Simple Summary
Insect pollinators such as bees contribute to crop production, food security, ecosystem stability and biodiversity in agroecosystems. However, intensification of agricultural practices jeopardizes pollination services in agricultural landscapes mainly through the decline in flower resources for pollinators in farmlands. The study examines a scheme to provide floral resources for insect pollinators in apple orchards potentially contributing to their conservation and enhancing crop pollination. For this reason, flowering mixtures including legume landraces were sown in patches inside apple orchards and compared to the orchard’s weed flora in respect of attraction of pollinators. Pollinators recorded on the sown and wild plant patches were honey bees, wild bees, syrphids and beeflies. The most abundant pollinator of apple was the honey bee but wild bees were also recorded. The sown mixture attracted greater numbers of pollinators and more wild bee taxa compared to the wild plants, but it did not have an effect on pollinators visiting apple flowers. Flowering patches with mixtures of suitable plants in groundcover can enhance pollinator conservation in apple orchards.
Abstract
Apples depend on insect pollination but intensification of agriculture jeopardizes pollination services in agroecosystems. Concerns about the dependency of crop pollination exclusively on honey bees increase the interest in agricultural practices that safeguard wild pollinators in agroecosystems. The purpose of the study was to assess the potential of floral resource provision in apple orchards to enhance the conservation of hymenopterous pollinating insects and potentially the pollination service to the crop. For this reason, flowering plant mixtures sown in patches inside apple orchards were tested against wild plant patches. Pollinator taxa recorded on the sown and wild plant patches were honey bees, wild bees (Andrena, Anthophora, Eucera, Halictus, Lasioglossum, Megachilidae on both; Systropha only on wild plants; Bombus, Hylaeus, Sphecodes, Nomada, Xylocopa only on sown mixture), syrphids, bee flies. The most abundant pollinator of apple was A. mellifera but wild bees were also recorded (Andrena, Anthophora, Bombus, Xylocopa, Lasioglossum, Megachilidae). The sown mixture attracted a more diverse taxa of pollinators and in greater numbers compared to the weed flora, but it did not have an effect on pollinators visiting apple flowers. Groundcover management with patches of suitable flowering mixtures can enhance pollinator conservation in apple orchards.
1. Introduction
Insects play a major role in several fundamental ecological processes of ecosystem functioning and particularly of ecosystem services, including pollination [1,2,3]. Approximately 75% of global food crops and nearly 90% of wild flowering plants are to some degree dependent on animal pollination [4,5], with insects being the most important pollinators in both natural and agricultural settings [6,7]. Pollination of crops is one of the main ecosystem services that affects agriculture [8,9], thus insect pollinators contribute to crop production, food security as well as ecosystem stability and biodiversity in agroecosystems [10,11].
Intensification of agricultural practices jeopardizes pollination services in agricultural landscapes through side effects such as fragmentation or even complete elimination of natural or semi-natural habitats [6,12,13]. Floral resources (pollen, nectar) are vital for pollinators [14,15,16,17], thus the deterioration of flower-rich habitats in farmlands, including hedgerows, species-rich grasslands and legume-rich leys, has been strongly linked with decline in pollinator populations in agroecosystems [18,19,20,21,22,23]. This decline in wild and managed pollinator populations in several regions of the European Union [24] and worldwide [13,25] are detrimental for crop production and biodiversity. Nevertheless, the available literature shows a lack of long-term data on diversity and abundance of bees and crop pollinators, indicating the importance of monitoring them over time [26,27,28,29,30] to enable assessment of declines in pollinating insects both in natural and intensively managed environments and to develop strategies for their support where needed.
In agricultural practice, hives of the domesticated European honey bee (Apis mellifera L.) have been utilized during the flowering period of many crops (e.g., almond, apple, cherry, pear, blueberry, melon, mustard) to ensure adequate pollination [31,32,33,34]. However, there are concerns about the dependency of global crop pollination on a single pollinator species [35] as well as on the hive rental costs [36,37]. Moreover, studies have shown that the contribution of wild pollinators (bees other than honey bee) to crop yields may be equal to, or even surpass, that of honey bees [38,39,40,41], hence the growing interest in the development of agricultural practices that safeguard wild pollinators in agroecosystems to ensure the delivery of pollination services [40,42,43,44].
For all the aforementioned reasons, the importance of pollinator conservation in agricultural areas has been acknowledged by the Food and Agriculture Organization and the Institute of European Environmental Policy, which enact policies that comply with the enhancement of ecosystem services, including the International Pollinator Initiative plan of action 2018–2030 [45,46] and the EU Common Agricultural Policy. In fact, approximately 40% of the EU land consists of agricultural production land [47], thus agriculture can contribute significantly to pollinator conservation in the EU. Monitoring of pollinator taxa [48] and conservation measures are being implemented to preserve pollinators in agroecosystems and enhance pollination services [49,50,51,52].
Agri-environmental interventions for insect pollinator conservation typically focus on re-establishing floral resources [53], e.g., restoring species-rich grasslands, sowing field margins with nectar and pollen-rich mixes, and inclusion of flowering species such as legumes in rotations [54,55]. The use of sown flower strips in crops to provide resources for pollinators (mainly Hymenoptera: Apoidea and Diptera: Syrphidae) has been examined by several researchers [8,56,57,58,59,60,61,62,63,64].
Apples, Malus domestica Borkh. (Rosaceae), are among the insect pollinated crops as most of their varieties are self-incompatible, and hence they are greatly dependent on insect pollination for high crop yields, fruit set, high fruit quality and economic value [65,66,67,68,69,70]. For this reason, apple growers usually hire hives of A. mellifera or Melipona spp., add bumblebee (Bombus terrestris L.) hives or utilize bee species such as Osmia spp. during apple blossom to ensure adequate crop pollination [71,72,73,74]. In addition, studies have shown that wild pollinators, e.g., Andrena spp., Bombus spp., Lasioglossum spp. and Syrphidae, are present in apple orchards [75,76,77,78,79]. However, the need for further research on insect pollinators in apple orchards is emphasized, particularly on the effect of local management practices on apple pollination [80]. Apples are one of the most important fruit crops both globally [81] and in Greece, where they are covering a cultivated area of 9.440 ha with a respective production of 285.9 thousand tons [82].
‘Delicious Pilafa Tripoleos’, hereafter ‘Pilafa’, is a Protected Designation of Origin (PDO) apple landrace, which is produced in all communities of the Prefecture of Arcadia with an altitude higher than 600 m and especially in the region of Tegea, Peloponnese, Greece [83,84]. ‘Pilafa’ apples vary in fruit shape and have a relatively short life of storage [85,86], but they have excellent organoleptic characteristics [87]. Nevertheless, their fruit setting can be limited due to the very short period of flowering and the weather that is usually unstable in Tegea plateau. For this reason, efforts to enhance the attraction of insect pollinators in these orchards can offer a valuable service for the crop sustainability and support the on-farm conservation and valorization of ‘Pilafa’ apples [88,89].
Floral resources for pollinators in perennial crops such as apples are preferably provided in mixtures of plant species of several families such as Apiaceae, Fabaceae, Brassicaceae, etc. [60,62,90]. In this kind of mixtures, the inclusion of traditional varieties would offer a dual benefit since they are part of each country’s cultural heritage, which should be well conserved [91], and their exploitation in agricultural practices would offer an extra incentive to the producer to preserve them on farm. In the present study, two Fabaceae landraces (a landrace of Vicia faba L. of Episkopi village in Tegea plateau and a landrace of Lathyrus sativus L. of Feneos village in the Peloponnese, Greece) from the region of Tegea were included in a flowering mixture for groundcover in apple orchards. In fact, Fabaceae plants are considered great food resources for the conservation of wild pollinators, such as Bombus spp., because of their high-quality pollen and the association of their flower with the bee’s glossa anatomy (e.g., length) [18,92,93,94,95]. Fabaceae pollen is particularly protein-rich, and for this reason nutritious for bee reproduction and larval development [94,96].
The present study tests the hypothesis that sown flowering patches in the groundcover of apple orchards could enhance pollinating insects, mainly pollinating bees, in apple orchards by providing extra flowering resources compared to wild plant flora, also to the benefit of the crop. The aim of the study further extends to contributing to the conservation of insect pollinators in agroecosystems as well as an apple landrace (‘Pilafa’) and two legume landraces (extra flower resources in the groundcover).
2. Materials and Methods
2.1. Experimental Orchards and Design
The study was carried out in three apple orchards, landrace ‘Pilafa’, located in Tegea plateau, Arcadia, Peloponnese, Greece, during three consecutive growing seasons (2019, 2020, 2021). Two orchards were organic, one palmette (OP) and one goblet (OG) training systems (0.25 ha each) and one IPM (palmette, 0.45 ha) (37.43705, 22.46942; 37.43658, 22.46895; 37.4415, 22.40492). The IPM orchard was located 6 km from the organic orchards while the distance between OG and OP was 100 m (Figure S1). Thus, the study included both temporal and spatial replications, including orchards with different surrounding semi-natural habitats, training system and pest management. Trees in the apple IPM and OG orchards were 14 years old, whereas in the OP orchard, the trees were 5 years old. During the first, baseline year (2019), no intervention was made and the effect of the wild plants/weed flora present in the orchard groundcover on attraction of pollinating insects was determined. The terms wild plants/weed flora will be used interchangeably hereafter, referring to the plant species occurring in the natural, i.e., not sown vegetation. During the next growing seasons (2020, 2021), a mixture of selected annual flowering plant species was established by sowing, to compare with the flowering wild plant species. In each orchard, four strips of the selected flowering mixture and four strips of wild plants were defined (each strip 24 m × 0.80 m) between the tree rows, where plots (patches) for measurements were selected at random from within these strips. The sites of the sown and wild plant strips were located 70–170 m apart (Figure S1).
The experimental design was completely randomized with two treatments (intervention with sown flower mixture and control with wild plants) with six replications (plots of 6.4 m2 (8 m × 0.8 m) selected on the strips) per treatment. The plot width (0.80 m) was determined by the wheel axis length of the tractor and other equipment used by the farmers for soil preparation and plant protection applications, which should be able to move freely without compressing the plot, as described in [97].
2.2. Establishment of Flower Patches
Nine plant species were selected for the flower mixtures, including 7 broadleaves from 3 families (Apiaceae, Brassicaceae, Fabaceae) and 2 grasses (Poaceae) with an expected combined flowering period extending from March until June (Table 1). The plant species selection was based on criteria such as flower traits that are correlated with pollinator visitation [98], presence of their cultivation in the examined region, exclusion of species that might act as noxious weeds. The grasses, which were wheat landraces, were used (one each year) to investigate their role in facilitating the establishment of ground-dwelling predatory arthropods, which will not be analyzed in the present study, but also in the cohesion of the mixture of flowering plants. There is currently no seed house in Greece that would provide seeds of indigenous plant species in large quantities. Therefore, commercial varieties and landraces of Tegea region (V. faba ‘Episkopi’, L. sativus ‘Fava Feneou’ PGI) were utilized.

Table 1.
Selected plant species used in sown seed mixtures in patches at the groundcover of apple orchards and seed percentage of each species in the two experimental years.
A mixture was preferred over single plant species to ensure longer periods of flowering and to provide greater diversity of suitable flowers for both honey bees and wild bees, thus avoiding behavioral competition in foraging between them over a single species [99]. The composition of the mixture was optimized in the third year (2021) with the appropriate replacement or exclusion of certain plant species based on their establishment and general performance (flowering period and duration, attractiveness to pollinators). General criteria in the mixtures’ composition included relative species growth and flowering characteristics, but also the cost and availability of the seed material. The seed weight for each species in the mixture (Ws) was calculated according to the equation by [63]:
Ws = Ps × (1/ESR) × (1/Pg) × Tp × A × TGW/1000, where Ps = Target percentage of plants per species (see Table 1); ESR = Estimated survival rate of germinated seeds (ranging between 50 and 70% depending on the plant species); Pg = Seed germination percentage (petri dish assays); Tp = Total target number of plants/m2; A = Area to be sown (m2); TGW = Thousand grain weight (g).
A rotary tiller was used for soil preparation prior to sowing of the mixtures in the flowering plots while the control plots were not tilled. Broadcast sowing of the mixtures was performed by hand in autumn (November 2020, 2021). Seed volume was augmented with a bulking material (river sand) to facilitate a uniform sowing. Seeds were covered with shallow raking and the soil surface was rolled to ensure good seed/soil contact. No irrigation was required since the soil moisture was adequate for seed germination and seedling establishment. It should be mentioned that the growers followed the usual agricultural practice regarding weed management in the control plots. There was no application of herbicides in the sown or wild plant patches in any of the orchards; instead, farmers managed the weeds with one to two rotary tillers, from March to June, without affecting the sown mixture patches.
