Simple Summary
A. m. jemenetica occurs naturally on the Arabian Peninsula and in tropical Africa, and this honeybee subspecies has acquired several morphological, behavioral and molecular adaptations to extreme summer temperatures in Saudi Arabia. In this study, expression levels of different heat-shock protein (hsp) genes in forager A. m. jemenetica (a thermotolerant honeybee subspecies) and A. m. carnica (a thermosusceptible subspecies) were explored and compared under desert and semi-arid climates within Saudi Arabia. The results revealed higher expression levels of hsp mRNAs in A. m. jemenetica compared to A. m. carnica. The expression levels of small- as well as large-molecular-weight heat-shock proteins were higher under desert climate conditions in the Riyadh region compared to the semi-arid conditions in Baha. It is clear that the expression of heat-shock proteins is a key molecular mechanism of A. m. jemenetica adaptation to extreme summer conditions.
Abstract
A. m. jemenetica is the indigenous honeybee of the Arabian Peninsula. It is highly adapted to extreme temperatures exceeding 40 °C, yet important molecular aspects of its adaptation are not well documented. In this study we quantify relative expression levels of small- and large-molecular-weight heat-shock proteins (hsp10, hsp28, hsp70, hsp83, hsp90 and hsc70 (mRNAs)) in the thermos-tolerant A. m. jemenetica and thermosusceptible A. m. carnica forager honeybee subspecies under desert (Riyadh) and semi-arid (Baha) summer conditions. The results showed significant day-long higher expression levels of hsp mRNAs in A. m. jemenetica compared to A. m. carnica under the same conditions. In Baha, the expression levels were very modest in both subspecies compared those in Riyadh though the expression levels were higher in A. m. jemenetica. The results also revealed a significant interaction between subspecies, which indicated milder stress conditions in Baha. In conclusion, the higher expression levels of hsp10, hsp28, hsp70ab, hsp83 and hsp90 mRNAs in A. m. jemenetica are key elements in the adaptive nature of A. m. jemenetica to local conditions that enhance its survival and fitness in high summer temperatures.
1. Introduction
Scientists do not know exactly how global warming will influence beekeeping, but it is likely to be an additional stress factor [1,2] that may necessitate unique beekeeping practices and alter the natural distribution of the honeybee subspecies (A. mellifera) worldwide. According to the National Oceanic and Atmospheric Administration (NOAA) [3], uneven warming across the Earth will cause more regions to experience rising rather than cooling temperature trends. In regions such as Saudi Arabia, a significant increase in the maximum average daily temperature (0.71 °C/decade) has been reported [4], with the average summer temperature exceeding 40 °C [5]. Furthermore, the general drought conditions that dominate most of the Arabian Peninsula may exacerbate the influence of increasing temperatures on beekeeping and beekeeping practices in this region.
The common honeybee Apis mellifera occurs naturally in Asia, Europe and Africa and has spread worldwide because of modern beekeeping practices [6,7]. Thirty geographical honeybee subspecies of Apis mellifera have been identified in diverse climatic zones and thermal gradients [6,8]. These subspecies display different thermal responses to extreme temperatures that are associated with adaptation to warmer climates. This leads to better fitness of these populations at higher temperatures relative to populations from temperate areas [9]. Recently, an Asian origin of A. mellifera was supported via an adaptive radiation involving selection on a few genomic “hotspots” [10]. These findings emphasized the importance of Middle Eastern honeybee subspecies such as A. m. syriaca and A. m. jemenetica in the evolution and radiation of A. mellifera [10].
Because the Apis mellifera honeybee colony has a eusocial structure, it exhibits unique responses to extreme temperatures, such as fanning [11,12,13,14], clustering [6,14], reduced foraging, stinging or even migratory swarming [6]. Thermal homeostasis of the honeybee colony is also essential for brood development because A. mellifera larvae and pupae are extremely stenothermic [15,16]. In addition to eusocial responses, thermotolerant honeybees have acquired specific morphological and molecular modifications that enhance their ability to withstand extreme temperatures. Body size and heat-shock protein synthesis are two of the documented adaptive traits responsible for heat tolerance in worker bees [6,10,17]. These traits can significantly diminish the impact of extreme temperatures on thermotolerant honey bee subspecies along with the unique social behavior of A. mellifera colony [18,19]. Therefore, A. mellifera is viewed as a super-organism and a model for functional homoeothermic insects [11,16,20,21].
