A Novel Nitrogen-Fixing Bacterium Raoultella electrica Isolated from the Midgut of the Leafhopper Recilia dorsalis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of the Bacterial Strain and Culture Condition
2.2. Bacterial Identification and Phylogenetic Analysis
2.3. De Novo Sequencing, Genome Assembly and Annotation
2.4. Fluorescence In Situ Hybridization (FISH)
2.5. Transmission Electron Microscopy
2.6. Nitrogen Fixation Capacity
3. Results
3.1. Isolation, Identification, and Localization of R. electrica in the Digestive Tract of Leafhoppers
3.2. General Genome Features of R. electrica Rd210413
3.3. Nitrogen Metabolism in R. electrica Rd210413
3.4. Phylogenetic Analysis of nifH
3.5. Nitrogen Fixation Capacity of R. electrica Rd210413
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sudakaran, S.; Kost, C.; Kaltenpoth, M. Symbiont Acquisition and Replacement as a Source of Ecological Innovation. Trends Microbiol. 2017, 25, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.K.; Moran, N.A. The Impact of Microbial Symbionts on Host Plant Utilization by Herbivorous Insects. Mol. Ecol. 2014, 23, 1473–1496. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.M.; Martinez, A.J. How Resident Microbes Modulate Ecologically-Important Traits of Insects. Curr. Opin. Insect Sci. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. The Microbial Dimension in Insect Nutritional Ecology. Funct. Ecol. 2009, 23, 38–47. [Google Scholar] [CrossRef]
- Douglas, A.E. Multiorganismal Insects: Diversity and Function of Resident Microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef]
- Bar-Shmuel, N.; Behar, A.; Segoli, M. What Do We Know About Biological Nitrogen Fixation in Insects? Evidence and Implications for the Insect and the Ecosystem. Insect Sci. 2020, 27, 392–403. [Google Scholar] [CrossRef]
- Benemann, J.R. Nitrogen Fixation in Termites. Science 1973, 181, 164–165. [Google Scholar] [CrossRef]