2.3. Landscape Elements around the Experimental Orchards
Landscape elements, such as semi-natural habitats, around the experimental orchards were recorded as such areas are a key factor for bee conservation [100,101,102]. Therefore, habitats (including semi-natural) in the proximity (at the border line) of the experimental apple orchards were recorded and are presented below. The annual plant species mentioned were in bloom after apple flowering (mid-May).
Neighboring habitats to the organic palmette apple (OP) orchard. These habitats included: (a) uncultivated area with Cardaria draba (L.) Desv. (Brassicaceae), Vicia sativa L. (Fabaceae), Galium aparine L. (Rubiaceae), Euphorbia sp. (Euphorbiaceae), Scabiosa sp. (Caprifoliaceae), Medicago polymorpha L. (Fabaceae), Cirsium sp. (Asteraceae), Brassicaceae (e.g., Sinapis sp., Raphanus sp.), Daucus carota L. (Apiaceae), Ranunculus sp. (Ranunculaceae), Echium sp. (Boraginaceae); (b) uncultivated area mainly with Trifolium repens L. (Fabaceae) and Avena sterilis L. (Poaceae), and small numbers of Lamium amplexicaule L. (Lamiaceae), C. draba, Euphorbia sp., Ranunculus sp., Brasicaceae and V. sativa; (c) a cultivated cherry orchard; (d) an abandoned old cherry orchard; (e) natural hedges of wild Prunus avium L. (Rosaceae) and Rubus sp. (Rosaceae); (f) hedge-field margin of Rubus sp., Poaceae (e.g., A. sterilis), L. amplexicaule, Vicia villosa Roth. (Fabaceae), and Scabiosa sp.
Neighboring habitats of the organic goblet apple (OG) orchard. These semi-natural habitats included: (a) a cherry orchard and groundcover mainly with T. repens and M. polymorpha; (b) forest trees of hawthorns, walnuts wild sour cherries, brambles, wild roses and annual plants of V. villosa, Scabiosa sp., T. repens, Anthemis sp. (Asteraceae), Vinca sp. (Apocynaceae), Papaver sp. (Papaveraceae), D. carota, A. sterilis, Cirsium sp., M. polymorpha; (c) an abandoned old cherry orchard.
Neighboring habitats of the IPM apple orchard. These habitats included: (a) an apple orchard and groundcover mainly with Matricaria chamomilla L. (Asteraceae); (b) a cherry orchard; (c) a cultivated area with cabbage, cauliflower and other vegetables such as peppers, eggplants, rocket, potatoes and plants such as M. chamomilla, Capsella bursa-pastoris L. (Brassicaceae), Veronica persica Poir. (Plantaginaceae), Ranunculus sp.
2.4. Flowering and Insect Presence Measurements
Measurements included recording of plant and flower cover and attracted insect pollinators. The measurements were performed during the flowering period of the sown plants (from April to June), the wild plants in the control plots (from April to June), and the apple trees (mid to late April). The methodology was as described before [62,63,64] adapted for the experimental design of the current study. More specifically, the plant cover (total) and flower cover (total and per plant species) were visually estimated and expressed as percentage cover of the plot area (6.4 m2), in all examined plots (sown and control (wild plants)). Plant species were identified in situ or, when necessary, in the laboratory using the botanical identification key Flora Europaea [103]. Visits of insect-pollinators (with a focus on bees, wild bees, syrphid flies and bee flies) at the flowers of the sown mixture, the wild plants as well as the apple blossom in trees adjacent to sown and control plots were recorded by visual observation, for 1 min per plot (6.4 m2 or 3 trees) (hereafter visitation will refer to mean number of visits/6.4 m2/min). The measurements of pollinator visitation on apple blossoms were taken on flowers of three consecutive trees of a row located between sown or wild plant patches. In the palmette orchards, the one-minute visual observation on apple flowers was conducted on the vertical leaf wall area of the trees. In the goblet orchard, each tree was perceived as a cube and the measurements were taken on the surface area that was in the same cardinal point as the examined groundcover plot. All counts were conducted between 10:00 and 16:00 h at temperatures in the range of 11 to 33 °C and no more than half the sky was covered with clouds, when possible, e.g., in year 2019 the weather was cloudy (more than half the sky covered with clouds) during the period (10 days) of apple flowering. Wind velocity was also recorded to ensure that it did not exceed 4.35 m/s. The pollinators that could not be identified in genus level in situ were collected by sweeping net sampling, stored in the freezer (−18 °C) and identified by the first author in generic level (or species level when possible) under a stereo-microscope (OLYMPUS SZ61, U.K) using taxonomic keys [16,104].
During the baseline year (spring 2019), plant cover and flower cover of wild plants as well as pollinator visits on flowers of wild plants and apple trees were recorded. These measurements were conducted at one site with six plots in each organic orchard, and at two sites (IPMa, IPMb—six plots each) in the IPM orchard due to its larger total area and variability in plant species across the groundcover area. The baseline recordings were deemed necessary in order to assess the effect of the intervention in the following years.
2.5. Statistical Analysis
The effect of groundcover (sown flowering mixture/wild plants), recording date and their interaction on the visitation of honey bees, wild bees in the flower patches and apple blossom were determined using 2-way ANOVA (α = 0.05) for each orchard (IPM, OP, OG) and for each experimental year separately. The visitation of Syrphidae and Bombyliidae was too low to allow a statistical analysis. When the effect of both factors and their interaction was significant or either factor and the interaction was significant, the means for the respective factors were separated using Tukey’s HSD test (α = 0.05). The statistical analyses were performed using the statistical package JMP [105].
3. Results
3.1. Plant Mixture Establishment and Flowering
The flowering wild plant species recorded in each apple orchard during the baseline year (2019) were mainly Veronica spp., C. bursa-pastoris, Calepina irregularis (Asso) Thell. (Brassicaceae), Stellaria apetala Ucria (Caryophyllaceae) and Ranunculus repens L. (Ranunculaceae) in early May, C. draba in mid-May, and Convolvulus arvensis L. (Convolvulaceae) in late June (Figure 1). Plant cover in the baseline year ranged between 60 and 100%. In year 2020, in the patches of sown mixtures, the broadleaved species that germinated and reached flowering were V. sativa, V. faba, Eruca sativa Mill. (Brassicaceae). Anethum graveolens L. (Apiaceae) germinated in small numbers but did not reach flowering and L. sativus reached flowering in small numbers, while Coriandrum sativum L. (Apiaceae) reached flowering only in the IPM orchard. Lathyrus sativus, which is cultivated as an annual spring crop in the region of Tegea plateau but was selected to participate in the sown mixture as a winter crop in our effort to utilize landraces in the sown mixture, did not establish well. Therefore, in year 2021, L. sativus was excluded from the mixture. In year 2021, V. sativa, V. faba, E. sativa and C. sativum germinated and reached flowering in all experimental orchards. Anethum graveolens and Foeniculum vulgare Mill. (Apiaceae) did not reach flowering. Triticum aestivum L. (Poaceae) germinated and fulfilled its cohesion role in the patches of the sown mixture in both years. The broadleaved sown plant species reached flowering in the successive order: V. faba > E. sativa > V. sativa > L. sativus > C. sativum. The duration of the flowering in the plant mixtures was from late March/early April to June/July depending on the experimental year. The plant species in the weed patches and the sown mixtures which reached the flowering stage in each orchard are presented in Figure 1, Figure 2 and Figure 3. These mainly included V. persica, Veronica hederifolia L. (Plantaginaceae), C. bursa-pastoris, C. irregularis, S. apetala, C. draba, L. amplexicaule, C. arvensis. Plant cover in the sown flower patches ranged between 70 and 100% and in the wild plant patches between 40 and 100%; flower cover is shown in Figure 1, Figure 2 and Figure 3. The apple trees were in bloom from mid- to late April.
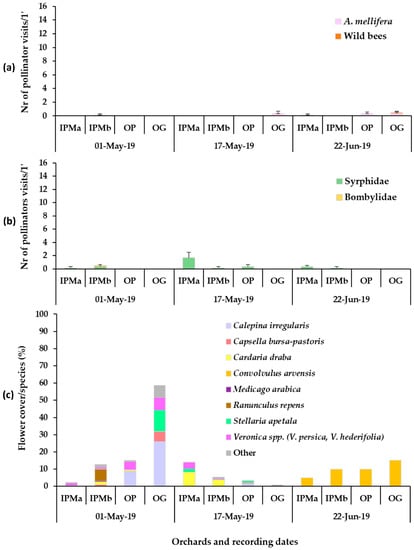
Figure 1.
Mean numbers of (a) Hymenoptera (honey bees, wild bees), (b) Diptera pollinator visits recorded on groundcover patches of natural vegetation (wild plants) (1 min/6.4 m2) and (c) mean flower cover percentage per plant species in the patches in apple orchards in the baseline year, 2019. IPMa: IPM field site a, IPMb: IPM field site b, OP: organic palmette field, OG: organic goblet field. Vertical bars represent standard error of means. Other species: Senecio vulgaris, Taraxacum officinale, Cardamine hirsute, Raphanus raphanistrum, Cerastium glomeratum, Euphorbia sp., Medicago polymorpha, Trifolium repens, Geranium disectum, Alopecurus myosuroides, Poa annua, Galium aparinae.
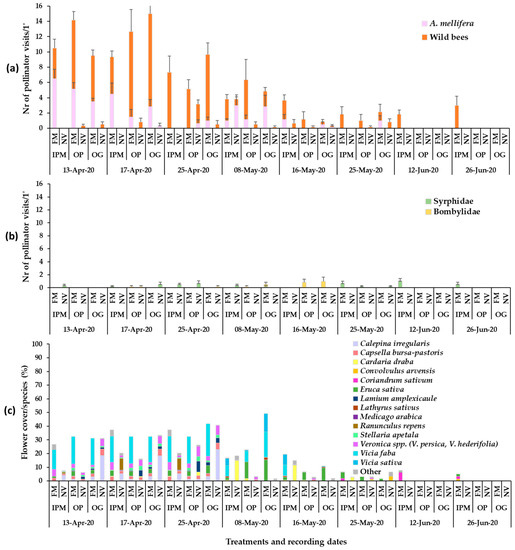
Figure 2.
Mean numbers of (a) Hymenoptera pollinator visits (honey bees, wild bees) and (b) Diptera visits on ground cover patches (1 min/6.4 m2) in apple orchards and (c) mean flower cover percentage per plant species in the patches in year 2020. FM: sown flowering mixture; NV: natural vegetation (wild plants); IPM: IPM orchard, OP: organic palmette orchard, OG: organic goblet orchard. Vertical bars represent standard error of means. Other species: Brassica nigra, Cardamine hirsuta, Geranium disectum, Malva spp., Matricaria chamomila, Medicago polymorpha, Poa annua, Raphanus sp., Senecio vulgaris, Sonchus oleraceus, Taraxacum officinale, Trifolium repens, Cerastium glomeratum, Draba muralis, Euphorbia sp., Galium aparinae, Lythospermum arvense.
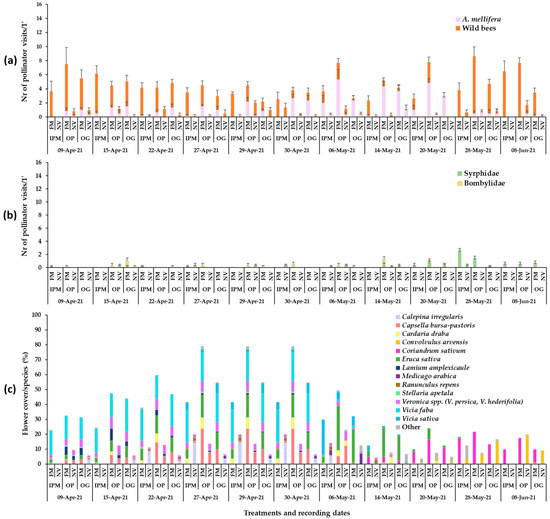
Figure 3.
Mean numbers of (a) Hymenoptera, (b) Diptera pollinator visits (honey bees, wild bees) and Diptera visits on ground cover patches (1 min/6.4 m2) in apple orchards and (c) mean flower cover percentage per plant species in the patches in year 2021. FM: sown flowering mixture; NV: natural vegetation (wild plants); IPM: IPM orchard, OP: organic palmette orchard, OG: organic goblet orchard. Vertical bars represent standard error of means. Other species: Brassica nigra, Cardamine hirsuta, Geranium disectum, Malva spp., Matricaria chamomila, Medicago polymorpha, Poa annua, Raphanus sp., Senecio vulgaris, Sonchus oleraceus, Taraxacum officinale, Trifolium repens, Alopecurus myosuroides, Campanula sp., Chrysanthemum sp., Fumaria officinalis, Papaver rhoeas, Picris hieracioides, Vicia incisa.
