Simple Summary
Coccoidea (scale insects) are sap-sucking hemipterous insects with a high diversity of species. They exhibit dramatically variable appearance and sexual dimorphism and are also closely related to human beings as important economic pests and resource insects. However, we know very little about the phylogenetic relationship within Coccoidea. We reconstructed that relationship among five coccoid families (e.g., Aclerdidae, Cerococcidae, Coccidae, Eriococcidae, and Kerriidae) based on mitogenomes. Aclerdidae and Coccidae were recovered as the sister group, successively sister to Cerococcidae, Kerriidae, and Eriococcidae. In addition, there were gene rearrangements occurring in all mitogenomes of coccoid species studied here. The novel gene rearrangement ND6-trnP and trnI-ND2-trnY supported the monophyly of Coccoidea and the sister relationship of Aclerdidae and Coccidae.
Abstract
Coccoidea (scale insects) are important plant parasites with high diversity of species. However, the phylogenetic relationship within Coccoidea has not been fully determined. In this study, we sequenced mitogenomes of six species belonging to five coccoid families. With the addition of three previously published mitogenomes, a total of 12 coccoid species were adopted for the phylogenetic reconstruction based on the maximum likelihood and Bayesian inference. The monophyly of Coccoidea was recovered and Aclerdidae and Coccidae were recovered as the sister group, successively sister to Cerococcidae, Kerriidae, and Eriococcidae. In addition, there were gene rearrangements occurring in all mitogenomes of coccoid species studied here. The novel gene rearrangement ND6-trnP and trnI-ND2-trnY supported the monophyly of Coccoidea and the sister relationship of Aclerdidae and Coccidae. This implies that data from the mitogenome can provide new insight for clarifying the deeper level of phylogenetic relationship within Coccoidea.
1. Introduction
Scale insects (Coccoidea) are small and sap-sucking hemipterans and closely related to Aphidoidea (aphids), Aleyrodoidea (whiteflies), and Psylloidea (jumping plant lice), belonging to the suborder Sternorrhyncha. Currently, there are more than 8000 species described in all zoogeographical fauna, belonging to 36 extant families [1]. Interestingly, they are extremely sexually dimorphic, adult females are paedomorphic and highly reduced while males have a more “typical” insect appearance and display complete metamorphosis [2,3].
The current classification of Coccoidea is mainly based on the morphology of adult females. However, the morphological features of adult females of coccoids have undergone significant reduction, providing very limited phylogenetic information; therefore, little is known about the relationship among coccoid families and the monophyly of some families remains verified, especially Eriococcidae [2]. Subsequently, Cook et al. [4] and Gullan and Cook [5] adopted a nuclear RNA gene (SSU rRNA or 18S) to infer the phylogenetic relationship among the coccoid families. These studies verified the monophyly of various families but Eriococcidae and recovered the sister relationship between Coccidae and Kerriidae without Aclerdidae involved. The morphology of adult males was believed to improve our understanding of evolutionary relationships among coccoid families [6,7,8,9]. Hodgson and Hardy [10] estimated the phylogeny of Coccoidea based on the morphological characters of adult males, supporting the sister relationship between Aclerdidae and Coccidae. Cerococcidae was recovered as a sister group with the clade of Aclerdidae and Coccidae. Vea and Grimaldi [11] reconstructed the relationship at the family level within Coccoidea combining the morphology and gene fragments (e.g., 18S, 28S, and EF-1a), also indicating the closest relationship between Coccidae and Aclerdidae. However, Kerriidae was found to have a closer affinity with Cerococcidae, inconsistent with previous results based on either several molecular markers or morphology. Furthermore, Eriococcidae was consistently found to be paraphyletic by the above studies both based on the molecular and morphological data [4,5,10,11]. Liu et al. also reconstructed the phylogenetic relationship based on the ultraconserved element (UCE) [12]. However, Diaspididae was found to be closer to Eriococcidae, represented by Acanthococcus lagerstroemiae, this was contrary to the morphology of male adults [10]. Accordingly, the relationship within Coccoidea was not fully understood using morphological or limited gene fragments [5], therefore further study is necessary.
The mitogenome is a circular and double-stranded DNA molecule. It has been widely adopted as a powerful molecular marker for phylogenetic and evolutionary analysis in various insect groups, especially high taxonomic levels, because of the relatively high evolutionary rate and rare recombination [13,14,15]. Moreover, there are gene rearrangements frequently occurring in the mitogenomes of insects. This gene structure information is also useful in inferring phylogenetic relationships [16,17,18,19,20]. There have been thirteen coccoid mitogenomes recorded in GenBank [21,22,23,24,25]; however, eight of which were regarded as unverified data (e.g., Ceroplastes japonicus, C. rubens, Drosicha corpulenta, Ericerus pela, Nipponaclerda biwakoensis, Planococcus citri, Saissetia coffeae, and Unaspis yanonensis). Moreover, the putative “P. citri and U. yanonensis” mitogenomes reported by Liu et al. [23] were lately considered not to originate from Coccoidea but from parasitic wasps in the Chalcidoidea [26]. Recently, there were gene rearrangements occurring in six valid coccoid mitogenomes and the phylogenetic results based on the mitogenomic sequence frequently found the monophyly of Coccoidea [24,25], thus indicating that the mitogenomes may have the potential in determining the phylogenetic relationship within Coccoidea. In this study, we sequenced four species from Cerococcidae, Eriococcidae, and Kerriidae, and re-sequenced two from Aclerdidae and Coccidae based on the next-generation sequencing method.