A. m. jemenetica, an indigenous honeybee subspecies to the Arabian Peninsula and tropical Africa [22,23,24], is the only honeybee subspecies that occurs naturally in Asia and Africa. On the Arabian Peninsula, it is believed that A. m. jemenetica beekeeping goes back about three thousand years [25]. It exhibits very distinctive morphological and behavioral features compared with other honeybee subspecies. Its comparatively small body [6], higher capability to forage at extreme temperatures [26] and ability to survive drought conditions with minimal food storage [25] are some of its unique characteristics [6]. Consequently, many scholars have characterized A. m. jemenetica as a highly thermotolerant subspecies [6,27,28,29]; specifically, its populations in Saudi Arabia and Sudan have been documented as being the most heat tolerant among A. mellifera subspecies [6]. However, its thermotolerance might diminish because of intensified climate change in Saudi Arabia [4,30]. Moreover, more than 1.3 million exotic honeybee packages of other subspecies and hybrids are imported annually into Saudi Arabia to fulfil seasonal beekeeping demand [31], which has a negative impact on local population structures [32] and a direct cost exceeding USD 40 million [31]. Most of these imported packages die in the summer months due to extreme temperature and drought [29]. Therefore, determining the heat-resistance mechanisms and tolerance thresholds of different honeybee subspecies would be very useful and may necessitate the conservation of many native A. mellifera subspecies and result in the reconsideration of importing exotic honeybee subspecies to regions that have well-established honeybee subspecies and extreme climatic conditions.
The heat-resistance mechanisms of Apis mellifera can include genetic or epigenetic modifications of the individual honeybee [20,21,33]. Heat-shock proteins (HSPs) are expressed in the cells of all organisms [34] and play a key role in the thermoregulation of insects, including Apis mellifera [35,36,37]. The expression of hsp genes can be induced in response to environmental cues such as heat shock, pesticides, oxidants, ultraviolet radiation and biotic stresses [35]. Exposure to sub-lethal heat stress suppresses protein synthesis in the cell and stimulates the transcription of heat-shock protein synthesis, while exposure to a lethally high temperature leads to apoptosis, which HSPs prevent by inducing protein thermal stability [38].
Based on their molecular mass and function, HSPs are classified into six families: HSP20 (small HSPS), HSP40 (J-proteins), HSP60, HSP70, HSP90 and HSP100 [37]. The expression of heat-shock proteins to increased ambient temperatures [39] is typically rapid, but unstressed cells keep the transcription of the inducible heat-shock proteins (HSPs) inactive. In a recent study, the exposure of A. m. jemnetica nurse bees to sublethal heat induced the transcriptional activation of many heat-shock protein genes by remodeling histone methylation states [33]. While some heat-shock proteins (HSPs) are induced by environmental stressors, others are continuously expressed during normal cell function [35]. For example, heat-shock cognate 70 (Hsc70) is constitutively expressed in the organism’s cells, while hsp70 is triggered only by stressors [40,41]. Under heat stress situations, HSP70 and all other inducible heat-shock proteins go into fast cap-independent translation pathways to increase the efficiency of heat-shock protein synthesis and to refold the large amount of misfolded proteins [42]. When the temperature returns to normal, the newly synthesized HSPs continue refolding misfolded proteins for some time and then resume regular transcription pathways [42].
The increased expression of hsps may contribute to species-specific stress tolerance and may involve one or more stress proteins [17]. For example, their differential expression in Apis mellifera, A. cerana, A. florea and A. dorsata in response to heat stress has been reported [43]. The expression of hsps may increase survival under heat or other environmental stressors and may also influence individual fitness in specific habitats [17,44]. In relation to the common honeybee A. mellifera, which has 30 geographical subspecies, the expression of heat-shock proteins could be subspecies-specific and may explain the disparity in heat tolerance and ability to survive extreme temperatures. In a two-year observational study in Saudi Arabia, survival rates among three different honeybee subspecies (A. m. jemenetica, A. m. carnica and A. m. ligustica) were highly associated with temperature range, and most colony losses (92% for A. m. carnica, 84% for A. m. ligustica and 48% for A. m. jemenetica) occurred in the summer [29]. Furthermore, A. m. jemenetica showed higher heat response thresholds compared to A. m. carnica or A. m. ligustica [45,46]. The Carniolan honeybee A. m. carnica is native to the temperate European climate, ranging from the Alps to the Carpathian Mountains [6], and it adapts poorly to temperature extremes. Nevertheless, the mechanisms for how A. m. jemenetica can withstand extreme summer temperatures are not well clarified. Indeed, Saudi Arabia is ideal for conducting comparative studies among honeybee subspecies and exploring their adaptation to extreme ambient temperatures and drought.
In this study, the quantitative expression levels of Hsp10, Hsp28, Hsp70, Hsp83 and Hsp90 in the indigenous thermotolerant A. m. jemenetica were measured and compared with expression levels in the thermosusceptible A. m. carnica under summer foraging conditions. The study was conducted in two climatic regions—Riyadh (desert) and Baha (semi-arid) (https://en.wikipedia.org/wiki/Climate_of_Saudi_Arabia, accessed on 1 February 2023)—and expression levels were measured at three-day intervals. We hypothesized that the levels of HSP gene expression would be much higher in A. m. jemenetica compared to A. m carnica under both conditions.