3.2. Presence of Pollinators in Sown/Wild Plant Patches and on Apple Blossom
In the baseline year (2019), low visitation (mean number of visits/6.4 m2/min) of honey bees was recorded on the wild plant patches in all orchards. In fact, the only wild bee recorded that year appeared in the OG orchard, where one visit of Systropha curvicornis Scopoli (Halictidae) was recorded on C. arvensis in late June. No statistical differences were indicated between the recording dates in all orchards (Table S1, Figure 1).
In the first year of groundcover management with the selected flowering mixture (2020), the groundcover, the recording date, and their interaction had a significant effect on the mean number of both honey bee and wild bee visits in all orchards (two-way ANOVA; Honey bees: IPM: Groundcover: F1,80 = 19.40, p < 0.0001; Date: F7,80 = 10.12, p < 0.0001; Groundcover*Date: F7,80 = 11.78, p < 0.0001; OP: Groundcover: F1,70 = 25.46, p < 0.0001; Date: F6,70 = 11.81, p < 0.0001; Groundcover*Date: F6,70 = 13.54, p < 0.0001; OG: Groundcover: F1,70 = 20.52, p < 0.0001; Date: F6,70 = 3.30, p < 0.0001; Groundcover*Date: F6,70 = 4.74, p < 0.0001; Wild bees: IPM: Groundcover: F1,80 = 66.59, p < 0.0001; Date: F7,80 = 2.38, p = 0.0285; Groundcover*Date: F7,80 = 2.93, p = 0.0089; OP: Groundcover: F1,70 = 38.62, p < 0.0001; Date: F6,70 = 7.25, p < 0.0001; Groundcover*Date: F6,70 = 5.63, p < 0.0001; OG: Groundcover: F1,70 = 51.82, p < 0.0001; Date: F6,70 = 10.58, p < 0.0001; Groundcover*Date: F6,70 = 11.23, p < 0.0001). For this reason, the results for the mean number of honey bee and wild bee visits were examined separately for each recording date.
The mean numbers of honey bee and wild bee visits on the sown flowering mixture and the wild plant patches in 2020 are shown in Figure 2. The flowering sown patches in the apple orchards attracted primarily wild bees and fewer honey bees. In the first recording date (13 April), mean honey bee visits on the flowering mixture were significantly higher compared to the visits on the wild plant patches in all orchards. In the second recording date (17 April), a significant difference between the flowering mixture and the wild plant patches was evident in IPM and OG orchards, whereas in the third recording date (25 April), a significant difference was recorded only in the OG orchard. In the following five recording dates (early May to late June), no significant differences were recorded in honey bee visits between the sown and wild plant patches (Tukey’s HSD test, a = 0.05 for each orchard/date; Supplementary Table S1). Honey bee visitation in the sown patches was higher compared to the wild plant patches in mid-April when in all orchards the main flowering species was V. faba (E. sativa, Veronica spp., C. bursa-pastoris also present), Figure 2. In the IPM orchard, higher visitation of honeybees in wild plant patches was recorded in early May when the main flowering species was C. draba and in the OG orchard when the main flower cover was from C. arvensis (Tukey’s HSD test, a = 0.05 for each orchard/groundcover; Supplementary Table S1).
Regarding wild bees, patches with the flowering mixture attracted a significantly higher number of visits compared to those with the wild plants. This was evident in all orchards just before full blooming of apple trees (13 April) and continued throughout apple blossom (17 April, 25 April). In later measurements, after the crop flowering (8 May), this was also evident in the IPM and the OG orchards; the main flowering species in the sown patches during all that three-week period were V. faba, V. sativa and E. sativa, the two latter species at the end of the period (Tukey’s HSD test, a = 0.05, for each orchard/date; Supplementary Table S1). In addition, significantly higher wild bee visits were recorded in the flowering mixture of the IPM orchard in June (12 June, 26 June) on flowers of C. sativum (coriander, the only flowering species in that period, failed to establish in the organic orchards) (Tukey’s HSD test, a = 0.05, for each orchard/groundcover; Supplementary Table S1).
In 2021, the groundcover type, the recording date and their interaction had a significant effect on the mean number of honey bee visits in the patches in both organic orchards, whereas in the IPM orchard, no significant differences were recorded in honey bee visitation between the flowering mixture and the wild plant patches (two-way ANOVA; Honey bees: IPM: Groundcover: F1,110 = 7.04, p = 0.0091; Date: F10,110 = 13.17, p = 0.0013; Groundcover*Date: F10,110 = 8.90, p = 0.055; OP: Groundcover: F1,110 = 91.22, p < 0.0001; Date: F10,110 = 7.37, p < 0.0001; Groundcover*Date: F10,110 = 8.16, p < 0.0001; OG: Groundcover: F1,110 = 49.35, p = 0.0004; Date: F10,110 = 3.59, p < 0.0001; Groundcover*Date: F10,110 = 2.32, p = 0.0161). Overall, the sown patches in the organic orchards had a higher visitation of honey bees compared to the wild plant patches from mid- / late April to late May. In the OP orchard, higher bee visitation was recorded in sown patches from the end of April (main flower cover from V. faba = C. bursa pastoris = E. sativa > Veronica sp. = C. draba) until 20 May (main flower cover from E. sativa and E. sativa + C. sativum at the end of this period) (Figure 3; Tukey’s HSD test, a = 0.05, for each groundcover/date; Supplementary Table S1).
The effect of groundcover management, recording date and their interaction was significant also on the wild bee visitation in the patches (two-way ANOVA; Wild bees: IPM: Groundcover: F1,110 = 122.74, p < 0.0001; Date: F10,110 = 2.38, p = 0.0135; Groundcover*Date: F10,110 = 2.95, p = 0.0025; OP: Groundcover: F1,110 = 101.27, p < 0.0001; Date: F10,110 = 6.39, p < 0.0001; Groundcover*Date: F10,110 = 6.06, p < 0.0001; OG: Groundcover: F1,110 = 79.85, p < 0.0001; Date: F10,110 = 6.09, p < 0.0001; Groundcover*Date: F10,110 = 5.01, p < 0.0001). Higher wild bee visitation was recorded on the sown patches compared to the wild plant patches throughout the observation period in the IPM orchard (mixed flower cover), while in the organic orchards, higher visitation of wild bees was observed during the middle weeks of April (mixed flower cover) and the end of May–early June (mainly coriander flowering) (Figure 3; Tukey’s HSD test, a = 0.05, Supplementary Table S1).
During the visual observations, a small number of visits of Diptera pollinators of Syrphidae and Bombyliidae was also recorded on the flowers of the sown mixture and wild flora (Figure 1b, Figure 2b and Figure 3b). Eruca sativa and C. sativum were the main plants to attract Diptera pollinators in the sown flowering mixture patches, while Veronica spp., C. bursa-pastoris, C. irregularis, C. draba and C. arvensis were the main plants to attract syrphids and bee flies in the wild plant patches.
The apple landrace ‘Pilafa’ has a very short period of flowering, approximately 10 to 15 days, usually mid- to late April, depending on the weather conditions. At that time of the season, the weather is usually unstable in Tegea plateau affecting the bee presence on the apple blossoms (Figure S2).
The honey bee visits on apple flowers (e.g., range of means in full bloom: 9–11 visits/1 min/plot in the three orchards) in the baseline year (2019) did not differ between the fields (two-way ANOVA; Honey bees: Orchard: F3,40 = 1.06, p = 0.3763; Date: F1,40 = 11.67, p = 0.0015; Orchard*Date: F3,40 = 1.89, p = 0.1465). No wild bees were recorded on apple blossoms in any of the orchards (Figure 4). In the experimental years with sown patches in the orchard, both honey bee visits (e.g., range of means in full bloom in the three orchards: year 2020/ IPM: 36- 37, OP: 28–31, OG: 24–26 visits/1 min/plot; year 2021/ IPM: 21–23, OP: 13–23, OG: 31–36 visits/1 min/plot) and few wild bee visits (e.g., range of means in full bloom in the three orchards: year 2020/ IPM: 2.66–3.33, OP: 0.33–0.67, OG: 0.67–2.83 visits/1 min/plot; year 2021/ IPM: 0.17 – 1.00, OP: 0.00–2.67, OG: 1.00–1.17 visits/1 min/ plot) were recorded on apple flowers of trees adjacent to the sown flowering patches and to the wild plant patches. Overall, the groundcover did not have a predominant significant effect on honey bee visits on apple flowers while the effect of ‘recording date’ was significant in all orchards indicating the role of the abundance of apple flowers in the attraction of A. mellifera.
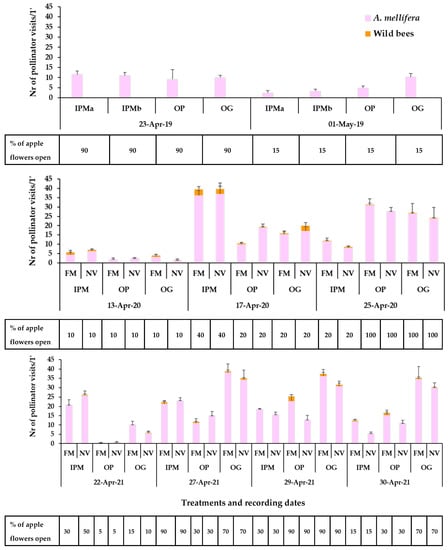
Figure 4.
Mean numbers of Hymenoptera pollinator visits (honey bees, wild bees) on apple flowers recorded for 1 min/6.4 m2 in the baseline year 2019, 2020, 2021. The horizontal bar below each graph represents the percentage of apple flowers open in each plot. IPM: IPM orchard with site (a) and (b) for the baseline year, OP: organic palmette orchard, OG: organic goblet orchard, FM: sown flowering mixture; NV: natural vegetation (wild plants). Vertical bars represent standard error of means.
More specifically, regarding the honey bee visitation on the apple blossom, groundcover did not have a significant effect, while the effect of recording date was significant as well as, in some cases, their interaction (two-way ANOVA; Year 2020: IPM: Groundcover: F1,30 = 0.009, p = 0.9248; Date: F2,30 = 69.99, p < 0.0001; Groundcover*Date: F2,30 = 0.51, p = 0.6034; OP: Groundcover: F1,30 = 1.68, p= 0.2042; Date: F2,30 = 99.02, p < 0.0001; Groundcover*Date: F2,30 = 5.29, p = 0.0107; OG: Groundcover: F1,30 = 0.09, p = 0.7669; Date: F2,30 = 19.87, p < 0.0001; Groundcover*Date: F2,30 = 0.21, p = 0.8068; Year 2021: IPM: Groundcover: F1,50 = 0.38, p = 0.5382; Date: F4,50 = 69.73, p < 0.0001; Groundcover*Date: F4,50 = 3.93, p = 0.0074; OP: Groundcover: F1,50 = 2.75, p = 0.1029; Date: F4,50 = 31.04, p < 0.0001; Groundcover*Date: F4,50 = 3.51, p = 0.0132; OG: Groundcover: F1,50 = 2.69, p = 0.1068; Date: F4,50 = 42.38, p < 0.0001; Groundcover*Date: F4,50 = 0.18, p = 0.9467). Looking at the cases of significant groundcover × recording date interaction, in spring 2020, honey bee visits in the OP orchard were significantly higher on the apple blossom of the trees adjacent to the wild plant patches on 17 April (Tukey’s HSD test, a = 0.05, Supplementary Table S2). Ιn spring 2021, honey bee visitation in OP and IPM orchards was higher on apple blossom of the trees next to the sown flowering patches at the end of April (Tukey’s HSD test, a = 0.05, Supplementary Table S2).
Concerning wild bees, the effect of groundcover was not significant on wild bee visitation on apple blossom except for the OP orchard in 2021 at the end of April, when a higher number of wild bee visits was recorded on flowers of trees adjacent to the sown flowering patches compared to wild plant patches (two-way ANOVA; Year 2020: IPM: Groundcover: F1,30 = 0.21, p = 0.6481; Date: F2,30 = 3.43, p = 0.0452; Groundcover*Date: F2,30 = 0.10, p = 0.8976; OP: Groundcover: F1,30 = 1.14, p = 0.2928; Date: F2,30 = 1.23, p = 0.3042; Groundcover*Date: F2,30 = 0.32, p = 0.7278; OG: Groundcover: F1,30 = 0.67, p = 0.4182; Date: F2,30 = 2.40, p = 0.1075; Groundcover*Date: F2,30 = 1.97, p = 0.1568. (Year 2021: OP: Groundcover: F1,50 = 20.69, p = 0.0001; Date: F4,50 = 7.31, p = 0.0001; Groundcover*Date: F4,50 = 7.28, p = 0.0001; Tukey’s HSD test, a = 0.05, Supplementary Table S2.)
