planche, C.; Whitshaw, C.D.; Scheidegger, A.; Mitrano, D.; Mathys, A.; Van Der Borght, M. Ingestion and Egestion Dynamics of Micro-and Nanoplastics in Black Soldier Fly Larvae. In Proceedings of the Insects to Feed The World, Singapore, 19–22 June 2024. [Google Scholar]
- Xu, Z.; Lin, Z.; Huang, R.; Chen, X.; Wang, L.; Deng, X.; Du, R.; Gu, J.; Wang, Y.; Yu, R. Microplastics Impair Black Soldier Fly Bioconversion of Pigeon Manure: Physiological and Transcriptomic Insights. Int. Biodeterior. Biodegrad. 2025, 205, 106181. [Google Scholar] [CrossRef]
- Tettamanti, G.; Van Campenhout, L.; Casartelli, M. A Hungry Need for Knowledge on the Black Soldier Fly Digestive System. J. Insects Food Feed 2022, 8, 217–222. [Google Scholar] [CrossRef]
- Bruno, D.; Bonelli, M.; Cadamuro, A.G.; Reguzzoni, M.; Grimaldi, A.; Casartelli, M.; Tettamanti, G. The Digestive System of the Adult Hermetia illucens (Diptera: Stratiomyidae): Morphological Features and Functional Properties. Cell Tissue Res. 2019, 378, 221–238. [Google Scholar] [CrossRef]
- Azzollini, D.; van Iwaarden, A.; Lakemond, C.M.M.; Fogliano, V. Mechanical and Enzyme Assisted Fractionation Process for a Sustainable Production of Black Soldier Fly (Hermetia illucens) Ingredients. Front. Sustain. Food Syst. 2020, 4, 80. [Google Scholar] [CrossRef]
- Li, X.; Dong, Y.; Sun, Q.; Tan, X.; You, C.; Huang, Y.; Zhou, M. Growth and Fatty Acid Composition of Black Soldier Fly Hermetia illucens (Diptera: Stratiomyidae) Larvae Are Influenced by Dietary Fat Sources and Levels. Animals 2022, 12, 486. [Google Scholar] [CrossRef]
- Šobotník, J.; Weyda, F.; Hanus, R.; Cvačka, J.; Nebesářová, J. Fat Body of Prorhinotermes simplex (Isoptera: Rhinotermitidae): Ultrastructure, Inter-Caste Differences and Lipid Composition. Micron 2006, 37, 648–656. [Google Scholar] [CrossRef]
- Farina, P.; Bedini, S.; Conti, B. Multiple Functions of Malpighian Tubules in Insects: A Review. Insects 2022, 13, 1001. [Google Scholar] [CrossRef]
- Nocelli, R.C.F.; Cintra-Socolowski, P.; Roat, T.C.; Silva-Zacarin, E.C.M.; Malaspina, O. Comparative Physiology of Malpighian Tubules: Form and Function. Open Access Insect Physiol. 2016, 6, 13–23. [Google Scholar] [CrossRef]
- Beyenbach, K.W. Transport Mechanisms of Diuresis in Malpighian Tubules of Insects. J. Exp. Biol. 2003, 206, 3845–3856. [Google Scholar] [CrossRef] [PubMed]
- Bruno, D.; Montali, A.; Gariboldi, M.; Wrońska, A.K.; Kaczmarek, A.; Mohamed, A.; Tian, L.; Casartelli, M.; Tettamanti, G. Morphofunctional Characterization of Hemocytes in Black Soldier Fly Larvae. Insect Sci. 2023, 30, 912–932. [Google Scholar] [CrossRef] [PubMed]
- Bruno, D.; Montali, A.; Mastore, M.; Brivio, M.F.; Mohamed, A.; Tian, L.; Grimaldi, A.; Casartelli, M.; Tettamanti, G. Insights into the Immune Response of the Black Soldier Fly Larvae to Bacteria. Front. Immunol. 2021, 12, 745160. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Heryanto, C.; Bassal, T.; Zhang, W.; Tettamanti, G.; Mohamed, A. Haemocyte-mediated Immunity in Insects: Cells, Processes and Associated Components in the Fight against Pathogens and Parasites. Immunology 2021, 164, 401–432. [Google Scholar] [CrossRef]
