Validation of an Optogenetic Approach to the Study of Olfactory Behavior in the T-Maze of Drosophila melanogaster Adults
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fly Stocks
2.2. Immunostaining
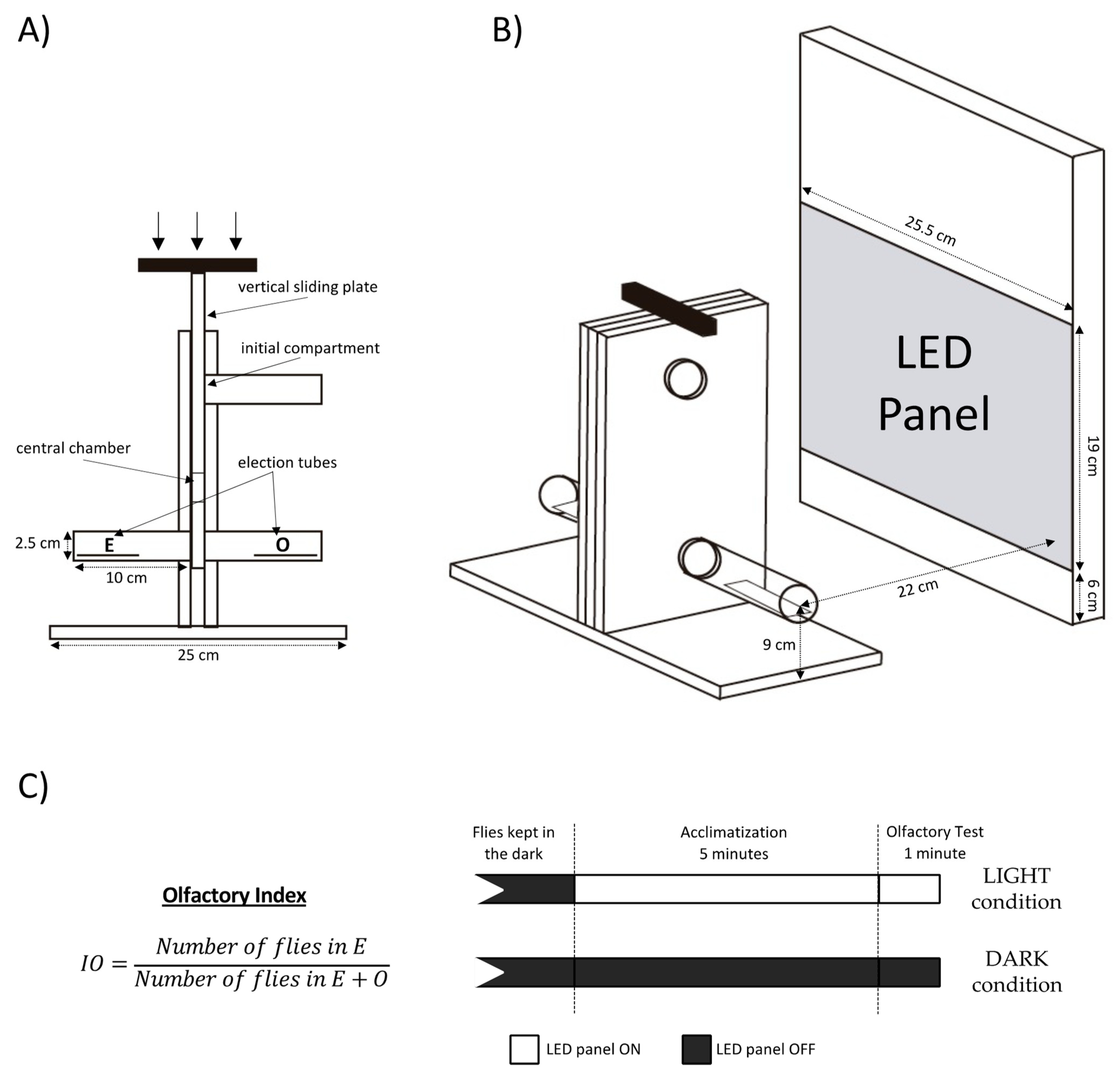
2.3. Behavioral Assay
2.4. Optogenetics
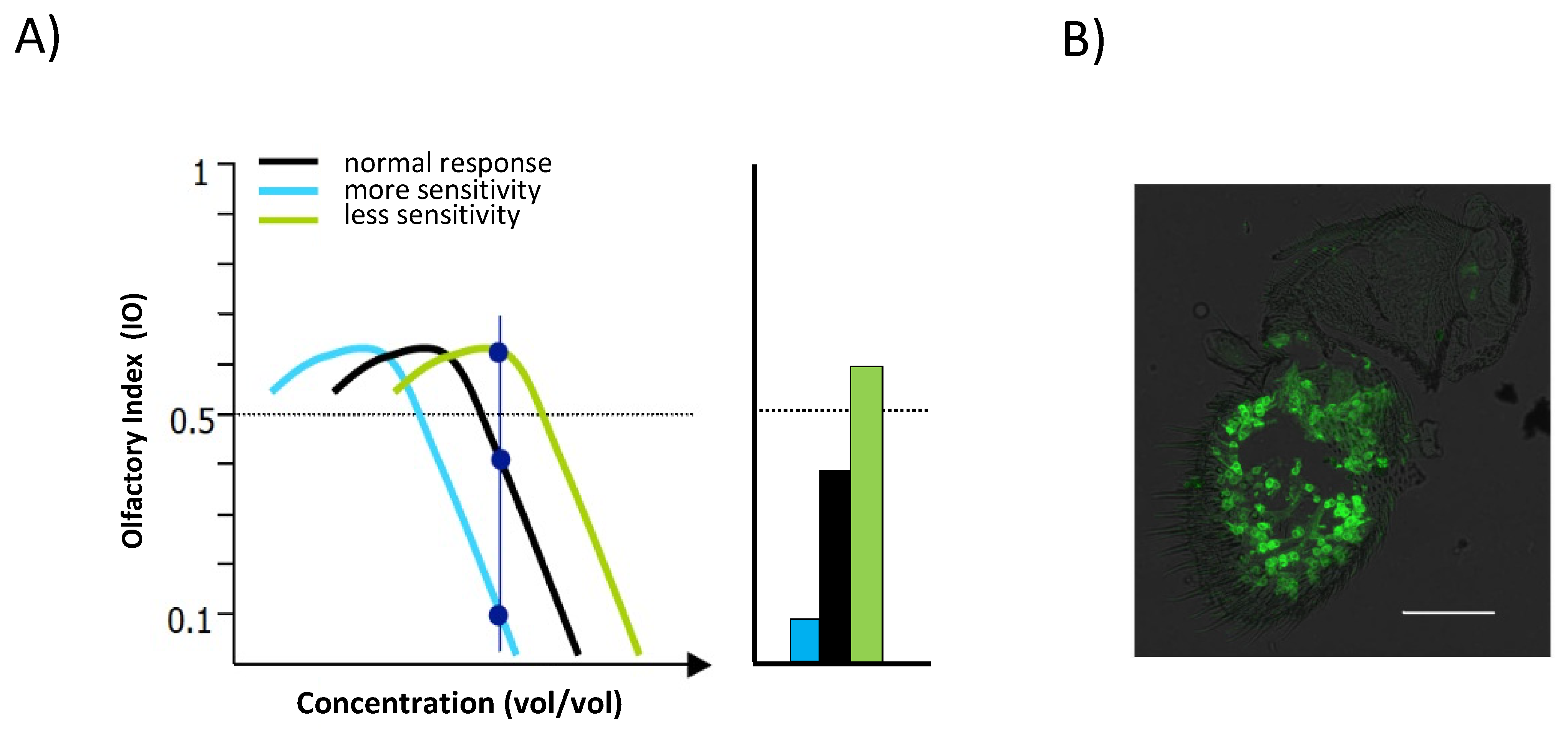
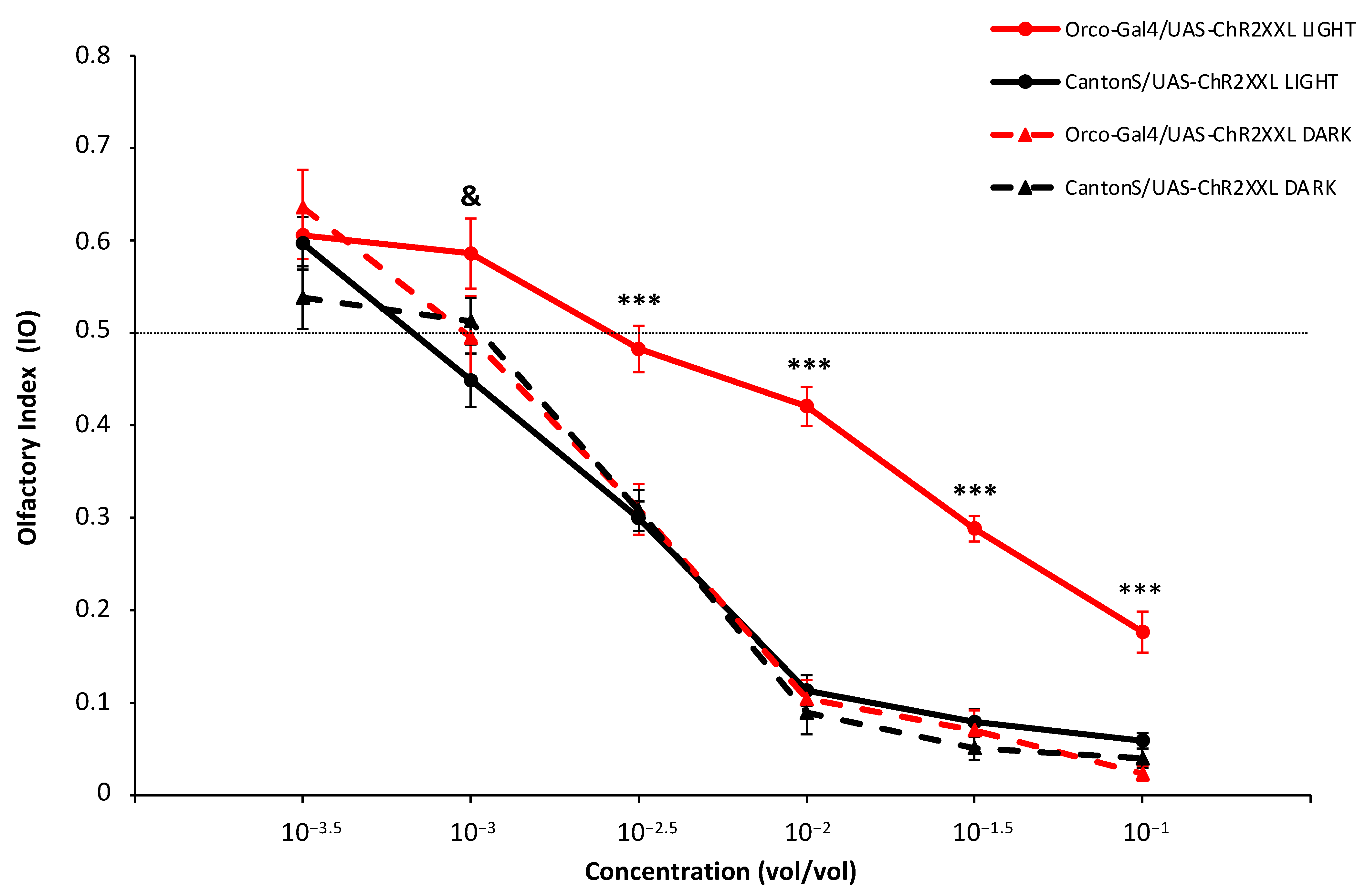
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiala, A.; Suska, A.; Schlüter, O.M. Optogenetic approaches in neuroscience. Cur. Biol. 2010, 20, R897–R903. [Google Scholar] [CrossRef] [Green Version]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef]
- Lima, S.Q.; Miesenböck, G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell 2005, 121, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Schroll, C.; Riemensperger, T.; Bucher, D.; Ehmer, J.; Völler, T.; Erbguth, K.; Gerber, B.; Hendel, T.; Nagel, G.; Buchner, E.; et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr. Biol. 2006, 16, 1741–1747. [Google Scholar] [CrossRef] [Green Version]
- Andersson, M.N.; Newcomb, R.D. Pest control compounds targeting insect chemoreceptors: Another silent spring? Front. Ecol. Evol. 2017, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Venthur, H.; Zhou, J.-J. Odorant receptors and odorant-binding proteins as insect pest control targets: A comparative analysis. Front. Physiol. 2018, 9, 1163. [Google Scholar] [CrossRef]
- Su, C.Y.; Menuz, K.; Carlson, J.R. Olfactory perception: Receptors, cells, and circuits. Cell 2009, 139, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Martin, F.; Boto, T.; Gomez-Diaz, C.; Alcorta, E. Elements of olfactory reception in adult Drosophila melanogaster. Anat. Rec. 2013, 296, 1477–1488. [Google Scholar] [CrossRef]