2. Materials and Methods
2.1. Sampling and Genomic DNA Extraction
Six species belonging to six families of Coccoidea were collected in China and Australia and were preserved in 95% ethanol (Tianjin Huihang Chemical Technology Co, Tianjin, China) under −20 °C at the Department of Forestry Protection, Beijing Forestry University (Table 1). The total genomic DNA was extracted from the whole body using QIAamp DNA Micro Kit following the manufacturer’s instructions.

Table 1.
Collection information of Coccoidea in this study.
2.2. Mitogenome Sequencing and Assembly
We sequenced six mitogenomes using a high-throughput sequencing platform with Illumina Hiseq 2500 at Berry Genomics (Beijing, China) with 6× sequencing depth and with 250 bp paired-end sequencing reads. An Illumina TruSeq library with single species was constructed from total genomic DNA with an average insert size of 450 bp. After removing adapters and low-quality reads, high-quality reads were used in de novo assembly with IDBA-UD [27]. Assemblies with IDBA-UD used a similarity threshold of 98% and minimum and maximum k values of 80 and 240 bp, respectively.
Additionally, COI and srRNA, two molecular fragments from mitogenomes, were amplified as bait sequences by standard PCR reactions using primers designed with reference [28], and then were adopted to identify the mitogenome assemblies. The mitogenomic sequences were identified by Geneious 10.1.3 (http://www.geneious.com/, accessed on 15 March 2019) with a BLASTn search [29] against the reference of bait sequences. Only hits with a 100% pairwise identity were regarded as successful identification. The identified mitogenomic sequences were manually checked in Geneious 10.1.3 for identical or near-identical overlapping terminal regions and were circularized where possible.
2.3. Gene Annotation and Bioinformatic Analysis
The annotation of protein-coding genes (PCGs) was implemented with MITOs WebServer [30] and, subsequently, the accurate position was further determined in MEGA v6.06 [31].
The tRNA genes were detected by MITOs WebServer, ARWEN [32], and the manual method with the reference of other hemipteran species (see the Supplementary Materials for detailed annotation information). The boundaries of rRNAs were determined by the upstream and downstream of tRNAs, and by the alignment with the homologous genes of other hemipteran species. The base composition, codon usages of PCGs, and relative synonymous codon usage (RSCU) values were calculated by MEGA v6.06. The number of synonymous substitutions per synonymous site (Ks), the number of nonsynonymous substitutions per nonsynonymous site (Ka), the effective number of codons (ENC), and the codon bias index (CBI) for PCGs were calculated by the DnaSP 5.0 software [33].
2.4. Phylogenetic Analysis
There were 40 species included in the phylogenetic analysis, including 12 coccoid species and 24 species of Sternorrhyncha with other four species of Auchenorrhyncha as outgroups (Table 2). A total of 13 PCGs and two rRNAs were merged into a dataset. Because of unverified data and incomplete numbers of PCG, five coccoid species (e.g., Ceroplastes rubens, Drosicha corpulenta, Ericerus pela, Planococcus citri, and Unaspis yanonensis), were excluded from this study.

Table 2.
Species involved in the phylogenetic analysis.
The heterogeneity of sequence divergence within the dataset was evaluated by AliGROOVE with the default settings. In addition, the indels in the dataset were scored by AliGROOVE as ambiguities.
The workflow of the data matrix and phylogenetic analysis was implemented in PHYLOSUITE v1.2.2 [34]. The phylogenetic relationship was reconstructed with the maximum likelihood (ML) method and Bayesian inference (BI). The sequences of PCGs were aligned in MAFFT [35] with the codon alignment model and G-INS-i (accurate) strategy. The nucleotide sequences of rRNAs were aligned in MAFFT with the normal alignment model and G-INS-i (accurate) strategy. Then, the poorly aligned sites of PCGs and rRNAs were removed by Gblocks [36] with the default parameter settings. The modified sequences of PCGs and rRNAs were eventually concatenated in PHYLOSUITE v1.2.2.
The optimal partitioning schemes for ML analysis were calculated by PartitionFinder 2 [37] with default settings. The best model was automatically determined by IQ-TREE 1.6.8 [38] and maximum likelihood phylogenies were inferred using IQ-TREE under the Edge-linked partition model for 8000 ultrafast [39] bootstraps. In addition, the result from AliGROOVE analysis showed a high degree of compositional heterogeneity (Figure S1), which can mislead the reconstruction of the phylogenetic tree [40]. Therefore, the Bayesian tree was reconstructed using Phylobayes MPI 1.4f [41] with the site-heterogeneous model CAT + GTR and a discrete gamma distribution with four rate categories [42,43]. Two independent chains were run each with 10,000 generations, and a consensus tree was calculated with a burn-in of 7500, taking one every 10 trees, up to the end of each chain.
2.5. Gene Rearrangement Analysis
The gene rearrangements of coccoid mitogenomes were analyzed by the CREx program [44], employing the common interval measurement with that of Drosophila yakuba as the reference.
3. Results
3.1. General Features and Nucleotide Composition
The length of six mitogenomes of Coccoidea ranged from 15,529 bp in Ceroplastes japonicus to 17,405 bp in Albotachaedina sinensis (Figure 1). The mitogenomes of Ap. munita, C. japonicus, and N. biwakoensis contain 37 typical genes. However, only 36 genes were detected in those of the other three species, of which trnV was not in Antecerococcus theydoni and Acanthococcus coriaceus, and trnC was not in Al. sinensis. These coccoid mitogenomes all have high A + T content ranging from 81% in N. biwakoensis to 90% in Al. sinensis, consistent with the strong bias toward A + T in the findings that Aclerda takahashii, D. koreanus, and S. coffeae previously published [22,24,25].
