Efficacy of Helicoverpa Armigera Nucleopolyhedrovirus on Soybean for Control of Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) in Arkansas Agriculture
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
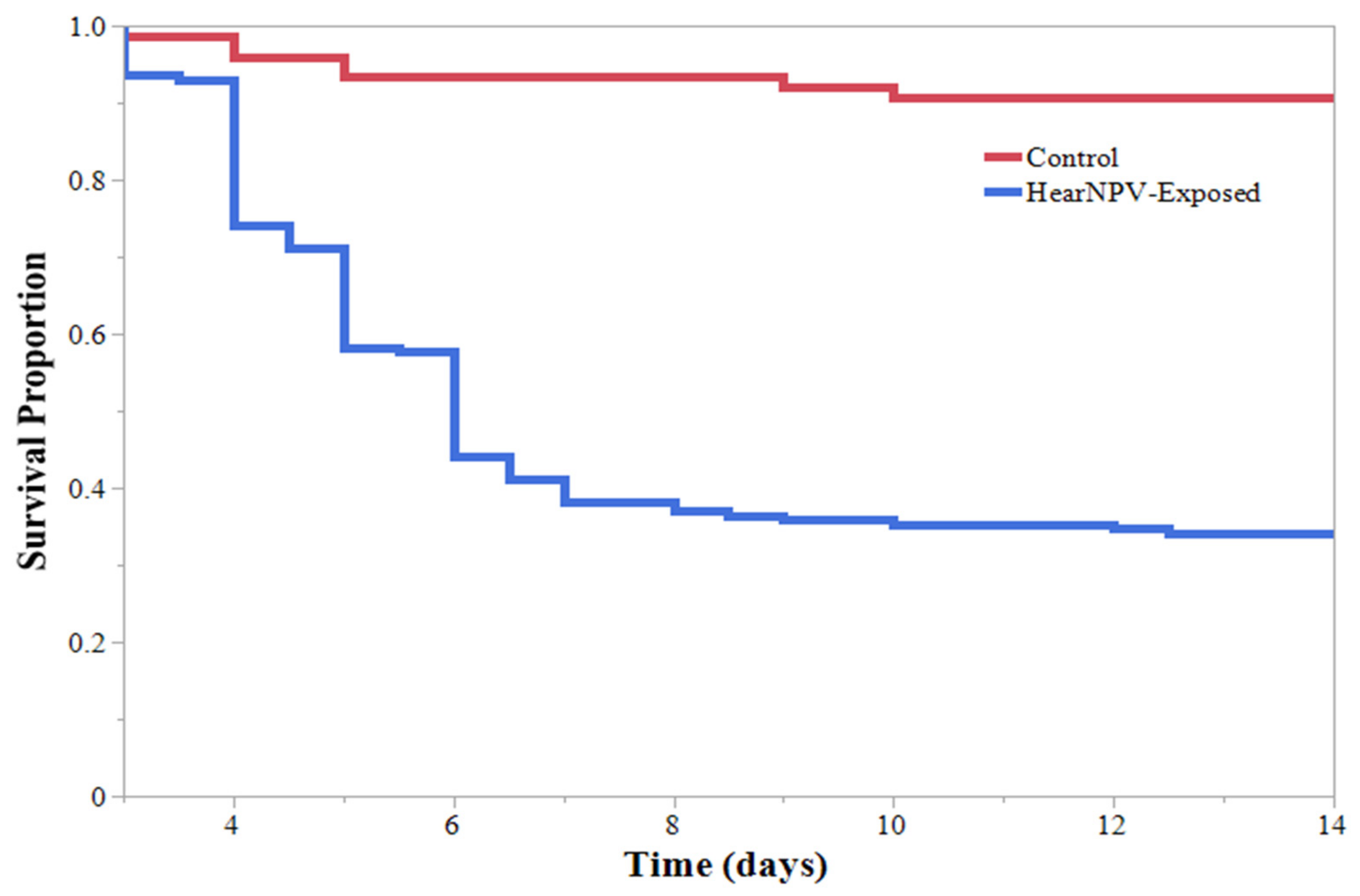
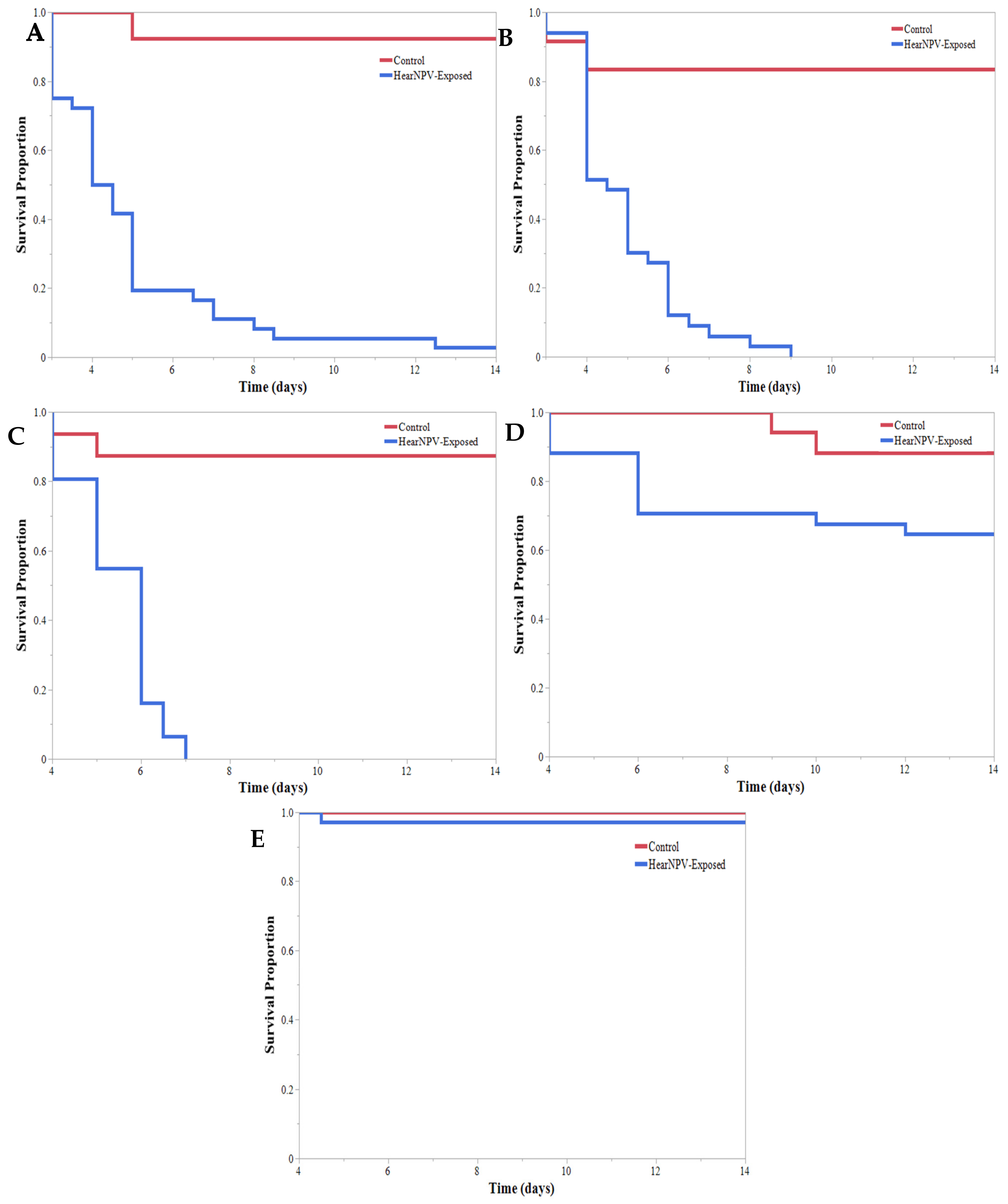
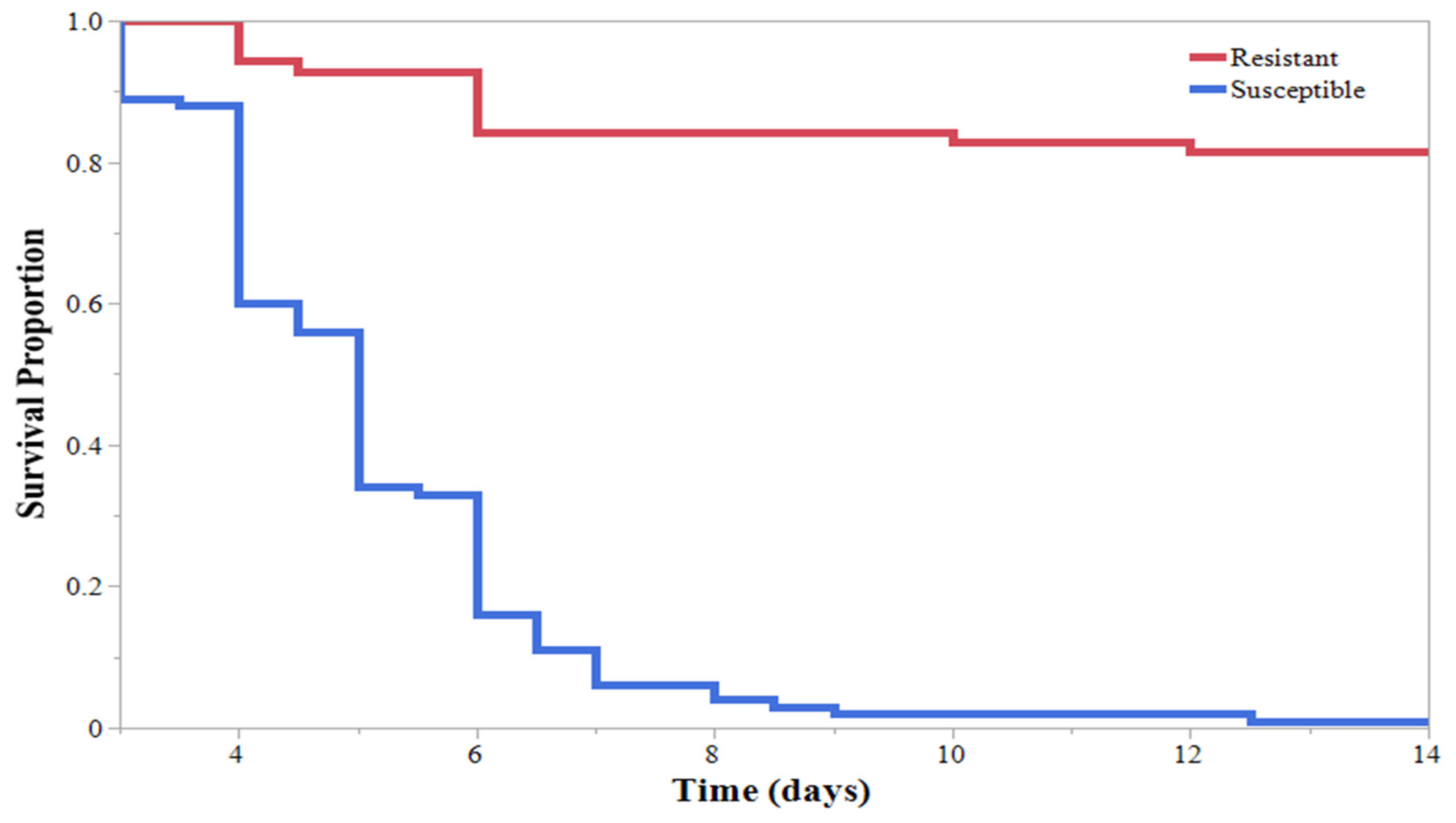
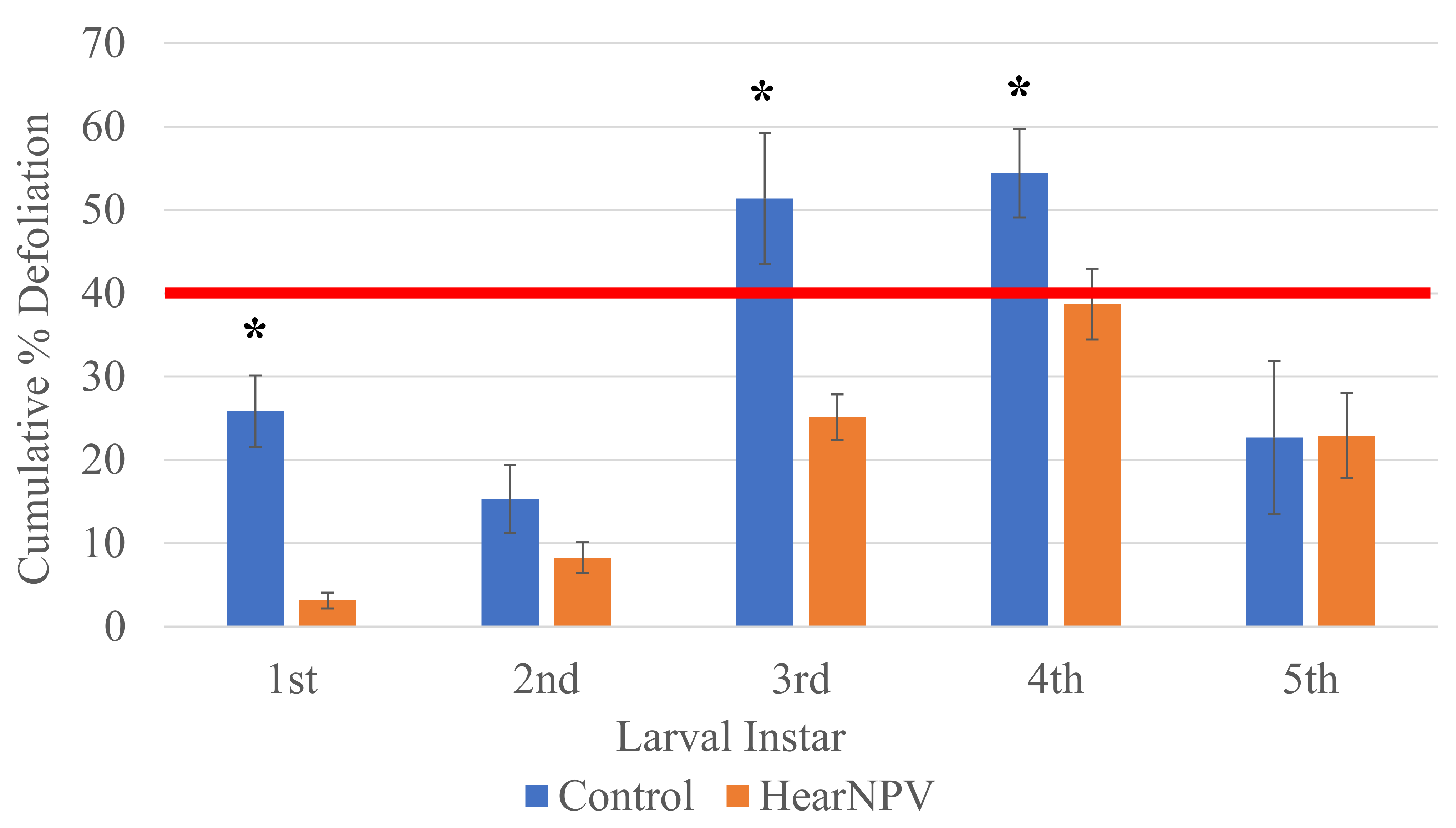
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Musser, F.R.; Catchot, A.L., Jr.; Davis, J.A.; Lorenz, G.M.; Reed, T.; Reisig, D.D.; Stewart, S.D.; Taylor, S. 2016 Soybean insect losses in the southern US. Midsouth Entomol. 2017, 10, 1–13. [Google Scholar]
- Young, S.Y.; McNew, R.W. Persistence and efficacy of four nuclear polyhedrosis viruses for corn earworm (Lepidoptera: Noctuidae) on heading grain sorghum. J. Entomol. Sci. 1994, 29, 370–380. [Google Scholar] [CrossRef]
- Black, J.B.; Lorenz, G.M.; Cato, A.J.; Faske, T.R.; Popham, H.J.; Paddock, K.J.; Bateman, N.R.; Seiter, N.J. Field studies on the horizontal transmission potential by voluntary and involuntary carriers of Helicoverpa armigera nucleopolyhedrovirus (Baculoviridae). J. Econ. Ent. 2019, 112, 1098–1104. [Google Scholar] [CrossRef]
