The Parasitoid Complex of Aleurothrixus floccosus (Hemiptera: Aleyrodidae) in the Citrus Groves of Central–Southern Italy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Collections
2.2. Parasitization Rate and Phenology
2.3. Morphological Analysis
2.4. Molecular Analysis
3. Results
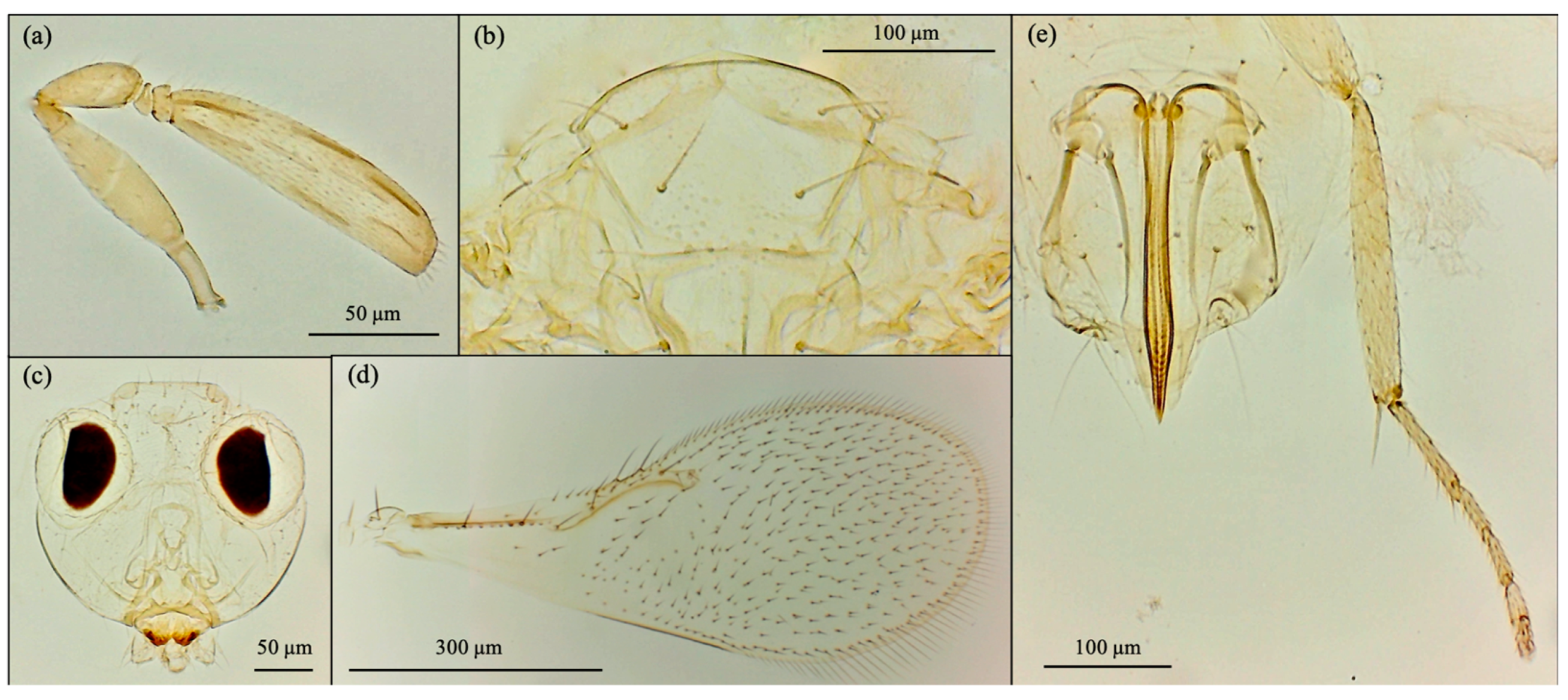