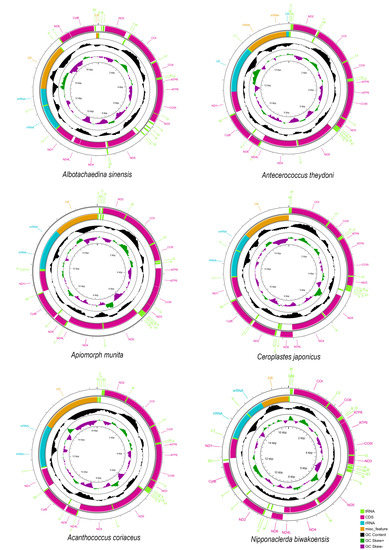
Figure 1.
Circular maps of the mitogenomes of Coccoidea.
3.2. Protein-Coding Genes
All 13 PCGs were detected in mitogenomes of Coccoidea newly sequenced here, the length of which ranged from 10,500 bp in Acanthococcus coriaceus to 10,641 bp in N. biwakoensis. The A + T content in PCGs in six newly sequenced coccoid species was high, ranging from 81% in Aclerdidae represented by N. biwakoensis to 89% in Kerriidae represented by Al. sinensis. In addition, the relative synonymous codon usage (RSCU) of coccoid mitogenomes was examined and shown in Figure 2. The codon ended with A or T was preferred for each amino acid in coccoid mitogenomes newly sequenced here, this is consistent with the strong bias towards A + T content of PCGs in coccoid mitogenomes previously published [22,24]. All PCGs in mitogenomes of Coccoidea studied here initiate with the typical start codon ATN and all terminate with the stop codon TAA or TAG, except for the COII in C. japonicus and ND4 in Acanthococcus coriaceus with a T residue as the stop codon.
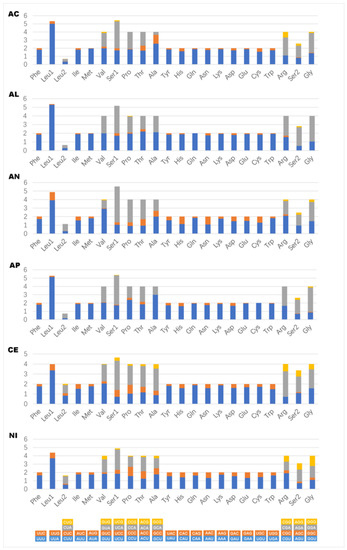
Figure 2.
The relative synonymous codon usage (RSCU) in the mitogenomes of Coccoidea. AC, Acanthococcus coriaceus; AL, Albotachaedina sinensis; AN, Antecerococcus theydoni; AP, Apiomorpha munita; CE, Ceroplastes japonicus; NI, Nipponaclerda biwakoensis.
The correlations among ENC (effective number codon), CBI (codon bias index), GC content of all codons, and GC content of the 3rd codon positions were analyzed to further investigate the codon usage bias (Figure 3a–e). A positive correlation was observed between ENC and GC content of all codons (R2 = 0.7133) (Figure 3a) and GC content of the 3rd codon positions (R2 = 0.7468) (Figure 3b), whereas a negative correlation was observed between CBI and GC content of all codons (R2 = 0.9741) (Figure 3c) and GC content of the 3rd codon positions (R2 = 0.9953) (Figure 3d) and ENC (R2 = 0.7513) (Figure 3e). These results show that the GC content is significant for the codon usage bias in Coccoidea, consistent with the neutral mutational theories [45,46].
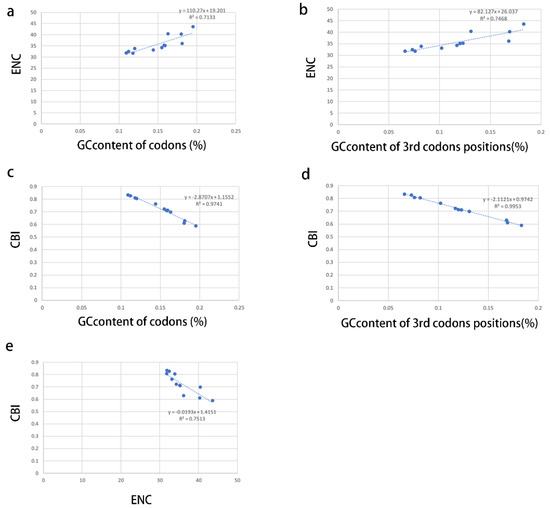
Figure 3.
Evaluation of codon bias across 12 coccoid mitogenomes. ENC, effective number of codons (out of a maximum of 61); CBI, codon bias index; G + C, GC content of codons; (G + C)3, GC content of 3rd codon positions. (a), relevance of GC content of codons to ENC; (b), relevance of GC content of 3rd codons positions to ENC; (c), relevance of GC content of codons to CBI; (d), relevance of GC content of 3rd codons positions to CBI; (e), relevance of ENC to CBI.
The rate of non-synonymous substitutions (Ka), the rate of synonymous substitutions (Ks), and the ratio of Ka/Ks were calculated for PCGs (Figure 4) to diagnose the evolutionary rates of protein-coding genes of coccoid mitogenomes [47,48]. All ratios of Ka/Ks for PCGs of each coccoid species were consistently higher than one (Table S1). The average ratios of Ka/Ks ranged from 2.11 for Cerococcidae to 2.68 for Eriococcidae were consistently higher than one (Figure 4), indicating that all PCGs of coccoid mitogenomes are evolving under positive selection.