- Schmidt, H.R.; Benton, R. Molecular mechanisms of olfactory detection in insects: Beyond receptors. Open Biol. 2020, 10, 200252. [Google Scholar] [CrossRef]
- Fleischer, J.; Pregitzer, P.; Breer, H.; Krieger, J. Access to the odor world: Olfactory receptors and their role for signal transduction in insects. Cell. Mol. Life Sci. 2018, 75, 485–508. [Google Scholar] [CrossRef]
- Devaud, J.M. Experimental studies of adult Drosophila chemosensory behaviour. Behav. Process. 2003, 64, 177–196. [Google Scholar] [CrossRef]
- Martín, F.; Alcorta, E. Novel genetic approaches to behavior in Drosophila. J. Neurogenet. 2017, 31, 288–299. [Google Scholar] [CrossRef]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118, 401–415. [Google Scholar] [CrossRef]
- Störtkuhl, K.F.; Fiala, A. The smell of blue light: A new approach toward understanding an olfactory neuronal network. Front. Neurosci. 2011, 5, 72. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Nunez, L.; Belina, J.; Klein, M.; Si, G.; Claus, L.; Carlson, J.R.; Samuel, A.D. Reverse-correlation analysis of navigation dynamics in Drosophila larva using optogenetics. eLife 2015, 4, e06225. [Google Scholar] [CrossRef]
- Schulze, A.; Gomez-Marin, A.; Rajendran, V.G.; Lott, G.; Musy, M.; Ahammad, P.; Deogade, A.; Sharpe, J.; Riedl, J.; Jarriault, D.; et al. Dynamical feature extraction at the sensory periphery guides chemotaxis. eLife 2015, 4, e06694. [Google Scholar] [CrossRef] [Green Version]
- Clark, D.A.; Odell, S.R.; Armstrong, J.M.; Turcotte, M.; Kohler, D.; Mathis, A.; Schmidt, D.R.; Mathew, D. Behavior responses to chemical and optogenetic stimuli in Drosophila larvae. Front. Behav. Neurosci. 2018, 12, 324. [Google Scholar] [CrossRef] [Green Version]