- Fuxa, J.R. Importance of epizootiology to biological control of insects with viruses. Mem. Inst. Oswaldo Cruz 1989, 84, 81–89. [Google Scholar] [CrossRef][Green Version]
- Yearian, W.C.; Lorenz, G.M. Efficacy of Elcar against Heliothis species. Ark. Farm Res. 1983, 32, 5. [Google Scholar]
- Young, S.Y.; Yearian, W.C. Movement of a nuclear polyhedrosis virus from soil to soybean and transmission in Anticarsia gemmatalis (Hübner) (Lepidoptera: Noctuidae) populations on soybean. Environ. Entomol. 1986, 15, 573–580. [Google Scholar] [CrossRef]
- Usta, C. Microorganisms in biological pest control—A review (Bacterial toxin application and effect of environmental factors. In Current Progress in Biological Research; Silva-Opps, M., Ed.; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Luttrell, R.G.; Yearian, W.C.; Young, S.Y. Effects of Elcar (Heliothis zea nuclear polyhedrosis virus) treatments on Heliothis spp. J. Ga. Entomol. Soc. 1982, 17, 211–221. [Google Scholar]
- Luttrell, R.G.; Yearian, W.C.; Young, S.Y. Mortality of Heliothis spp. larvae treated with Heliothis zea nuclear polyhedrosis virus-spray adjuvant combinations on cotton and soybean. J. Ga. Entomol. Soc. 1982, 17, 447–453. [Google Scholar]