3.3. Bee Fauna Composition and Other Main Fauna of Flower Visitors in Association to Flower Species
The bee fauna visiting the flowers in the flowering mixture consisted of several taxa: Apis mellifera, wild bees of the genera Andrena, Anthophora, Bombus, Eucera, Nomada, Hylaeus, Halictus, Lasioglossum, Sphecodes, Systropha, Xylocopa, and the family Megachilidae. Honey bees were recorded visiting flowers of all broadleaved plant species of the sown mixture. Regarding the wild bee taxa composition in association to flower species, V. faba attracted Eucera spp. (mainly E. nigrescens), A. plumipes, Bombus spp. and X. violaceae; Vicia sativa attracted mainly X. violaceae and Eucera spp.; L. sativus attracted a few bees of the families Megachilidae and Halictidae; E. sativa attracted mainly Halictidae, Anthophora spp., and Andrena spp.; C. sativum attracted mainly Hylaeus, Lasioglossum, Halictus, Andrena. Bumblebees, X. violaceae and Hylaeus spp. were recorded only in the sown flowering patches and the mean numbers of Anthophora and Eucera visits on the sown mixture were higher compared to those of the wild plant patches (Figure 5).
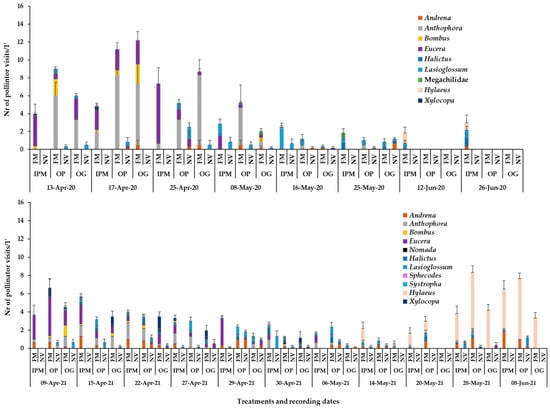
Figure 5.
Mean numbers of wild bees recorded in groundcover patches (1 min/6.4 m2) in apple orchards in 2020 and 2021. Wild bee taxa are indicated by different colour in each column. FM: sown flowering mixture; NV: natural vegetation (wild plants). IPM: IPM orchard, OP: organic palmette orchard, OG: organic goblet orchard. Vertical bars represent standard error of means.
Flowers of wild plant species also attracted several taxa of pollinators (plant sp.—pollinator taxa): Veronica spp.—A. mellifera, Lasioglossum, Andrena, Syrphidae; Capsella bursa-pastoris—Lasioglossum, Andrena, Bombyliidae, Syrphidae; S. apetala—Lasioglossum and Syrphidae; C. draba—A. mellifera, Andrena, Lasioglossum, Syrphidae; Cerastium glomeratum Thuill. (Caryophyllaceae)—Lasioglossum; Ranunculus repens—A. mellifera and Lasioglossum; T. repens—A. mellifera, Eucera, Andrena; Taraxacum officinale L. (Asteraceae)—Megachilidae (only one visit recorded); Sonchus oleraceus L. (Asteraceae)—Lasioglossum and Syrphidae; C. irregularis—mainly syrphid flies as well as few Lasioglossum and honey bees; L. amplexicaule (present only in the organic orchards)—honey bees and wild bees of the genera Anthophora, Eucera and Andrena; Medicago arabica L. (Fabaceae)—Andrena, Eucera, Lasioglossum; C. arvensis—A. mellifera, S. curvicornis, Lasioglossum, Halictus, Andrena (Figure 5). Most of these plant species were also present in the sown mixture patches (Veronica spp., C. irregularis, C. bursa-pastoris, S. apetala, C. glomeratus, C. draba), increasing their flower diversity.
Wild bees recorded on apple flowers belong to the following taxa: Andrena, Anthophora, Bombus (e.g., B. terrestris, B. argilaceus), Xylocopa, Lasioglossum (e.g., L. marginatum), Megachilidae. The majority of wild bee visits in the sown patches of the OP orchard at the end of April in 2021 was from Lasioglossum spp. (Figure 6). Examples of insect pollinators recorded on the apple blossoms, the sown flowering mixture and the wild plants of the apple orchards are shown in Figure 7 and Table 2.
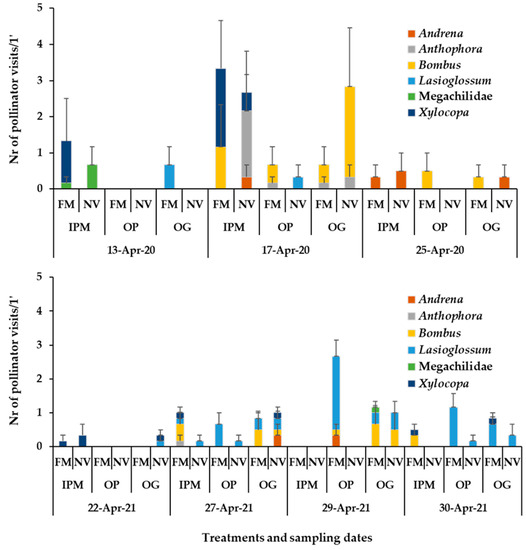
Figure 6.
Mean numbers of wild bees recorded on apple flowers (1 min/6.4 m2) in 2020 and 2021. Wild bee taxa are indicated by different colour in each column. FM: sown flowering mixture; NV: natural vegetation (wild plants); IPM: IPM orchard, OP: organic palmette orchard, OG: organic goblet orchard. Vertical bars represent standard error of means.

Figure 7.
Insect pollinators in apple orchards in Tegea, Peloponnese, Greece, 2019–2021: on wild plant flowers (1st column): Andrena sp.—Veronica persica, Lasioglossum sp.—C. draba, S. curvicornis—C. arvensis, Syrphidae—C. arvensis, Syrphidae—Ranunculus repens; on sown mixture flowers (2nd—4th column): Hylaeus (Dentigera)—C. sativum, Hylaeus variegatus—C. sativum, A. mellifera—C. sativum, Syrphidae—C. sativum, Eucera sp.—V. sativa, Andrena sp.—E. sativa, Lasioglossum sp.—E. sativa, Anthophora sp.—E. sativa, Syrphidae—E. sativa, Bombyliidae—E. sativa, A. plumipes—V. faba, E. nigrescens—V. faba, B. argilaceus—V. faba, X. violaceae—V. faba, A. plumipes and Eucera sp.—V. faba; on apple blossoms (5th column): A. mellifera-M. domestica, B. terrestris—M. domestica, Lasioglossum—M. domestica, X. violaceae—M. domestica.

Table 2.
Hymenopterous pollinators and associated flowering in sown flowering mixtures and the natural vegetation (wild plants) in apple orchards in Tegea, Peloponnese, Greece, 2019–2021.
4. Discussion
Establishment of flower strips inside the crop or in field margins has been an agricultural practice in orchards for the support of functional biodiversity in agroecosystems, especially in relation to pollinators [106,107]. The present study tested the hypothesis that patches of flowering mixtures could enhance pollinators in apple orchards. In this respect, baseline recordings on the weed flora, before the intervention, were deemed necessary. The baseline fauna of pollinating insects on the wild plants of the orchards included few visits of A. mellifera mainly on R. repens and C. arvensis on IPM orchard, on C. draba and C. arvensis in OG orchard and on C. arvensis in OP orchard. The only wild bee visit on the wild plant patches in the baseline year was from S. curvicornis on flowers of C. arvensis in late June in the OG orchard. This observation aligns with the pollen preference of S. curvicornis as it is a specialist on plants of the Convolvulaceae family [16]. This species was also recorded in 2021 on C. arvensis on the wild plant patches, indicating the contribution of the weed flora in the conservation of wild pollinators. Syrphid flies were recorded on Veronica spp., C. draba and R. repens while the latter also attracted a few visits of bee flies (Bombyliidae).
The introduction of sown flowering patches in the two following years increased pollinator visitation on the groundcover of the apple orchards compared to the wild plants, while at the same time it attracted pollinators of diverse taxa. While similar findings were reported before [62,63], most of the published studies compare the pollinator visits on the flowers of the sown plants to regularly mown grassy field patches or margins [60,63], practically with null flowering resources. Thus, in the present study, the comparison is more challenging, yet the higher records of pollinator visits on the flower resources of the sown flowering patches vs. of the wild plant patches in the apple orchards were evident and statistically significant.
From the Fabaceae, the V. faba ‘Episkopi’ attracted A. mellifera, Eucera spp. (mainly E. nigrescens), A. plumipes, Bombus spp. (e.g., B. argillaceus, B. terrestris) and X. violaceae. Faba bean has been reported to attract honey bees and mainly long-tongued wild bees such as Bombus, Xylocopa, A. plumipes, as well as small-tongued bees because of its extrafloral nectar or through nectar robbing [55,108,109,110,111]. In addition, V. sativa attracted small numbers of A. mellifera, X. violaceae, Eucera spp. and fewer visits of bees in the genera Andrena and Anthophora. Vicia sativa started flowering approximately one month after V. faba, indicating that V. sativa extends the provision of legume flowering resources in the flowering mixture despite its low attraction of pollinators as it is also mentioned in [96]. Lathyrus sativus is cultivated as an annual spring crop due to the climatological conditions of Tegea plateau, which explains why it did not establish well when sown in the mixture as a winter crop. However, scattered plants of L. sativus PGI ‘Fava Feneou’ which reached flowering attracted a few bees of the families Megachilidae and Halictidae (genus Lasioglossum). Lathyrus sativus was the only plant species of the flowering mixture to attract bees of the family Megachilidae which has been reported to include pollinators of the apple crop [112,113,114,115]. Thus, its contribution to a spring flowering mixture should be well taken into consideration.
Despite the valuable source of foraging for pollinators that legumes can provide [92,93,95], their complex flower structures may not support the full range of pollinators present in agricultural landscapes as they might restrict accessibility of resources to pollinating taxa with short mouthparts [116,117,118,119]. Thus, it is necessary to include plants other than Fabaceae in the sown mixture to provide accessible floral resources for a diversity of pollinators. Brassicaceae and Apiaceae are two plant families that have been used in sown mixtures to attract insect pollinators [62].
From the Brassicaceae, E. sativa attracted wild bees of Halictidae (Lasioglossum spp., Halictus spp.) and honey bees. Lower visitation by Anthophora spp., Eucera spp., Hylaeus spp., Andrena (which has also been recorded on E. sativa flowers by [120]) were noted. Eruca sativa was the main plant of the flowering mixture to attract Syrphidae and Bombylidae. Syrphid flies are one of the most frequent visitors of E. sativa flowers [121] and have been reported to contribute to ecosystem services in agroecosystems through their supporting roles as crop pollinators and predators of pests [122].
From the Apiaceae, A. graveolens and F. vulgare had a poor establishment and did not reach flowering probably due to the prolonged low temperatures [123] after sowing (November–March), common in Tegea plateau, hence these species are probably not suitable for a winter sown mixture in areas with harsh weather conditions. Coriandrum sativum was the last species to bloom, extending the window of flowering period of the sown mixtures in the season when flower resources in the apple orchards were scarce (late May–June), hence adding extra value to the overall performance of the flowering mixtures. Coriander was attractive to both honey bees and wild bees. The wild bees which were recorded on C. sativum flowers in descending order belong to the genera Hylaeus (e.g., H. variegatus), Lasioglossum, Halictus, Andrena, Sphecodes, and have been reported before as pollinators of coriander [124,125]. Furthermore, C. sativum had many visits of syrphid flies in late May–June which have also been reported to visit this plant species by [57]. In the first year of introduction of sown flower patches (2020), coriander established only in the IPM orchard in patches where the interspecific competition with V. faba plants was lower. Considering that V. faba is a competitive species which can influence the ability of less competitive plants to persist and flower in a mixture [96], the seed percentage of V. faba in the sown mixture was almost halved to ensure the good establishment of coriander in all the experimental orchards in the second year of mixture estabishment (2021).
The number of honey bee and wild bee visits on the flowers of wild plant patches was low, yet the attracted pollinators included several genera. Previous studies have highlighted the role of weed flora in field margins in supporting pollinators and addressed the need to protect it and utilize this function in intensive farming systems [126,127]. However, the concerns of introducing species in the mixture which could spread into the crops and become noxious weeds still remain [128]. In fact, it has been suggested to include many plant families of the weed flora in the present study, such as Brassicaceae and Fabaceae, in wildflower seed mixes aiming to attract wild bees [129]. From Brassicaceae, C. draba in the present study attracted syrphids, honey bees and wild bees of the genera Andrena and Lasioglossum, while from Fabaceae, T. repens and M. arabica attracted honey bees and wild bees of Eucera, Andrena and Lasioglossum.
Veronica hederifolia and V. persica in the present study attracted A. mellifera, Lasioglossum (which have been also recorded on V. persica by [130]) and Andrena. Moreover, V. hederifolia has been reported to be an attractive nectar source for pollinators [127]. Here, Veronica spp. also attracted syrphid flies that have been documented on its flowers before [131]. Stellaria apetala attracted Lasioglossum sp. together with syrphid flies. Ara et al. [130] mention Apis spp. and Lasioglosum spp. on Stellaria media flowers, while wild bees of the genus Lasioglossum have also been reported on Stellaria spp. flowers by [132]. Ranunculus repens attracted small numbers of A. mellifera (which have been also recorded to visit Ranunculus sp. flowers by [130]) and Lasioglossum.
