Deciphering the Biological Enigma—Genomic Evolution Underlying Anhydrobiosis in the Phylum Tardigrada and the Chironomid Polypedilum vanderplanki
Simple Summary
Abstract
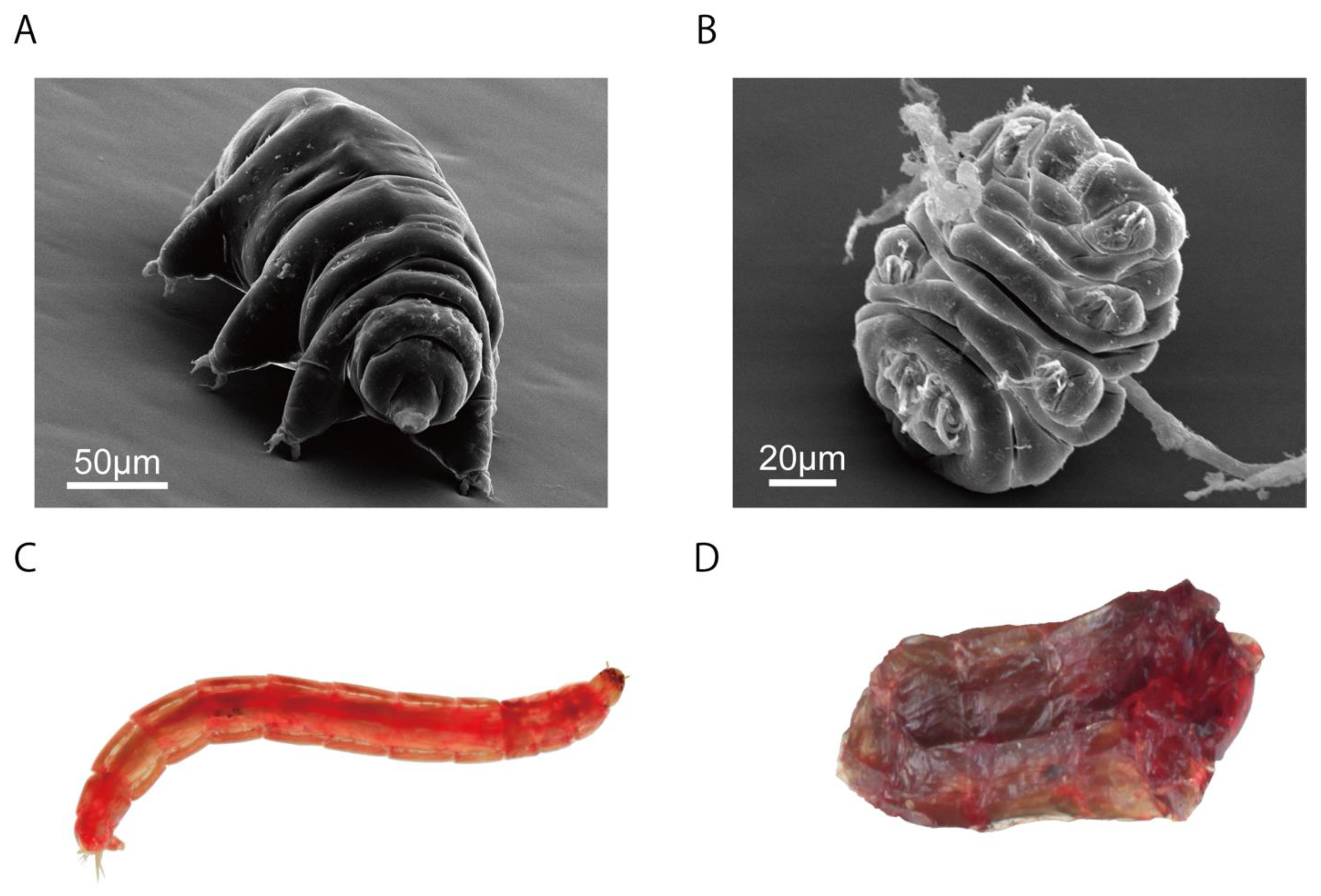
1. Anhydrobiosis—A State with No Visible Signs of Life
2. Candidate Protective Molecules in Anhydrobiosis, from Trehalose to Intrinsically Disordered Proteins
2.1. “Traditional” Protective Molecules Accumulating in Anhydrobiotes
2.2. Abundant Proteins in an Anhydrobiotic Tardigrade Are Lineage-Specific Intrinsically Disordered Proteins
3. Conserved Cellular Maintenance and Repair Pathways Contributing to Anhydrobiosis
4. Examples of Genomic Evolution Underlying Anhydrobiosis at Species (Pol. vanderplanki) and Phylum Levels (Tardigrada)
4.1. Tardigrada: A Phylum Showing Complicated Evolution of Anhydrobiosis
4.2. Polypedilum vanderplanki and Polypedilum pembai: The Only Insects Capable of Anhydrobiosis
5. Considerations for Future Development in Anhydrobiosis Research
5.1. Complex Evolution Sometimes Calls for Elaborate Methods
5.2. Functional Analysis of Anhydrobiosis Genes In Vivo
5.3. Preconditioning Determines the Survival of Anhydrobiosis
5.4. Desiccation-Induced Quiescence and Cryptobiotic Anhydrobiosis
5.5. The Conundrum of Latent Life—Comparison of Distinct Anhydrobiotic Mechanisms across Phyla
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franks, F. Water: A Matrix of Life, 2nd ed.; The Royal Society of Chemistry: Cambridge, UK, 2000; 225p. [Google Scholar]
- Sheng, H.P.; Huggins, R.A. A review of body composition studies with emphasis on total body water and fat. Am. J. Clin. Nutr. 1979, 32, 630–647. [Google Scholar] [CrossRef]
- Van Leeuwenhoek, A. On certain animalcules found in the sediment in gutters of the roofs of houses. In The Select Works of Antony van Leeuwenhoekm, Letter 144; BiblioBazaar: London, UK, 1702; Volume 2, pp. 207–213. [Google Scholar]
- Keilin, D. The problem of anabiosis or latent life: History and current concept. Proc. R. Soc. Lond. B Biol. Sci. 1959, 150, 149–191. [Google Scholar] [CrossRef]
- Clegg, J.S. Cryptobiosis—A peculiar state of biological organization. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 128, 613–624. [Google Scholar] [CrossRef]
- Clegg, J.S. Desiccation tolerance in encysted embryos of the animal extremophile, Artemia. Integr. Comp. Biol. 2005, 45, 715–724. [Google Scholar] [CrossRef]
- Cornette, R.; Yamamoto, N.; Yamamoto, M.; Kobayashi, T.; Petrova, N.A.; Gusev, O.; Shimura, S.; Kikawada, T.; Pemba, D.; Okuda, T. A new anhydrobiotic midge from Malawi, Polypedilum pembai sp. n. (Diptera: Chironomidae), closely related to the desiccation tolerant midge, Polypedilum vanderplanki Hinton. Syst. Entomol. 2017, 42, 814–825. [Google Scholar] [CrossRef]
- Hinton, H.E. Cryptobiosis in the Larva of Polypedilum vanderplanki Hint. (Chironomidae). J. Insect Physiol. 1960, 5, 286–300. [Google Scholar] [CrossRef]
- Needham, J. Concerning chalky tubulous concretions called Malm: With some microscopical observations on the Farina of Red Lily, and of Worms discovered in Smutty Corn. Philos. Trans. 1743, 42, 634–641. [Google Scholar]
- Corti, B. Osservazioni Microscopiche Sulla Tremella e Sulla Circulazione del Fluido in una Pianta Acquajuola (Chara); G. Rocchi: Lucca, Italy, 1774. [Google Scholar]
- Wright, J.; Westh, P.; Ramlov, H. Cryptobiosis in Tardigrada. Biol. Rev. 1991, 67, 1–29. [Google Scholar] [CrossRef]
- Gadd, G.M.; Chalmers, K.; Reed, R.H. The Role of Trehalose in Dehydration Resistance of Saccharomyces cerevisiae. FEMS Microbiol. Lett. 1987, 48, 249–254. [Google Scholar] [CrossRef]
- Ratnakumar, S.; Tunnacliffe, A. Intracellular trehalose is neither necessary nor sufficient for desiccation tolerance in yeast. FEMS Yeast Res. 2006, 6, 902–913. [Google Scholar] [CrossRef]
- Tapia, H.; Koshland, D.E. Trehalose is a versatile and long-lived chaperone for desiccation tolerance. Curr. Biol. 2014, 24, 2758–2766. [Google Scholar] [CrossRef]
- Tapia, H.; Young, L.; Fox, D.; Bertozzi, C.R.; Koshland, D. Increasing intracellular trehalose is sufficient to confer desiccation tolerance to Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2015, 112, 6122–6127. [Google Scholar] [CrossRef]
- Higa, L.M.; Womersley, C.Z. New insights into the anhydrobiotic phenomenon: The effects of trehalose content and differential rates of evaporative water loss on the survival of Aphelenchus avenae. J. Exp. Zool. 1993, 267, 120–129. [Google Scholar] [CrossRef]
- Erkut, C.; Penkov, S.; Khesbak, H.; Vorkel, D.; Verbavatz, J.M.; Fahmy, K.; Kurzchalia, T.V. Trehalose renders the dauer larva of Caenorhabditis elegans resistant to extreme desiccation. Curr. Biol. 2011, 21, 1331–1336. [Google Scholar] [CrossRef]
- Ricci, C. Anhydrobiotic capabilities of bdelloid rotifers. Hydrobiologia 1998, 387, 321–326. [Google Scholar] [CrossRef]
- Lapinski, J.; Tunnacliffe, A. Anhydrobiosis without trehalose in bdelloid rotifers. FEBS Lett. 2003, 553, 387–390. [Google Scholar] [CrossRef]
- Watanabe, M.; Kikawada, T.; Minagawa, N.; Yukuhiro, F.; Okuda, T. Mechanism allowing an insect to survive complete dehydration and extreme temperatures. J. Exp. Biol. 2002, 205, 2799–2802. [Google Scholar] [CrossRef]
- Sakurai, M.; Furuki, T.; Akao, K.; Tanaka, D.; Nakahara, Y.; Kikawada, T.; Watanabe, M.; Okuda, T. Vitrification is essential for anhydrobiosis in an African chironomid, Polypedilum vanderplanki. Proc. Natl. Acad. Sci. USA 2008, 105, 5093–5098. [Google Scholar] [CrossRef]
- Watanabe, K.; Imanishi, S.; Akiduki, G.; Cornette, R.; Okuda, T. Air-dried cells from the anhydrobiotic insect, Polypedilum vanderplanki, can survive long term preservation at room temperature and retain proliferation potential after rehydration. Cryobiology 2016, 73, 93–98. [Google Scholar] [CrossRef]
- Horikawa, D.D.; Kunieda, T.; Abe, W.; Watanabe, M.; Nakahara, Y.; Yukuhiro, F.; Sakashita, T.; Hamada, N.; Wada, S.; Funayama, T.; et al. Establishment of a rearing system of the extremotolerant tardigrade Ramazzottius varieornatus: A new model animal for astrobiology. Astrobiology 2008, 8, 549–556. [Google Scholar] [CrossRef]