- Luttrell, R.G.; Yearian, W.C.; Young, S.Y. Effect of spray adjuvants on Heliothis zea (Lepidoptera: Noctuidae) nuclear polyhedrosis virus efficacy. J. Econ. Entomol. 1983, 76, 162–167. [Google Scholar] [CrossRef]
- Stacey, A.L.; Yearian, W.C.; Young, S.Y., III. Evaluation of Baculovirus heliothis with feeding stimulants for control of Heliothis larvae on cotton. J. Econ. Entomol. 1977, 70, 779–784. [Google Scholar] [CrossRef]
- Abd-Elghafar, S.F.; Knowles, C.O.; Wall, M.L. Pyrethroid resistance in two field strains of Helicoverpa zea (Lepidoptera: Noctuidae). J. Econ. Entomol. 1993, 86, 1651–1655. [Google Scholar] [CrossRef]
- Kanga, L.H.B.; Plapp, F.W.; McCutchen, B.F.; Bagwell, R.D.; Lopez, J.D. Tolerance to cypermethrin and endosulfan in field populations of the bollworm (Lepidoptera: Noctuidae) from Texas. J. Econ. Entomol. 1966, 89, 583–589. [Google Scholar] [CrossRef]
- Musser, F.R.; Greene, J.K.; Herbert, D.A.; Jones, M.; Kerns, D.; Lorenz, G.M.; Parajulee, M.N.; Reisig, D.; Roberts, P.M.; Stewart, S.D. Update on bollworm pyrethroid resistance monitoring. In Proceedings of the 2015 Beltwide Cotton Conferences, San Antonio, TX, USA, 5–7 January 2015; TX National Cotton Council of America: Memphis, TN, USA, 2015. [Google Scholar]
- Pieters, E.P.; Young, S.Y.; Yearian, W.C.; Sterling, W.L.; Clower, D.F.; Melville, D.R.; Gilliland, F.R., Jr. Efficacy of microbial pesticide and chlordimeform mixtures for control of Heliothis spp. on cotton. Southwest. Entomol. 1978, 3, 237–240. [Google Scholar]
- Young, S.Y.; Yearian, W.C. Nabis roseipennis adults (Hemiptera: Nabidae) as disseminators of nuclear polyhedrosis virus to Anticarsia gemmatalis (Lepidoptera: Noctuidae) larvae. Environ. Entomol. 1987, 16, 1330–1333. [Google Scholar] [CrossRef]
- Young, S.Y.; Yearian, W.C. Nuclear polyhedrosis virus transmission by Microplitis croceipes (Hymenoptera: Braconidae) adult females reared in infected Heliothis virescens (Lepidoptera: Noctuidae) larvae. J. Entomol. Sci. 1989, 24, 500–506. [Google Scholar] [CrossRef]
- Young, S.Y.; Yearian, W.C. Transmission of nuclear polyhedrosis virus by the parasitoid Microplitis croceipes (Hymenoptera: Braconidae) to Heliothis virescens (Lepidoptera: Noctuidae) on soybean. Environ. Entomol. 1990, 19, 251–256. [Google Scholar] [CrossRef]