- Breznak, J.A.; Brill, W.J.; Mertins, J.W.; Coppel, H.C. Nitrogen Fixation in Termites. Nature 1973, 244, 577–580. [Google Scholar] [CrossRef]
- Ren, X.; Guo, R.; Akami, M.; Niu, C. Nitrogen Acquisition Strategies Mediated by Insect Symbionts: A Review of Their Mechanisms, Methodologies, and Case Studies. Insects 2022, 13, 84. [Google Scholar] [CrossRef]
- Weihrauch, D.; O’Donnell, M.J. Mechanisms of Nitrogen Excretion in Insects. Curr. Opin. Insect Sci. 2021, 47, 25–30. [Google Scholar] [CrossRef]
- Tamayo, D.O.; Heil, M. N Fixation in Insects: Its Potential Contribution to N Cycling in Ecosystems and Insect Biomass. In Biological Nitrogen Fixation; De Bruijn, F.J., Ed.; Wiley Online Library: New York, NY, USA, 2015; pp. 1143–1152. [Google Scholar]
- Drancourt, M.; Bollet, C.; Carta, A.; Rousselier, P. Phylogenetic Analyses of Klebsiella Species Delineate Klebsiella and Raoultella Gen. Nov., with Description of Raoultella ornithinolytica Comb. Nov., Raoultella terrigena Comb. Nov. And Raoultella planticola Comb. Nov. Int. J. Syst. Evol. Microbiol. 2001, 51, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Bagley, S.T.; Seidler, R.J.; Brenner, D.J. Klebsiella planticola sp. Nov.: A New Species of Enterobacteriaceae Found Primarily in Nonclinical Environments. Curr. Microbiol. 1981, 6, 105–109. [Google Scholar] [CrossRef]
- Izard, D.; Ferragut, C.; Gavini, F.; Kersters, K.; De Ley, J.; Leclerc, H. Klebsiella terrigena, a New Species from Soil and Water. Int. J. Syst. Bacteriol. 1981, 31, 116–127. [Google Scholar] [CrossRef]
- Sakazaki, R.; Tamura, K.; Kosako, Y.; Yoshizaki, E. Klebsiella ornithinolytica sp. Nov., Formerly Known as Ornithine-Positive Klebsiella oxytoca. Curr. Microbiol. 1989, 18, 201–206. [Google Scholar] [CrossRef]
- Kimura, Z.-i.; Chung, K.M.; Itoh, H.; Hiraishi, A.; Okabe, S. Raoultella electrica sp. Nov., Isolated from Anodic Biofilms of a Glucose-Fed Microbial Fuel Cell. Int. J. Syst. Evol. Microbiol. 2014, 64, 1384–1388. [Google Scholar] [CrossRef] [PubMed]