- Suh, G.S.; de Leon, S.B.-T.; Tanimoto, H.; Fiala, A.; Benzer, S.; Anderson, D.J. Light activation of an innate olfactory avoidance response in Drosophila. Curr. Biol. 2007, 17, 905–908. [Google Scholar] [CrossRef] [Green Version]
- Bell, J.S.; Wilson, R.I. Behavior reveals selective summation and max pooling among olfactory processing channels. Neuron 2016, 91, 425–438. [Google Scholar] [CrossRef] [Green Version]
- Tumkaya, T.; Burhanudin, S.; Khalilnezhad, A.; Stewart, J.; Choi, H.; Claridge-Chang, A. Most primary olfactory neurons have individually neutral effects on behavior. eLife 2022, 11, e71238. [Google Scholar] [CrossRef]
- Dawydow, A.; Gueta, R.; Ljaschenko, D.; Ullrich, S.; Hermann, M.; Ehmann, N.; Gao, S.; Fiala, A.; Langenhan, T.; Nagel, G.; et al. Channelrhodopsin-2–XXL, a powerful optogenetic tool for low-light applications. Proc. Natl. Acad. Sci. USA 2014, 111, 13972–13977. [Google Scholar] [CrossRef] [Green Version]
- Meloni, I.; Sachidanandan, D.; Thum, A.S.; Kittel, R.J.; Murawski, C. Controlling the behaviour of Drosophila melanogaster via smartphone optogenetics. Sci. Rep. 2020, 10, 17614. [Google Scholar] [CrossRef]
- Inagaki, H.K.; Jung, Y.; Hoopfer, E.D.; Wong, A.M.; Mishra, N.; Lin, J.Y.; Tsien, R.Y.; Anderson, D.J. Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nat. Methods 2014, 11, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Klapoetke, N.C.; Murata, Y.; Kim, S.S.; Pulver, S.R.; Birdsey-Benson, A.; Cho, Y.K.; Morimoto, T.K.; Chuong, A.S.; Carpenter, E.J.; Tian, Z.; et al. Independent optical excitation of distinct neural populations. Nat. Methods 2014, 11, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Silbering, A.F.; Rytz, R.; Grosjean, Y.; Abuin, L.; Ramdya, P.; Jefferis, G.S.; Benton, R. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J. Neurosci. 2011, 31, 13357–13375. [Google Scholar] [CrossRef]