- Young, S.Y.; Yearian, W.C. Contamination of arthropod predators with Heliothis nuclear polyhedrosis virus after Elcar applications to soybean for control of Heliothis spp. (Lepidoptera: Noctuidae). J. Entomol. Sci. 1990, 25, 486–492. [Google Scholar] [CrossRef]
- Ali, M.I.; Young, S.Y.; Yearian, W.C. Transmission of a nuclear polyhedrosis virus in Heliothis zea (Lep.: Noctuidae) larval populations on soybean. Entomophaga 1985, 30, 167–175. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E.; Burmood, D.T.; Pennington, J.S. Stage of development descriptions for soybeans, Glycine max (L.) Merril. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Neunzig, H.H. The eggs and early-instar larvae of Heliothis zea and Heliothis virescens (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 1964, 57, 98–102. [Google Scholar] [CrossRef]
- Neunzig, H.H. The biology of the tobacco budworm and the corn earworm in North Carolina. NC AES Tech. Bul. 1969, 196, 1–76. [Google Scholar]
- Ali, M.I.; Young, S.Y.; Yearian, W.C. Nuclear polyhedrosis virus transmission by infected Heliothis zea (Boddie) (Lepidoptera: Noctuidae) prior to death. J. Entomol. Sci. 1987, 22, 289–294. [Google Scholar] [CrossRef]
- Alam, M.Z.; Yearian, W.C.; Young, S.Y.; Mueller, A.J. Soybean foliage consumption by Pseudoplusia includes (Walker) (Lepidoptera: Noctuidae) larvae infected with nuclear polyhedrosis virus. J. Entomol. Sci. 1987, 22, 212–223. [Google Scholar] [CrossRef]
- Flusche, N.E.; Yearian, W.C.; Mueller, A.J.; Young, S.Y. Effect of the Heliothis nuclear polyhedrosis virus infection on food consumption by Heliothis zea. J. Entomol. Sci. 1986, 21, 118–126. [Google Scholar] [CrossRef]
- Ignoffo, C.M.; Bradley, J.R., Jr.; Gilliland, F.R., Jr.; Harris, F.A.; Falcon, L.A.; Larson, L.V.; McGarr, R.L.; Skiorowski, P.P.; Watson, T.F.; Yearian, W.C. Field studies on stability of the Heliothis nucleopolyhedrosis virus at various sites throughout the cotton belt. Environ. Entomol. 1972, 1, 388–390. [Google Scholar] [CrossRef]
- McLeod, P.J.; Yearian, W.C.; Young, S.Y., III. Inactivation of Baculovirus heliothis by ultraviolet irradiation, dew, and temperature. J. Invertebr. Pathol. 1977, 30, 237–241. [Google Scholar] [CrossRef]