3.1. Morphological Analysis
3.2. Molecular Analysis
3.3. Parasitization Rate
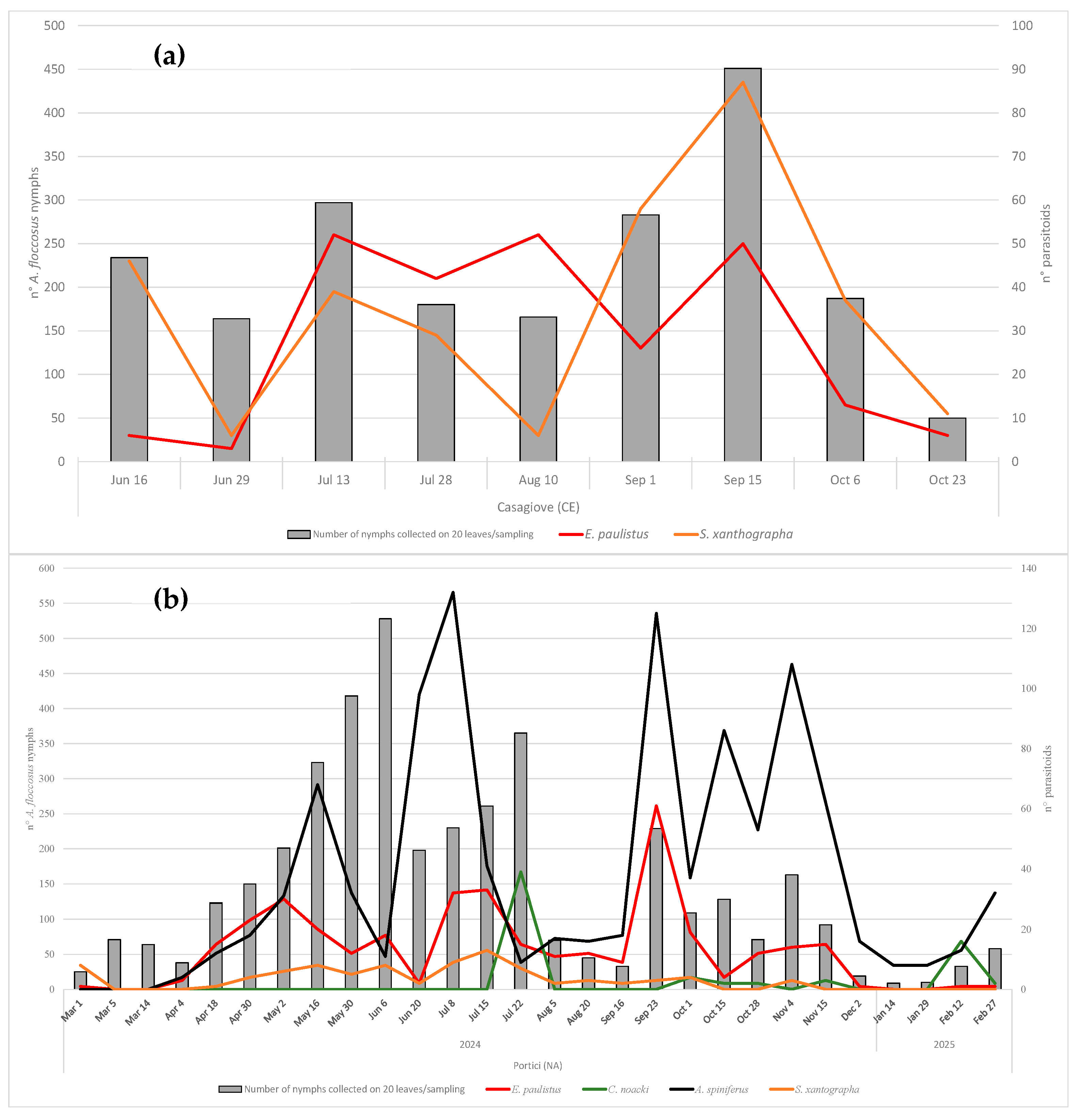
3.4. Phenology
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EPPO. EPPO Global Database. 2025. Available online: https://gd.eppo.int/taxon/ALTHFL (accessed on 24 June 2025).