- Kondo, K.; Kubo, T.; Kunieda, T. Suggested Involvement of PP1/PP2A Activity and De Novo Gene Expression in Anhydrobiotic Survival in a Tardigrade, Hypsibius dujardini, by Chemical Genetic Approach. PLoS ONE 2015, 10, e0144803. [Google Scholar] [CrossRef]
- Poprawa, I.; Bartylak, T.; Kulpla, A.; Erdmann, W.; Roszkowska, M.; Chajec, L.; Kaczmarek, L.; Karachitos, A.; Kmita, H. Verification of Hypsibius exemplaris Gasiorek et al., 2018 (Eutardigrada; Hypsibiidae) application in anhydrobiosis research. PLoS ONE 2022, 17, e0261485. [Google Scholar] [CrossRef]
- Hara, Y.; Shibahara, R.; Kondo, K.; Abe, W.; Kunieda, T. Parallel evolution of trehalose production machinery in anhydrobiotic animals via recurrent gene loss and horizontal transfer. Open Biol. 2021, 11, 200413. [Google Scholar] [CrossRef]
- Horikawa, D.D.; Higashi, S. Desiccation tolerance of the tardigrade Milnesium tardigradum collected in Sapporo, Japan, and Bogor, Indonesia. Zool. Sci. 2004, 21, 813–816. [Google Scholar] [CrossRef]
- Hygum, T.L.; Clausen, L.K.B.; Halberg, K.A.; Jørgensen, A.; Møbjerg, N. Tun formation is not a prerequisite for desiccation tolerance in the marine tidal tardigrade Echiniscoides sigismundi. Zool. J. Linn. Soc. 2016, 178, 907–911. [Google Scholar] [CrossRef]
- Jönsson, K.I.; Borsari, S.; Rebecchi, L. Anhydrobiotic Survival in Populations of the Tardigrades Richtersius coronifer and Ramazzottius oberhaeuseri from Italy and Sweden. Zool. Anz. 2001, 240, 419–423. [Google Scholar] [CrossRef]
- Gade, V.R.; Traikov, S.; Oertel, J.; Fahmy, K.; Kurzchalia, T.V. C. elegans possess a general program to enter cryptobiosis that allows dauer larvae to survive different kinds of abiotic stress. Sci. Rep. 2020, 10, 13466. [Google Scholar] [CrossRef]
- Jonsson, K.I. Causes and consequences of excess resistance in cryptobiotic metazoans. Physiol. Biochem. Zool. 2003, 76, 429–435. [Google Scholar] [CrossRef][Green Version]
- Becquerel, P. La suspension de la vie au dessous de 1/20 K absolu par demagnetization adiabatique de l’alun de fer dans le vide les plus eléve. CR Hebd. Seance. Acad. Sci. Paris 1950, 231, 261–264. [Google Scholar]
- Hengherr, S.; Reuner, A.; Brummer, F.; Schill, R.O. Ice crystallization and freeze tolerance in embryonic stages of the tardigrade Milnesium tardigradum. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 156, 151–155. [Google Scholar] [CrossRef]
- Hengherr, S.; Worland, M.R.; Reuner, A.; Brummer, F.; Schill, R.O. Freeze tolerance, supercooling points and ice formation: Comparative studies on the subzero temperature survival of limno-terrestrial tardigrades. J. Exp. Biol. 2009, 212, 802–807. [Google Scholar] [CrossRef]
- Hengherr, S.; Worland, M.R.; Reuner, A.; Brummer, F.; Schill, R.O. High-temperature tolerance in anhydrobiotic tardigrades is limited by glass transition. Physiol. Biochem. Zool. 2009, 82, 749–755. [Google Scholar] [CrossRef]
- Persson, D.; Halberg, K.A.; Jorgensen, A.; Ricci, C.; Mobjerg, N.; Kristensen, R.M. Extreme stress tolerance in tardigrades: Surviving space conditions in low earth orbit. J. Zool. Syst. Evol. Res. 2011, 49, 90–97. [Google Scholar] [CrossRef]
- Horikawa, D.D.; Iwata, K.; Kawai, K.; Koseki, S.; Okuda, T.; Yamamoto, K. High hydrostatic pressure tolerance of four different anhydrobiotic animal species. Zool. Sci. 2009, 26, 238–242. [Google Scholar] [CrossRef]
- Ono, F.; Mori, Y.; Takarabe, K.; Fujii, A.; Saigusa, M.; Matsushima, Y.; Yamazaki, D.; Ito, E.; Galas, S.; Saini, N.L.; et al. Effect of ultra-high pressure on small animals, tardigrades and Artemia. Cogent Phys. 2016, 3, 1167575. [Google Scholar] [CrossRef]
- Seki, K.; Toyoshima, M. Preserving tardigrades under pressure. Nature 1998, 395, 853–854. [Google Scholar] [CrossRef]
- Ramløv, H.; Westh, P. Cryptobiosis in the Eutardigrade Adorybiotus (Richtersius) coronifer: Tolerance to Alcohols, Temperature and de novo Protein Synthesis. Zool. Anz. 2001, 240, 517–523. [Google Scholar] [CrossRef]
- Hygum, T.L.; Fobian, D.; Kamilari, M.; Jorgensen, A.; Schiott, M.; Grosell, M.; Mobjerg, N. Comparative Investigation of Copper Tolerance and Identification of Putative Tolerance Related Genes in Tardigrades. Front. Physiol. 2017, 8, 95. [Google Scholar] [CrossRef]
- Halberg, K.A.; Persson, D.; Ramlov, H.; Westh, P.; Kristensen, R.M.; Mobjerg, N. Cyclomorphosis in Tardigrada: Adaptation to environmental constraints. J. Exp. Biol. 2009, 212, 2803–2811. [Google Scholar] [CrossRef]
- Heidemann, N.W.T.; Smith, D.K.; Hygum, T.L.; Stapane, L.; Clausen, L.K.B.; Jørgensen, A.; Hélix-Nielsen, C.; Møbjerg, N. Osmotic stress tolerance in semi-terrestrial tardigrades. Zool. J. Linn. Soc. 2016, 178, 912–918. [Google Scholar] [CrossRef]
- Bonifacio, A.; Guidetti, R.; Altiero, T.; Sergo, V.; Rebecchi, L. Nature, source and function of pigments in tardigrades: In Vivo raman imaging of carotenoids in Echiniscus blumi. PLoS ONE 2012, 7, e50162. [Google Scholar] [CrossRef]
- Altiero, T.; Guidetti, R.; Caselli, V.; Cesari, M.; Rebecchi, L. Ultraviolet radiation tolerance in hydrated and desiccated eutardigrades. J. Zool. Syst. Evol. Res. 2011, 49, 104–110. [Google Scholar] [CrossRef]
- Horikawa, D.D.; Cumbers, J.; Sakakibara, I.; Rogoff, D.; Leuko, S.; Harnoto, R.; Arakawa, K.; Katayama, T.; Kunieda, T.; Toyoda, A.; et al. Analysis of DNA repair and protection in the Tardigrade Ramazzottius varieornatus and Hypsibius dujardini after exposure to UVC radiation. PLoS ONE 2013, 8, e64793. [Google Scholar] [CrossRef]
- May, R.M.; Maria, M.; Gumard, J. Action différentielle des rayons x et ultraviolets sur le tardigrade Macrobiotus areolatus, a l’état actif et desséché. Bull. Biol. Fr. Belg. 1964, 98, 349–367. [Google Scholar]
- Beltran-Pardo, E.; Jonsson, K.I.; Harms-Ringdahl, M.; Haghdoost, S.; Wojcik, A. Tolerance to Gamma Radiation in the Tardigrade Hypsibius dujardini from Embryo to Adult Correlate Inversely with Cellular Proliferation. PLoS ONE 2015, 10, e0133658. [Google Scholar] [CrossRef]
- Beltran-Pardo, E.; Jonsson, K.I.; Wojcik, A.; Haghdoost, S.; Harms-Ringdahl, M.; Bermudez-Cruz, R.M.; Bernal Villegas, J.E. Effects of ionizing radiation on embryos of the tardigrade Milnesium cf. tardigradum at different stages of development. PLoS ONE 2013, 8, e72098. [Google Scholar] [CrossRef]