- McWilliams, J.M. Relationship of soybean pod development to bollworm and tobacco budworm damage. J. Econ. Entomol. 1983, 76, 502–506. [Google Scholar] [CrossRef]
- Young, S.Y.; Yearian, W.C. Persistence of Heliothis NPV on foliage of cotton, soybean, and tomato. Environ. Entomol. 1974, 3, 253–255. [Google Scholar] [CrossRef]
- Young, S.Y.; Yearian, W.C.; Kim, K.S. Effect of dew from cotton and soybean foliage on activity of Heliothis nuclear polyhedrosis virus. J. Invertebr. Pathol. 1977, 29, 105–111. [Google Scholar] [CrossRef]
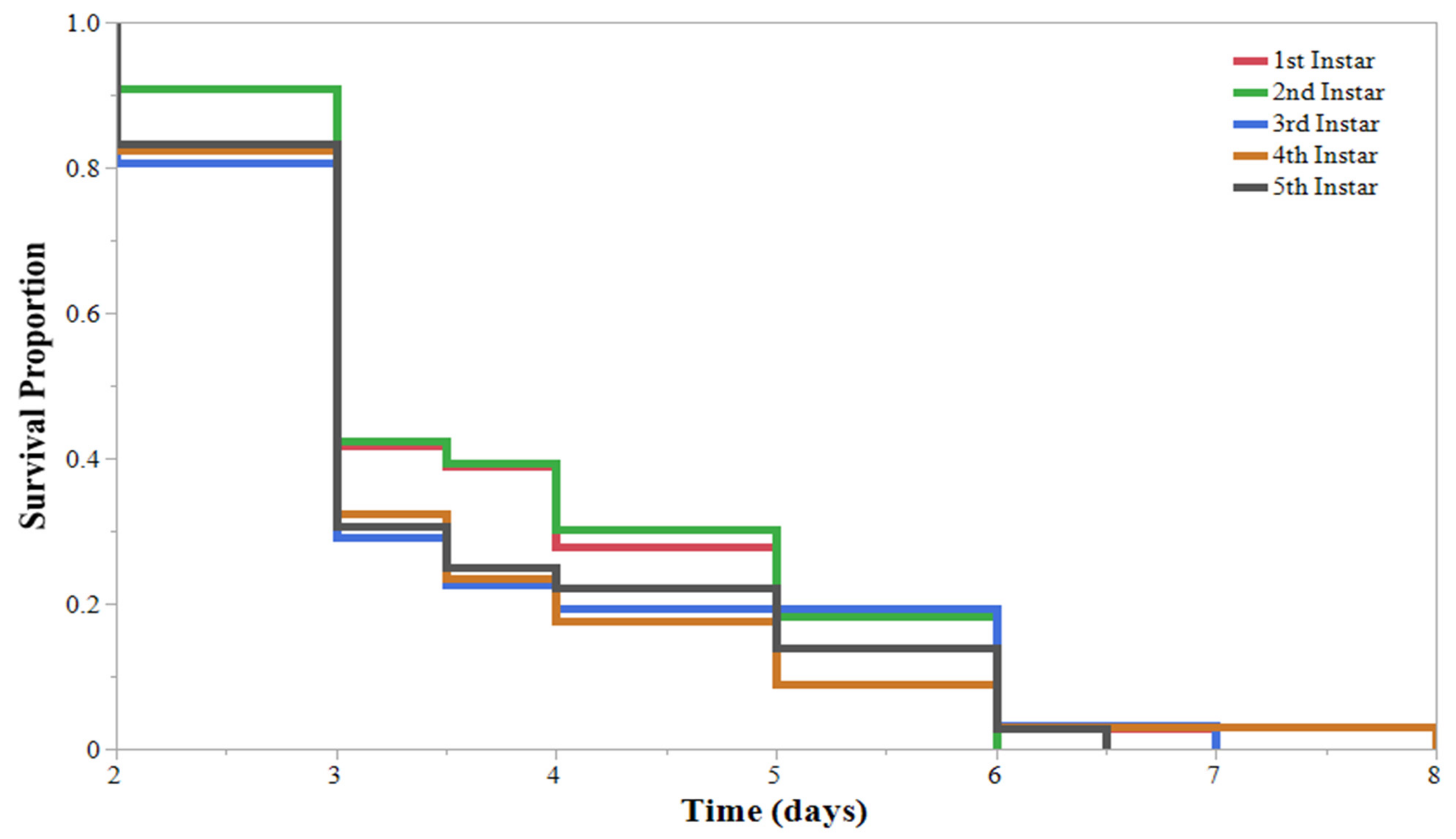
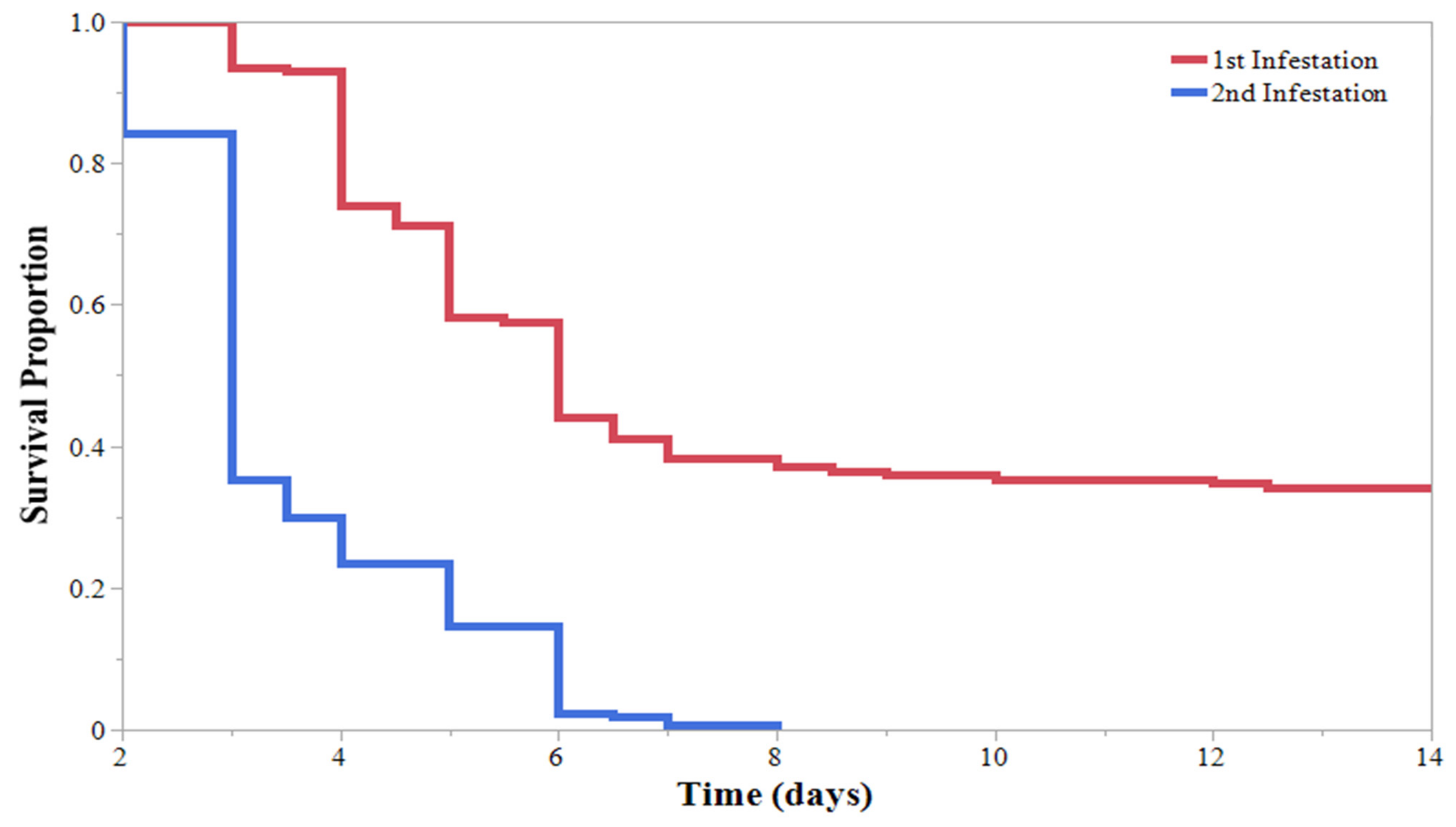
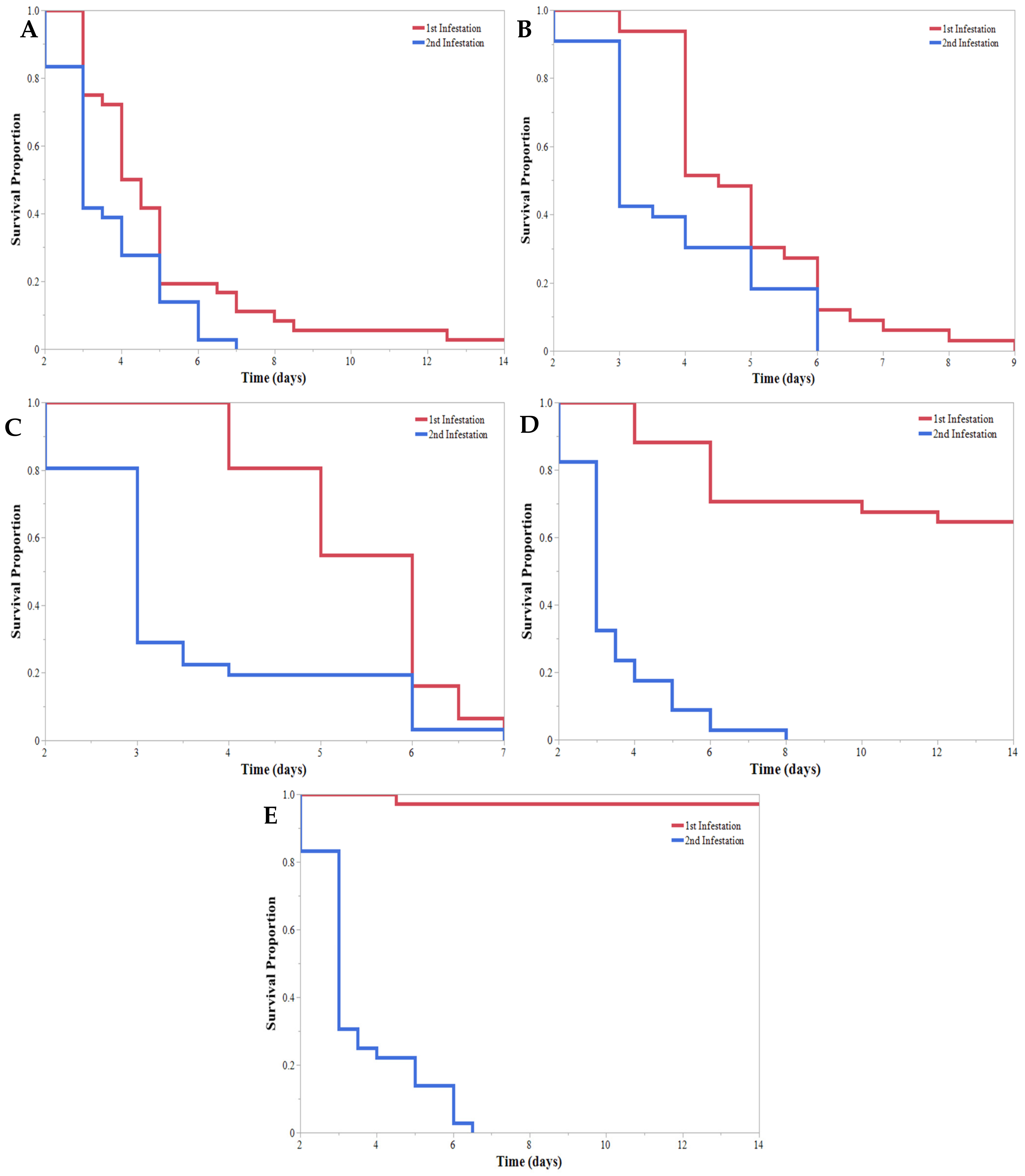
| Initial Instar | % Mortality * | % Pupated * | Mortality (DAA) ** | Pupation (DAA) ** | 2nd Infestation Mortality (DAA) ** |
|---|---|---|---|---|---|
| 1 | 97 a | 3 c | 4.7 b | 14 b | 3.7 a |
| 2 | 100 a | 0 c | 4.9 ab | - | 3.8 a |
| 3 | 100 a | 0 c | 5.5 ab | - | 3.5 a |
| 4 | 35 b | 65 b | 6.2 a | 5.3 a | 3.4 a |