- Onillon, J.C. A propos de la presence en France d’une nouvelle espece d’Aleurode nuisible aux Citrus, Aleurothrixus floccosus Maskell (Homopt. Aleurodidae). C. R. Acad. Agric. Fr. 1969, 55, 937–940. [Google Scholar]
- Mifsud, D.; Coquempot, C.; Mühlethaler, R.; Wilson, M.; Streito, J.C. Other Hemiptera Sternorrhyncha (Aleyrodidae, Phylooxeroidea, and Psylloidea) and Hemiptera Auchenorrhyncha. BioRisk 2010, 4, 511–552. [Google Scholar] [CrossRef]
- CABI. Aleurothrixus floccosus (woolly whitefly). In CABI Compendium; CAB International: Wallingford, UK, 2021. [Google Scholar] [CrossRef]
- Evans, G.A. The Whiteflies (Hemiptera: Aleyrodidae) of the World and Their Host Plants and Natural Enemies; USDA/Animal SDA/Animal Plant Health Inspection Service: Riverdale, MD, USA, 2007.
- Ripa, R.; Rodríguez, F.; Rojaa, S.; Larral, P.; Castro, L.; Ortúzar, J.; Carmona, P.; Vargas, R. Citrus Pests, Their Natural Enemies and Management; INIA Books Collection N°3: Santiago de Chile, Chile, 1999; p. 151. [Google Scholar]
- Giliomee, J.H.; Millar, I.M. The woolly whitefly, Aleurothrixus floccosus (Maskell) (Hemiptera: Aleyrodidae), a potentially serious citrus pest, recorded from South Africa. Afr. Entomol. 2009, 17, 232–233. [Google Scholar] [CrossRef]
- DeBach, P.; Rose, M. Biological control of Woolly Whitefly. Calif. Agric. 1976, 30, 4–7. [Google Scholar]
- DeBach, P.; Rosen, D. Biological Control by Natural Enemies, 2nd ed.; Cambridge University Press: London, UK, 1991. [Google Scholar]
- Onillon, J.; Abbassi, M. Notes bio-ecologiques sur l’aleurode flocconeux des agrumes Aleurothrixus floccosus Mask. (Homop., Aleurodidae) et moyens de lutte. Al-Awamia 1973, 49, 99–116. [Google Scholar]
- Katsoyannos, P. First record of Aleurothrixus floccosus (Mask.) (Homoptera: Aleyrodidae) in Greece and some observations οn its phenology. Entomol. Hell. 1991, 9, 69–72. [Google Scholar] [CrossRef]
- Barbagallo, S.; Longo, S.; Patti, I.; Rapisarda, C. Efficiency of biological control against citrus whiteflies in Italy. Boll. Zool. Agrar. Bachic. 1992, 24, 121–135. [Google Scholar]
- Katsoyannos, P.; Ifantis, K.; Kontodimas, D.C. Phenology, population trend and natural enemies of Aleurothrixus floccosus (Hom.:Aleyrodidae) at a newly invaded area in Athens, Greece. BioControl 1997, 42, 619–628. [Google Scholar] [CrossRef]
- Santamaría, A.; Costa-Comelles, J.; Alonso, A.; Rodríguez, J.M.; Ferrer, J. Ensayo del hongo entomopatógeno Beauveria bassiana (Balsamo) Vuillemin para el control de la mosca blanca de los cítricos Aleurothrixus floccosus (Maskell) (Homoptera: Aleyrodidae) y su acción sobre el parásito Cales noacki (Howard) (Hymenoptera: Aphelinidae). Bol. San. Veg. Plagas 1998, 24, 695–706. [Google Scholar]
- Ulusoy, M.R.; Vatansever, G.; Erkılıç, L.; Uygun, N. Studies on Aleurothrixus floccosus (Maskell) (Homoptera, Aleyrodidae) and its parasitoid, Cales noacki Howard (Hymenoptera, Aphelinidae) in the East Mediterranean Region of Turkey. J. Pest Sci. 2003, 76, 163–169. [Google Scholar] [CrossRef]
- Rodas-Martínez, C.E.; Galindo-Alcántara, A.; del Carmen Ruiz-Acosta, S.; Sánchez-Hernández, R. Methods for the control of whitefly (Aleyrodidae) in citrus: A systematic review. Agro Product. 2023, 16, 37–45. [Google Scholar] [CrossRef]
- Noyes, J.S. Universal Chalcidoidea Database. 2019. Available online: https://www.nhm.ac.uk (accessed on 28 January 2025).