- Semelidou, O.; Acevedo, S.F.; Skoulakis, E. Accessing Olfactory Habituation in Drosophila melanogaster with a T-maze Paradigm. Bio-Protocol 2019, 9, e3259. [Google Scholar] [CrossRef]
- Wang, J.W.; Wong, A.M.; Flores, J.; Vosshall, L.B.; Axel, R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 2003, 112, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Benton, R.; Sachse, S.; Michnick, S.W.; Vosshall, L.B. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006, 4, e20. [Google Scholar] [CrossRef] [Green Version]
- Riveron, J.; Boto, T.; Alcorta, E. The effect of environmental temperature on olfactory perception in Drosophila melanogaster. J. Insect Physiol. 2009, 55, 943–951. [Google Scholar] [CrossRef]
- Devaud, J.M.; Keane, J.; Ferrús, A. Blocking sensory inputs to identified antennal glomeruli selectively modifies odorant perception in Drosophila. J. Neurobiol. 2003, 56, 1–12. [Google Scholar] [CrossRef]
- Nagel, K.I.; Wilson, R.I. Biophysical mechanisms underlying olfactory receptor neuron dynamics. Nat. Neurosci. 2011, 14, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.H.; Jing, B.Y.; Yang, D.; Zeng, X.; Shen, Y.; Tu, Y.; Luo, D.G. Distinct signaling of Drosophila chemoreceptors in olfactory sensory neurons. Proc. Natl. Acad. Sci. USA 2016, 113, E902–E911. [Google Scholar] [CrossRef] [Green Version]
- Borst, A.; Heisenberg, M. Osmotropotaxis in Drosophila melanogaster. J. Comp. Physiol. A 1982, 147, 479–484. [Google Scholar] [CrossRef]
- Ali, M.Z.; Anushree; Bilgrami, A.L.; Ahsan, J. Drosophila melanogaster chemosensory pathways as potential targets to curb the insect menace. Insects 2022, 13, 142. [Google Scholar] [CrossRef]