- Bentzon-Tilia, M.; Severin, I.; Hansen, L.H.; Riemann, L. Genomics and Ecophysiology of Heterotrophic Nitrogen-Fixing Bacteria Isolated from Estuarine Surface Water. mBio 2015, 6, e00929-15. [Google Scholar] [CrossRef] [PubMed]
- Morales-Jiménez, J.; de León, A.V.-P.; García-Domínguez, A.; Martínez-Romero, E.; Zúñiga, G.; Hernández-Rodríguez, C. Nitrogen-Fixing and Uricolytic Bacteria Associated with the Gut of Dendroctonus rhizophagus and Dendroctonus valens (Curculionidae: Scolytinae). Microb. Ecol. 2013, 66, 200–210. [Google Scholar] [CrossRef]
- Schicklberger, M.; Shapiro, N.; Loqué, D.; Woyke, T.; Chakraborty, R. Draft Genome Sequence of Raoultella terrigena R1gly, a Diazotrophic Endophyte. Genome Announc. 2015, 3, e00607-15. [Google Scholar] [CrossRef]
- Levorova, J.; Machon, V.; Guha, A.; Foltan, R. Septic Arthritis of the Temporomandibular Joint Caused by Rare Bacteria Raoultella ornithinolytica. Int. J. Oral Maxillofac. Surg. 2017, 46, 111–115. [Google Scholar] [CrossRef]
- Li, F.; Huang, S.-S. Isolation and Identification of Lignin-Degrading Bacteria from the Gut of Termite and Their Degradation Characteristics. Biotechnol. Bull. 2020, 36, 61–68. [Google Scholar]
- Yang, X.; Zhang, T.; Chen, B.; Zhou, G. Transmission Biology of Rice Stripe Mosaic Virus by an Efficient Insect Vector Recilia dorsalis (Hemiptera: Cicadellidae). Front. Microbiol. 2017, 8, 2457. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, C.; Li, M.; Wu, W.; Zhou, G.; Wei, T. Adverse Effects of Rice Gall Dwarf Virus Upon Its Insect Vector Recilia dorsalis (Hemiptera: Cicadellidae). Plant Dis. 2016, 100, 784–790. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, J.P.; Moran, N.A. Functional Convergence in Reduced Genomes of Bacterial Symbionts Spanning 200 My of Evolution. Genome Biol. Evol. 2010, 2, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Gonella, E.; Mandrioli, M.; Tedeschi, R.; Crotti, E.; Pontini, M.; Alma, A. Activation of Immune Genes in Leafhoppers by Phytoplasmas and Symbiotic Bacteria. Front. Physiol. 2019, 10, 795. [Google Scholar] [CrossRef] [PubMed]