- Arzone, A.; Vidano, C. Indagini sui parassiti di Aleurothrixus floccosus in Liguria (Italia). Inf. Tore Fitopatol. 1983, 6, 11–18. [Google Scholar]
- Longo, S.; Rapisarda, C.; Russo, A. Risultati del controllo biologico dell’Aleurothrixus floccosus (Maskell) in agrumeti della Sicilia orientale. In Proceedings of the Atti del XIV Congresso Nazionale Italiano di Entomologia, Palermo, Erice, Bagheria, Italy, 28 May–1 June 1985; pp. 841–848. [Google Scholar]
- Ippolito, R.; Laccone, G. Distribuzione e parassiti di Aleurothrixus floccosus Mask. e Dialeurodes citri Ashm. (Hom. Aleyrodidae) su agrumi in Puglia. Entomologica 1987, 22, 157–164. [Google Scholar]
- Guerrieri, E.; Viggiani, G. Osservazioni sull’Aleurothrixus floccosus (Mask.) (Homoptera: Aleyrodidae) e sul suo antagonista Cales noacki How. (Hymenoptera: Aphelinidae) in Campania. Ann. Fac. Se. Agr. Univ. Napoli 1988, 22, 11–17. [Google Scholar]
- Miklasiewicz, T.J.; Walker, G.P. Population dynamics and biological control of the woolly whitefly (Homoptera: Aleyrodidae) on citrus. Environ. Entomol. 1990, 19, 1485–1490. [Google Scholar] [CrossRef]
- Katsoyannos, P.; Kontodimas, D.C.; Stathas, G.J. The inundative release of Cales noacki Howard (Hymenoptera: Aphelinidae), for curative treatment of Aleurothrixus floccosus (Maskell)(Homoptera: Aleyrodidae) on heavily infested citrus in Greece. Ann. Inst. Phytopathol. Benaki 1998, 18, 111–122. [Google Scholar]
- Soto, A.; Ohlenschlager, F.; García-Marí, F. Dinámica poblacional y control biológico de las moscas blancas Aleurothrixus floccosus, Dialeurodes citri y Parabemisia myricae (Homoptera: Aleyrodidae) en los cítricos valencianos. Bol. San. Veg. Plagas 2001, 29, 3–20. [Google Scholar]
- Tello Mercado, V.; Fernández, E.S.; Gillomee, J.H. Life table parameters of the woolly whitefly Aleurothrixus floccosus (Hemiptera: Aleyrodidae) and its parasitoid Cales noacki (Hymenoptera: Aphelinidae). Eur. J. Entomol. 2014, 111, 251–256. [Google Scholar]
- Rose, M.; Woolley, J. Previously imported parasite may control invading whitefly. Calif. Agric. 1984, 38, 24–25. [Google Scholar]
- Onillon, J.C.; Onillon, J. Contribution à l’étude de la dynamique des populations d’Homoptères inféodés aux agrumes. III. Introduction, dans les Alpes-Maritimes de Cales noacki How. (Hymenopt., Aphelinidae), parasite d’Aleurothrixus floccosus Mask. (Homopt. Aleurodidae). C. R. Acad. Agric. Fr. 1972, 58, 365–370. [Google Scholar]
- Viggiani, G.; Laudonia, S. Osservazioni sulla fenologia e sui parassiti di Aleurotuba jelineki (Frauenf.) (Homoptera; Aleyrodidae) in Italia. Bol. Lab. Entomol. Agrar. “Filippo Silvestri” 1984, 41, 225–234. [Google Scholar]
- Liotta, G.; Maniglia, G. Introduzione, allevamento e diffusione di nemici naturali indigeni ed esotici di Aleurothrixus floccosus (Mask.) (Horn., Aleyrodidae). II. Ruolo di Cales noacki How. (Hym., Aphelinidae) nel controllo di Aleurothrixus floccosus (Mask.) in Sicilia. Phytophaga 1983, 1, 133–142. [Google Scholar]