2. Materials and Methods
2.1. Study Sites
The study was conducted in two thermogeographical regions within Saudi Arabia: Riyadh (latitude: 24.7742 N, longitude: 46.7385 E), which provided a perfect hot, dry desert habitat, and Baha (latitude: 20.0046 N, longitude: 41.283.6 E), which has a semiarid climate (Table 1). Riyadh experiences extremely hot summers with an average maximum temperature of around 43 °C. Baha, a highland area in the Sarawat Mountain Range (called Sarah) at an altitude of 2270 m above sea level, has milder summers and may offer a better habitat for A. m. carnica (www.latlong.net, accessed on 1 March 2022).

Table 1.
Some environmental data from the two study locations, Riyadh and Baha (Source: climate-data.org, accessed on 1 January 2023).
2.2. Honeybee Colonies Preparations
Sixteen colonies of A. m. jemenetica were obtained from purebred certified local breeders (chair of bee research, King Saud University). Another sixteen colonies of A. m. carnica were established by heading 16 queenless package bees with imported purebred certified Carniolan queens (LOKACIJA, Slovenia). After three months of colony establishment, subspecies affiliation for each colony was confirmed by our lab at the university’s Bee Research Unit by conducting a morphometric analysis of body size, wing angles and the color of the abdominal tergites using 15 honeybees per colony [47,48]. Reference data for both subspecies were obtained from the Oberursel Bee Research Institute (Frankfurt, Germany). Eight colonies of each subspecies were kept in each of the two thermogeographical regions. After standardization, each colony consisted of 3–4 brood frames and 7–8 adult bee frames. Colonies were established in each location in March and were treated according to the International Federation of Beekeepers’ Associations (Apimondia) guidelines for performance testing until sampling [22].
2.3. Forager Honeybee Sampling
In June, each of the 32 established colonies (16 A. m. carnica and 16 A. m. jemenetica) was sampled 3 times. The first samples were collected in the morning one hour after sunrise (about 7:00 a.m.); the second group about midday (12:00 p.m.); and the third group at 5:00 p.m. Samples of 10 foraging bees were taken at the entrance of each honeybee colony using small forceps, dipped directly in liquid nitrogen and stored in a freezer at −80 °C. Then, the samples representing the same region, race and sampling time were mixed together to form a single pooled sample. In total, 12 pooled samples were set, 6 from Riyadh and 6 from Baha, each comprising 3 samples for each subspecies. A pooled sample consisted of 80 bees from 8 colonies to be used for gene expression analysis. Ambient temperatures were recorded in the study locations at sampling times.
2.4. RNA Extraction and cDNA Synthesis
Total RNA was extracted from each pooled sample (bee heads and thoraces) using TRIzol™ Plus RNA Purification Kit following the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA) and was further purified using Qiagen RNeasy column (Qiagen, Germantown, TN, USA). First-strand cDNA was synthesized using the SuperScriptTM III First-Strand Synthesis Super Mix (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s guidelines. The synthesized cDNA was used as a template in real PCR reactions.
2.5. Primer Design and Real-Time PCR
Public databases (NCBI, Honeybee Genome Consortium) were explored for known heat-shock protein (HSP) genes. The genes included were: Hsp10, Hsp28, Hsp70, Hsp83 and Hsp90, and heat-shock cognate-70 Hsc70cb. The gene sequences were downloaded into Geneious® Prime v.2019.2.3 (https://www.geneious.com, Biomatters Ltd., Newark, NJ, USA, accessed on 1 January 2020) for analysis and primer design. The designed primers are listed in Table 2. Briefly, real-time PCR tests were performed using SYBR GREEN (SYBR® GREEN PCR Master Mix; API: Applied Biosystems, Carlsbad, CA, USA) and the 7500 Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA). The real time reaction mix (25 μL) was prepared from 13 μL of the master mix, 2 μL of forward primer (2 pmol), 2 μL of reverse primer (2 pmol), 2 μL of the cDNA of the sample and 7 μL of nuclease-free water. The A. mellifera β-actin gene was used as an endogenous control for relative expression data analyses. Reactions were conducted in triplicate. Amplification was performed under the following qPCR thermocycling conditions: 95 °C for 5 min, 40 cycles of 95 °C for 10 s, 30 s at 57 °C, 72 °C for 10 s and 95 °C for 20 s.

Table 2.
Primer sequences designed to amplify different heat-shock proteins with gene names, gene subcellular location and NCBI gene ID. Heat-shock cognate (hsc70cb) genes were used in the present study. (*) subcellular locations of different heat shock genes were obtained from the official website of the University of Port Harcourt: UniPort website (www.uniprot.org, accessed on 1 January 2023).