- Rosales, C.; Vonnie, S. Cellular and Molecular Mechanisms of Insect Immunity. Insect Physiol. Ecol. 2017, 10, 182–190. [Google Scholar]
- Tsakas, S.; Marmaras, V.J. Insect Immunity and Its Signalling: An Overview. Invertebr. Surviv. J. 2010, 7, 228–238. [Google Scholar]
- Shah, P.N.; Maistrou, S.; van Loon, J.J.A.; Dicke, M. Effect of the Bacterial Pathogen Pseudomonas Protegens Pf-5 on the Immune Response of Larvae of the Black Soldier Fly, Hermetia illucens L. J. Invertebr. Pathol. 2025, 209, 108272. [Google Scholar] [CrossRef]
- Liu, S.; Raheel Tariq, M.; Zhang, Q.; Wang, H.; Wang, F.; Zheng, C.; Li, K.; Zhuang, Z.; Wang, L. Dietary Influence on Growth, Physicochemical Stability, and Antimicrobial Mechanisms of Antimicrobial Peptides in Black Soldier Fly Larvae. Insects 2024, 15, 872. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of Organic Material by Black Soldier Fly Larvae: Establishing Optimal Feeding Rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef]
- Lincoln Bastos Farias, J.; da Cunha Barreto-Vianna, A.R.; de Mello, M.D.; Dos Santos, A.L.; da Fonte Ramos, C.; Fontoura, P. Comparison of Diff-Quick and Spermac Staining Methods for Sperm Morphology Evaluation. J. Reprod. Infertil. 2023, 24, 166. [Google Scholar] [CrossRef]
- Shao, W.; Kang, Y.; Yu, W.; Kong, Y.; Huo, Y.; He, Z.; Hu, X.; Zhang, X. Ingestion of Polystyrene Microplastics by Bombyx Mori Larvae Disrupts Midgut Epithelial Barrier Integrity and Potentially Promotes Susceptibility to BmNPV Infection. J. Invertebr. Pathol. 2025, 214, 108463. [Google Scholar] [CrossRef] [PubMed]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and Intestinal Effects of Nano-and Microplastics: A Review of the Literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Zhou, X.; He, J.; Zhang, N.; Shen, X.; Sun, C.; Yan, B.; Shao, Y. Toxic Effects of Acute Exposure to Polystyrene Microplastics and Nanoplastics on the Model Insect, Silkworm Bombyx Mori. Environ. Pollut. 2021, 285, 117255. [Google Scholar] [CrossRef]
- Malafaia, G.; da Luz, T.M.; Guimarães, A.T.B.; da Costa Araújo, A.P. Polyethylene Microplastics Are Ingested and Induce Biochemical Changes in Culex quinquefasciatus (Diptera: Culicidae) Freshwater Insect Larvae. Ecotoxicol. Environ. Contam. 2020, 15, 79–89. [Google Scholar] [CrossRef]
- Shen, J.; Liang, B.; Jin, H. The Impact of Microplastics on Insect Physiology and the Indication of Hormesis. TrAC Trends Anal. Chem. 2023, 165, 117130. [Google Scholar] [CrossRef]
- Parenti, C.C.; Binelli, A.; Caccia, S.; Della Torre, C.; Magni, S.; Pirovano, G.; Casartelli, M. Ingestion and Effects of Polystyrene Nanoparticles in the Silkworm Bombyx Mori. Chemosphere 2020, 257, 127203. [Google Scholar] [CrossRef]