- Ortu, S.; Prota, R. Brevi considerazioni sulle recenti introduzioni in Sardegna di entomofagi a protezione della coltura agrumicola. Frustula Entomol. 1986, 7–8, 115–123. [Google Scholar]
- Maniglia, G. Osservazioni biologiche su Amitus spiniferus (Bréthes) (Hym. Platygastridae) parassitoide di Aleurothrixus floccosus (Mask.) (Hom. Aleyrodidae). In Proceedings of the Atti del XV Congresso Nazionale Italiano di Entomologia, L’Aquila, Italy, 13–17 June 1988; pp. 1007–1012. [Google Scholar]
- Mottern, J.L.; Polaszek, A. Calesidae. In Chalcidoidea of the World; Heraty, J.M., Woolley, J.B., Eds.; CABI Press: Wallingford, UK, 2025; pp. 233–240. [Google Scholar]
- Mottern, J.L.; Heraty, J.M. Revision of the Cales noacki species complex (Hymenoptera, Chalcidoidea, Aphelinidae). Syst. Entomol. 2014, 39, 354–379. [Google Scholar] [CrossRef]
- Naranjo, S.E. Displacement of native natural enemies by introduced biological control agents in agro-ecosystems: A serious non-target effect or not? In Proceedings of the 5th International Symposium on Biological Control of Arthropods, Langkawi, Malaysia, 11–15 September 2017; CAB International: Wallingford, UK, 2017; pp. 46–47. [Google Scholar]
- Castella, C.; Orsat, C.; Marcdargent, M.; Malausa, T.; Desneux, N.; De Clercq, P.; Pappas, M.; Stenberg, J.A.; Roques, N. Study on the Union’s Situation and Options Regarding Invertebrate Biological Control Agents for the Use in Plant Health and Plant Protection; European Union. 2022. Available online: https://hal.science/hal-04228764 (accessed on 23 July 2025).
- Viggiani, G. Lotta Biologica e Integrata nella Difesa Fitosanitaria: Volume Primo, 1st ed.; Liguori: Napoli, Italy, 1994; pp. 401–404. [Google Scholar]
- Noyes, J.S. Collecting and preserving chalcid wasps (Hymenoptera: Chalcidoidea). J. Nat. Hist. 1982, 16, 315–334. [Google Scholar] [CrossRef]
- Viggiani, G.; Mazzone, P. The Amitus Hald. (Hym. Platygastridae) of Italy, with descriptions of three new species. Bol. Lab. Entomol. Agrar. “Filippo Silvestri” 1982, 39, 59–69. [Google Scholar]
- Rose, M. Eretmocerus Haldeman (Hymenoptera: Aphelinidae) reared from Aleurothrixus floccosus (Maskell) (Homoptera: Aleyrodidae) in the Americas. Vedalia 2000, 7, 3–46. [Google Scholar]
- Myartseva, S.N.; Coronado-Blanco, J.M. Especies de Eretmocerus Haldeman (Hymenoptera: Aphelinidae) parasitoides de Aleurothrixus floccosus (Maskell) (Homoptera: Aleyrodidae) de México, con la descripción de una nueva especie. Acta Zool. Mex. 2007, 23, 37–46. [Google Scholar] [CrossRef][Green Version]
- Myartseva, S.N.; Ruiz-Cancino, E.; Coronado-Blanco, J.M. Species of Eretmocerus Haldeman (Hymenoptera: Aphelinidae) from Mexico with short club, key and description of a new species. Acta Zool. mex. 2011, 27, 583–590. [Google Scholar] [CrossRef]