2.6. Statistical Analysis
Relative expression and fold change analyses were performed using qPCR Ct values for each gene according to the sampling plan. Relevant actin cycle threshold (Ct) values were used to calculate the relative expression (relative expression = 2 ct (actin-gene)). Average ct values of A. m. carnica samples from Baha were used as a calibrator to calculate gene expression fold changes in different heat-shock protein genes (fold change = 2 ct (reference-(actin-gene)). Significant fold change differences were determined based on the average gene expression of each group using SAS Statistical Analysis System software suite (SAS Institute: www.sas.com, accessed on 15 February 2023). Mixed models included the random effects of honeybee subspecies, location and sampling times followed by Multiple Student–Newman–Keuls tests. Differences were considered statistically significant at p < 0.05, and the figures were prepared using GraphPad Prism 9 (www.graphstates.net, accessed on 5 January 2023).
3. Results
Significantly higher expression levels of five hsp mRNAs (hsp70ab, hsc70cb, hsp83, hsp90 and hsp28) were found in A. m. jemenetica compared to A. m. carnica (p values for all comparisons are listed in Table 3). The expression levels of hsp10 mRNA were higher in A. m. jemenetica as well, but the variation was not significant (P > F = 0.0520). The highest variation in the hsp mRNA fold change occurred in the Hsp70 mRNAs (more than 150×) using the expression levels of A. m. carnica in Baha as a calibrator, which revealed the highest absolute expression levels among all other heat-shock protein genes (Figure 1). Nevertheless, the smaller-molecular-weight hsp10 and hsp28 exhibited higher expression fold changes compared to the larger-molecular-weight hsp83 and hsp90 in this study. Fold changes in the relative expression of hsc70cb mRNAs were the lowest in both subspecies.

Table 3.
Summary of SAS PROC GLM analyses for different heat-shock proteins (hsps mRNA) relative expression levels. Hypothesis testing was performed using three-way ANOVA (subspecies: A. m. jemenetica or A. m. carnica; location: Riyadh or Baha; sampling time: morning, midday or evening. F-ratios were calculated from type III mean squares for all statistics. p < 0.05 was deemed statistically significant and p < 0.001 as highly significant.
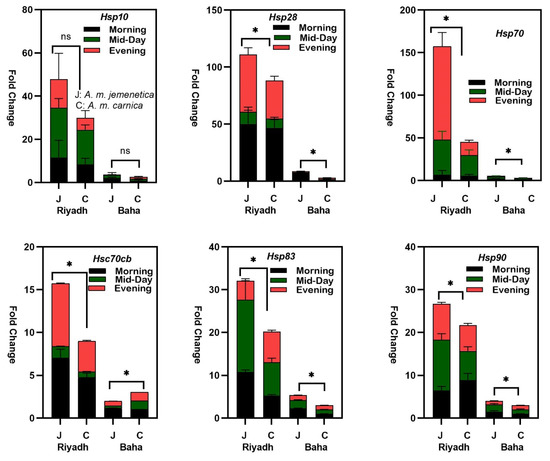
Figure 1.
Fold change in Hsp28, Hsp10, Hsp83, Hsp90, Hsp70 and Hsc70cb (mRNA) relative expression levels in A. m. jemenetica (J) and A. m. carnica (C) at the two thermogeographical regions (Riyadh and Baha) and the three foraging times (7:00 a.m.: after ≈ 1 h of foraging; 12:00 p.m.; and 5:00 p.m. (≈1 h before sunset)). Actine was used as endogenous control to calculate ∆ct, and relative expression in A. m. carnica in Baha was used as a reference to calculate the fold change (fold change = 2 ct (reference-treatment)). Significant fold change difference was determined based on average gene expression of each group using a three-way ANOVA analysis followed by Multiple Student–Newman–Keuls tests. Differences were considered statistically significant at p < 0.05. (*) = significant variation and (ns) = non-significant variation.
The results also revealed significantly higher relative expression levels of all hsp mRNAs under desert conditions (Riyadh) compared to semi-arid conditions (Baha) (Table 2). Within the same region, relative expression levels were higher in A. m. jemenetica except in the only case of hsc70cb in Baha, which was significantly higher in A. m. carnica (Figure 1). Although variations in the relative expression levels between A. m. jemenetica and A. m. carnica within the same region were significant for both Riyadh and Baha, variations in the relative expression levels between both subspecies were far higher under desert conditions (Figure 1). The relative expression levels of all hsp mRNAs (using the expression levels of A. m. carnica in Baha as a calibrator) were very modest in Baha mainly for hsp70ab, hsp10 and hsp28 (Figure 1). The highest difference in the relative expression levels within the same region was calculated for hsp70ab. The results also revealed a significant interaction between subspecies and region in the relative expression levels of hsp70ab, hsp90, hsp83 and hsc70cb (Table 1).