- Cho, S.; Kim, C.-H.; Kim, M.-J.; Chung, H. Effects of Microplastics and Salinity on Food Waste Processing by Black Soldier Fly (Hermetia illucens) Larvae. J. Ecol. Environ. 2020, 44, 7. [Google Scholar] [CrossRef]
- Silva, C.J.M.; Beleza, S.; Campos, D.; Soares, A.M.V.M.; Silva, A.L.P.; Pestana, J.L.T.; Gravato, C. Immune Response Triggered by the Ingestion of Polyethylene Microplastics in the Dipteran Larvae Chironomus Riparius. J. Hazard. Mater. 2021, 414, 125401. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Cheng, Z.; Wang, M.; Wei, C.; Liu, H.; Yang, Y. A Multi-Levels Analysis to Evaluate the Toxicity of Microplastics on Aquatic Insects: A Case Study with Damselfly Larvae (Ischnura elegans). Ecotoxicol. Environ. Saf. 2025, 289, 117447. [Google Scholar] [CrossRef] [PubMed]


| Groups | Sacrifice | Way of Preservation | Hemolymph Collection |
|---|---|---|---|
| Experimental Group 1 | 1 h | 10% formalin | + |
| ice | − | ||
| Experimental Group 2 | 6 h | 10% formalin | + |
| ice | − | ||
| Experimental Group 3 | 24 h | 10% formalin | + |
| ice | − | ||
| Experimental Group 4 | 48 h | 10% formalin | + |
| ice | − | ||
| Experimental Group 5 | 7 days | 10% formalin | + |
| ice | − | ||
| Control Group 6 | 7 days | 10% formalin | + |
| ice | − |
| Groups | Larvae | Body Weight Before Administration | Body Weight Before Sacrifice |
|---|---|---|---|
| Experimental Group 1 | L1 | 0.0640 g | - |
| L2 | 0.0444 g | - | |
| Experimental Group 2 | L3 | 0.0749 g | - |
| L4 | 0.0513 g | - | |
| Experimental Group 3 | L5 | 0.0721 g | - |
| L6 | 0.0687 g | - | |
| Experimental Group 4 | L7 | 0.0480 g | 0.0390 g |
| L8 | 0.0599 g | 0.0482 g | |
| Experimental Group 5 | L9 | 0.0547 g | 0.1475 g |
| L10 | 0.0976 g | 0.1268 g | |
| Control Group | L11 | 0.0102 g | 0.1116 g |
| L12 | 0.0920 g | 0.1117 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionica, C.-N.; Pop, R.; Hodor, D.; Constantin, I.; Hiruta, A.; Hota, A.-T.; Tabaran, A.F.; Daina, S.; Szakacs, A.-R.; Macri, A. Cellular Uptake and Tissue Retention of Microplastics in Black Soldier Fly Larvae. Insects 2025, 16, 1169. https://doi.org/10.3390/insects16111169
Ionica C-N, Pop R, Hodor D, Constantin I, Hiruta A, Hota A-T, Tabaran AF, Daina S, Szakacs A-R, Macri A. Cellular Uptake and Tissue Retention of Microplastics in Black Soldier Fly Larvae. Insects. 2025; 16(11):1169. https://doi.org/10.3390/insects16111169
Chicago/Turabian StyleIonica, Claudiu-Nicusor, Romelia Pop, Dragos Hodor, Irina Constantin, Ana Hiruta, Alexia-Teodora Hota, Alexandru Flaviu Tabaran, Sorana Daina, Andrei-Radu Szakacs, and Adrian Macri. 2025. "Cellular Uptake and Tissue Retention of Microplastics in Black Soldier Fly Larvae" Insects 16, no. 11: 1169. https://doi.org/10.3390/insects16111169
APA StyleIonica, C.-N., Pop, R., Hodor, D., Constantin, I., Hiruta, A., Hota, A.-T., Tabaran, A. F., Daina, S., Szakacs, A.-R., & Macri, A. (2025). Cellular Uptake and Tissue Retention of Microplastics in Black Soldier Fly Larvae. Insects, 16(11), 1169. https://doi.org/10.3390/insects16111169