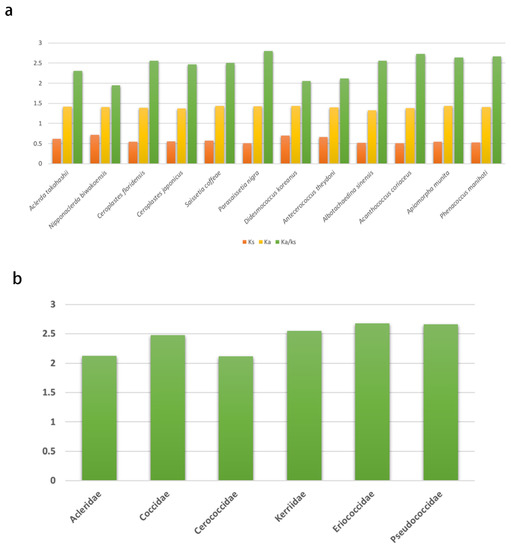
Figure 4.
Evolutionary rates of all protein-coding genes (PCGs) in the mitogenomes of 12 coccoid species with Aphis citricidus as the reference. The Y-axis provides the substitution rate of mitochondrial PCGs. (a) Evolutionary rates of PCGs of each coccoid species; (b) average evolutionary rates of PCGs of coccoid families.
3.3. Transfer and Ribosomal RNA Genes
The length of tRNA genes in coccoid mitogenomes ranged from 43 bp to 74 bp. There are only a few tRNA genes in each coccoid species studied here that can fold into typical cloverleaf secondary structures (e.g., trnA, trnI, and trnL2 in Al. sinensis, trnK, trnL2, and trnW in Antecerococcus theydoni, trnK, trnL1, trnL2, trnT, and trnV in Ap. munita, trnK, trnL1, trnL2, and trnM in C. japonicus, trnF, trnK, and trnM in Acanthococcus coriaceus, trnK, trnI, trnL2, and trnM in N. biwakoensis), while most of the tRNA genes in these six mitogenomes of Aclerdidae, Cerococcidae, Eriococcidae, and Kerriidae were truncated. Similarly, this phenomenon of truncated tRNA genes is also prevalent in mitogenomes of Coccidae reported before [21,22,24].
The rRNA boundaries were determined by alignment with corresponding sequences from Sternorrhyncha previously published. As in other insect mitogenomes, both lrRNA and srRNA in six coccoid species were encoded on the light strand. The length of lrRNA ranged from 1062 bp in Ap. munita to 1243 bp in N. biwakoensis and that of srRNA ranged from 723 bp in Antecerococcus theydoni to 792 bp in N. biwakoensis. All lrRNA of six coccoid species had a high AT content, ranging from 85% in Antecerococcus theydoni to 91% in Ap. munita and Al. sinensis. Likely, all srRNA of six coccoid species had a strong bias to AT content, ranging from 83% in N. biwakoensis to 92% in Ap. munita, Acanthococcus coriaceus, and Al. sinensis.
3.4. Gene Rearrangements Analysis
There were gene rearrangements occurring in six coccoid species sequenced relative to the putative ancestral gene order, including the rearrangement of tRNA genes and protein-coding genes. Based on the result from CREx analysis, the common intervals ranged from 160 in Aclerda takahashii to 872 in Acanthococcus coriaceus (Figure 5). This means that the highest degree of gene rearrangement relative to ancestral gene order was observed in the Aclerda takahashii of the Aclerdidae family, while the lowest degree of that was observed in the Acanthococcus coriaceus of the Eriococcidae family. In addition, C. floridensis, C. japonicus, D. koreanus, and P. nigra shared the same pattern of gene rearrangement.
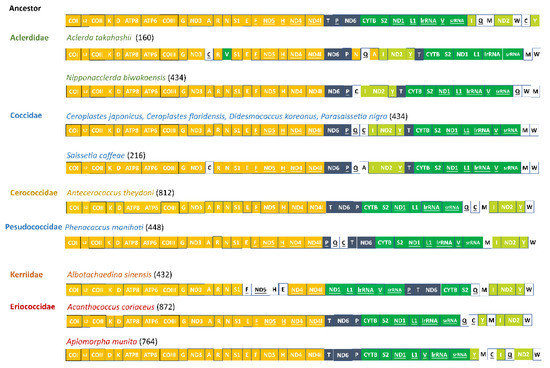
Figure 5.
Gene orders in mitogenomes of Coccoidea and the putative ancestor. The boxes with colors indicate relatively conserved gene orders. The genes encoded on the light strand were indicated with underlines. The numbers in parentheses were the value of common intervals.
Relative to the putative ancestral gene order, there were two relatively conserved gene blocks in mitogenomes of Coccoidea (e.g., COI-trnL2-COII-trnK-trnD-ATP8-ATP6-COIII-trnG-ND3-trnA-trnR-trnN-trnS1-trnE-trnF-ND5-trnH-ND4-ND4l and CYTB-trnS2-ND1-trnL1-lrRNA-trnV-srRNA). Among the rearranged genes, the trnP-ND6 ancestral gene block underwent a transposition event to form ND6-trnP, a novel gene boundary. This novel gene cluster was frequently present in coccoid species but absent in Pseudococcidae, Kerriidae, and three other sternorrhynchan superfamilies, implying that it may be an apomorphy in Coccoidea. In addition, except for in Eriococcidae, ND2 was rearranged between trnI and trnY in Aclerdidae, Coccidae, Cerococcidae, and Kerriidae, forming a novel gene boundary. Additionally, it is notable that ND2 was translocated between the ND6 and CytB in both Aclerdidae and Coccidae but not in the other three families.