- Noda, H.; Watanabe, K.; Kawai, S.; Yukuhiro, F.; Miyoshi, T.; Tomizawa, M.; Koizumi, Y.; Nikoh, N.; Fukatsu, T. Bacteriome-Associated Endosymbionts of the Green Rice Leafhopper Nephotettix cincticeps (Hemiptera: Cicadellidae). Appl. Entomol. Zool. 2012, 47, 217–225. [Google Scholar] [CrossRef]
- Iasur-Kruh, L.; Weintraub, P.G.; Mozes-Daube, N.; Robinson, W.E.; Perlman, S.J.; Zchori-Fein, E. Novel Rickettsiella Bacterium in the Leafhopper Orosius albicinctus (Hemiptera: Cicadellidae). Appl. Environ. Microbiol. 2013, 79, 4246–4252. [Google Scholar] [CrossRef]
- Crotti, E.; Rizzi, A.; Chouaia, B.; Ricci, I.; Favia, G.; Alma, A.; Sacchi, L.; Bourtzis, K.; Mandrioli, M.; Cherif, A. Acetic Acid Bacteria, Newly Emerging Symbionts of Insects. Appl. Environ. Microbiol. 2010, 76, 6963–6970. [Google Scholar] [CrossRef]
- Aguin-Pombo, D.; Rodrigues, M.C.P.A.; Voetdijk, B.; Breeuwer, J.A.J. Parthenogenesis and Sex-Ratio Distorting Bacteria in Empoasca (Hemiptera: Cicadellidae) Leafhoppers. Ann. Entomol. Soc. Am. 2021, 114, 738–749. [Google Scholar] [CrossRef]
- Hansen, A.K.; Pers, D.; Russell, J.A. Symbiotic Solutions to Nitrogen Limitation and Amino Acid Imbalance in Insect Diets. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–205. [Google Scholar]
- Bennett, G.M.; Moran, N.A. Small, Smaller, Smallest: The Origins and Evolution of Ancient Dual Symbioses in a Phloem-Feeding Insect. Genome Biol. Evol. 2013, 5, 1675–1688. [Google Scholar] [CrossRef]
- Gallon, J.R. The Oxygen Sensitivity of Nitrogenase: A Problem for Biochemists and Micro-Organisms. Trends Biochem. Sci. 1981, 6, 19–23. [Google Scholar] [CrossRef]
- Phillips, D.A. Efficiency of Symbiotic Nitrogen Fixation in Legumes. Annu. Rev. Plant Physiol. 1980, 31, 29–49. [Google Scholar] [CrossRef]