- Carey, A.F.; Carlson, J.R. Insect olfaction from model systems to disease control. Proc. Natl. Acad. Sci. USA 2011, 108, 12987–12995. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Zhao, X.; Liang, X.; Gao, X.; Liu, Y.; Wang, G. Identification of candidate chemosensory genes in Mythimna separata by transcriptomic analysis. BMC Genom. 2018, 19, 518. [Google Scholar] [CrossRef] [Green Version]
- Olafson, P.U.; Saski, C.A. Chemosensory-related gene family members of the horn fly, Haematobia irritans irritans (Diptera: Muscidae), identified by transcriptome analysis. Insects 2020, 11, 816. [Google Scholar] [CrossRef]
- Krieger, J.; Klink, O.; Mohl, C.; Raming, K.; Breer, H. A candidate olfactory receptor subtype highly conserved across different insect orders. J. Comp. Physiol. A 2003, 189, 519–526. [Google Scholar] [CrossRef]
- Pitts, R.J.; Fox, A.N.; Zwiebel, L.J. A highly conserved candidate chemoreceptor expressed in both olfactory and gustatory tissues in the malaria vector Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2004, 101, 5058–5063. [Google Scholar] [CrossRef] [Green Version]
- Smadja, C.; Shi, P.; Butlin, R.K.; Robertson, H.M. Large gene family expansions and adaptive evolution for odorant and gustatory receptors in the pea aphid, Acyrthosiphon pisum. Mol. Biol. Evol. 2009, 26, 2073–2086. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Krieger, J.; Zhang, L.; Breer, H. The olfactory co-receptor orco from the migratory locust (Locusta migratoria) and the desert locust (Schistocerca gregaria): Identification and expression pattern. Int. J. Biol. Sci. 2012, 8, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Homem, R.A.; Davies, T. An overview of functional genomic tools in deciphering insecticide resistance. Curr. Opin. Insect Sci. 2018, 27, 103–110. [Google Scholar] [CrossRef]