- Woolley, J.B.; Dal Molin, A. Taxonomic revision of the flavopalliata species group of Signiphora (Hymenoptera: Signiphoridae). Zootaxa 2017, 4315, 1–150. [Google Scholar] [CrossRef]
- Thongjued, K.; Chotigeat, W.; Bumrungsri, S.; Thanakiatkrai, P.; Kitpipit, T. A new cost-effective and fast direct PCR protocol for insects based on PBS buffer. Mol. Ecol. Resour. 2019, 19, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Gebiola, M.; Bernardo, U.; Monti, M.M.; Navone, P.; Viggiani, G. Pnigalio agraules (Walker) and Pnigalio mediterraneus Ferriere and Delucchi (Hymenoptera: Eulophidae): Two closely related valid species. J. Nat. Hist. 2009, 43, 2465–2480. [Google Scholar] [CrossRef]
- Hajibabaei, M.; Smith, M.A.; Janzen, D.H.; Rodriguez, J.J.; Whitfield, J.B.; Hebert, P.D.N. A minimalist barcode can identify a specimen whose DNA is degraded. Mol. Ecol. Notes 2006, 6, 959–964. [Google Scholar] [CrossRef]
- Simon, S.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entom. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Warbroek, T.; Cottyn, B.; Gottsberger, R. PM 7/129 (2) DNA barcoding as an identification tool for a number of regulated pests. EPPO Bull. 2021, 51, 100–143. [Google Scholar] [CrossRef]
- Campbell, B.C.; Steffen-Campbell, J.D.; Werren, J.H. Phylogeny of the Nasonia species complex (Hymenoptera: Pteromalidae) inferred from an internal transcribed spacer (ITS2) and 28S rDNA sequences. Insect Mol. Biol. 1994, 2, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, E.; Formisano, G.; Noyes, J.S. Redescription of Microterys chalcostomus (Dalman) (Hymenoptera: Chalcidoidea: Encyrtidae), a parasitoid associated with Phenacoccus aceris (Signoret) (Hemiptera: Pseudococcidae) and Kermes spp. (Hemiptera: Kermesidae), with comments on its host relationship. J. Nat. Hist. 2020, 54, 1213–1222. [Google Scholar] [CrossRef]
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large data sets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef]
- Andreason, S.A.; Triapitsyn, S.V.; Perring, T.M. Untangling the Anagyrus pseudococci species complex (Hymenoptera: Encyrtidae), parasitoids of worldwide importance for biological control of mealybugs (Hemiptera: Pseudococcidae): Genetic data corroborates separation of two new, previously misidentified species. Biol. Control 2019, 129, 65–82. [Google Scholar]
- Miller, K.E.; Polaszek, A.; Evans, D.M. A dearth of data: Fitting parasitoids into ecological networks. Trends Parasitol. 2021, 37, 863–874. [Google Scholar] [CrossRef]
- Hempel, A. Notas sobre dois inimigos da laranjeira. Bol. Secr. Agr. Ind. E Com. Est. S. Paulo Ser. 1904, 5, 10–21. [Google Scholar]
- UCD Community. Universal Chalcidoidea Database. 2023. Available online: https://ucd.chalcid.org (accessed on 29 May 2025).