- Bentley, B.L. Nitrogen Fixation in Termites: Fate of Newly Fixed Nitrogen. J. Insect Physiol. 1984, 30, 653–655. [Google Scholar] [CrossRef]
- Meuti, M.E.; Jones, S.C.; Curtis, P.S. 15n Discrimination and the Sensitivity of Nitrogen Fixation to Changes in Dietary Nitrogen in Reticulitermes flavipes (Isoptera: Rhinotermitidae). Environ. Entomol. 2010, 39, 1810–1815. [Google Scholar] [CrossRef] [PubMed]
- Reid, N.M.; Lloyd-Jones, G. Symbiotic Nitrogen Fixation in the New Zealand Dampwood Termite (Stolotermes ruficeps). N. Z. J. Ecol. 2009, 33, 90–95. [Google Scholar]
- Ulyshen, M.D. Insect-Mediated Nitrogen Dynamics in Decomposing Wood. Ecol. Entomol. 2015, 40, 97–112. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hosokawa, T.; Fukatsu, T. An Ancient but Promiscuous Host–Symbiont Association between Burkholderia Gut Symbionts and Their Heteropteran Hosts. ISME J. 2011, 5, 446–460. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Meng, X.-Y.; Fukatsu, T. Gut Symbiotic Bacteria of the Genus Burkholderia in the Broad-Headed Bugs Riptortus clavatus and Leptocorisa chinensis (Heteroptera: Alydidae). Appl. Environ. Microbiol. 2005, 71, 4035–4043. [Google Scholar] [CrossRef]
- Vera-Ponce de León, A.; Ormeno-Orrillo, E.; Ramírez-Puebla, S.T.; Rosenblueth, M.; Esposti, M.D.; Martínez-Romero, J.; Martínez-Romero, E. Candidatus Dactylopiibacterium Carminicum, a Nitrogen-Fixing Symbiont of Dactylopius Cochineal Insects (Hemiptera: Coccoidea: Dactylopiidae). Genome Biol. Evol. 2017, 9, 2237–2250. [Google Scholar] [CrossRef]
- Dos Santos, P.C.; Fang, Z.; Mason, S.W.; Setubal, J.C.; Dixon, R. Distribution of Nitrogen Fixation and Nitrogenase-Like Sequences Amongst Microbial Genomes. BMC Genom. 2012, 13, 162. [Google Scholar] [CrossRef]
- Hardy, R.W.F.; Holsten, R.D.; Jackson, E.K.; Burns, R.C. The Acetylene-Ethylene Assay for N2 Fixation: Laboratory and Field Evaluation. Plant Physiol. 1968, 43, 1185–1207. [Google Scholar] [CrossRef]