3.5. Phylogenetic Analysis
The phylogenetic analyses were performed on the nucleotide sequences of 13 PCGs and two rRNAs from mitogenomes of 40 hemipteran species. The phylogenetic relationship generated by BI and ML methods was found (Figure 6 and Figure 7). Two topologies are identical with the exception of the relationship within Aphidoidea and the position of the Aclerdidae coccoid family in this study. The monophyly of Sternorrhyncha was determined (bootstrap value = 100%, posterior possibility = 1) and, within Sternorrhyncha, the monophyly of Coccoidea was supported and other three sternorrhynchan superfamilies (Aleyrodoidea, Aphidoidea, and Psylloidea) were also found as monophyletic groups. Psylloidea was determined to be a sister to Aleyrodoidea with high statistical support. In addition, Coccoidea was grouped with Aphidoidea. Within Coccoidea, a phylogenetic relationship of (Pseudococcidae + (Eriococcidae + (Kerriidae + (Cerococcidae + (Aclerdidae + Coccidae))))) was found with high statistical support.
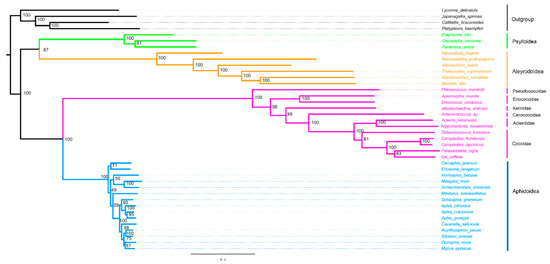
Figure 6.
Phylogenetic trees inferred from mitogenomes of Coccoidea under ML method (right). The values at nodes indicate ML bootstrap values.
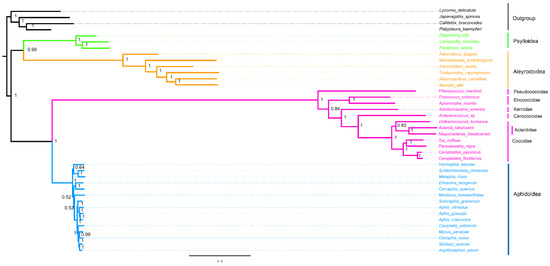
Figure 7.
Phylogenetic trees inferred from mitogenomes of Coccoidea under BI. The values at nodes indicate Bayesian posterior probabilities.
4. Discussion
The relationship within Sternorrhyncha has been controversial. Aleyrodoidea or Psylloidea was found to be at a basal position of Sternorrhyncha based on molecular evidence [43,49]. Aleyrodoidea was grouped with Psylloidea and Coccoidea was sister to Aphidoidea based on morphological characters from both extant and extinct taxa in the latest study [50]. The topology resulting from mitogenomic sequences agreed with the result-based morphological characters.
Within Coccoidea, the Pseudococcidae family has been regarded as a sister to the rest of the neococcoids [5,10,51]. This family was also sister to other coccoid families studied here. The monophyly and systematic position of Eriococcidae has been uncertain. It was believed to be close to Pseudococcidae based on the morphology of adult males [7] and, subsequently, was frequently recovered to be a paraphyly with Dactylopiidae, Beesoniidae, and Stictococcidae based on both morphology and molecular markers [4,5,9]. This family represented by single species Acanthococcus lagerstroemiae, belonging to the acanthococcid group defined by the study of Gullan and Cook [5], was grouped with Diaspididae based on the ultraconserved elements (UCEs), excluding the above three families [12]. Two eriococcid species adopted in phylogenetic analysis (e.g., Acanthococcus coriaceus and Apiomorpha munita) were also members of the acanthococcid group defined by the study of Gullan and Cook [5]. However, the Eriococcidae family represented by the two species above was placed to be sister to other families studied here (e.g., Aclerdidae, Cerococcidae, Coccidae, and Kerriidae). To some extent, this supported the conclusion based on the morphology of adult males [7]. Because the samplings in this study were too limited, the relationship between Eriococcidae with other coccoid families and within this family reflects just a partial portion of the big picture. The relationship between the morphologically peculiar Kerriidae and the other three related families (e.g., Aclerdidae, Cerococcidae, and Coccidae) remains uncertain [52]. It was believed to be closer to Coccidae based on SSU rRNA without Aclerdidae and Cerococcidae included [4,5] or closer to Cerococcidae based on the total evidence [11]. Although this family was found to be sister to the clade of Aclerdidae, Cerococcidae, and Coccidae based on the morphology of adult males, this clade was weakly supported with just 0.58 posterior possibilities [10]. In the present analysis, Kerriidae was recovered to be sister to the clade of Aclerdidae, Cerococcidae, and Coccidae in both ML and Bayesian trees with 99% bootstrapping support and 1 posterior possibility, respectively. In addition, Cerococcidae was supported by the result based on the mitogenomes as the sister group to the clade of Aclerdidae and Coccidae. Coccidae was found to be a sister to Aclerdidae in the ML tree with 100% bootstrapping support (Figure 6), consistent with the previous conclusion based on the morphology of adult females and males and DNA sequences [10,11,53,54]. This novel gene boundary trnI-ND2-trnY supported the phylogenetic result of the grouping of these four families based on the mitogenomic sequences. In addition, the translocation of ND2 also supported the closest relationship of Aclerdidae and Coccidae as a synapomorphy, as indicated by the result based on the mitogenomic sequences above. Although Coccidae was found to be paraphyletic with the inclusion of Aclerdidae in the Bayesian tree, the closer relationship between both families than other families were supported with 1 posterior possibility (Figure 7). Within Coccidae, the topology of ML and the Bayesian tree was largely similar, except for the Aclerdidae nested in Coccidae in the Bayesian tree. The Ceroplastinae represented by C. floridensis and C. japonicus had a closer affinity with Coccinae, represented by P. nigra and S. coffeae in both ML and Bayesian tree, then grouped with Eulecaniinae, represented by D. koreanus, corresponding with the topology (Eulecaniinae + (Coccinae B + Ceroplastinae)) [55].