- Horikawa, D.D.; Yamaguchi, A.; Sakashita, T.; Tanaka, D.; Hamada, N.; Yukuhiro, F.; Kuwahara, H.; Kunieda, T.; Watanabe, M.; Nakahara, Y.; et al. Tolerance of anhydrobiotic eggs of the Tardigrade Ramazzottius varieornatus to extreme environments. Astrobiology 2012, 12, 283–289. [Google Scholar] [CrossRef]
- Horikawa, D.D.; Sakashita, T.; Katagiri, C.; Watanabe, M.; Kikawada, T.; Nakahara, Y.; Hamada, N.; Wada, S.; Funayama, T.; Higashi, S.; et al. Radiation tolerance in the tardigrade Milnesium tardigradum. Int. J. Radiat. Biol. 2006, 82, 843–848. [Google Scholar] [CrossRef]
- Jonsson, K.I.; Hygum, T.L.; Andersen, K.N.; Clausen, L.K.; Mobjerg, N. Tolerance to Gamma Radiation in the Marine Heterotardigrade, Echiniscoides sigismundi. PLoS ONE 2016, 11, e0168884. [Google Scholar] [CrossRef]
- Jönsson, I.; Beltran-Pardo, E.; Haghdoost, S.; Wojcik, A.; Bermúdez-Cruz, R.M.; Bernal Villegas, J.E.; Harms-Ringdahl, M. Tolerance to gamma-irradiation in eggs of the tardigrade Richtersius coronifer depends on stage of development. J. Limnol. 2013, 72, 73–79. [Google Scholar] [CrossRef]
- Jonsson, K.I.; Harms-Ringdahl, M.; Torudd, J. Radiation tolerance in the eutardigrade Richtersius coronifer. Int. J. Radiat. Biol. 2005, 81, 649–656. [Google Scholar] [CrossRef]
- Jonsson, K.I.; Wojcik, A. Tolerance to X-rays and Heavy Ions (Fe, He) in the Tardigrade Richtersius coronifer and the Bdelloid Rotifer Mniobia russeola. Astrobiology 2017, 17, 163–167. [Google Scholar] [CrossRef]
- Nilsson, E.J.; Jonsson, K.I.; Pallon, J. Tolerance to proton irradiation in the eutardigrade Richtersius coronifer—A nuclear microprobe study. Int. J. Radiat. Biol. 2010, 86, 420–427. [Google Scholar] [CrossRef]
- Crowe, J.H.; Hoekstra, F.A.; Crowe, L.M. Anhydrobiosis. Annu. Rev. Physiol. 1992, 54, 579–599. [Google Scholar] [CrossRef]
- Oliver, M.J.; Farrant, J.M.; Hilhorst, H.W.M.; Mundree, S.; Williams, B.; Bewley, J.D. Desiccation Tolerance: Avoiding Cellular Damage during Drying and Rehydration. Annu. Rev. Plant Biol. 2020, 71, 435–460. [Google Scholar] [CrossRef]
- Lebre, P.H.; De Maayer, P.; Cowan, D.A. Xerotolerant bacteria: Surviving through a dry spell. Nat. Rev. Microbiol. 2017, 15, 285–296. [Google Scholar] [CrossRef]
- Crowe, L.M. Lessons from nature: The role of sugars in anhydrobiosis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 131, 505–513. [Google Scholar] [CrossRef]
- Guo, N.; Puhlev, I.; Brown, D.R.; Mansbridge, J.; Levine, F. Trehalose expression confers desiccation tolerance on human cells. Nat. Biotechnol. 2000, 18, 168–171. [Google Scholar] [CrossRef]
- Eroglu, A.; Russo, M.J.; Bieganski, R.; Fowler, A.; Cheley, S.; Bayley, H.; Toner, M. Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat. Biotechnol. 2000, 18, 163–167. [Google Scholar] [CrossRef]
- De Castro, A.G.; Lapinski, J.; Tunnacliffe, A. Anhydrobiotic engineering. Nat. Biotechnol. 2000, 18, 473. [Google Scholar] [CrossRef]
- Madin, K.A.C.; Crowe, J.H. Anhydrobiosis in nematodes: Carbohydrate and lipid metabolism during dehydration. J. Exp. Zool. 1975, 193, 335–342. [Google Scholar] [CrossRef]
- Crowe, J.H.; Crowe, L.M.; Chapman, D. Preservation of membranes in anhydrobiotic organisms: The role of trehalose. Science 1984, 223, 701–703. [Google Scholar] [CrossRef]
- Nakahara, Y.; Imanishi, S.; Mitsumasu, K.; Kanamori, Y.; Iwata, K.; Watanabe, M.; Kikawada, T.; Okuda, T. Cells from an anhydrobiotic chironomid survive almost complete desiccation. Cryobiology 2010, 60, 138–146. [Google Scholar] [CrossRef]
- Kikawada, T.; Saito, A.; Kanamori, Y.; Nakahara, Y.; Iwata, K.; Tanaka, D.; Watanabe, M.; Okuda, T. Trehalose transporter 1, a facilitated and high-capacity trehalose transporter, allows exogenous trehalose uptake into cells. Proc. Natl. Acad. Sci. USA 2007, 104, 11585–11590. [Google Scholar] [CrossRef]
- Ryabova, A.; Cornette, R.; Cherkasov, A.; Watanabe, M.; Okuda, T.; Shagimardanova, E.; Kikawada, T.; Gusev, O. Combined metabolome and transcriptome analysis reveals key components of complete desiccation tolerance in an anhydrobiotic insect. Proc. Natl. Acad. Sci. USA 2020, 117, 19209–19220. [Google Scholar] [CrossRef]
- Westh, P.; Ramlov, H. Trehalose Accumulation in the Tardigrade Adorybiotus coronifer during Anhydrobiosis. J. Exp. Zool. 1991, 258, 303–311. [Google Scholar] [CrossRef]
- Hengherr, S.; Heyer, A.G.; Kohler, H.R.; Schill, R.O. Trehalose and anhydrobiosis in tardigrades—Evidence for divergence in responses to dehydration. FEBS J. 2008, 275, 281–288. [Google Scholar] [CrossRef]
- Arakawa, K. Comparative metabolomics of anhydrobiosis in tardigrade Ramazzottius varieornatus. J. Jpn. Soc. Extrem. 2013, 11, 30–37. [Google Scholar]
- Sugiura, K.; Matsumoto, M.; Kunieda, T. Description of a model tardigrade Paramacrobiotus metropolitanus sp. nov. (Eutardigrada) from Japan with a summary of its life history, reproduction and genomics. Zootaxa 2022, 5134, 92–112. [Google Scholar] [CrossRef]
- Yoshida, Y.; Koutsovoulos, G.; Laetsch, D.R.; Stevens, L.; Kumar, S.; Horikawa, D.D.; Ishino, K.; Komine, S.; Kunieda, T.; Tomita, M.; et al. Comparative genomics of the tardigrades Hypsibius dujardini and Ramazzottius varieornatus. PLoS Biol. 2017, 15, e2002266. [Google Scholar] [CrossRef]
- Crowe, J.H.; Oliver, A.E.; Hoekstra, F.A.; Crowe, L.M. Stabilization of dry membranes by mixtures of hydroxyethyl starch and glucose: The role of vitrification. Cryobiology 1997, 35, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H. Trehalose as a “chemical chaperone”: Fact and fantasy. Adv. Exp. Med. Biol. 2007, 594, 143–158. [Google Scholar] [CrossRef]
- Cuming, A.C. LEA Proteins. In Seed Proteins; Shewry, P.R., Casey, R., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 753–780. [Google Scholar]
- Tunnacliffe, A.; Wise, M.J. The continuing conundrum of the LEA proteins. Naturwissenschaften 2007, 94, 791–812. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Olvera-Carrillo, Y.; Garciarrubio, A.; Campos, F.; Covarrubias, A.A. The enigmatic LEA proteins and other hydrophilins. Plant Physiol. 2008, 148, 6–24. [Google Scholar] [CrossRef]
- Roberts, J.K.; DeSimone, N.A.; Lingle, W.L.; Dure, L., 3rd. Cellular Concentrations and Uniformity of Cell-Type Accumulation of Two Lea Proteins in Cotton Embryos. Plant Cell 1993, 5, 769–780. [Google Scholar] [CrossRef]