| 5 | 3 c | 97 a | 4.5 c | 4.5 a | 3.5 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Black, J.L.; Lorenz, G.M.; Cato, A.J.; Bateman, N.R.; Seiter, N.J. Efficacy of Helicoverpa Armigera Nucleopolyhedrovirus on Soybean for Control of Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) in Arkansas Agriculture. Insects 2022, 13, 91. https://doi.org/10.3390/insects13010091
Black JL, Lorenz GM, Cato AJ, Bateman NR, Seiter NJ. Efficacy of Helicoverpa Armigera Nucleopolyhedrovirus on Soybean for Control of Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) in Arkansas Agriculture. Insects. 2022; 13(1):91. https://doi.org/10.3390/insects13010091
Chicago/Turabian StyleBlack, Joseph L., Gus M. Lorenz, Aaron J. Cato, Nick R. Bateman, and Nicholas J. Seiter. 2022. "Efficacy of Helicoverpa Armigera Nucleopolyhedrovirus on Soybean for Control of Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) in Arkansas Agriculture" Insects 13, no. 1: 91. https://doi.org/10.3390/insects13010091
APA StyleBlack, J. L., Lorenz, G. M., Cato, A. J., Bateman, N. R., & Seiter, N. J. (2022). Efficacy of Helicoverpa Armigera Nucleopolyhedrovirus on Soybean for Control of Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) in Arkansas Agriculture. Insects, 13(1), 91. https://doi.org/10.3390/insects13010091