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/insects14030257/s1, Supplementary File S1: mitogenomic annotations of Acanthococcus coriaceus; Supplementary File S2: mitogenomic annotations of Albotachaedina sinensis; Supplementary File S3: mitogenomic annotations of Antecerococcus theydoni; Supplementary File S4: mitogenomic annotations of Apiomorpha munita; Supplementary File S5: mitogenomic annotations of Ceroplastes japonicus; Supplementary File S6: mitogenomic annotations of Nipponaclerda biwakoensis. Figure S1. Heterogeneous sequence divergence within hemipteran mitochondrial genomes in this study. The obtained mean similarity score between sequences was represented by a colored square. The scores were ranging from −1, indicating full random similarity, to +1, non-random similarity. The darker red indicated the higher randomized accordance between pairwise sequence comparisons. Blue indicated the opposite. All taxa names were listed on top and the right-hand side of the matrix. Table S1. Evolutionary rates of all protein-coding genes (PCGs) in the mitogenomes of nine coccoid species with Aphis citricidus as the reference.
Author Contributions
Conceptualization, H.X. and S.-a.W.; methodology, X.L. and P.W.; software, X.L., P.W., and H.L.; validation, H.X.; data curation, H.X. and X.L.; writing—original draft preparation, H.X.; writing—review and editing, S.-a.W.; visualization, H.X.; supervision, S.-a.W.; project administration, S.-a.W.; funding acquisition, H.X., P.W., and S.-a.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundamental Research Funds for the Central Universities (Grants 2021ZY10), the Beijing Natural Science Foundation (Grants 5224042), the National Natural Science Foundation of China (Grants 32270476), and the Guiding project of science and technology plan of Fujian (Grants 2021N0023).
Data Availability Statement
The data that support the findings of this study are openly available in the National Center for Biotechnology Information at https://www.ncbi.nlm.nih.gov/nuccore (accessed on 1 December 2021), reference numbers OP351521–OP351526.
Acknowledgments
We thank the editor and anonymous reviewers for valuable comments and improvement of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- ScaleNet: A Literature-Based Model of Scale Insect Biology and Systematics|Database|Oxford Academic. Available online: https://academic.oup.com/database/article/doi/10.1093/database/bav118/2630093 (accessed on 26 November 2022).
- Gullan, P.J.; Kosztarab, M. Adaptations in Scale Insects. Annu. Rev. Entomol. 1997, 42, 23–50. [Google Scholar] [CrossRef]
- Gullan, P.J.; Martin, J.H. Sternorrhyncha (jumping plant-lice, whiteflies, aphids, and scale Insects). In Encyclopedia of Insects; Academic Press: Cambridge, MA, USA, 2003; pp. 1079–1089. [Google Scholar]
- Cook, L.G.; Gullan, P.J.; Trueman, H.E. A Preliminary Phylogeny of the Scale Insects (Hemiptera: Sternorrhyncha: Coccoidea) Based on Nuclear Small-Subunit Ribosomal DNA. Mol. Phylogenet. Evol. 2002, 25, 43–52. [Google Scholar] [CrossRef]
- Gullan, P.J.; Cook, L.G. Phylogeny and Higher Classification of the Scale Insects (Hemiptera: Sternorrhyncha: Coccoidea). Zootaxa 2007, 1668, 413–425. [Google Scholar] [CrossRef]
- Boratyński, K.; Davies, R.G. Taxonomic Value of Male Coccoidea (Homoptera) with an Evaluation of Some Numerical Techniques. Biol. J. Linn. Soc. 1971, 3, 57–102. [Google Scholar] [CrossRef]
- Hodgson, C.J. Preliminary Phylogeny of Some Non-Margarodid Coccoidea (Hemiptera) Based on Adult Male Characters. Boll Zool Agrar. Bachic. 2002, 33, 129–137. [Google Scholar]
- Hodgson, C.J.; Foldi, I. Preliminary Phylogenetic Analysis of the Margarodidae Sensu Morrison and Related Taxa (Hemiptera: Coccoidea) Based on Adult Male Morphology. In Proceedings of the X International Symposium of Scale Insect Studies, Adana, Turkey, 19–23 April 2004; pp. 35–47. [Google Scholar]
- Hodgson, C.; Foldi, I. A Review of the Margarodidae Sensu Morrison (Hemiptera: Coccoidea) and Some Related Taxa Based on the Morphology of Adult Males. Zootaxa 2006, 1263, 1–250. [Google Scholar] [CrossRef]
- Hodgson, C.J.; Hardy, N.B. The Phylogeny of the Superfamily Coccoidea (Hemiptera: Sternorrhyncha) Based on the Morphology of Extant and Extinct Macropterous Males: Phylogeny of Coccoidea via Macropterous Males. Syst. Entomol. 2013, 38, 794–804. [Google Scholar] [CrossRef]