Similarly to this study, pollinator visits have been reported before on C. bursa-pastoris flowers by solitary bees and especialy Andrena spp. [133] and on T. officinale by megachilids [134,135]. Lamium sp. have been reported for visits by both long-tongued and short-tongued bees similarly to this case (Anthophora, Eucera and Andrena). However, Bombus spp. which are referred to as the main visiting pollinators of Lamium spp. [136] were not recorded here. Convolulus arvensis attracted mainly Halictidae [137] and honeybees. Convolvulaceae and in particular C. arvensis is an important floral resource for wild bees, especially those in the genus Systropha, [16,129]. However, C. arvensis and C. bursa-pastoris are listed among the most troublesome weeds in agriculture [138,139], which renders them undesirable in the field despite their capacity for attracting pollinators.
Overall, in the present study, the sown plants attracted pollinators from diverse genera with higher visitation of wild bees compared to the weed flora of the orchards. The superior effect of the sown patches was evident in all orchards and various conditions, i.e., rich (organic orchards) and poor (IPM) surrounding semi-natural habitats in respect to flower resources and nesting sites, different canopy architecture (goblette/palmete) and plant protection systems (organic/IPM). It is noteworthy that the usual practice in the area is avoiding the use of pesticides during the flowering period of the apple trees to avoid harming the pollinators visiting the apple flowers. Indeed, as Albrecht et al. [140] and McKerchar et al. [141] highlight, the sustainable use of pesticides in fields where conservation agronomic practices of wild pollinators are implemented is of outmost importance.
On the other hand, the contribution of the wild plant flower resources to attracting a diverse range of wild bee genera should not be ignored but exploited when planning groundcover management for the enhancement of pollinators. Wood et al. [142] conducted an experiment on agri-environment pollinator-friendly schemes and reported that the majority of bee species preferred wild plants that are not included in a flower-rich mixture, stressing the significance of the natural vegetation flowering resources inside an agroecosystem. In this study, most of the wild plant species (Veronica spp., C. irregularis, C. bursa-pastoris, S. apetala, C. glomeratus, C. draba) were also present in the sown mixtures, although in a lower density. Their presence increased the plant diversity of the sown flowering patches and subsequently the provided floral resources. The differences in the pollinator abundance and the genera recorded among the apple orchards suggest that groundcover management recommendations must be site-specific to ensure the long-term availability of diverse floral resources for pollinators [134].
To return to our hypothesis, that sown flowering patches could enhance pollinators’ presence and diveristy also to the benefit of the crop, A. mellifera was the most frequent pollinator on the apple blossoms in all years. The lower range of means of honey bee visits during apple full bloom in the baseline year compared to the two following years is attributed to the prolonged rainy and cloudy weather during the short flowering period (10 days) of ‘Pilafa’ in Tegea plateau in 2019 and is not linked with the groundcover management. However, when the sown flower patches were present in the orchards, visits of wild bees were also recorded on apple blossoms. Previous studies have stated that honey bees outnumber wild bees in apple orchards [143,144] or that wild pollinators were not observed to visit apple flowers and honey bee was the only apple flower visitor [145]. Nevertheless, published studies highlight the importance of management for diverse pollinator communities to apple crop pollination, even in the presence of large populations of managed honey bees, which may also decrease reliance on managed honey bees for pollination services and enhance crop yields [146,147]. The common wild bee taxa recorded on apple flowers and on the sown/wild plant patches include Halictidae, Megachilidae, Andrena, Anthophora as well as Bombus and Xylocopa (only in sown mixture). In 2020, Bombus and Xylocopa visits on apple flowers could be associated with their attractance by V. faba in the sown patches. In 2021, higher visitation of Lasioglossum spp. on the apple flowers of the trees adjacent to the sown mixture could be attributed to E. sativa plants which attracted high numbers of Lasioglossum spp. However, there is no clear pattern to link wild bee visitation on apple flowers and the sown patches. Lowe et al. [148] and Albrecht et al. [140] conducted review meta-analyses which showed that flower plantings in agroecosystems are highly effective at increasing pollinator richness and abundance in the intervention area, but the influence of these plantings on crop pollination and yield is inconsistent.
The landscape elements around the apple orchards could have also influenced the pollinator taxa that visited the apple flowers [149]. For example, the organic goblet orchard attracted Bombus spp. in trees adjacent to both sown mixture and wild plant patches. The flowering resources on undisturbed land around the organic goblet orchard included T. repens, M. polymorpha, V. villosa, all natural vegetation of Fabaceae plant species, which are the major pollen source for most bumblebee species [45], as well as wild forest trees and bushes which prevent human passage and might contribute to the formation of protected pollinator nesting sites. This might explain the presence of Bombus spp. on apple flowers regardless of the ground management inside the organic goblet orchard in 2020 and 2021. Földesi et al. [145] mention that the maintenance of semi-natural habitats within 500 m around apple orchards is highly recommended to enhance wild pollinator communities and apple production. Moreover, Gervais et al. [150] also report that landscape enhancements improve bumble bee queen presence and diversity in apple orchards and should therefore be considered by growers as a means to enhance and ensure the pollination and diversity of beneficial insects in their orchards. Therefore, policy measures for pollinators should reffer not only to agroecosystems but to various landscapes and neighbouring specialised habitats as maintaining species diversity is crucial in providing ecosystem resilience. Furthermore, the environental policies on pollinators should have a holistic ecosystem approach taking into acount the fact that ecosystem service management, such as establishment of sown flowering patches in perenial crops, does not equal biodiversity conservation, but that these terms are interlinked [151].
5. Conclusions
Honey bee (A. mellifera) is the main pollinator of the apple crop in ‘Pilafa’ apple orchards in Tegea, Peloponnese, Greece. Wild plants in the groundcover provided floral supplies for different genera of pollinators, but these were not notably attractive to pollinators as indicated by the small number of visits mainly of honey bees, Halictidae and Syrphidae. The introduction of sown flowering patches of V. faba, V. sativa, E. sativa, C. sativum in mixtures attracted honey bees and wild bees in greater numbers and more diverse wild bee taxa indicating the importance of the floral abundance and diversity as well as flowering period of the resources for the pollinating insects. The plant species E. sativa and V. faba of the sown mixture attracted high numbers and diverse pollinator fauna (A. mellifera, Eucera spp., Anthophora spp., Bombus spp., X. violaceae, Halictidae, Andrena spp.) which also visited apple flowers except for Eucera spp., whereas C. sativum provided resources for pollinators (mainly Hylaeus spp., Halictidae, Andrena spp. and honey bees) in a period when the available flowering reservoirs in the apple orchards were scarce. The results are valuable for a better understanding of the flora–pollinator fauna relationships in apple orchards and for the design of future sustainable management strategies mainly in relation to ecosystem pollination services in this crop. The tested sown mixture, including the legume landraces, in patches provides floral sources for honey bees, wild bees, syrphid flies and bee flies in the studied area and can serve as a good agricultural tool/practice to attract insect pollinators and potentially enhance pollination if adjusted appropriately to meet site-specific parameters in respect to pollinator conservation and exploitation of wild flora in agroecosystems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects14020208/s1, Figure S1: Experimentation sites of two organic orchards (OP: organic palmette orchard, OG: organic goblet orchard) and one IPM orchard (37.43705, 22.46942; 37.43658, 22.46895; 37.4415, 22.40492) in Tegea plateau, Peloponnese, Greece, and example of the layout of the sown flowering mixture patches (FM) (pink blocks) and the natural vegetation patches (NV) (green blocks) in the apple orchards where the presence of pollinators was recorded; Figure S2: Meteorological data [Temperature (°C), rain (mm), wind speed (km/hr)] for years 2019, 2020, 2021 from the National Observatory of Athens. Meteorological station ‘Tripoli (LG83)’, elevation: 646 m latitude: 37°30′34″ N, longitude: 22°25′04″ E; Table S1: Mean number of honey bee and wild bee visits/patch/1′ (±s.e.m.) in the sown flowering mixture (FM) patches and the natural vegetation (NV) patches of groundcover, at three apple orchards (IPM, OP, OG), in three consecutive years 2019, 2020, 2021 (recordings from April to June); Table S2: Mean number of honey bee and wild bee visits/plot/1′ (±s.e.m.) on the apple tree blossoms adjacent to the sown flowering mixture (FM) patches and the natural vegetation (NV) patches of groundcover, at three apple orchards (IPM, OP, OG), in years 2019, 2020, 2021 (recordings from April to June).
Author Contributions
Conceptualization, F.K. and M.B.; methodology, F.K., V.K. and M.B.; formal analysis, M.B.; investigation, M.B.; resources, F.K. and V.K.; data curation, M.B.; writing—original draft preparation, M.B.; writing—review and editing, F.K., V.K. and D.P.; visualization, M.B, F.K. and D.P.; supervision, D.P. and F.K.; project administration, F.K. and D.P.; funding acquisition, F.K., D.P. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank all those who have contributed in this work, and specifically the farmers and agronomists Antonios Mitropoulos, Dimitrios Mitropoulos, and farmer Nikolaos Karras for providing the apple orchards, performing the soil preparation for sowing, and for sharing their invaluable experience and knowledge on the legume landraces used in the flowering mixtures, the duration and performance of apple flowering of ‘Pilafa’ apples in Tegea; the Agricultural Livestock Cooperation of Arcadia ‘H Ένωση’ and Elisavet Katsini, Georgios Markopoulos for donating the landrace Vicia faba ‘Episkopis’; Theodoros Stathakis and Leonidas Economou for their indispensable support; the Laboratory of Agricultural Zoology and Entomology of the Agricultural University of Athens and the Benaki Phytopathological Institute for providing appropriate documentation in order to travel to the fields during the COVID-19 pandemic lockdowns. The first Author would also like to thank Benaki Phytopathological Institute, Laboratory of Efficacy Control of Pesticides, for providing the facilities and equipment for the laboratory work. Special thanks to Jelle Devalez and Mike Edwards for their guidance on taxonomy issues of bees and the confirmation of genus in some of the specimens by Jelle Devalez. The authors would like to thank the reviewers for their useful and constructive comments towards the improvement of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Losey, J.E.; Vaughan, M. The economic value of ecological services provided by insects. Bioscience 2006, 56, 311–323. [Google Scholar] [CrossRef]