- Solomon, A.; Salomon, R.; Paperna, I.; Glazer, I. Desiccation stress of entomopathogenic nematodes induces the accumulation of a novel heat-stable protein. Parasitology 2000, 121, 409–416. [Google Scholar] [CrossRef]
- Browne, J.; Tunnacliffe, A.; Burnell, A. Anhydrobiosis—Plant desiccation gene found in a nematode. Nature 2002, 416, 38. [Google Scholar] [CrossRef]
- Browne, J.A.; Dolan, K.M.; Tyson, T.; Goyal, K.; Tunnacliffe, A.; Burnell, A.M. Dehydration-specific induction of hydrophilic protein genes in the anhydrobiotic nematode Aphelenchus avenae. Eukaryot Cell 2004, 3, 966–975. [Google Scholar] [CrossRef]
- Tunnacliffe, A.; Lapinski, J.; McGee, B. A Putative LEA Protein, but no Trehalose, is Present in Anhydrobiotic Bdelloid Rotifers. Hydrobiologia 2005, 546, 315–321. [Google Scholar] [CrossRef]
- Kikawada, T.; Nakahara, Y.; Kanamori, Y.; Iwata, K.; Watanabe, M.; McGee, B.; Tunnacliffe, A.; Okuda, T. Dehydration-induced expression of LEA proteins in an anhydrobiotic chironomid. Biochem. Biophys. Res. Commun. 2006, 348, 56–61. [Google Scholar] [CrossRef]
- Hand, S.C.; Jones, D.; Menze, M.A.; Witt, T.L. Life without water: Expression of plant LEA genes by an anhydrobiotic arthropod. J. Exp. Zool. A Ecol. Genet. Physiol. 2007, 307, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Tanaka, J.; Miwa, Y.; Horikawa, D.D.; Katayama, T.; Arakawa, K.; Toyoda, A.; Kubo, T.; Kunieda, T. Novel mitochondria-targeted heat-soluble proteins identified in the anhydrobiotic Tardigrade improve osmotic tolerance of human cells. PLoS ONE 2015, 10, e0118272. [Google Scholar] [CrossRef]
- Chakrabortee, S.; Boschetti, C.; Walton, L.J.; Sarkar, S.; Rubinsztein, D.C.; Tunnacliffe, A. Hydrophilic protein associated with desiccation tolerance exhibits broad protein stabilization function. Proc. Natl. Acad. Sci. USA 2007, 104, 18073–18078. [Google Scholar] [CrossRef]
- Hatanaka, R.; Hagiwara-Komoda, Y.; Furuki, T.; Kanamori, Y.; Fujita, M.; Cornette, R.; Sakurai, M.; Okuda, T.; Kikawada, T. An abundant LEA protein in the anhydrobiotic midge, PvLEA4, acts as a molecular shield by limiting growth of aggregating protein particles. Insect Biochem. Mol. Biol. 2013, 43, 1055–1067. [Google Scholar] [CrossRef]
- Li, S.; Chakraborty, N.; Borcar, A.; Menze, M.A.; Toner, M.; Hand, S.C. Late embryogenesis abundant proteins protect human hepatoma cells during acute desiccation. Proc. Natl. Acad. Sci. USA 2012, 109, 20859–20864. [Google Scholar] [CrossRef]
- Marunde, M.R.; Samarajeewa, D.A.; Anderson, J.; Li, S.; Hand, S.C.; Menze, M.A. Improved tolerance to salt and water stress in Drosophila melanogaster cells conferred by late embryogenesis abundant protein. J. Insect Physiol. 2013, 59, 377–386. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Tanaka, S.; Yamaguchi, S.; Kuwahara, H.; Takamura, C.; Imajoh-Ohmi, S.; Horikawa, D.D.; Toyoda, A.; Katayama, T.; Arakawa, K.; et al. Two novel heat-soluble protein families abundantly expressed in an anhydrobiotic tardigrade. PLoS ONE 2012, 7, e44209. [Google Scholar] [CrossRef]
- Yagi-Utsumi, M.; Aoki, K.; Watanabe, H.; Song, C.; Nishimura, S.; Satoh, T.; Yanaka, S.; Ganser, C.; Tanaka, S.; Schnapka, V.; et al. Desiccation-induced fibrous condensation of CAHS protein from an anhydrobiotic tardigrade. Sci. Rep. 2021, 11, 21328. [Google Scholar] [CrossRef]
- Malki, A.; Teulon, J.M.; Camacho-Zarco, A.R.; Chen, S.W.; Adamski, W.; Maurin, D.; Salvi, N.; Pellequer, J.L.; Blackledge, M. Intrinsically Disordered Tardigrade Proteins Self-Assemble into Fibrous Gels in Response to Environmental Stress. Angew. Chem. Int. Ed. Engl. 2022, 61, e202109961. [Google Scholar] [CrossRef] [PubMed]
- Hesgrove, C.S.; Nguyen, K.H.; Biswas, S.; Childs, C.A.; Shraddha, K.C.; Medina, B.X.; Alvarado, V.; Yu, F.; Sukenik, S.; Malferrari, M.; et al. Tardigrade CAHS Proteins Act as Molecular Swiss Army Knives to Mediate Desiccation Tolerance through Multiple Mechanisms. bioRxiv 2021. [Google Scholar] [CrossRef]
- Veling, M.T.; Nguyen, D.T.; Thadani, N.N.; Oster, M.E.; Rollins, N.J.; Brock, K.P.; Bethel, N.P.; Lim, S.; Baker, D.; Way, J.C.; et al. Natural and Designed Proteins Inspired by Extremotolerant Organisms Can Form Condensates and Attenuate Apoptosis in Human Cells. ACS Synth. Biol. 2022, 11, 1292–1302. [Google Scholar] [CrossRef]
- Tanaka, A.; Nakano, T.; Watanabe, K.; Masuda, K.; Honda, G.; Kamata, S.; Yasui, R.; Kozuka-Hata, H.; Watanabe, C.; Chinen, T.; et al. Stress-dependent cell stiffening by tardigrade tolerance proteins through reversible formation of cytoskeleton-like filamentous network and gel-transition. bioRxiv 2022. [Google Scholar] [CrossRef]
- Boothby, T.C.; Tapia, H.; Brozena, A.H.; Piszkiewicz, S.; Smith, A.E.; Giovannini, I.; Rebecchi, L.; Pielak, G.J.; Koshland, D.; Goldstein, B. Tardigrades Use Intrinsically Disordered Proteins to Survive Desiccation. Mol. Cell 2017, 65, 975–984.e5. [Google Scholar] [CrossRef]
- Tsuboyama, K.; Osaki, T.; Matsuura-Suzuki, E.; Kozuka-Hata, H.; Okada, Y.; Oyama, M.; Ikeuchi, Y.; Iwasaki, S.; Tomari, Y. A widespread family of heat-resistant obscure (Hero) proteins protect against protein instability and aggregation. PLoS Biol. 2020, 18, e3000632. [Google Scholar] [CrossRef]
- Hashimoto, T.; Horikawa, D.D.; Saito, Y.; Kuwahara, H.; Kozuka-Hata, H.; Shin, I.T.; Minakuchi, Y.; Ohishi, K.; Motoyama, A.; Aizu, T.; et al. Extremotolerant tardigrade genome and improved radiotolerance of human cultured cells by tardigrade-unique protein. Nat. Commun. 2016, 7, 12808. [Google Scholar] [CrossRef]
- Chavez, C.; Cruz-Becerra, G.; Fei, J.; Kassavetis, G.A.; Kadonaga, J.T. The tardigrade damage suppressor protein binds to nucleosomes and protects DNA from hydroxyl radicals. eLife 2019, 8, e47682. [Google Scholar] [CrossRef]
- Ni, K.; Spiess, A.N.; Schuppe, H.C.; Steger, K. The impact of sperm protamine deficiency and sperm DNA damage on human male fertility: A systematic review and meta-analysis. Andrology 2016, 4, 789–799. [Google Scholar] [CrossRef]
- Belott, C.; Janis, B.; Menze, M.A. Liquid-liquid phase separation promotes animal desiccation tolerance. Proc. Natl. Acad. Sci. USA 2020, 117, 27676–27684. [Google Scholar] [CrossRef]