- Broughton, W.J.; Wool, K.C.; Hoh, C.H. Acetylene Reduction by Legume Root Nodule Protoplasts. Nature 1967, 262, 208–209. [Google Scholar] [CrossRef]
- Kuranouchi, T.; Nakamura, T.; Shimamura, S.; Kojima, H.; Goka, K.; Okabe, K.; Mochizuki, A. Nitrogen Fixation in the Stag Beetle, Dorcius (Macrodorcus) Rectus (Motschulsky) (Col., Lucanidae). J. Appl. Entomol. 2006, 130, 471–472. [Google Scholar] [CrossRef]
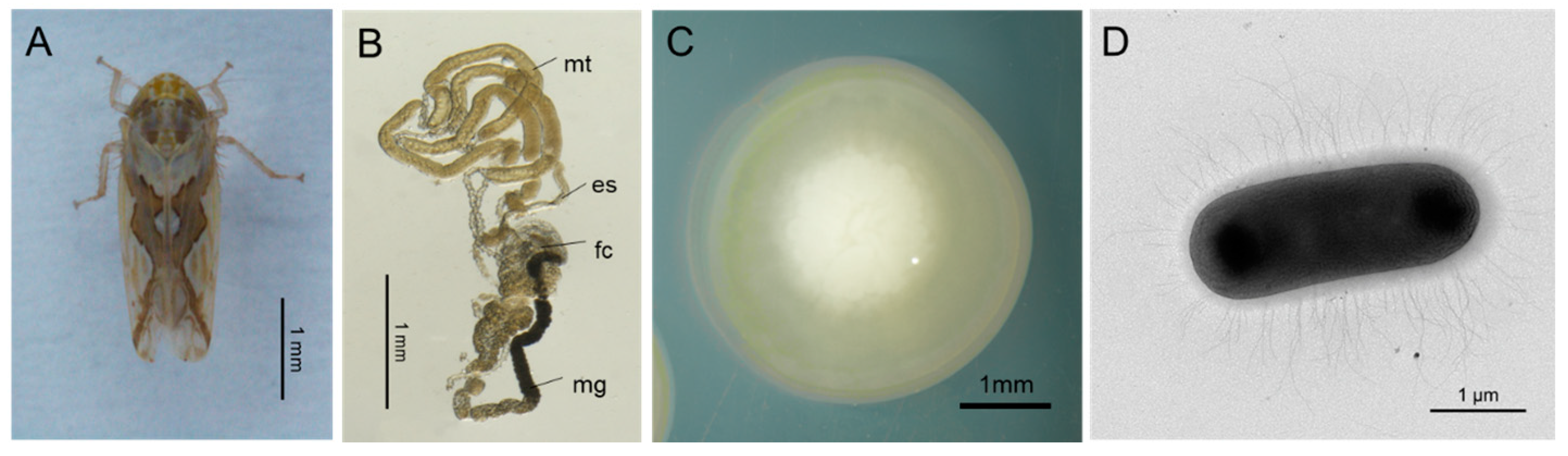


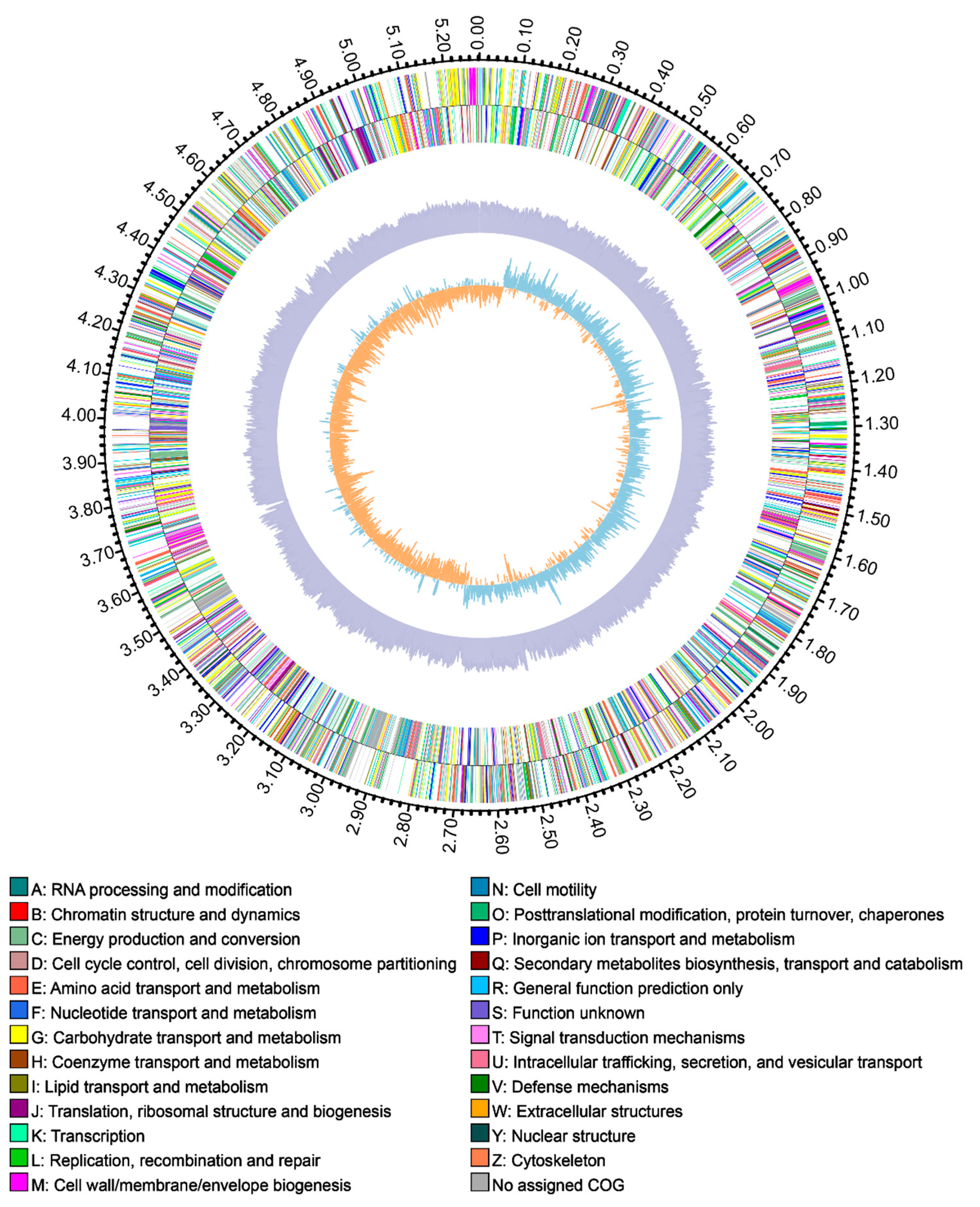
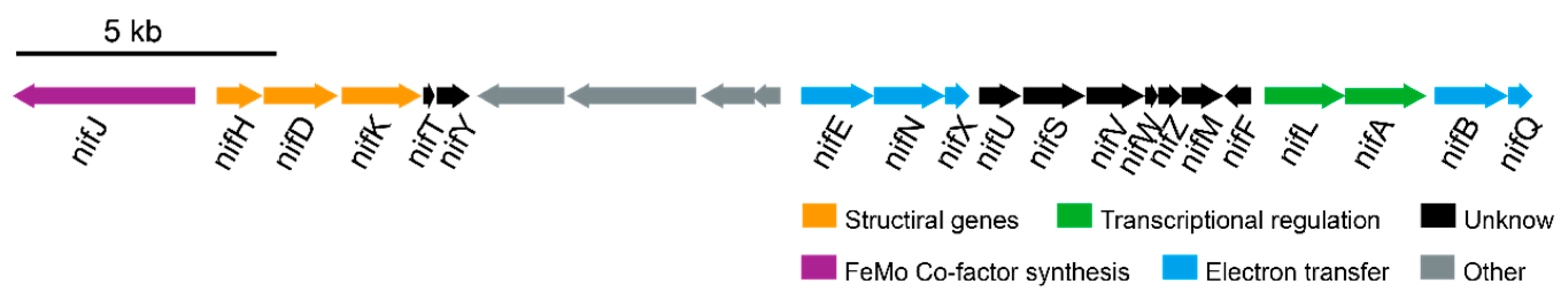

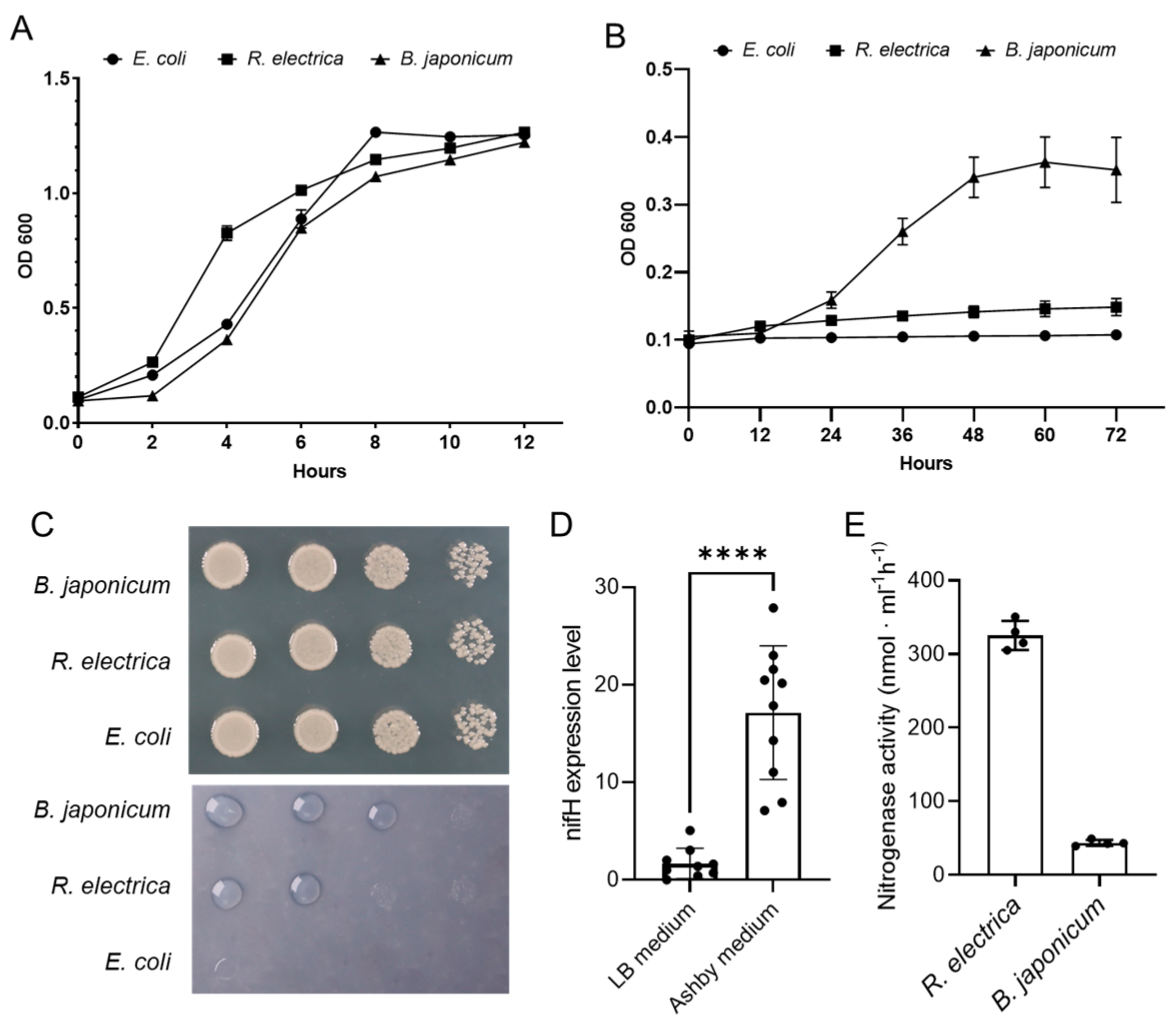
| Features | Chromosome |
|---|---|
| Genome size (bp) | 5,282,709 |
| G + C content (%) | 55.58 |
| Protein-coding genes (CDS) | 4890 |
| CDS average length (bp) | 936 |
| Percent of coding region (%) | 86.68 |
| rRNA (5S, 16S, 23S) | 9, 8, 8 |
| tRNA | 86 |
| sRNA | 121 |
| Genes with function prediction | 4865 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Feng, Y.; Shan, H.-W.; Chen, J.-P.; Wu, W. A Novel Nitrogen-Fixing Bacterium Raoultella electrica Isolated from the Midgut of the Leafhopper Recilia dorsalis. Insects 2023, 14, 431. https://doi.org/10.3390/insects14050431
Huang Q, Feng Y, Shan H-W, Chen J-P, Wu W. A Novel Nitrogen-Fixing Bacterium Raoultella electrica Isolated from the Midgut of the Leafhopper Recilia dorsalis. Insects. 2023; 14(5):431. https://doi.org/10.3390/insects14050431
Chicago/Turabian StyleHuang, Qiuyan, Yilu Feng, Hong-Wei Shan, Jian-Ping Chen, and Wei Wu. 2023. "A Novel Nitrogen-Fixing Bacterium Raoultella electrica Isolated from the Midgut of the Leafhopper Recilia dorsalis" Insects 14, no. 5: 431. https://doi.org/10.3390/insects14050431
APA StyleHuang, Q., Feng, Y., Shan, H.-W., Chen, J.-P., & Wu, W. (2023). A Novel Nitrogen-Fixing Bacterium Raoultella electrica Isolated from the Midgut of the Leafhopper Recilia dorsalis. Insects, 14(5), 431. https://doi.org/10.3390/insects14050431