In Riyadh, the ambient sampling temperatures were 31, 42 and 39 °C (morning, midday and evening, respectively). In Baha, they were 22, 30 and 25 °C. Variation in the expression levels of all hsp mRNAs were also significant among sampling times (Table 1). However, relative expression levels of hsp mRNAs were not consistent for either subspecies within the same sampling time (Figure 1). The highest relative expression levels were not always at the highest day temperature; some were in the morning then evening in both locations (hsc70cb and hsp28), while others exhibited the highest expression levels at midday (hsp10 and hsp83) and in the evening (hsp70ab) in Riyadh (Figure 1).
4. Discussion
The study shows day-long higher expression levels of hsp mRNAs in the thermo-tolerant honeybee subspecies A. m. jemenetica compared to the thermosusceptible A. m. carnica under the same conditions. This indicates a higher adaptation of A. m. jemenetica to local conditions at both locations. Under the milder ambient temperatures in Baha, the expression levels were very modest in both honeybee subspecies though the expression levels for A. m. jemenetica were still higher. The higher expression levels of hsp10, hsp28, hsp70ab, hsp83 and hsp90 mRNAs in A. m. jemenetica in Baha can be associated with the adaptation of A. m. jemenetica to start heat-shock protein synthesis even at lower foraging temperatures, which enhances survival and fitness at higher temperatures and might be associated with longer foraging trips. The accumulation of hsp70 mRNAs at a low foraging temperature was reported in the desert ant Cataglyphis bombycina, which can forage at an ambient temperature exceeding 50 °C [49]. Furthermore, the real body temperature of foraging A. m. carnica and Melipona panamica was found to be higher than the reported ambient temperatures [50,51], and this may be the same for Apis mellifera. The foragers’ body temperature could also be affected by foraging duration distance and conditions. Nevertheless, the modest expression levels in Baha and the significant interaction between subspecies and location indicated less heat-stress-inducing conditions to A. m. carnica compared to Riyadh. Consequently, keeping A. m. carnica in Baha can be more successful compared to Riyadh, where extremely low year-round survival rates (8%) were reported in the summer months [29]. Obviously, the higher expression levels of hsp mRNAs in A. m. jemenetica under desert foraging conditions can be proposed as a key component of higher adaptation to extreme temperatures compared to A. m. carnica. Both honeybee subspecies exhibited significantly different expression levels of hsp mRNAs at different sampling times, which indicated the fast and continuous adjustment of hsp expression in response to daily changes in ambient foraging temperatures.
In this study, the relative expression levels of hsp70ab mRNA were the highest among the other hsps. Heat-shock proteins of the HSP70 family demonstrated a very fast response and exhibited robust transcriptional activation and expression in many insects after heat stress [33,42,45]. Transcriptional induction of the HSP70 family was associated with the overall transcriptional downregulation of constitutively expressed genes such as heat-shock cognate 70Cb (Hsc70cb). Under desert conditions, the fold change in Hsp70 mRNA was more than 50× higher in A. m. jemenetica than A. m. carnica and might be considered the main heat-shock protein to be involved in thermotolerance. This could also explain the generally lower expression levels of the constitutively expressed hsc70cb in both regions. The fold change differences in Hsc70cb mRNA were the lowest between both subspecies as well as locations, which could be related to the relatively small transcriptional activation response of Hsc70cb as a constitutive stress gene to the increase in ambient foraging temperatures, and its expression is associated with other biological processes [52]. The special cap-independent translation pathway of hsp70 [42] may explain the high fold change in the relative expression of hsp70 mRNA under hot foraging in Riyadh compared to the relatively normal foraging temperature in Baha. The relative amounts of hsp70 mRNA increased during the day along with the increase in the ambient temperature, but they resumed regular transcription pathways at night when all forager bees had returned to the colony and hsp70 mRNA began to decay. This was also applicable to the inducible small heat-shock proteins (HSPs). In a previous study [45] using the SDS-PAGE method, the expression of the large-molecular-weight heat-shock proteins hsp70 and hsp83 were reported in nurses of Apis mellifera jemenetica after exposure to 40 °C, but only hsp70 in foragers after exposure to 45 °C [45]. In this study, samples were collected when forager bees returned to their colonies with body temperatures normally higher than the reported ambient temperature (14-44°C) [51], which may explain the detection of hsp70 and hsp83 mRNAs at lower ambient temperatures in both subspecies. This deviation might also be explained by the inherent technical limitations associated with the WB method such as incomplete protein transfers [53]. Fold changes in the relative expression levels of hsp28 and hsp10 mRNAs between hot foraging (Riyadh) and normal foraging (Baha) conditions demonstrated the significant contribution of these heat-shock proteins in enabling both subspecies to overcome heat stress.
On the other hand, the inconsistency in the relative expression patterns of hsp mRNAs at different sampling times might be associated with a variable quantitative response and the interaction of different hsp genes with short-term environmental changes. We do not know if each hsp or hsp family has the same response threshold for heat stress and if their interaction among different honeybee subspecies is similar.