- Chakrabortee, S.; Meersman, F.; Kaminski Schierle, G.S.; Bertoncini, C.W.; McGee, B.; Kaminski, C.F.; Tunnacliffe, A. Catalytic and chaperone-like functions in an intrinsically disordered protein associated with desiccation tolerance. Proc. Natl. Acad. Sci. USA 2010, 107, 16084–16089. [Google Scholar] [CrossRef]
- Daly, M.J. Death by protein damage in irradiated cells. DNA Repair. 2012, 11, 12–21. [Google Scholar] [CrossRef]
- Daly, M.J.; Gaidamakova, E.K.; Matrosova, V.Y.; Vasilenko, A.; Zhai, M.; Leapman, R.D.; Lai, B.; Ravel, B.; Li, S.M.; Kemner, K.M.; et al. Protein oxidation implicated as the primary determinant of bacterial radioresistance. PLoS Biol. 2007, 5, e92. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, I.; Boothby, T.C.; Cesari, M.; Goldstein, B.; Guidetti, R.; Rebecchi, L. Production of reactive oxygen species and involvement of bioprotectants during anhydrobiosis in the tardigrade Paramacrobiotus spatialis. Sci. Rep. 2022, 12, 1938. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.M.; Negroni, M.; Altiero, T.; Montorfano, G.; Corsetto, P.; Berselli, P.; Berra, B.; Guidetti, R.; Rebecchi, L. Antioxidant defences in hydrated and desiccated states of the tardigrade Paramacrobiotus richtersi. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 156, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, C.C.S.; Guidelli, G.V.; Borges, G.; Araujo, T.F.; Souza, T.A.J.; Neves, U.; Tunnacliffe, A.; Pereira, T.C. Multiple genes contribute to anhydrobiosis (tolerance to extreme desiccation) in the nematode Panagrolaimus superbus. Genet. Mol. Biol. 2017, 40, 790–802. [Google Scholar] [CrossRef]
- Murai, Y.; Yagi-Utsumi, M.; Fujiwara, M.; Tanaka, S.; Tomita, M.; Kato, K.; Arakawa, K. Multiomics study of a heterotardigrade, Echinisicus testudo, suggests the possibility of convergent evolution of abundant heat-soluble proteins in Tardigrada. BMC Genom. 2021, 22, 813. [Google Scholar] [CrossRef]
- Yoshida, Y.; Satoh, T.; Ota, C.; Tanaka, S.; Horikawa, D.D.; Tomita, M.; Kato, K.; Arakawa, K. Time-series transcriptomic screening of factors contributing to the cross-tolerance to UV radiation and anhydrobiosis in tardigrades. BMC Genom. 2022, 23, 405. [Google Scholar] [CrossRef]
- Yoshida, Y.; Horikawa, D.D.; Sakashita, T.; Yokota, Y.; Kobayashi, Y.; Tomita, M.; Arakawa, K. RNA sequencing data for gamma radiation response in the extremotolerant tardigrade Ramazzottius varieornatus. Data Brief 2021, 36, 107111. [Google Scholar] [CrossRef]
- Kamilari, M.; Jorgensen, A.; Schiott, M.; Mobjerg, N. Comparative transcriptomics suggest unique molecular adaptations within tardigrade lineages. BMC Genom. 2019, 20, 607. [Google Scholar] [CrossRef]
- Cornette, R.; Kanamori, Y.; Watanabe, M.; Nakahara, Y.; Gusev, O.; Mitsumasu, K.; Kadono-Okuda, K.; Shimomura, M.; Mita, K.; Kikawada, T.; et al. Identification of anhydrobiosis-related genes from an expressed sequence tag database in the cryptobiotic midge Polypedilum vanderplanki (Diptera; Chironomidae). J. Biol. Chem. 2010, 285, 35889–35899. [Google Scholar] [CrossRef]
- Gusev, O.; Suetsugu, Y.; Cornette, R.; Kawashima, T.; Logacheva, M.D.; Kondrashov, A.S.; Penin, A.A.; Hatanaka, R.; Kikuta, S.; Shimura, S.; et al. Comparative genome sequencing reveals genomic signature of extreme desiccation tolerance in the anhydrobiotic midge. Nat. Commun. 2014, 5, 4784. [Google Scholar] [CrossRef]
- Ryabova, A.; Mukae, K.; Cherkasov, A.; Cornette, R.; Shagimardanova, E.; Sakashita, T.; Okuda, T.; Kikawada, T.; Gusev, O. Genetic background of enhanced radioresistance in an anhydrobiotic insect: Transcriptional response to ionizing radiations and desiccation. Extremophiles 2017, 21, 109–120. [Google Scholar] [CrossRef]
- Yamada, T.G.; Suetsugu, Y.; Deviatiiarov, R.; Gusev, O.; Cornette, R.; Nesmelov, A.; Hiroi, N.; Kikawada, T.; Funahashi, A. Transcriptome analysis of the anhydrobiotic cell line Pv11 infers the mechanism of desiccation tolerance and recovery. Sci. Rep. 2018, 8, 17941. [Google Scholar] [CrossRef] [PubMed]
- Tyson, T.; O’Mahony Zamora, G.; Wong, S.; Skelton, M.; Daly, B.; Jones, J.T.; Mulvihill, E.D.; Elsworth, B.; Phillips, M.; Blaxter, M.; et al. A molecular analysis of desiccation tolerance mechanisms in the anhydrobiotic nematode Panagrolaimus superbus using expressed sequenced tags. BMC Res. Notes 2012, 5, 68. [Google Scholar] [CrossRef] [PubMed]
- Gusev, O.; Nakahara, Y.; Vanyagina, V.; Malutina, L.; Cornette, R.; Sakashita, T.; Hamada, N.; Kikawada, T.; Kobayashi, Y.; Okuda, T. Anhydrobiosis-associated nuclear DNA damage and repair in the sleeping chironomid: Linkage with radioresistance. PLoS ONE 2010, 5, e14008. [Google Scholar] [CrossRef]
- Neumann, S.; Reuner, A.; Brummer, F.; Schill, R.O. DNA damage in storage cells of anhydrobiotic tardigrades. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 153, 425–429. [Google Scholar] [CrossRef]
- Hespeels, B.; Knapen, M.; Hanot-Mambres, D.; Heuskin, A.C.; Pineux, F.; Lucas, S.; Koszul, R.; Van Doninck, K. Gateway to genetic exchange? DNA double-strand breaks in the bdelloid rotifer Adineta vaga submitted to desiccation. J. Evol. Biol. 2014, 27, 1334–1345. [Google Scholar] [CrossRef]
- Rebecchi, L.; Cesari, M.; Altiero, T.; Frigieri, A.; Guidetti, R. Survival and DNA degradation in anhydrobiotic tardigrades. J. Exp. Biol. 2009, 212, 4033–4039. [Google Scholar] [CrossRef]
- Schill, R.O.; Steinbruck, G.H.; Kohler, H.R. Stress gene (hsp70) sequences and quantitative expression in Milnesium tardigradum (Tardigrada) during active and cryptobiotic stages. J. Exp. Biol. 2004, 207, 1607–1613. [Google Scholar] [CrossRef]
- Jonsson, K.I.; Schill, R.O. Induction of Hsp70 by desiccation, ionising radiation and heat-shock in the eutardigrade Richtersius coronifer. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 146, 456–460. [Google Scholar] [CrossRef]
- Gusev, O.; Cornette, R.; Kikawada, T.; Okuda, T. Expression of heat shock protein-coding genes associated with anhydrobiosis in an African chironomid Polypedilum vanderplanki. Cell Stress Chaperones 2011, 16, 81–90. [Google Scholar] [CrossRef]
- Mazin, P.V.; Shagimardanova, E.; Kozlova, O.; Cherkasov, A.; Sutormin, R.; Stepanova, V.V.; Stupnikov, A.; Logacheva, M.; Penin, A.; Sogame, Y.; et al. Cooption of heat shock regulatory system for anhydrobiosis in the sleeping chironomid Polypedilum vanderplanki. Proc. Natl. Acad. Sci. USA 2018, 115, E2477–E2486. [Google Scholar] [CrossRef]