- Vea, I.M.; Grimaldi, D.A. Putting Scales into Evolutionary Time: The Divergence of Major Scale Insect Lineages (Hemiptera) Predates the Radiation of Modern Angiosperm Hosts. Sci. Rep. 2016, 6, 23487. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Niu, M.; Lu, Y.; Wei, J.; Zhang, H. Taxon-Specific Ultraconserved Element Probe Design for Phylogenetic Analyses of Scale Insects (Hemiptera: Sternorrhyncha: Coccoidea). Front. Ecol. Evol. 2022, 10, 984396. [Google Scholar] [CrossRef]
- Cameron, S.L. Insect Mitochondrial Genomics: Implications for Evolution and Phylogeny. Annu. Rev. Entomol. 2014, 59, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Li, H.; Jiang, P.; Zhou, X.; Liu, J.; Sun, C.; Vogler, A.P.; Cai, W. Capturing the Phylogeny of Holometabola with Mitochondrial Genome Data and Bayesian Site-Heterogeneous Mixture Models. Genome Biol. Evol. 2016, 8, 1411–1426. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Garzón-Orduña, I.J.; Winterton, S.L.; Yan, Y.; Aspöck, U.; Aspöck, H.; Yang, D. Mitochondrial Phylogenomics Illuminates the Evolutionary History of Neuropterida. Cladistics 2017, 33, 617–636. [Google Scholar] [CrossRef] [PubMed]
- Kômoto, N.; Yukuhiro, K.; Tomita, S. Novel Gene Rearrangements in the Mitochondrial Genome of a Webspinner, Aposthonia Japonica (Insecta: Embioptera). Genome 2012, 55, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, H.; Winterton, S.L.; Liu, Z. Ancestral Gene Organization in the Mitochondrial Genome of Thyridosmylus Langii (McLachlan, 1870) (Neuroptera: Osmylidae) and Implications for Lacewing Evolution. PLoS ONE 2013, 8, e62943. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.; Babbucci, M.; Pauletto, M.; Riginella, E.; Patarnello, T.; Negrisolo, E. The Highly Rearranged Mitochondrial Genomes of the Crabs Maja Crispata and Maja Squinado (Majidae) and Gene Order Evolution in Brachyura. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.; Gan, H.M.; Lee, Y.P.; Linton, S.; Grandjean, F.; Bartholomei-Santos, M.L.; Miller, A.D.; Austin, C.M. ORDER within the Chaos: Insights into Phylogenetic Relationships within the Anomura (Crustacea: Decapoda) from Mitochondrial Sequences and Gene Order Rearrangements. Mol. Phylogenet. Evol. 2018, 127, 320–331. [Google Scholar] [CrossRef]
- Song, F.; Li, H.; Liu, G.-H.; Wang, W.; James, P.; Colwell, D.D.; Tran, A.; Gong, S.; Cai, W.; Shao, R. Mitochondrial Genome Fragmentation Unites the Parasitic Lice of Eutherian Mammals. Syst. Biol. 2018, 68, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Lu, C.; Huang, X. The First Mitochondrial Genome of Scale Insects (Hemiptera: Coccoidea). Mitochondrial DNA Part B 2019, 4, 2094–2095. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Huang, X.; Deng, J. The Challenge of Coccidae (Hemiptera: Coccoidea) Mitochondrial Genomes: The Case of Saissetia Coffeae with Novel Truncated TRNAs and Gene Rearrangements. Int. J. Biol. Macromol. 2020, 158, 854–864. [Google Scholar] [CrossRef]
- Liu, H.-L.; Chen, Q.-D.; Chen, S.; Pu, D.-Q.; Chen, Z.-T.; Liu, Y.-Y.; Liu, X. The Highly Rearranged Mitochondrial Genomes of Three Economically Important Scale Insects and the Mitochondrial Phylogeny of Coccoidea (Hemiptera: Sternorrhyncha). PeerJ 2020, 8, e9932. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, X.; Li, H.; Wu, S. The Mitogenome of the Scale Insect Didesmococcus Koreanus Borchsenius, 1955 (Coccoidea: Coccidae). Mitochondrial DNA Part B 2021, 6, 1298–1299. [Google Scholar] [CrossRef]
- Deng, J.; Wang, G.; Lu, C.; Zhang, J.T.; Huang, X. Sequencing and Analysis of the Complete Mitochondrial Genome of Aclerda Takahashii (Hemiptera: Aclerdidae). Acta Entomol. Sin. 2022, 65, 451–459. [Google Scholar]
- Xu, H.; Wu, S. Parasitized Wasp Mitogenomes Mistaken for Scale Insect Host Mitogenome Sequences. Entomotaxonomia 2022, 44, 24–29. [Google Scholar]
- Peng, Y.; Leung, H.C.M.; Yiu, S.M.; Chin, F.Y.L. IDBA-UD: A de novo Assembler for Single-Cell and Metagenomic Sequencing Data with Highly Uneven Depth. Bioinformatics 2012, 28, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Buckley, T.R.; Frati, F.; Stewart, J.B.; Beckenbach, A.T. Incorporating Molecular Evolution into Phylogenetic Analysis, and a New Compilation of Conserved Polymerase Chain Reaction Primers for Animal Mitochondrial DNA. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 545–579. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C. Geneious Basic: An Integrated and Extendable Desktop Software Platform for the Organization and Analysis of Sequence Data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Donath, A.; Jühling, F.; Al-Arab, M.; Bernhart, S.H.; Reinhardt, F.; Stadler, P.F.; Middendorf, M.; Bernt, M. Improved Annotation of Protein-Coding Genes Boundaries in Metazoan Mitochondrial Genomes. Nucleic Acids Res. 2019, 47, 10543–10552. [Google Scholar] [CrossRef]