- Blanchard, E.E. Apuntes sobre calcidoideos argentinos, nuevos y conocidos. Rev. Soc. Entomol. Arg. 1936, 8, 18–20. [Google Scholar]
- De Santis, L. Catálogo de los Himenópteros Argentinos de la Serie Parasitica, Incluyendo Bethyloidea; Publicación Comisión Investigación Científica Provincia de Buenos Aires: Buenos Aires, Argentina, 1967; pp. 124–148. [Google Scholar]
- Onillon, J.C. Biological and integrated control in citrus groves in the Mediterranean region. Entomophaga 1989, 33, 481–494. [Google Scholar] [CrossRef]
- Amouroux, P.; Crochard, D.; Correa, M.; Groussier, G.; Kreiter, P.; Roman, C.; Guerrieri, E.; Garonna, A.; Malausa, T.; Zaviezo, T. Natural enemies of armored scales (Hemiptera: Diaspididae) and soft scales (Hemiptera: Coccidae) in Chile: Molecular and morphological identification. PLoS ONE 2019, 14, e0205475. [Google Scholar] [CrossRef]
- Costa Lima, A.M. Insetos do Brasil, Volume 3, Homopteros; Serie Didática N°4; Escola Nacional de Agronomia: Rio de Janeiro, Brazil, 1942; p. 186. [Google Scholar]
- Rodrigues, W.C.; Cassino, P.C.R. Parasitismo de Aleurothrixus floccosus (Homoptera, Aleyrodidae) por Encarsia sp. (Hymenoptera, Aphelinidae) e Signiphora sp. (Hymenoptera, Signiphoridae) em tangerina (Citrus reticulata) cv. Poncã. Rev. Univ. Rural. Série Ciências Da Vida 2003, 23, 31–37. [Google Scholar]
- Tello Mercado, V.; Zarzar-Maza, M. Fluctuación poblacional y parasitismo de Aleurothrixus floccosus (Hemiptera: Aleyrodidae) en cítricos del Desierto de Atacama, Chile. Rev. Colomb. Entomol. 2021, 47, e7806. [Google Scholar] [CrossRef]
- Anonymous. La lucha en España contra la «mosca blanca» de los cítricos Aleurothrixus floccosus Mask. Bol. Serv. Plagas 1977, 3, 87–100. [Google Scholar]
- Myartseva, S.N.; Ruíz-Cancino, E.; Coronado-Blanco, J.M. Aphelinidae (Hymenoptera) parasitoides de los principales hemípteros plaga (Hemiptera: Aleyrodoidea: Coccoidea) de los cítricos en México. Folia Entomol. Mex. 2017, 3, 32–41. [Google Scholar]
- Greathead, D.J. A review of biological control in western and southern Europe. In Technical Communication N°7; Commonwealth Agricultural Bureaux, Institute of Biological Control: Wallingford, UK, 1976. [Google Scholar]
- Gerber, E.; Schaffner, U. Exotic insect biocontrol agents released in Europe. In Review of Invertebrate Biological Control Agents Introduced into Europe; CAB International: Wallingford, UK, 2016; pp. 9–117. [Google Scholar]
- Viscarret, M.M.; Botto, E.N.; Polaszek, A. Whiteflies (Hemiptera: Aleyrodidae) of economic importance and their natural enemies (Hymenoptera: Aphelinidae, Signiphoridae) in Argentina. Rev. Chil. Entomol. 2000, 26, 5–12. [Google Scholar]
- Woolley, J.B.; Hanson, P. Familia Signiphoridae. In Hymenoptera de la Region Neotropical. Memories of the American Entomological Institute; Hanson, P., Gauld, I.D., Eds.; AEI: Gainesville, FL, USA, 2006; Volume 77, pp. 422–425. [Google Scholar]
- Marsaro Júnior, A.L.; Racca Filho, F.; Raga, A.; Costa, V.A. Nuevos registros de moscas blancas (Hemiptera: Aleyrodidae) en Rio Grande do Sul, Brasil. Idesia 2015, 33, 143–145. [Google Scholar] [CrossRef]
- Ramírez-Ahuja, M.D.L.; Dal Molin, A.; González-Hernández, A.; Woolley, J.B. Sinopsis y clave para la identificación de las especies de Signiphora (Hymenoptera: Signiphoridae) de México, con notas sobre biología y distribución. Rev. Mex. Biodivers. 2015, 86, 337–347. [Google Scholar]