- Yamada, T.G.; Hiki, Y.; Hiroi, N.F.; Shagimardanova, E.; Gusev, O.; Cornette, R.; Kikawada, T.; Funahashi, A. Identification of a master transcription factor and a regulatory mechanism for desiccation tolerance in the anhydrobiotic cell line Pv11. PLoS ONE 2020, 15, e0230218. [Google Scholar] [CrossRef]
- Kondo, K.; Mori, M.; Tomita, M.; Arakawa, K. AMPK activity is required for the induction of anhydrobiosis in a tardigrade Hypsibius exemplaris, and its potential up-regulator is PP2A. Genes Cells 2019, 24, 768–780. [Google Scholar] [CrossRef]
- Nelson, D.R. Current status of the tardigrada: Evolution and ecology. Integr. Comp. Biol. 2002, 42, 652–659. [Google Scholar] [CrossRef]
- Degma, P.; Bertolani, R.; Guidetti, R. Actual Checklist of Tardigrada Species; Università di Modena e Reggio Emilia: Modena, Italy, 2019; Volume 48. [Google Scholar]
- Rahm, G. Eine neue Tardigraden-Ordnung aus den heissen Quellen von Unzen, Insel Kyushu, Japan. Zool. Anz. 1937, 120, 65–71. [Google Scholar]
- Grothman, G.T.; Johansson, C.; Chilton, G.; Kagoshima, H.; Tsujimoto, M.; Suzuki, A.C. Gilbert Rahm and the Status of Mesotardigrada Rahm, 1937. Zool. Sci. 2017, 34, 5–10. [Google Scholar] [CrossRef]
- Renaud-Mornant, J. Species diversity in marine Tardigrada. In Proceedings of the Third International Symposium Tardigrada, Johnson City, TN, USA, 3–6 August 1980; pp. 149–178. [Google Scholar]
- May, M. L’évolution des Tardigrades de la vie aquatique à la vie terrestre. Bull. Fr. Piscic. 1953, 168, 93–100. [Google Scholar] [CrossRef]
- Regier, J.C.; Shultz, J.W.; Kambic, R.E.; Nelson, D.R. Robust support for tardigrade clades and their ages from three protein-coding nuclear genes. Invertebr. Biol. 2004, 123, 93–100. [Google Scholar] [CrossRef]
- Sanders, K.L.; Lee, M.S.Y. Arthropod molecular divergence times and the Cambrian origin of pentastomids. Syst. Biodivers 2010, 8, 63–74. [Google Scholar] [CrossRef]
- Gąsiorek, P.; Stec, D.; Morek, W.; Michalczyk, Ł. An integrative redescription of Hypsibius dujardini (Doyère, 1840), the nominal taxon for Hypsibioidea (Tardigrada: Eutardigrada). Zootaxa 2018, 4415, 45–75. [Google Scholar] [CrossRef]
- Suzuki, A.C. Life history of Milnesium tardigradum Doyere (tardigrada) under a rearing environment. Zool. Sci. 2003, 20, 49–57. [Google Scholar] [CrossRef]
- Gabriel, W.N.; McNuff, R.; Patel, S.K.; Gregory, T.R.; Jeck, W.R.; Jones, C.D.; Goldstein, B. The tardigrade Hypsibius dujardini, a new model for studying the evolution of development. Dev. Biol. 2007, 312, 545–559. [Google Scholar] [CrossRef]
- Wang, C.; Grohme, M.A.; Mali, B.; Schill, R.O.; Frohme, M. Towards decrypting cryptobiosis—Analyzing anhydrobiosis in the tardigrade Milnesium tardigradum using transcriptome sequencing. PLoS ONE 2014, 9, e92663. [Google Scholar] [CrossRef]
- Forster, F.; Liang, C.; Shkumatov, A.; Beisser, D.; Engelmann, J.C.; Schnolzer, M.; Frohme, M.; Muller, T.; Schill, R.O.; Dandekar, T. Tardigrade workbench: Comparing stress-related proteins, sequence-similar and functional protein clusters as well as RNA elements in tardigrades. BMC Genom. 2009, 10, 469. [Google Scholar] [CrossRef]
- Mali, B.; Grohme, M.A.; Forster, F.; Dandekar, T.; Schnolzer, M.; Reuter, D.; Welnicz, W.; Schill, R.O.; Frohme, M. Transcriptome survey of the anhydrobiotic tardigrade Milnesium tardigradum in comparison with Hypsibius dujardini and Richtersius coronifer. BMC Genom. 2010, 11, 168. [Google Scholar] [CrossRef]
- Schokraie, E.; Hotz-Wagenblatt, A.; Warnken, U.; Mali, B.; Frohme, M.; Forster, F.; Dandekar, T.; Hengherr, S.; Schill, R.O.; Schnolzer, M. Proteomic analysis of tardigrades: Towards a better understanding of molecular mechanisms by anhydrobiotic organisms. PLoS ONE 2010, 5, e9502. [Google Scholar] [CrossRef]
- Schokraie, E.; Warnken, U.; Hotz-Wagenblatt, A.; Grohme, M.A.; Hengherr, S.; Forster, F.; Schill, R.O.; Frohme, M.; Dandekar, T.; Schnolzer, M. Comparative proteome analysis of Milnesium tardigradum in early embryonic state versus adults in active and anhydrobiotic state. PLoS ONE 2012, 7, e45682. [Google Scholar] [CrossRef]
- Forster, F.; Beisser, D.; Grohme, M.A.; Liang, C.; Mali, B.; Siegl, A.M.; Engelmann, J.C.; Shkumatov, A.V.; Schokraie, E.; Muller, T.; et al. Transcriptome analysis in tardigrade species reveals specific molecular pathways for stress adaptations. Bioinform. Biol. Insights 2012, 6, 69–96. [Google Scholar] [CrossRef]
- Beisser, D.; Grohme, M.A.; Kopka, J.; Frohme, M.; Schill, R.O.; Hengherr, S.; Dandekar, T.; Klau, G.W.; Dittrich, M.; Muller, T. Integrated pathway modules using time-course metabolic profiles and EST data from Milnesium tardigradum. BMC Syst. Biol. 2012, 6, 72. [Google Scholar] [CrossRef]
- Bertolani, R.; Kinchin, I.M. A New Species of Ramazzottius (Tardigrada, Hypsibiidae) in a Rain Gutter Sediment from England. Zool. J. Linn. Soc. 1993, 109, 327–333. [Google Scholar] [CrossRef]
- Doyère, L.-M.-F. Mémoire sur les tardigrades. Sur le facilité que possedent les tardigrades, les rotifers, les anguillules des toits et quelques autres animalcules, de revenir à la vieaprès été completement déssechées. Ann. Sci. Nat. Zool. Biol. Anim. 1842, 18, 5–35. [Google Scholar]
- Horikawa, D.D.; Arakawa, K. Tardigrades—Ultimate Animals Surviving Extreme Environments. SFC J. 2015, 15, 246–260. [Google Scholar]
- Ito, M.; Saigo, T.; Abe, W.; Kubo, T.; Kunieda, T. Establishment of an isogenic strain of the desiccation-sensitive tardigrade Isohypsibius myrops (Parachela, Eutardigrada) and its life history traits. Zool. J. Linn. Soc. 2016, 178, 863–870. [Google Scholar] [CrossRef]
- Bertolani, R.; Bartels, P.J.; Guidetti, R.; Cesari, M.; Nelson, D.R. Aquatic tardigrades in the Great Smoky Mountains National Park, North Carolina and Tennessee, U.S.A., with the description of a new species of Thulinius (Tardigrada, Isohypsibiidae). Zootaxa 2014, 3764, 524–536. [Google Scholar] [CrossRef]
- Sobczyk, M.; Michno, K.; Kosztyla, P.; Stec, D.; Michalczyk, L. Tolerance to Ammonia of Thulinius ruffoi (Bertolani, 1981), a Tardigrade Isolated from a Sewage Treatment Plant. Bull. Environ. Contam. Toxicol. 2015, 95, 721–727. [Google Scholar] [CrossRef]