- Laslett, D.; Canbäck, B. ARWEN: A Program to Detect TRNA Genes in Metazoan Mitochondrial Nucleotide Sequences. Bioinformatics 2008, 24, 172–175. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A Software for Comprehensive Analysis of DNA Polymorphism Data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An Integrated and Scalable Desktop Platform for Streamlined Molecular Sequence Data Management and Evolutionary Phylogenetics Studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Talavera, G.; Castresana, J. Improvement of Phylogenies after Removing Divergent and Ambiguously Aligned Blocks from Protein Sequence Alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New Methods for Selecting Partitioned Models of Evolution for Molecular and Morphological Phylogenetic Analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; von Haeseler, A. Ultrafast Approximation for Phylogenetic Bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Kück, P.; Meid, S.A.; Groß, C.; Wägele, J.W.; Misof, B. AliGROOVE—Visualization of Heterogeneous Sequence Divergence within Multiple Sequence Alignments and Detection of Inflated Branch Support. BMC Bioinformatics 2014, 15, 294. [Google Scholar] [CrossRef] [PubMed]
- Lartillot, N.; Rodrigue, N.; Stubbs, D.; Richer, J. PhyloBayes MPI: Phylogenetic Reconstruction with Infinite Mixtures of Profiles in a Parallel Environment. Syst. Biol. 2013, 62, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shao, R.; Song, N.; Song, F.; Jiang, P.; Li, Z.; Cai, W. Higher-Level Phylogeny of Paraneopteran Insects Inferred from Mitochondrial Genome Sequences. Sci. Rep. 2015, 5, 8527. [Google Scholar] [CrossRef]
- Li, H.; Leavengood, J.M.; Chapman, E.G.; Burkhardt, D.; Song, F.; Jiang, P.; Liu, J.; Zhou, X.; Cai, W. Mitochondrial Phylogenomics of Hemiptera Reveals Adaptive Innovations Driving the Diversification of True Bugs. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171223. [Google Scholar] [CrossRef] [PubMed]
- Perseke, M.; Fritzsch, G.; Ramsch, K.; Bernt, M.; Merkle, D.; Middendorf, M.; Bernhard, D.; Stadler, P.F.; Schlegel, M. Evolution of Mitochondrial Gene Orders in Echinoderms. Mol. Phylogenet. Evol. 2008, 47, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Hershberg, R.; Petrov, D.A. Selection on Codon Bias. Annu. Rev. Genet. 2008, 42, 287–299. [Google Scholar] [CrossRef]
- Plotkin, J.B.; Kudla, G. Synonymous but Not the Same: The Causes and Consequences of Codon Bias. Nat. Rev. Genet. 2011, 12, 32–42. [Google Scholar] [CrossRef]
- Nei, M.; Gojobori, T. Simple Methods for Estimating the Numbers of Synonymous and Nonsynonymous Nucleotide Substitutions. Mol. Biol. Evol. 1986, 3, 418–426. [Google Scholar]
- Hurst, L.D. The Ka/Ks Ratio: Diagnosing the Form of Sequence Evolution. Trends Genet. 2002, 18, 486–487. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.P.; Dietrich, C.H.; Friedrich, F.; Beutel, R.G.; Wipfler, B.; Peters, R.S.; Allen, J.M.; Petersen, M.; Donath, A.; Walden, K.K.O.; et al. Phylogenomics and the Evolution of Hemipteroid Insects. Proc. Natl. Acad. Sci. USA 2018, 115, 12775–12780. [Google Scholar] [CrossRef]
- Drohojowska, J.; Szwedo, J.; Żyła, D.; Huang, D.-Y.; Müller, P. Fossils Reshape the Sternorrhyncha Evolutionary Tree (Insecta, Hemiptera). Sci. Rep. 2020, 10, 11390. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R. Phylogeny and Classification of the Margarodidae and Related Groups (Homoptera: Coccoidea). In Proceedings of the 10th International Symposium of Central European Entomofaunistics, Budapest, Hungary, 15–20 August 1984; pp. 321–324. [Google Scholar]
- Hodgson, C.J. 1.1.3.4. Classification of the coccidae and related coccoid families. In World Crop Pests; Elsevier: Amsterdam, The Netherlands, 1997; Volume 7, pp. 157–201. ISBN 978-0-444-89303-1. [Google Scholar]
- Fernald, M.E. A Catalogue of the Coccidae of the World. Bull. Hatch Agric. Exp. Stn. Mass. Agric. Coll. 1903, 88, 1–360. [Google Scholar]
- Balachowsky, A.S. Essai Sur La Classification Des Cochenilles: Homoptera Coccoidea. Ann. Grignon Ecole Natl. Agric. Ser. 3 1943, 3, 34–48. [Google Scholar]
- Choi, J.; Lee, S. Molecular Phylogeny of the Family Coccidae (Hemiptera, Coccomorpha), with a Discussion of Their Waxy Ovisacs. Syst. Entomol. 2020, 45, 396–414. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).