- Kremen, C.; Williams, N.M.; Aizen, M.A.; Gemmill-Herren, B.; LeBuhn, G.; Minckley, R.; Packer, L.; Potts, S.G.; Roulston, T.A.; Steffan-Dewenter, I.; et al. Pollination and other ecosystem services produced by mobile organisms: A conceptual framework for the effects of land-use change. Ecol. Lett. 2007, 10, 299–314. [Google Scholar] [CrossRef]
- Porto, R.G.; de Almeida, R.F.; Cruz-Neto, O.; Tabarelli, M.; Viana, B.F.; Peres, C.A.; Lopes, A.V. Pollination ecosystem services: A comprehensive review of economic values, research funding and policy actions. Food Secur. 2020, 12, 1425–1442. [Google Scholar] [CrossRef]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Potts, S.G.; Imperatriz Fonseca, V.; Ngo, H.T.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.; Garibaldi, L.A.; Hill, R.; Settele, J.; Vanbergen, A.J.; et al. Summary for Policymakers of the Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on Pollinators, Pollination and Food Production; Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2016; 36p. [Google Scholar]
- Kearns, C.A.; Inouye, D.; Waser, N.M. Endangered mutualisms: The conservation of plant-pollinator interactions. Annu. Rev. Ecol. Syst. 1998, 29, 83–112. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Kremen, C.; Morales, J.M.; Bommarco, R.; Cunningham, S.A.; Carvalheiro, L.G.; Chacoff, N.P.; Dudenhöffer, J.H.; Greenleaf, S.S.; et al. Stability of pollination services decreases with isolation from natural areas despite honey bee visits. Ecol. Lett. 2011, 14, 1062–1072. [Google Scholar] [CrossRef]
- Isaacs, R.; Tuell, J.; Fiedler, A.; Gardiner, M.; Landis, D. Maximizing arthropod-mediated ecosystem services in agricultural landscapes: The role of native plants. Front. Ecol. Environ. 2009, 7, 196–203. [Google Scholar] [CrossRef]
- Bommarco, R.; Kleijn, D.; Potts, S.G. Ecological intensification: Harnessing ecosystem services for food security. Trends Ecol. Evol. 2013, 28, 230–238. [Google Scholar] [CrossRef]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef]
- Johnson, C.A.; Dutt, P.; Levine, J.M. Competition for pollinators destabilizes plant coexistence. Nature 2022, 607, 721–725. [Google Scholar] [CrossRef]
- Biesmeijer, J.C.; Roberts, S.P.; Reemer, M.; Ohlemuller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.J.M.C.; Thomas, C.D.; et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Meek, B.; Loxton, D.; Sparks, T.; Pywell, R.; Pickett, H.; Nowakowski, M. The effect of arable field margin composition on invertebrate biodiversity. Biol. Conserv. 2002, 106, 259–271. [Google Scholar] [CrossRef]
- Potts, S.G.; Vulliamy, B.; Dafni, A.; Ne’eman, G.; Willmer, P. Linking Bees and Flowers: How Do Floral Communities Structure Pollinator Communities? Ecology 2003, 84, 2628–2642. [Google Scholar] [CrossRef]
- Michener, C.D. The Bees of the World, 2nd ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2007. [Google Scholar]
- Van Rijn, P.C.J.; Kooijman, J.; Wäckers, F.L. The contribution of floral resources and honeydew to the performance of predatory hoverflies (Diptera: Syrphidae). Biol. Control 2013, 67, 32–38. [Google Scholar] [CrossRef]
- Goulson, D.; Hanley, M.E.; Darvill, B.; Ellis, J.S.; Knight, M.E. Causes of rarity in bumblebees. Biol. Conserv. 2005, 122, 1–8. [Google Scholar] [CrossRef]
- Winfree, R.; Aguilar, R.; Vázquez, D.P.; LeBuhn, G.; Aizen, M.A. A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 2009, 90, 2068–2076. [Google Scholar] [CrossRef]
- Bommarco, R.; Lundin, O.; Smith, H.G.; Rundlöf, M. Drastic historic shifts in bumble-bee community composition in Sweden. Proc. R. Soc. B Biol. Sci. 2012, 279, 309–315. [Google Scholar] [CrossRef]
- Schindler, M.; Diestelhorst, O.; Härtel, S.; Saure, C.; Schanowski, A.; Schwenninger, H.R. Monitoring agricultural ecosystems by using wild bees as environmental indicators. BioRisk 2013, 8, 53–71. [Google Scholar] [CrossRef]
- Baude, M.; Kunin, W.E.; Boatman, N.D.; Conyers, S.; Davies, N.; Gillespie, M.A.K.; Morton, R.D.; Smart, S.M.; Memmott, J. Historical nectar assessment reveals the fall and rise of floral resources in Britain. Nature 2016, 530, 85–88. [Google Scholar] [CrossRef]
- Cole, L.J.; Brocklehurst, S.; Robertson, D.; Harrison, W.; McCracken, D.I. Exploring the interactions between resource availability and the utilisation of semi-natural habitats by insect pollinators in an intensive agricultural landscape. Agric. Ecosyst. Environ. 2017, 246, 157–167. [Google Scholar] [CrossRef]
- Nieto, A.; Roberts, S.P.M.; Kemp, J.; Rasmont, P.; Kuhlmann, M.; García Criado, M.; Biesmeijer, J.C.; Bogusch, P.; Dathe, H.H.; De la Rúa, P.; et al. European Red List of Bees; Publication Office of the European Union: Luxembourg, 2014. [CrossRef]
- Reilly, J.R.; Artz, D.R.; Biddinger, D.; Bobiwash, K.; Boyle, N.K.; Brittain, C.; Brokaw, J.; Campbell, J.W.; Daniels, J.; Elle, E.; et al. Crop production in the USA is frequently limited by a lack of pollinators. Proc. R. Soc. B 2020, 287, 20200922. [Google Scholar] [CrossRef]
- Petanidou, T.; Kallimanis, A.S.; Tzanopoulos, J.; Sgardelis, S.P.; Pantis, J.D. Long-term observation of a pollination network: Fluctuation in species and interactions, relative invariance of network structure and implications for estimates of specialization. Ecol. Lett. 2008, 11, 564–575. [Google Scholar] [CrossRef]
- Patiny, S.; Rasmont, P.; Michez, D. A survey and review of the status of wild bees in the West-Palaearctic region. Apidologie 2009, 40, 313–331. [Google Scholar] [CrossRef]
- Petanidou, T.; Ståhls, G.; Vujić, A.; Olesen, J.M.; Rojo, S.; Thrasyvoulou, A.; Sgardelis, S.; Kallimanis, A.S.; Kokkini, S.; Tscheulin, T. Investigating plant—Pollinator relationships in the Aegean: The approaches of the project POL-AEGIS (The pollinators of the Aegean archipelago: Diversity and threats). J. Apic. Res. 2013, 52, 106–117. [Google Scholar] [CrossRef]
- Melin, A.; Rouget, M.; Midgley, J.J.; Donaldson, J.S. Pollination ecosystem services in South African agricultural systems. S. Afr. J. Sci. 2014, 110, 1–9. [Google Scholar] [CrossRef]
- Meiners, J.M.; Griswold, T.L.; Carril, O.M. Decades of native bee biodiversity surveys at Pinnacles National Park highlight the importance of monitoring natural areas over time. PLoS ONE 2019, 14, e0207566. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Aizen, M.A.; Cunningham, S.; Klein, A.M. Pollinator shortage and global crop yield: Looking at the whole spectrum of pollinator dependency. Commun. Integr. Biol. 2009, 2, 37–39. [Google Scholar] [CrossRef]
- Sagili, R.R.; Burgett, D.M. Evaluating Honey Bee Colonies for Pollination A Guide for Commercial Growers and Beekeepers; Oregon State University: Corvallis, OR, USA, 2011; Available online: https://catalog.extension.oregonstate.edu/pnw623 (accessed on 1 January 2023).
- DeGrandi-Hoffman, G.; Graham, H.; Ahumada, F.; Smart, M.; Ziolkowski, N. The Economics of Honey Bee (Hymenoptera: Apidae) Management and Overwintering Strategies for Colonies Used to Pollinate Almonds. J. Econ. Entomol. 2019, 112, 2524–2533. [Google Scholar] [CrossRef]
- Devkota, K.; Rijal, P.; Fernando dos Santos, C. The Use of Honeybee Hives May Boost Yields of Some Crops in Nepal. Psyche A J. Entomol. 2021, 2021, 8876388. [Google Scholar] [CrossRef]
- Breeze, T.D.; Vaissière, B.E.; Bommarco, R.; Petanidou, T.; Seraphides, N.; Kozák, L.; Scheper, J.; Biesmeijer, J.C.; Kleijn, D.; Gyldenkærne, S.; et al. Agricultural Policies Exacerbate Honeybee Pollination Service Supply-Demand Mismatches Across Europe. PLoS ONE 2014, 9, e82996. [Google Scholar] [CrossRef]
- Westerkamp, C.; Gottsberger, G. The Costly Crop Pollination Crisis. Pollinating Bees—The Conservation Link between Agriculture and Nature; Kevan, P., Fonseca, V.I., Eds.; Ministry of Environment: Brasilia, Brazil, 2022; pp. 51–56.
- Bond, J.; Plattner, K.; Hunt, K. Pollination-Services Market, Fruit and Tree Nuts Outlook; Situation and Outlook Report No. FTS-357SA; US Department of Agriculture: Washington, DC, USA; Economic Research Service: Washington, DC, USA, 2014. Available online: https://www.ers.usda.gov/publications/pub-details/?pubid=37060 (accessed on 21 December 2022).
- Winfree, R.; Williams, N.M.; Dushoff, J.; Kremen, C. Native bees provide insurance against ongoing honey bee losses. Ecol. Lett. 2007, 10, 1105–1113. [Google Scholar] [CrossRef]
- O’Toole, C. Mason Bees as Managed Pollinators of Orchard Crops. Bee World 2011, 88, 5–8. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O.; et al. Wild Pollinators Enhance Fruit Set of Crops Regardless of Honey Bee Abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef]
- Pérez-Méndez, N.; Andersson, G.K.S.; Requier, F.; Hipólito, J.; Aizen, M.A.; Morales, C.L.; García, N.; Gennari, G.P.; Garibaldi, L.A. The economic cost of losing native pollinator species for orchard production. J. Appl. Ecol. 2019, 57, 599–608. [Google Scholar] [CrossRef]
- Nicholls, C.I.; Altieri, M.A. Plant biodiversity enhances bees and other insect pollinators in agroecosystems. A review. Agron. Sustain. Dev. 2012, 33, 257–274. [Google Scholar] [CrossRef]
- Dicks, L.V.; Abrahams, A.; Atkinson, J.; Biesmeijer, J.; Bourn, N.; Brown, C.; Brown, M.J.; Carvell, C.; Connolly, C.; Cresswell, J.E.; et al. Identifying key knowledge needs for evidence-based conservation of wild insect pollinators: A collaborative cross-sectoral exercise. Insect Conserv. Divers. 2013, 6, 435–446. [Google Scholar] [CrossRef]
- Giovanetti, M.; Albertazzi, S.; Flaminio, S.; Ranalli, R.; Bortolotti, L.; Quaranta, M. Pollination in Agroecosystems: A Review of the Conceptual Framework with a View to Sound Monitoring. Land 2021, 10, 540. [Google Scholar] [CrossRef]
- Mottershead, D.; Underwood, E. Pollinators in the CAP: Integrating Pollinator Conservation into the Common Agricultural Policy; Institute for European Environmental Policy: Brussels, Belgium, 2020. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAO’s Global Action on Pollination Services for Sustainable Agriculture, Global Pollination Project. Available online: https://www.fao.org/common-pages/search/en/?q=pollinators (accessed on 19 July 2022).
- European Commission. Eurostat Regional Yearbook: 2020; European Commission: Brussels, Belgium, 2020.