- Mysore, K.; Li, P.; Duman-Scheel, M. Identification of Aedes aegypti cis-regulatory elements that promote gene expression in olfactory receptor neurons of distantly related dipteran insects. Parasit. Vectors 2018, 11, 406. [Google Scholar] [CrossRef] [Green Version]
- Tabuchi, M.; Sakurai, T.; Mitsuno, H.; Namiki, S.; Minegishi, R.; Shiotsuki, T.; Uchino, K.; Sezutsu, H.; Tamura, T.; Haupt, S.S.; et al. Pheromone responsiveness threshold depends on temporal integration by antennal lobe projection neurons. Proc. Natl. Acad. Sci. USA 2013, 110, 15455–15460. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coya, R.; Martin, F.; Calvin-Cejudo, L.; Gomez-Diaz, C.; Alcorta, E. Validation of an Optogenetic Approach to the Study of Olfactory Behavior in the T-Maze of Drosophila melanogaster Adults. Insects 2022, 13, 662. https://doi.org/10.3390/insects13080662
Coya R, Martin F, Calvin-Cejudo L, Gomez-Diaz C, Alcorta E. Validation of an Optogenetic Approach to the Study of Olfactory Behavior in the T-Maze of Drosophila melanogaster Adults. Insects. 2022; 13(8):662. https://doi.org/10.3390/insects13080662
Chicago/Turabian StyleCoya, Ruth, Fernando Martin, Laura Calvin-Cejudo, Carolina Gomez-Diaz, and Esther Alcorta. 2022. "Validation of an Optogenetic Approach to the Study of Olfactory Behavior in the T-Maze of Drosophila melanogaster Adults" Insects 13, no. 8: 662. https://doi.org/10.3390/insects13080662
APA StyleCoya, R., Martin, F., Calvin-Cejudo, L., Gomez-Diaz, C., & Alcorta, E. (2022). Validation of an Optogenetic Approach to the Study of Olfactory Behavior in the T-Maze of Drosophila melanogaster Adults. Insects, 13(8), 662. https://doi.org/10.3390/insects13080662