- Woolley, J.B. Higher classification of the Signiphoridae (Hymenoptera: Chalcidoidea) and a Revision of Signiphora Ashmead of the New World. Ph.D. Dissertation, University of California, Riverside, CA, USA, 1983. [Google Scholar]
- Mineo, G.; Viggiani, G. Notizie preliminari su una Signiphora (Hymenoptera: Signiphoridae) ottenuta da Aleurothrixus floccosus (Maskell) (Homoptera: Aleyrodidae) in Italia. Bol. Lab. Entomol. Agrar. “Filippo Silvestri” 2002, 57, 159–165. [Google Scholar]
- Stathas, G.J.; Kavallieratos, N.G.; Cheliotis, L.N.; Skouras, P.J.; Giakoumaki, M.V.; Milonas, P.G. New data on the parasitization of Aleurothrixus floccosus (Maskell) (Hemiptera: Aleyrodidae) in Greece. Hell. Plant Prot. J. 2023, 16, 79–82. [Google Scholar] [CrossRef]
- Pace, R.; Ascolese, R.; Miele, F.; Russo, E.; Griffo, R.V.; Bernardo, U.; Nugnes, F. The bugs in the bags: The risk associated with the introduction of small quantities of fruit and plants by airline passengers. Insects 2022, 13, 617. [Google Scholar] [CrossRef]
- Bertelsmeier, C.; Bonnamour, A.; Brockerhoff, E.G.; Pyšek, P.; Skuhrovec, J.; Richardson, D.M.; Liebhold, A.M. Global proliferation of nonnative plants is a major driver of insect invasions. BioScience 2024, 74, 770–781. [Google Scholar] [CrossRef]





| Localities | Coordinates | Host Plants |
|---|---|---|
| Casagiove (CE) | 41.078472 N, 14.316778 E | Clementine Citrus clementina Hort. ex Tanaka |
| Portici (NA) | 40.810556 N, 14.341944 E | Lemon Citrus limon (L.) Burm. f. Clementine Citrus clementina Hort. ex Tanaka |
| Grottammare (AP) | 42.988778 N, 13.869278 E | Bitter orange Citrus × aurantium L. Orange Citrus sinensis (L.) Osbeck |
| Parasitoid Species | Localities | Coordinates | Date of Sampling | COI Accession Number | 28S-D2 Accession Number |
|---|---|---|---|---|---|
| Eretmocerus paulistus | Casagiove (CE) | 41.078472 N, 14.316778 E | 01.IX.2024 | PX103238 | PX103257 |
| Signiphora xanthographa | Casagiove (CE) | 41.078472 N, 14.316778 E | 01.IX.2024 | PX103239 | PX103258 |
| Amitus spiniferus | Portici (NA) | 40.810556 N, 14.341944 E | 22.VII.2024 | PX118932 | PX121923 |
| Cales noacki | Portici (NA) | 40.810556 N, 14.341944 E | 22.VII.2024 | PX121924 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melone, G.; Andretta, L.; Guastaferro, V.M.; Romito, E.; Formisano, G.; Giorgini, M.; Laudonia, S. The Parasitoid Complex of Aleurothrixus floccosus (Hemiptera: Aleyrodidae) in the Citrus Groves of Central–Southern Italy. Insects 2025, 16, 1037. https://doi.org/10.3390/insects16101037
Melone G, Andretta L, Guastaferro VM, Romito E, Formisano G, Giorgini M, Laudonia S. The Parasitoid Complex of Aleurothrixus floccosus (Hemiptera: Aleyrodidae) in the Citrus Groves of Central–Southern Italy. Insects. 2025; 16(10):1037. https://doi.org/10.3390/insects16101037
Chicago/Turabian StyleMelone, Gianluca, Lucia Andretta, Valentino Maria Guastaferro, Eleonora Romito, Giorgio Formisano, Massimo Giorgini, and Stefania Laudonia. 2025. "The Parasitoid Complex of Aleurothrixus floccosus (Hemiptera: Aleyrodidae) in the Citrus Groves of Central–Southern Italy" Insects 16, no. 10: 1037. https://doi.org/10.3390/insects16101037
APA StyleMelone, G., Andretta, L., Guastaferro, V. M., Romito, E., Formisano, G., Giorgini, M., & Laudonia, S. (2025). The Parasitoid Complex of Aleurothrixus floccosus (Hemiptera: Aleyrodidae) in the Citrus Groves of Central–Southern Italy. Insects, 16(10), 1037. https://doi.org/10.3390/insects16101037