- Nowell, R.W.; Almeida, P.; Wilson, C.G.; Smith, T.P.; Fontaneto, D.; Crisp, A.; Micklem, G.; Tunnacliffe, A.; Boschetti, C.; Barraclough, T.G. Comparative genomics of bdelloid rotifers: Insights from desiccating and nondesiccating species. PLoS Biol. 2018, 16, e2004830. [Google Scholar] [CrossRef]
- Nowell, R.W.; Wilson, C.G.; Almeida, P.; Schiffer, P.H.; Fontaneto, D.; Becks, L.; Rodriguez, F.; Arkhipova, I.R.; Barraclough, T.G. Evolutionary dynamics of transposable elements in bdelloid rotifers. eLife 2021, 10, e63194. [Google Scholar] [CrossRef]
- Hashimoto, T.; Kunieda, T. DNA Protection Protein, a Novel Mechanism of Radiation Tolerance: Lessons from Tardigrades. Life 2017, 7, 26. [Google Scholar] [CrossRef]
- Carrero, D.; Perez-Silva, J.G.; Quesada, V.; Lopez-Otin, C. Differential mechanisms of tolerance to extreme environmental conditions in tardigrades. Sci. Rep. 2019, 9, 14938. [Google Scholar] [CrossRef]
- Bemm, F.M.; Burleigh, L.; Foerster, F.; Schmucki, R.; Ebeling, M.; Janzen, C.; Dandekar, T.; Schill, R.; Certa, U.; Schultz, J. Draft genome of the Eutardigrade Milnesium tardigradum sheds light on ecdysozoan evolution. bioRxiv 2017, 122309v2. [Google Scholar] [CrossRef]
- Cranston, P.S.; Hardy, N.B.; Morse, G.E. A dated molecular phylogeny for the Chironomidae (Diptera). Syst. Entomol. 2012, 37, 172–188. [Google Scholar] [CrossRef]
- Armitage, P.D.; Cranston, P.S.; Pinder, L.C.V. The Chironomidae; Springer: Dordrecht, The Netherlands, 1995. [Google Scholar]
- Cornette, R.; Kikawada, T. The induction of anhydrobiosis in the sleeping chironomid: Current status of our knowledge. IUBMB Life 2011, 63, 419–429. [Google Scholar] [CrossRef]
- Hinton, H.E. A new Chironomid from Africa, the larva of which can be dehydrated without injury. Proc. Zool. Soc. Lond. 1951, 121, 371–380. [Google Scholar] [CrossRef]
- Cornette, R.; Gusev, O.; Nakahara, Y.; Shimura, S.; Kikawada, T.; Okuda, T. Chironomid midges (Diptera, chironomidae) show extremely small genome sizes. Zool. Sci. 2015, 32, 248–254. [Google Scholar] [CrossRef]
- Yoshida, Y.; Shaikhutdinov, N.; Kozlova, O.; Itoh, M.; Tagami, M.; Murata, M.; Nishiyori-Sueki, H.; Kojima-Ishiyama, M.; Noma, S.; Cherkasov, A.; et al. High quality genome assembly of the anhydrobiotic midge provides insights on a single chromosome-based emergence of extreme desiccation tolerance. NAR Genom. Bioinform. 2022, 4, lqac029. [Google Scholar] [CrossRef]
- Shaikhutdinov, N.M.; Klink, G.V.; Garushyants, S.K.; Kozlova, O.S.; Cherkasov, A.V.; Kikawada, T.; Okuda, T.; Pemba, D.; Deviatiiarov, R.M.; Gazizova, G.R.; et al. Population genomics of two closely related anhydrobiotic midges reveals differences in adaptation to extreme desiccation. bioRxiv 2020. [Google Scholar] [CrossRef]
- Pinder, L.C.V. Biology of Freshwater Chironomidae. Annu. Rev. Entomol. 1986, 31, 1–23. [Google Scholar] [CrossRef]
- De Melo, D.B.; Dolbeth, M.; Paiva, F.F.; Molozzi, J. Extreme drought scenario shapes different patterns of Chironomid coexistence in reservoirs in a semi-arid region. Sci. Total Environ. 2022, 821, 153053. [Google Scholar] [CrossRef]
- Brown, C.J.; Johnson, A.K.; Dunker, A.K.; Daughdrill, G.W. Evolution and disorder. Curr. Opin. Struct. Biol. 2011, 21, 441–446. [Google Scholar] [CrossRef]
- Yoshida, Y.; Nowell, R.W.; Arakawa, K.; Blaxter, M. Horizontal Gene Transfer in Metazoa: Examples and Methods. In Horizontal Gene Transfer; Springer International Publishing: Cham, Switzerland, 2019; pp. 203–226. [Google Scholar]
- Eyres, I.; Boschetti, C.; Crisp, A.; Smith, T.P.; Fontaneto, D.; Tunnacliffe, A.; Barraclough, T.G. Horizontal gene transfer in bdelloid rotifers is ancient, ongoing and more frequent in species from desiccating habitats. BMC Biol. 2015, 13, 90. [Google Scholar] [CrossRef]
- Boschetti, C.; Carr, A.; Crisp, A.; Eyres, I.; Wang-Koh, Y.; Lubzens, E.; Barraclough, T.G.; Micklem, G.; Tunnacliffe, A. Biochemical diversification through foreign gene expression in bdelloid rotifers. PLoS Genet. 2012, 8, e1003035. [Google Scholar] [CrossRef]
- Boschetti, C.; Pouchkina-Stantcheva, N.; Hoffmann, P.; Tunnacliffe, A. Foreign genes and novel hydrophilic protein genes participate in the desiccation response of the bdelloid rotifer Adineta ricciae. J. Exp. Biol. 2011, 214, 59–68. [Google Scholar] [CrossRef]
- Boothby, T.C.; Tenlen, J.R.; Smith, F.W.; Wang, J.R.; Patanella, K.A.; Nishimura, E.O.; Tintori, S.C.; Li, Q.; Jones, C.D.; Yandell, M.; et al. Evidence for extensive horizontal gene transfer from the draft genome of a tardigrade. Proc. Natl. Acad. Sci. USA 2015, 112, 15976–15981. [Google Scholar] [CrossRef]
- Koutsovoulos, G.; Kumar, S.; Laetsch, D.R.; Stevens, L.; Daub, J.; Conlon, C.; Maroon, H.; Thomas, F.; Aboobaker, A.A.; Blaxter, M. No evidence for extensive horizontal gene transfer in the genome of the tardigrade Hypsibius dujardini. Proc. Natl. Acad. Sci. USA 2016, 113, 5053–5058. [Google Scholar] [CrossRef]
- Arakawa, K. No evidence for extensive horizontal gene transfer from the draft genome of a tardigrade. Proc. Natl. Acad. Sci. USA 2016, 113, E3057. [Google Scholar] [CrossRef]
- Bemm, F.; Weiss, C.L.; Schultz, J.; Forster, F. Genome of a tardigrade: Horizontal gene transfer or bacterial contamination? Proc. Natl. Acad. Sci. USA 2016, 113, E3054–E3056. [Google Scholar] [CrossRef]
- Boothby, T.C.; Goldstein, B. Reply to Bemm et al. and Arakawa: Identifying foreign genes in independent Hypsibius dujardini genome assemblies. Proc. Natl. Acad. Sci. USA 2016, 113, E3058–E3061. [Google Scholar] [CrossRef]
- Delmont, T.O.; Eren, A.M. Identifying contamination with advanced visualization and analysis practices: Metagenomic approaches for eukaryotic genome assemblies. PeerJ 2016, 4, e1839. [Google Scholar] [CrossRef]
- Arakawa, K.; Yoshida, Y.; Tomita, M. Genome sequencing of a single tardigrade Hypsibius dujardini individual. Sci. Data 2016, 3, 160063. [Google Scholar] [CrossRef]
- Hoencamp, C.; Dudchenko, O.; Elbatsh, A.M.O.; Brahmachari, S.; Raaijmakers, J.A.; van Schaik, T.; Cacciatore, A.S.; Contessoto, V.G.; van Heesbeen, R.G.H.P.; van den Broek, B.; et al. 3D genomics across the tree of life reveals condensin II as a determinant of architecture type. Science 2021, 372, 984–989. [Google Scholar] [CrossRef]