In conclusion, the higher expression levels of hsp10, hsp28, hsp70ab, hsp83 and hsp90 mRNAs in A. m. jemenetica can be strongly associated with adaptation to the extreme ambient desert temperatures which characterize the Riyadh region.
Author Contributions
Conceptualization, Y.Z.A. and A.A.A.; methodology, Y.Z.A.; software, Y.Z.A.; validation, Y.Z.A. and A.A.A.; formal analysis, Y.Z.A.; investigation, Y.Z.A.; resources, A.A.A.; data curation, Y.Z.A.; writing—original draft preparation, Y.Z.A.; writing—review and editing, Y.Z.A. and A.A.A.; visualization, Y.Z.A.; supervision, Y.Z.A. and A.A.A.; project administration, A.A.A.; funding acquisition A.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deputyship for Research and Innovation, “Ministry of Education”, in Saudi Arabia grant number IFKSURG-2-559.
Data Availability Statement
Data are available in tables and figures.
Acknowledgments
We wish to thank Oberursel Bee Research Institute (Frankfurt, Germany) for providing us with reference morphometric data for both subspecies used for comparison in this article. The authors also extend their appreciation the Deputyship for Research and Innovation, “Ministry of Education”, in Saudi Arabia grant number IFKSURG-2-559.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Porcelli, D.; Gaston, K.J.; Butlin, R.K.; Snook, R.R. Local adaptation of reproductive performance during thermal stress. J. Evol. Biol. 2017, 30, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Insolia, L.; Molinari, R.; Rogers, S.R.; Williams, G.R.; Chiaromonte, F.; Calovi, M. Honey bee colony loss linked to parasites, pesticides and extreme weather across the United States. Sci Rep. 2022, 12, 20787. [Google Scholar] [CrossRef] [PubMed]
- UNEP. Emission Gap Report: The Closing Window: Climate Crisis Calls for Rapid Transformation of Societies; United Nation Environmental Program: Nairobi, Kenya, 2022; ISBN 978-92-807-3979-4. [Google Scholar]
- Almazroui, M.; Nazrul, I.; Athar, H.; Jones, P.; Ashfaqur, M. Recent climate change in the Arabian Peninsula: Annual rainfall and temperature analysis of Saudi Arabia for 1978–2009. Int. J. Climatol. 2012, 32, 953–966. [Google Scholar] [CrossRef]
- POMEP. Climatic Data for Saudi Arabia: Presidency of Metrology and Environmental Protection; Ministry of Defense and Aviation: Riyadh, Saudi Arabia, 2020. [Google Scholar]
- Ruttner, F. Biogeography and Taxonomy of Honeybees, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1988; p. 284. ISBN 978-3-64272651-4. [Google Scholar]
- Han, F.; Wallberg, A.; Webster, M.T. From where did the Western honeybee (Apis mellifera) originate? Ecol. Evol. 2012, 2, 1949–1957. [Google Scholar] [CrossRef]
- Ilyasov, R.A.; Lee, M.; Takahashi, J.; Kwon, H.W.; Nikolenko, A.G. A revision of subspecies structure of western honeybee Apis mellifera. Saudi J. Biol. Sci. 2020, 27, 3615–3621. [Google Scholar] [CrossRef]
- Angilletta, M.J.; Wilson, R.S.; Navas, C.A.; James, R.S. Tradeoffs and the evolution of thermal reaction norms. Trends Ecol. Evol. 2003, 18, 234–240. [Google Scholar] [CrossRef]
- Dogantzis, K.A.; Tiwari, T.; Conflitti, I.M.; Dey, A.; Patch, H.M.; Muli, E.M.; Garnery, L.; Whitfield, C.W.; Stolle, E.; Alqarni, A.S. Thrice out of Asia and the adaptive radiation of the western honey bee. Sci. Adv. 2021, 7, eabj2151. [Google Scholar] [CrossRef]
- Severson, D.W.; Erickson, E.H.; Williamson, J.L.; Aiken, J.M. Heat stress induced enhancement of heat shock protein gene activity in the honey bee (Apis mellifera). Experientia 1990, 46, 737–739. [Google Scholar] [CrossRef]
- Binda, O. On your histone mark, SET, methylate! Epigenetica 2013, 8, 457–463. [Google Scholar] [CrossRef]
- Southwick, E.E. The honey bee cluster as a homeothermic superorganism. Comp. Biochem. Physiol. Part A Physiol. 1983, 75, 641–645. [Google Scholar] [CrossRef]
- Kronenberg, F.; Heller, H.C. Colonial thermoregulation in honey bees (Apis mellifera). J. Comp. Physiol. 1982, 148, 65–76. [Google Scholar] [CrossRef]
- Tautz, J.; Maier, S.; Groh, C.; Rossler, W.; Brockmann, A. Behavioral performance in adult honey bees is influenced by the temperature experienced during their pupal development. Proc. Natl. Acad. Sci. USA 2003, 100, 7343–7347. [Google Scholar] [CrossRef] [PubMed]