- Nielsen, A.; Steffan-Dewenter, I.; Westphal, C.; Messinger, O.; Potts, S.G.; Roberts, S.P.; Settele, J.; Szentgyörgyi, H.; Vaissière, B.E.; Vaitis, M.; et al. Assessing bee species richness in two Mediterranean communities: Importance of habitat type and sampling techniques. Ecol. Res. 2011, 26, 969–983. [Google Scholar] [CrossRef]
- Drossart, M.; Gérard, M. Beyond the Decline of Wild Bees: Optimizing Conservation Measures and Bringing Together the Actors. Insects 2020, 11, 649. [Google Scholar] [CrossRef]
- Krahner, A.; Schmidt, J.; Maixner, M.; Porten, M.; Schmitt, T. Evaluation of four different methods for assessing bee diversity as ecological indicators of agro-ecosystems. Ecol. Indic. 2021, 125, 107573. [Google Scholar] [CrossRef]
- Brandl, M.; Hussain, R.I.; Maas, B.; Rabl, D.; Pachinger, B.; Holzinger, W.; Krautzer, B.; Moser, D.; Frank, T. Improving insect conservation values of agri-environment schemes through diversified seed mixtures. Biol. Conserv. 2022, 269, 109530. [Google Scholar] [CrossRef]
- Cano, D.; Martínez-Núñez, C.; Pérez, A.J.; Salido, T.; Rey, P.J. Small floral patches are resistant reservoirs of wild floral visitor insects and the pollination service in agricultural landscapes. Biol. Conserv. 2022, 276, 109789. [Google Scholar] [CrossRef]
- Winfree, R. The conservation and restoration of wild bees. Ann. N. Y. Acad. Sci. 2010, 1195, 169–197. [Google Scholar] [CrossRef]
- Scheper, J.; Bommarco, R.; Holzschuh, A.; Potts, S.G.; Riedinger, V.; Roberts, S.P.M.; Rundlöf, M.; Smith, H.G.; Steffan-Dewenter, I.; Wickens, J.B.; et al. Local and landscape-level floral resources explain effects of wildflower strips on wild bees across four European countries. J. Appl. Ecol. 2015, 52, 1165–1175. [Google Scholar] [CrossRef]
- Beyer, N.; Gabriel, D.; Kirsch, F.; Schulz-Kesting, K.; Dauber, J.; Westphal, C. Functional groups of wild bees respond differently to faba bean Vicia faba L. cultivation at landscape scale. J. Appl. Ecol. 2020, 57, 2499–2508. [Google Scholar] [CrossRef]
- Pywell, R.F.; Warman, E.A.; Carvell, C.; Sparks, T.H.; Dicks, L.V.; Bennett, D.; Wright, A.; Critchley, C.N.R.; Sherwood, A. Providing foraging resources for bumblebees in intensively farmed landscapes. Biol. Conserv. 2005, 121, 479–494. [Google Scholar] [CrossRef]
- Ambrosino, M.D.; Luna, J.M.; Jepson, P.C.; Wratten, S.D. Relative frequencies of visits to selected insectary plants by predatory hoverflies (Diptera: Syrphidae), other beneficial insects, and herbivores. Environ. Entomol. 2006, 35, 394–400. [Google Scholar] [CrossRef]
- Carvell, C.; Meek, W.R.; Pywell, R.F.; Goulson, D.; Nowakowski, M. Comparing the efficacy of agri-environment schemes to enhance bumble bee abundance and diversity on arable field margins. J. Appl. Ecol. 2007, 44, 29–40. [Google Scholar] [CrossRef]
- Carvalheiro, L.G.; Seymour, C.L.; Nicolson, S.W.; Veldtman, R. Creating patches of native flowers facilitates crop pollination in large agricultural fields: Mango as a case study. J. Appl. Ecol. 2012, 49, 1373–1383. [Google Scholar] [CrossRef]
- Campbell, A.; Sutton, P.; Wilby, A.; Wackers, F. Improving pest control and pollination services in cider apple orchards by means of ’multi-functional’ flowering strips. Environ. Manag. Farml. 2013, 118, 283–290. [Google Scholar]
- Blaauw, B.R.; Isaacs, R. Flower plantings increase wild bee abundance and the pollination services provided to a pollination-dependent crop. J. Appl. Ecol. 2014, 51, 890–898. [Google Scholar] [CrossRef]
- Karamaouna, F.; Kati, V.; Volakakis, N.; Varikou, K.; Garantonakis, N.; Economou, L.; Birouraki, A.; Markellou, E.; Liberopoulou, S.; Edwards, M. Ground cover management with mixtures of flowering plants to enhance insect pollinators and natural enemies of pests in olive groves. Agric. Ecosyst. Environ. 2019, 274, 76–89. [Google Scholar] [CrossRef]
- Kati, V.; Karamaouna, F.; Economou, L.; Mylona, P.V.; Samara, M.; Mitroiu, M.-D.; Barda, M.; Edwards, M.; Liberopoulou, S. Sown Wildflowers Enhance Habitats of Pollinators and Beneficial Arthropod in a Tomato Field Margin. Plants 2021, 10, 1003. [Google Scholar] [CrossRef]
- Karamaouna, F.; Kati, V.; Economou, L.; Troyanos, G.; Samara, M.; Liberopoulou, S.; Barda, M.; Mitroiu, M.-D.; Edwards, M. Selected flowering plants as a habitat for pollinators and natural enemies in field margins of a watermelon crop—Implications for crop yield. Int. J. Pest Manag. 2022. [Google Scholar] [CrossRef]
- Delaplane, K.S.; Mayer, D.F. Crop Pollination by Bees; CABI Publishing: Wallingford, UK, 2000. [Google Scholar]
- Garratt, M.P.; Breeze, T.D.; Jenner, N.; Polce, C.; Biesmeijer, J.C.; Potts, S.G. Avoiding a bad apple: Insect pollination enhances fruit quality and economic value. Agric. Ecosyst. Environ. 2014, 184, 34–40. [Google Scholar] [CrossRef]
- Free, J.B. Pollinating efficiency of honey-bee visits to apple flowers. J. Hortic. Sci. Biotechnol. 1966, 41, 91–94. [Google Scholar] [CrossRef]
- Samnegård, U.; Hambäck, P.A.; Smith, H.G. Pollination treatment affects fruit set and modifies marketable and storable fruit quality of commercial apples. R. Soc. Open Sci. 2019, 6, 190326. [Google Scholar] [CrossRef]
- Vicens, N.; Bosch, J. Pollinating efficacy of Osmia cornuta and Apis mellifera (Hymenoptera: Megachilidae, Apidae) on ’red Delicious’ apple. Environ. Entomol. 2000, 29, 235–240. [Google Scholar] [CrossRef]
- Radzevičiūtė, R.; Theodorou, P.; Schlegel, M.; Paxton, R.J. A two-part modelling approach reveals a positive effect of pollinator biodiversity in boosting the pollination of apple flowers. Agric. Ecosyst. Environ. 2021, 306, 107197. [Google Scholar] [CrossRef]
- Sheffield, C.; Westby, S.; Smith, R.; Kevan, P. Potential of bigleaf lupine for building and sustaining Osmia lignaria populations for pollination of apple. Can. Entomol. 2008, 140, 589–599. [Google Scholar] [CrossRef]
- Sedivy, C.; Dorn, S. Towards a sustainable management of bees of the subgenus Osmia (Megachilidae; Osmia) as fruit tree pollinators. Apidologie 2013, 45, 88–105. [Google Scholar] [CrossRef]
- Viana, B.F.; da Encarnação Coutinho, J.G.; Garibaldi, L.A.; Castagnino, G.L.B.; Gramacho, K.P.; Silva, F.O. Stingless bees further improve apple pollination and production. J. Pollinat. Ecol. 2014, 14, 261–269. [Google Scholar] [CrossRef]
- Sapir, G.; Baras, Z.; Azmon, G.; Goldway, M.; Shafir, S.; Allouche, A.; Stern, E.; Stern, R.A. Synergistic effects between bumblebees and honey bees in apple orchards increase cross pollination, seed number and fruit size. Sci. Hortic. 2017, 219, 107–117. [Google Scholar] [CrossRef]
- Tepedino, V.J.; Alston, D.G.; Bradley, B.A.; Toler, T.R.; Griswold, T.L. Orchard pollination in Capitol Reef National Park, Utah, USA. Honey bees or native bees? Biodivers. Conserv. 2007, 16, 3083–3094. [Google Scholar] [CrossRef]
- Marini, L.; Quaranta, M.; Fontana, P.; Biesmeijer, J.C.; Bommarco, R. Landscape context and elevation affect pollinator communities in intensive apple orchards. Basic Appl. Ecol. 2012, 13, 681–689. [Google Scholar] [CrossRef]
- Campbell, A.J.; Wilby, A.; Sutton, P.; Wäckers, F.L. Do sown flower strips boost wild pollinator abundance and pollination services in a spring-flowering crop? A case study from UK cider apple orchards. Agric. Ecosyst. Environ. 2017, 239, 20–29. [Google Scholar] [CrossRef]
- Miñarro, M.; García, D. Complementarity and redundancy in the functional niche of cider apple pollinators. Apidologie 2018, 49, 789–802. [Google Scholar] [CrossRef]
- Porcel, M.; Andersson, G.K.S.; Pålsson, J.; Tasin, M. Organic management in apple orchards: Higher impacts on biological control than on pollination. J. Appl. Ecol. 2018, 55, 2779–2789. [Google Scholar] [CrossRef]
- Pardo, A.; Borges, P.A.V. Worldwide importance of insect pollination in apple orchards: A review. Agric. Ecosyst. Environ. 2020, 293, 106839. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook 2021; FAO: Rome, Italy, 2021. [CrossRef]
- Hellenic Statistical Authority. (24 June 2021) Areas and Production, Annual Agricultural Statistical Survey: Year 2019. Available online: https://www.statistics.gr/documents/20181/96929c5c-54d8-ccca-22fe-1c7af02f8bf3 (accessed on 18 July 2022).
- European Union, Commission Regulation (EC) No. 1065/97 of 12 June 1997 Supplementing the Annex to Regulation (EC) No. 1107/96 on the Registration of Geographical Indications and Designations of Origin under the Procedure Laid Down in Article 17 of Council Regulation (EEC) No. 2081/92. Available online: https://op.europa.eu/en/publication-detail/-/publication/01708409-532d-4e55-9f88-cb277630453f/language-en (accessed on 21 December 2022).
- Kizos, T.; Vakoufaris, H. Alternative agri-food geographies? Geographic indications in greece. Tijdschr. Voor Econ. En Soc. Geogr. 2011, 102, 220–235. [Google Scholar] [CrossRef]
- Mitropoulos, D.; Lambrinos, G. Changes in firmness of apples affected by moisture loss during storage. J. Hortic. Sci. Biotechnol. 2005, 80, 399–402. [Google Scholar] [CrossRef]
- Mitropoulos, D.; Lambrinos, G. “Delicious Pilafa” apple density changes as a quality index of mass loss degradation during storage. J. Food Qual. 2007, 30, 527–537. [Google Scholar] [CrossRef]
- Thanopoulos, R.; Chatzigeorgiou, T.; Argyropoulou, K.; Kostouros, N.-M.; Bebeli, P.J. State of Crop Landraces in Arcadia (Greece) and In-Situ Conservation Potential. Diversity 2021, 13, 558. [Google Scholar] [CrossRef]
- Villa, T.; Maxted, N.; Scholten, M.; Ford-Lloyd, B. Defining and identifying crop landraces. Plant Genet. Resour. 2005, 3, 373–384. [Google Scholar] [CrossRef]
- Veteläinen, M.; Negri, V.; Maxted, N. (Eds.) European Landraces: On-Farm Conservation, Management and Use; Bioversity Technical Bulletin; Bioversity International: Rome, Italy, 2009; Volume 15, p. 358. ISBN 978-92-9043-805-2. Available online: https://hdl.handle.net/10568/106154 (accessed on 27 January 2023).
- Park, M.; Danforth, B.; Losey, J.; Biddinger, D.; Vaughan, M.; Dollar, J.; Edwin Rajotte, E.; Agnello, A. Wild Pollinators of Eastern Apple Orchards and How to Conserve Them; College of Agriculture and Life Sciences, Cornell University: Ithaca, NY, USA, 2012; Available online: http://www.northeastipm.org/park2012 (accessed on 30 October 2020).
- Bellon, M.R.; Gotor, E.; Caracciolo, F. Conserving landraces and improving livelihoods: How to assess the success of on-farm conservation projects? Int. J. Agric. Sustain. 2014, 13, 167–182. [Google Scholar] [CrossRef]
- Goulson, D.; Lye, G.C.; Darvill, B. Decline and conservation of bumble bees. Annu. Rev. Entomol. 2008, 53, 191–208. [Google Scholar] [CrossRef]
- Hanley, M.E.; Franco, M.; Pichon, S.; Darvill, B.; Goulson, D. Breeding system, pollinator choice and variation in pollen quality in British herbaceous plants. Funct. Ecol. 2008, 22, 592–598. [Google Scholar] [CrossRef]
- Kleijn, D.; Raemakers, I. Ecological society of America A retrospective analysis of pollen host plant use by stable and declining bumble bee species. Ecol. Soc. Am. 2008, 89, 1811–1823. [Google Scholar] [CrossRef]
- Scheper, J.; Reemer, M.; van Kats, R.; Ozinga, W.A.; van der Linden, G.T.J.; Schaminée, J.H.J.; Siepel, H.; Kleijn, D. Museum specimens reveal loss of pollen host plants as key factor driving wild bee decline in The Netherlands. Proc. Natl. Acad. Sci. USA 2014, 111, 17552–17557. [Google Scholar] [CrossRef]
- Cole, L.J.; Baddeley, J.A.; Robertson, D.; Topp, C.F.E.; Walker, R.L.; Watson, C.A. Supporting wild pollinators in agricultural landscapes through targeted legume mixtures. Agric. Ecosyst. Environ. 2022, 323, 107648. [Google Scholar] [CrossRef]
- Pfiffner, L.; Laurent, J.; Cahenzli, F.; Maren, K.; Weronika, S.; Lene, S. Perennial Flower Strips—A Tool for Improving Pest Control in Fruit Orchards, Technical Guide No. 1096. 2018; 16p. Available online: www.fibl-shop.org (accessed on 16 March 2019).
- Cresswell, C.J.; Cunningham, H.M.; Wilcox, A.; Randall, N.P. A trait-based approach to plant species selection to increase functionality of farmland vegetative strips. Ecol. Evol. 2019, 9, 4532–4543. [Google Scholar] [CrossRef]
- Shavit, O.; Dafni, A.; Neeman, G. Competition between honey bees (Apis mellifera) and native solitary bees in the Mediterranean region of Israel—Implications for conservation. Isr. J. Plant Sci. 2009, 57, 171–183. [Google Scholar] [CrossRef]
- Kennedy, C.M.; Lonsdorf, E.; Neel, M.C.; Williams, N.M.; Ricketts, T.H.; Winfree, R.; Bommarco, R.; Brittain, C.; Burley, A.L.; Cariveau, D.; et al. A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol. Lett. 2013, 16, 584–599. [Google Scholar] [CrossRef]
- Gleiciani, P.-R.; Campos, M. Aspects of Landscape and Pollinators—What is Important to Bee Conservation? Diversity 2014, 6, 158–175. [Google Scholar] [CrossRef]
- Raderschall, C.A.; Bommarco, R.; Lindström, S.A.M.; Lundin, O. Landscape crop diversity and semi-natural habitat affect crop pollinators, pollination benefit and yield. Agric. Ecosyst. Environ. 2021, 306, 107189. [Google Scholar] [CrossRef]
- Tutin, T.G.E.A. (Ed.) Flora Europaea v. 5; Cambridge University Press: Cambridge, UK, 1980. [Google Scholar]
- Collins, A.G. Key to the Genera of British Bees; BWARS: London, UK, 2012; Available online: https://www.bwars.com/content/bees-britain (accessed on 5 September 2019).
- SAS Institute Inc. JMP v7; SAS Institute Inc.: Cary, NC, USA. Available online: https://www.jmp.com/en_ph/company/about-us.html (accessed on 27 January 2023).