- Tenlen, J.R.; McCaskill, S.; Goldstein, B. RNA interference can be used to disrupt gene function in tardigrades. Dev. Genes Evol. 2013, 223, 171–181. [Google Scholar] [CrossRef]
- Sogame, Y.; Okada, J.; Kikuta, S.; Miyata, Y.; Cornette, R.; Gusev, O.; Kikawada, T. Establishment of gene transfer and gene silencing methods in a desiccation-tolerant cell line, Pv11. Extremophiles 2017, 21, 65–72. [Google Scholar] [CrossRef]
- Schill, R.O.; Fritz, G.B. Desiccation tolerance in embryonic stages of the tardigrade. J. Zool. 2008, 276, 103–107. [Google Scholar] [CrossRef]
- Nakahara, Y.; Watanabe, M.; Fujita, A.; Kanamori, Y.; Tanaka, D.; Iwata, K.; Furuki, T.; Sakurai, M.; Kikawada, T.; Okuda, T. Effects of dehydration rate on physiological responses and survival after rehydration in larvae of the anhydrobiotic chironomid. J. Insect Physiol. 2008, 54, 1220–1225. [Google Scholar] [CrossRef]

| Species | Desiccation Condition | Preconditioning | Survival Rate | Reference | Notes |
|---|---|---|---|---|---|
| Fungi | |||||
| Saccharomyces cerevisiae | Freeze-dryer for 3 days | 2-week culture for stationary phase | ~100% + 500 mM trehalose/~10% + 0 mM trehalose | Gadd et al., 1987 [12] | Intracellular trehalose was about 300 mM in the stationary phase |
| Saccharomyces cerevisiae | Air, 30 °C, ~16 h | 72 h culture (late postdiauxic phase) | ~50%, BY4741 | Ratnakumar and Tunnacliffe, 2006 [13] | Intracellular trehalose was about 140 mM at the late postdiauxic phase |
| Saccharomyces cerevisiae | 60% RH, 23 °C, > 48 h | 5-day culture to saturation | <20%, WT, 2 days dry | Tapia and Koshland, 2014 [14] | Yeast had only 600 μg/mL trehalose after 5-day culture. Half of the trehalose degraded during the 30-day desiccation period, and more than 90% by 180 days |
| Saccharomyces cerevisiae | 60% RH, 23 °C, >48 h | - | ~1%, TDH3pr-AGT1, +1% trehalose | Tapia et al., 2015 [15] | AGT1 can transport extracellular trehalose. In 1% trehalose, intracellular trehalose was 157 μg/mL |
| Nematode | |||||
| Aphelenchus avenae | 80% RH, 24 h; 40% RH, 24 h; 0% RH, 24 h | 97% RH, 24–72 h | ~50% | Higa et al., 1993 [16] | About 7% trehalose of dry weight under all preconditioning conditions |
| Caenorhabditis elegans | 98% RH/23% RH/0% RH | 98% RH, 4 days | ~100%/~100%/~10%, daf-2 | Erkut et al., 2011 [17] | Intracellular trehalose was about 400 mM after preconditioning |
| Caenorhabditis elegans | 98% RH/23% RH/0% RH | - | ~100%/~0%/~0%, daf-2 | Erkut et al., 2011 [17] | Intracellular trehalose was about 80 mM without preconditioning |
| Rotifer | |||||
| Adineta vaga | 22 °C, 7 days | In a container at 22 °C, 24 h | ~80%, adults/~60%, juvenile/>80%, egg | Ricci, 1998 [18] | |
| Philodina roseola | Air (~33% RH), RT (~23 °C), 3 days | 100% RH, 2 days | ~75%, well fed | Lapinski and Tunnacliffe, 2003 [19] | Survival rate without preconditioning was less than 1% |
| Insect | |||||
| Polypedilum vanderplanki | <5% RH, RT (24–26 °C), >48 h | - | 100% | Watanabe et al., 2002 [20] | Trehalose was 35 μg/individual at 48 h |
| Polypedilum vanderplanki | 5% RH | 100% RH for first day, 76% RH for the second day, and 5% RH for a third day | 91% | Sakurai et al., 2008 [21] | The survival rate without preconditioning was 0%. Trehalose: 277 μg/mg dry weight with preconditioning; 4.2 μg/mg without preconditioning |
| Polypedilum vanderplanki, Pv11 | <10% RH, 25 °C, +600 mM trehalose, >48 h | Incubation with 600 mM trehalose, 48 h | 16% | Watanabe et al., 2016 [22] | |
| Tardigrade | |||||
| Ramazzottius varieornatus | 0% RH, 25 °C, 10 days | 85% RH, 25 °C, 24 h | ~100%, egg, juvenile, and adult | Horikawa et al., 2008 [23] | |
| Hypsibius exemplaris | 10%, RH 18 °C, 2 days | 95% RH, 18 °C, 4 days | ~100% | Kondo et al., 2015 [24] | For rehydration, specimens were transferred to 95% RH for 1 day |
| Hypsibius exemplaris | 40% RH, 24 h; 22%, 7 days, 20 °C | 92% RH, 20 °C, 16 h | ~2% | Poprawa et al., 2022 [25] | |
| Hypsibius exemplaris | 40–50% RH, 72 h; incubator,7 days, 20 °C | - | ~50% | Poprawa et al., 2022 [25] | |
| Paramacrobiotus metropolitanus | 10% RH, 22 °C, 2 days | 95% RH, 22 °C, 48 h | >60% | Hara et al., 2022 [26] | Trehalose was 70 ng/μg protein after 2 days desiccation |
| Milnesium tardigradum | 50–62% RH, 25 °C, 1 h | - | ~90% | Horikawa and Higashi, 2004 [27] | |
| Echiniscoides sigismundi | 62 or 39% RH, 22–23 °C, 48 h | - | ~99% | Hygum et al., 2016 [28] | |
| Richtersius coronifer | 65% RH, 23 °C, 12 days | - | ~40% | Jönsson et al., 2001 [29] | |
| Species | Life Stage with Desiccation Tolerance | Trehalose Accumulation | IDP | Genome Size | Regulation of Anhydrobiotic Genes |
|---|---|---|---|---|---|
| Polypedilum vanderplanki | Only larva | 35 μg/individual | LEA | 104 Mb | Expression induction through HSF and NFY-C |
| Ramazzottius varieornatus | Embryo, juvenile, adult | 300 μM/sample | CAHS, SAHS, MAHS, LEAM, Dsup | 56 Mb | Constitutive expression |
| Hypsibius exemplaris | Adult | (gene lost) | CAHS, SAHS, MAHS, LEAM, Dsup | 104 Mb | Regulation by AMPK and PP1/PP2A |
| (Hypsibius dujardini) | |||||
| Paramacrobiotus metropolitanus | Adult | 70 ng/μg protein | CAHS, SAHS, MAHS, LEAM | 170 Mb | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, Y.; Tanaka, S. Deciphering the Biological Enigma—Genomic Evolution Underlying Anhydrobiosis in the Phylum Tardigrada and the Chironomid Polypedilum vanderplanki. Insects 2022, 13, 557. https://doi.org/10.3390/insects13060557
Yoshida Y, Tanaka S. Deciphering the Biological Enigma—Genomic Evolution Underlying Anhydrobiosis in the Phylum Tardigrada and the Chironomid Polypedilum vanderplanki. Insects. 2022; 13(6):557. https://doi.org/10.3390/insects13060557
Chicago/Turabian StyleYoshida, Yuki, and Sae Tanaka. 2022. "Deciphering the Biological Enigma—Genomic Evolution Underlying Anhydrobiosis in the Phylum Tardigrada and the Chironomid Polypedilum vanderplanki" Insects 13, no. 6: 557. https://doi.org/10.3390/insects13060557
APA StyleYoshida, Y., & Tanaka, S. (2022). Deciphering the Biological Enigma—Genomic Evolution Underlying Anhydrobiosis in the Phylum Tardigrada and the Chironomid Polypedilum vanderplanki. Insects, 13(6), 557. https://doi.org/10.3390/insects13060557