- Stabentheiner, A.; Kovac, H.; Mandl, M.; Kaefar, H. Coping with the cold and fighting the heat: Thermal homeostasis of a superorganism, the honeybee colony. J. Comp. Physiol. A 2021, 207, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, G.; Guo, D.; Li, H.; Liu, Q.; Xu, B.; Guo, X. Response mechanisms to heat stress in bees. Apidologie 2021, 52, 388–399. [Google Scholar] [CrossRef]
- Winston, M.L. The Biology of the Honeybee, 1st ed.; Harvard University Press: Cambridge, MA, USA, 1991; p. 294. ISBN 9780674074095. [Google Scholar]
- Elekonich, M.M. Extreme thermotolerance and behavioral induction of 70-kDa heat shock proteins and their encoding genes in honey bees. Cell Stress Chaperones 2009, 14, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Dickman, M.J.; Kucharski, R.; Maleszka, R.; Hurd, P.J. Extensive histone post-translational modification in honey bees. Insect Biochem. Mol. Biol. 2013, 43, 125–137. [Google Scholar] [CrossRef]
- Bloch, G.; Grozinger, C.M. Social molecular pathways and the evolution of bee societies. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 2155–2170. [Google Scholar] [CrossRef]
- Ruttner, H. Technische Empfehlungen zur Leistungsprüfung von Bienenvölkern. In Proceedings of the Paarungskontrolle und Selektion bei der Honigbiene: Internationales Symposium, Lunz am See, Austria, 31 July–5 August 1972. [Google Scholar]
- Alattal, Y.; Alghamdi, A.; Alsharhi, M.; Fuchs, S. Morphometric characterisation of the native Honeybee, Apis mellifera Linnaeus, 1758, of Saudi Arabia. Zool. Middle East 2014, 60, 226–235. [Google Scholar] [CrossRef]
- Alattal, Y.; Alghamdi, A. Evidence for sub-populations of Apis mellifera jemenitica colonies along the Red Sea coast of Saudi Arabia. Bull. Insectology 2022, 71, 7–14. [Google Scholar]
- Alqarni, A.S.; Hannan, M.A.; Owayss, A.A.; Engel, M.S. The indigenous honey bees of Saudi Arabia (Hymenoptera, Apidae, Apis mellifera jemenitica Ruttner): Their natural history and role in beekeeping. ZooKeys 2011, 134, 83–98. [Google Scholar]
- Ali, M. Comparative study for evaluating two honey bee races, Apis mellifera jementica (indigenous race) and Apis mellifera carnica (Carniolan race) in brood production, population development and foraging activity under the environmental conditions of the central region of the Kingdom of Saudi Arabia. Ann. Agric. Sci. 2011, 56, 127–134. [Google Scholar]
- Alqarni, A.S. Tolerance of summer temperature in imported and indigenous honeybee Apis mellifera L. races in central Saudi Arabia. Saudi J. Biol. Sci. 2006, 13, 123–127. [Google Scholar]
- Ali, H.; Alqarni, A.S.; Owayss, A.A.; Hassan, A.M.; Smith, B.S. Osmotic concentration in three races of honey bee, Apis mellifera L. under environmental conditions of arid zone. Saudi J. Biol. Sci. 2017, 24, 1081–1085. [Google Scholar] [CrossRef]
- Alattal, Y.; Alghamdi, A. Impact of temperature extremes on survival of indigenous and exotic honey bee subspecies, Apis mellifera, under desert and semiarid climates. Bull. Insectology 2015, 68, 219–222. [Google Scholar]
- Chen, C.H.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.H.H. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- MoEP. Import Data of Honeybees from Different Sources; Central Department of Statistics and Information, Ministry of Economy and Planning: Riyadh, Saudi Arabia, 2022. [Google Scholar]
- Alattal, Y.; Alghamdi, A.; Alsharni, M. Population Structure of the Yemeni Honeybee (Apis mellifera jemenitica) Entails an Urgent Conservative Strategy. J. Entomol. 2014, 11, 163–169. [Google Scholar] [CrossRef]
- Alattal, Y.Z.; Alghamdi, A.A. Linking Histone Methylation States and hsp Transcriptional Regulation in Thermo-Tolerant and Thermo-Susceptible A. mellifera L. Subspecies in Response to Heat Stress. Insects 2023, 14, 225. [Google Scholar] [CrossRef]
- Madrigal-Matute, J.; Martin-Ventura, J.L.; Blanco-Colio, L.M.; Egido, J.; Michel, J.B.; Meilhac, O. Heat-shock proteins in cardiovascular disease. Adv. Clin. Chem. 2011, 54, 3–29. [Google Scholar]
- Zhao, L.; Jones, W.A. Expression of heat shock protein genes in insect stress responses. Invert. Survival. J. 2012, 9, 93–101. [Google Scholar]