- Feltham, H.; Park, K.; Minderman, J.; Goulson, D. Experimental evidence that wildflower strips increase pollinator visits to crops. Ecol. Evol. 2015, 5, 3523–3530. [Google Scholar] [CrossRef]
- Königslöw, V.; Fornoff, F.; Klein, A.M. Wild bee communities benefit from temporal complementarity of hedges and flower strips in apple orchards. J. Appl. Ecol. 2022, 59, 2814–2824. [Google Scholar] [CrossRef]
- Free, J.B. Insect Pollination of Crops By J. B. Free London: Academic Press (1970), pp. 544, £7.25. Exp. Agric. 1971, 7, 367–368. [Google Scholar] [CrossRef]
- Kirk, W.D.J. Faba bean: Vicia faba. Bee World 2004, 85, 60–62. [Google Scholar] [CrossRef]
- Aouar-Sadli, M.; Louadi, K.; Doumandji, S. Pollination of the broad bean (Vicia faba L. var. major) (Fabaceae) by wild bees and honey bees (Hymenoptera: Apoidea) and its impact on the seed production in the Tizi-Ouzou area (Algeria). Afr. J. Agric. Res. 2018, 3, 266–272. [Google Scholar]
- Marzinzig, B.; Brünjes, L.; Biagioni, S.; Behling, H.; Link, W.; Westphal, C. Bee pollinators of faba bean (Vicia faba L.) differ in their foraging behaviour and pollination efficiency. Agric. Ecosyst. Environ. 2018, 264, 24–33. [Google Scholar] [CrossRef]
- Boyle, R.M.D.; Philogène, B.J.R. The native pollinators of an apple orchard: Variations and significance. J. Hortic. Sci. 1983, 58, 355–363. [Google Scholar] [CrossRef]
- Wei, S.G.; Wang, R.; Smirle, M.J.; Xu, H.L. Release of Osmia excavata and Osmia jacoti (Hymenoptera: Megachilidae) for apple pollination. Can. Entomol. 2002, 134, 369–380. [Google Scholar] [CrossRef]
- Adamson, N.L.; Roulston, T.H.; Fell, R.D.; Mullins, D.E. From April to August—Wild bees pollinating crops through the growing season in Virginia, USA. Environ. Entomol. 2012, 41, 813–821. [Google Scholar] [CrossRef]
- Campbell, A.; Wilby, A.; Sutton, P.; Wäckers, F. Getting More Power from Your Flowers: Multi-Functional Flower Strips Enhance Pollinators and Pest Control Agents in Apple Orchards. Insects 2017, 8, 101. [Google Scholar] [CrossRef]
- Carvell, C.; Osborne, J.L.; Bourke, A.F.G.; Freeman, S.N.; Pywell, R.F.; Heard, M.S. Bumble bee species’ responses to a targeted conservation measure depend on landscape context and habitat quality. Ecol. Appl. 2011, 21, 1760–1771. [Google Scholar] [CrossRef]
- Underwood, E.; Tucker, G. Ecological Focus Area choices and their potential impacts on biodiversity. In Report for BirdLife Europe and the European Environmental Bureau; Institute for European Environmental Policy: London, UK, 2016. [Google Scholar] [CrossRef]
- Hart, K.; Mottershead, D.; Tucker, G.; Underwood, E.; Mar´echal, A.; Menet, L.; Martin, I.; Dayde, C.; Bresson, C.; Deniel, E.; et al. European Commission, Directorate-General for Agriculture and Rural Development, Evaluation Study of the Payment for Agricultural Practices Beneficial for the Climate and the Environment; Final Report. Publications Office: Luxembourg, 2017. Available online: https://data.europa.eu/doi/10.2762/71725 (accessed on 28 December 2022).
- Cole, L.J.; Kleijn, D.; Dicks, L.V.; Stout, J.C.; Potts, S.G.; Albrecht, M.; Balzan, M.V.; Bartomeus, I.; Bebeli, P.J.; Bevk, D.; et al. A critical analysis of the potential for EU Common Agricultural Policy measures to support wild pollinators on farmland. J. Appl. Ecol. 2020, 57, 681–694. [Google Scholar] [CrossRef]
- Shakeel, M.; Ali, H.; Ahmad, S.; Said, F.; Khan, K.A.; Bashir, M.A.; Anjum, S.I.; Islam, W.; Ghramh, H.A.; Ansari, M.J.; et al. Insect pollinators diversity and abundance in Eruca sativa Mill. (Arugula) and Brassica rapa L. (Field mustard) crops. Saudi J. Biol. Sci. 2018, 26, 1704–1709. [Google Scholar] [CrossRef]
- El-Berry, A.A.; Moustafa, M.A.; Abdel-Gawaad, A.A.; El-Bialey, S. Pollinators other than honey bees visiting certain vegetable plants in Egypt. Z. Für Angew. Entomol. 1974, 77, 106–110. [Google Scholar] [CrossRef]
- Dunn, L.; Lequerica, M.; Reid, C.R.; Latty, T. Dual ecosystem services of syrphid flies (Diptera: Syrphidae): Pollinators and biological control agents. Pest Manag. Sci. 2020, 76, 1973–1979. [Google Scholar] [CrossRef]
- Boroumand, R.Z.; Kouchaki, A. Evaluation of cardinal temperature for three species of medicinal plants, Ajowan (Trachyspermum ammi), Fennel (Foeniculum vulgare) and Dill (Anethum graveolens). Desert J. 2006, 11, 11–16. [Google Scholar]
- Hussein, M.H.; Abdel-Aal, S.A. Wild and honeybees as pollinators of 10 plant species in Assiut area. Egypt. Z. Fur Angew. Entomol. 1982, 93, 342–346. [Google Scholar] [CrossRef]
- Bendifallah, L.; Louadi, K.; Doumandji, S. Bee fauna potential visitors of coriander flowers Coriandrum sativum L. (Apiaceae) in the Mitidja area (Algeria). J. Apic. Sci. 2013, 57, 59–70. [Google Scholar] [CrossRef]
- Bretagnolle, V.; Gaba, S. Weeds for bees? A review. Agron. Sustain. Dev. 2015, 35, 891–909. [Google Scholar] [CrossRef]
- Štefanić, E.; Rašić, S.; Panjković, B.; Kovačević, V.; Zima, D.; Antunović, S.; Štefanić, I. The role of weeds from field margins in supporting crop pollinators. J. Cent. Eur. Agric. 2020, 21, 602–608. [Google Scholar] [CrossRef]
- Carvell, C.; Meek, W.R.; Pywell, R.F.; Nowakowski, M. The response of foraging bumblebees to successional change in newly created arable field margins. Biol. Conserv. 2004, 118, 327–339. [Google Scholar] [CrossRef]
- Nichols, R.N.; Goulson, D.; Holland, J.M. The best wildflowers for wild bees. J. Insect Conserv. 2019, 23, 819–830. [Google Scholar] [CrossRef]
- Ara, S.; Rather, Z.A.; Paray, M.A. Hang around flora-the pollination enhancers of apple, of Kashmir Himalaya. J. Pharmacogn. Phytochem. 2018, 7, 1462–1467. [Google Scholar]
- Lysenkov, S.N. Difficulties in Studies of the Ecological Links between Plants and Flower-Visiting Insects: One and a Half Centuries of Research on Veronica chamaedrys L. Biol. Bull. Rev. 2021, 11, 488–497. [Google Scholar] [CrossRef]
- Pesenko, Y.A. Contributions to the halictid fauna of the Eastern Palaearctic Region: Genus Lasioglossum Curtis (Hymenoptera: Halictidae, Halictinae). Zoosystematica Ross. 2006, 15, 133–166. [Google Scholar] [CrossRef]
- Nutt, P.; Ziermann, J.; Hintz, M.; Neuffer, B.; Theißen, G. Capsella as a model system to study the evolutionary relevance of floral homeotic mutants. Plant Syst. Evol. 2006, 259, 217–235. [Google Scholar] [CrossRef]
- Rosa García, R.; Miñarro, M. Role of floral resources in the conservation of pollinator communities in cider-apple orchards. Agric. Ecosyst. Environ. 2014, 183, 118–126. [Google Scholar] [CrossRef]
- Lynn, A.; Piotter, E.; Harrison, E.; Galen, C. Sexual and natural selection on pollen morphology in Taraxacum. Am. J. Bot. 2020, 107, 364–374. [Google Scholar] [CrossRef]
- Hattori, M.; Nagano, Y.; Itino, T. Geographic Variation in Flower Size and Flower-Visitor Composition of Two Bumblebee-Pollinated, Spring-Flowering Herbs, Lamium album L. var. barbatum (Lamiaceae) and Meehania urticifolia (Lamiaceae). Am. J. Plant Sci. 2015, 6, 737–745. [Google Scholar] [CrossRef]
- Waddington, K.D. Foraging patterns of halictid bees at flowers of Convolvulus arvensis. Psyche 1976, 83, 112–119. [Google Scholar] [CrossRef]
- Holm, L.G.; Plucknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weeds. Distribution and Biology; University Press of Hawaii: Honolulu, HI, USA, 1977. [Google Scholar]
- Skinner, K.; Smith, L.; Rice, P. Using noxious weed lists to prioritize targets for developing weed management strategies. Weed Sci. 2000, 48, 640–644. [Google Scholar] [CrossRef]
- Albrecht, M.; Kleijn, D.; Williams, N.M.; Tschumi, M.; Blaauw, B.R.; Bommarco, R.; Campbell, A.J.; Dainese, M.; Drummond, F.A.; Entling, M.H.; et al. The effectiveness of flower strips and hedgerows on pest control, pollination services and crop yield: A quantitative synthesis. Ecol. Lett. 2020, 23, 1488–1498. [Google Scholar] [CrossRef]
- McKerchar, M.; Potts, S.G.; Fountain, M.T.; Garratt, M.P.D.; Westbury, D.B. The potential for wildflower interventions to enhance natural enemies and pollinators in commercial apple orchards is limited by other management practices. Agric. Ecosyst. Environ. 2020, 301, 107034. [Google Scholar] [CrossRef]
- Wood, T.J.; Holland, J.M.; Goulson, D. Pollinator-friendly management does not increase the diversity of farmland bees and wasps. Biol. Conserv. 2015, 187, 120–126. [Google Scholar] [CrossRef]
- Morse, R.A.; Calderone, N.W. The Value of Honey Bees as Pollinators of US Crops in 2000. Bee Cult. 2000, 128, 1–15. [Google Scholar]
- Prendergast, K.S.; Leclercq, N.; Vereecken, N.J. Honey bees (Hymenoptera: Apidae) outnumber native bees in Tasmanian apple orchards: Perspectives for balancing crop production and native bee conservation. Austral Entomol. 2021, 60, 422–435. [Google Scholar] [CrossRef]
- Geslin, B.; Aizen, M.A.; Garcia, N.; Pereira, A.-J.; Vaissière, B.E.; Garibaldi, L.A. The impact of honey bee colony quality on crop yield and farmers’ profit in apples and pears. Agric. Ecosyst. Environ. 2017, 248, 153–161. [Google Scholar] [CrossRef]
- Blitzer, E.J.; Gibbs, J.; Park, M.G.; Danforth, B.N. Pollination services for apple are dependent on diverse wild bee communities. Agric. Ecosyst. Environ. 2016, 221, 1–7. [Google Scholar] [CrossRef]
- Földesi, R.; Kovács-Hostyánszki, A.; Kőrösi, Á.; Somay, L.; Elek, Z.; Markó, V.; Sárospataki, M.; Bakos, R.; Varga, Á.; Nyisztor, K.; et al. Relationships between wild bees, hoverflies and pollination success in apple orchards with different landscape contexts. Agric. For. Entomol. 2016, 18, 68–75. [Google Scholar] [CrossRef]
- Lowe, E.B.; Groves, R.; Gratton, C. Impacts of field-edge flower plantings on pollinator conservation and ecosystem service delivery—A meta-analysis. Agric. Ecosyst. Environ. 2021, 310, 107290. [Google Scholar] [CrossRef]
- Watson, J.C.; Wolf, A.T.; Ascher, J.S. Forested landscapes promote richness and abundance of native bees (Hymenoptera: Apoidea: Anthophila) in Wisconsin apple orchards. Environ. Entomol. 2011, 40, 621–632. [Google Scholar] [CrossRef]
- Gervais, A.; Bélisle, M.; Mazerolle, M.J.; Fournier, V. Landscape Enhancements in Apple Orchards: Higher Bumble Bee Queen Species Richness, But No Effect on Apple Quality. Insects 2021, 12, 421. [Google Scholar] [CrossRef]
- Senapathi, D.; Biesmeijer, J.C.; Breeze, T.D.; Kleijn, D.; Potts, S.G.; Carvalheiro, L.G. Pollinator conservation—The difference between managing for pollination services and preserving pollinator diversity. Curr. Opin. Insect Sci. 2015, 12, 93–101. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).