- Perez, R.; Aron, S. Adaptations to thermal stress in social insects: Recent advances and future directions. Biol. Rev. 2020, 95, 1535–1553. [Google Scholar] [CrossRef]
- Jing, X.Y.; Li, F.M. Identifying Heat Shock Protein Families from Imbalanced Data by Using Combined Features. Comput. Math. Methods Med. 2020, 2020, 8894478. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, P.; Stepanenko, I.; Nikolay, K. Heat Shock Proteins. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Maloy, S., Hughes, K., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 402–405. ISBN 9780080961569. [Google Scholar]
- Khadir, A.; Kavalakatt, S.; Cherian, P.; Warsame, S.; Abubaker, J.A.; Dehbi, M.; Tiss, A. Physical exercise enhanced heat shock protein 60 expression and attenuated inflammation in the adipose tissue of human diabetic obese. Front. Endocrinol. 2018, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.M.; Zhang, Q.; Zhang, Y.L.; Zhang, G.Z.; Zhang, Z.; Yu, Q.Y. Heat Shock Protein 70 Family in Response to Multiple Abiotic Stresses in the Silkworm. Insects 2021, 12, 928. [Google Scholar] [CrossRef] [PubMed]
- Aquino, D.A.; Klipfel, A.A.; Brosnan, C.F.; Norton, W.T. The 70-kDa heat shock cognate protein (HSC70) is a major constituent of the central nervous system and is up-regulated only at the mRNA level in acute experimental autoimmune encephalomyelitis. J. Neurochem. 1993, 61, 1340–1348. [Google Scholar] [CrossRef]
- Alagar-Boopathy, L.R.; Jacob-Tomas, S.; Alecki, C.; Vera, M. Mechanisms tailoring the expression of heat shock proteins to proteostasis challenges. J. Biol. Chem. 2022, 298, 101796. [Google Scholar] [CrossRef]
- Sen-Sarma, M.; Whitfield, C.W.; Robinson, G.E. Species differences in brain gene expression profiles associated with adult behavioral maturation in honey bees. BMC Genome 2007, 8, 200. [Google Scholar] [CrossRef]
- Feder, M.E.; Blair, N.; Figeuras, H. Natural thermal stress and heat-shock protein expression in Drosophila larvae and pupae. Funct. Ecol. 2007, 11, 90–100. [Google Scholar] [CrossRef]
- Alqarni, A.S.; Ali, H.; Iqbal, J.; Owayss, A.A.; Smith, B.H. Expression of heat shock proteins in adult honey bee (Apis mellifera L.) workers under hot-arid subtropical ecosystems. Saudi J. Biol. Sci. 2019, 26, 1372–1376. [Google Scholar] [CrossRef]
- Kovac, H.; Käfer, H.; Stabentheiner, A.; Costa, C. Metabolism and upper thermal limits of Apis mellifera carnica and A. m. ligustica. Apidologie 2014, 45, 664–677. [Google Scholar] [CrossRef]
- Meixner, M.D.; Pinto, M.A.; Bouga, M.; Kryger, P.; Ivanova, E.; Fuchs, S. Standard methods for characterising subspecies and ecotypes of Apis mellifera. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Bouga, M.; Alaux, C.; Bienkowska, M.; Büchler, R.; Carreck, N.L.; Cauia, E.; Chlebo, R.; Dahle, B.; Dall’Olio, R.; De la Rúa, P.; et al. A review of methods for discrimination of honey bee populations as applied to European beekeeping. J. Apic. Res. 2011, 50, 51–84. [Google Scholar] [CrossRef]
- Gehring, W.J.; Wehner, R. Heat shock protein synthesis and thermotolerance in Cataglyphis, an ant from the Sahara desert. Proc. Natl. Acad. Sci. USA 1995, 92, 2994–2998. [Google Scholar] [CrossRef] [PubMed]
- Felipe, A.L.; Contrera, J.; Nieh, C. The effect of ambient temperature on forager sound production and thoracic temperature in the stingless bee, Melipona panamica. Behav. Ecol. Sociobiol. 2007, 61, 887–897. [Google Scholar]
- Kovac, H.; Stabentheiner, A. Thermoregulation of foraging honeybees on flowering plants: Seasonal variability and influence of radiative heat gain. Ecol. Entomol. 2011, 36, 686–699. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.S.; Moyle, L.C. Constitutive and Plastic Gene Expression Variation Associated with Desiccation Resistance Differences in the Drosophila americana Species Group. Genes 2020, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Maria, E. A Comparative Study of qPCR, Western Blot and Mass Spectrometry for the Estimation of Protein Concentrations; KTH Royal Institute of Technology School of Biotechnology: Stockholm, Sweden, 2016; p. 39. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).