Five-Year Monitoring of a Desert Burrow-Dwelling Spider Following an Environmental Disaster Indicates Long-Term Impacts
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
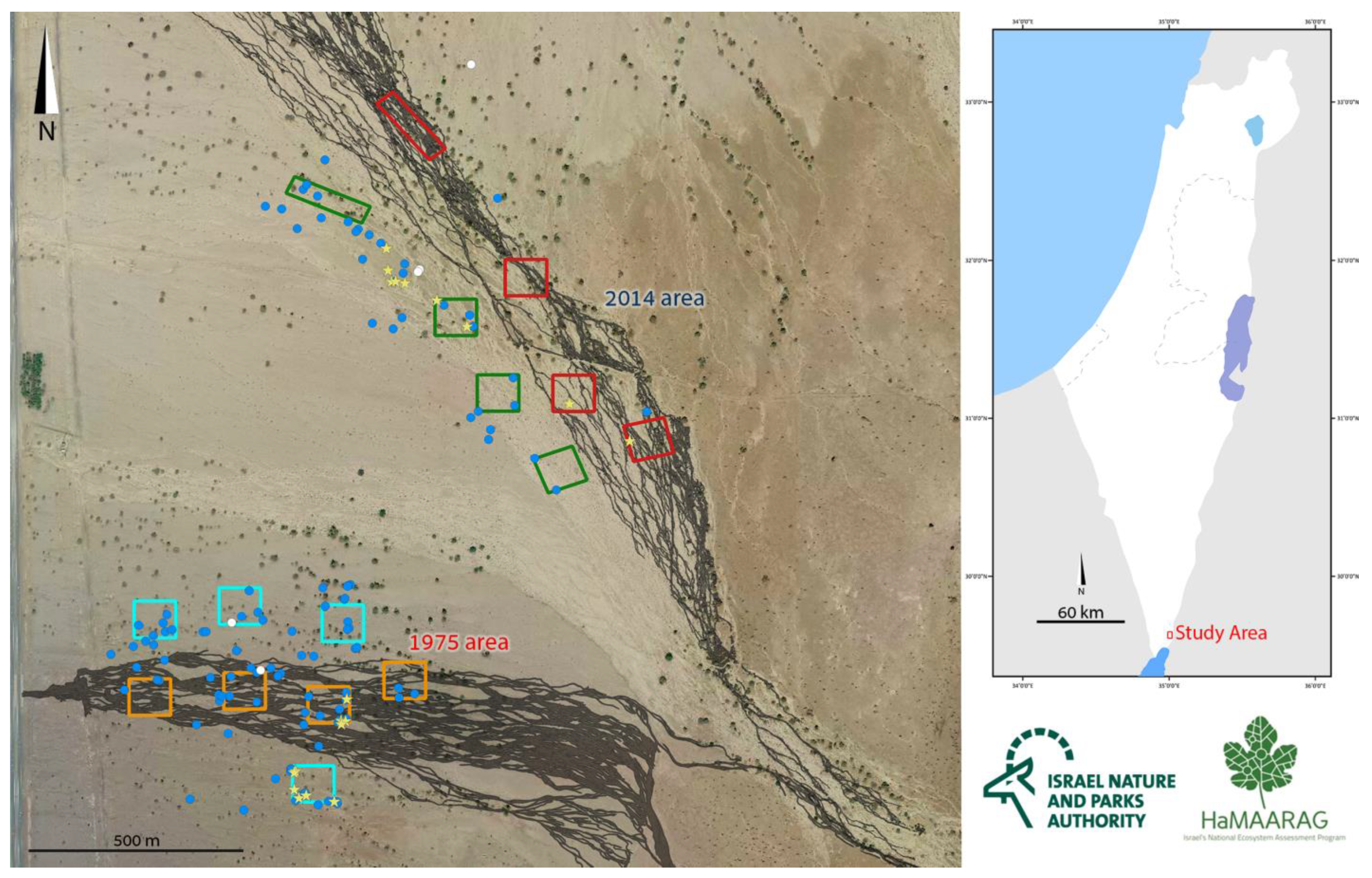
2.1. Monitoring
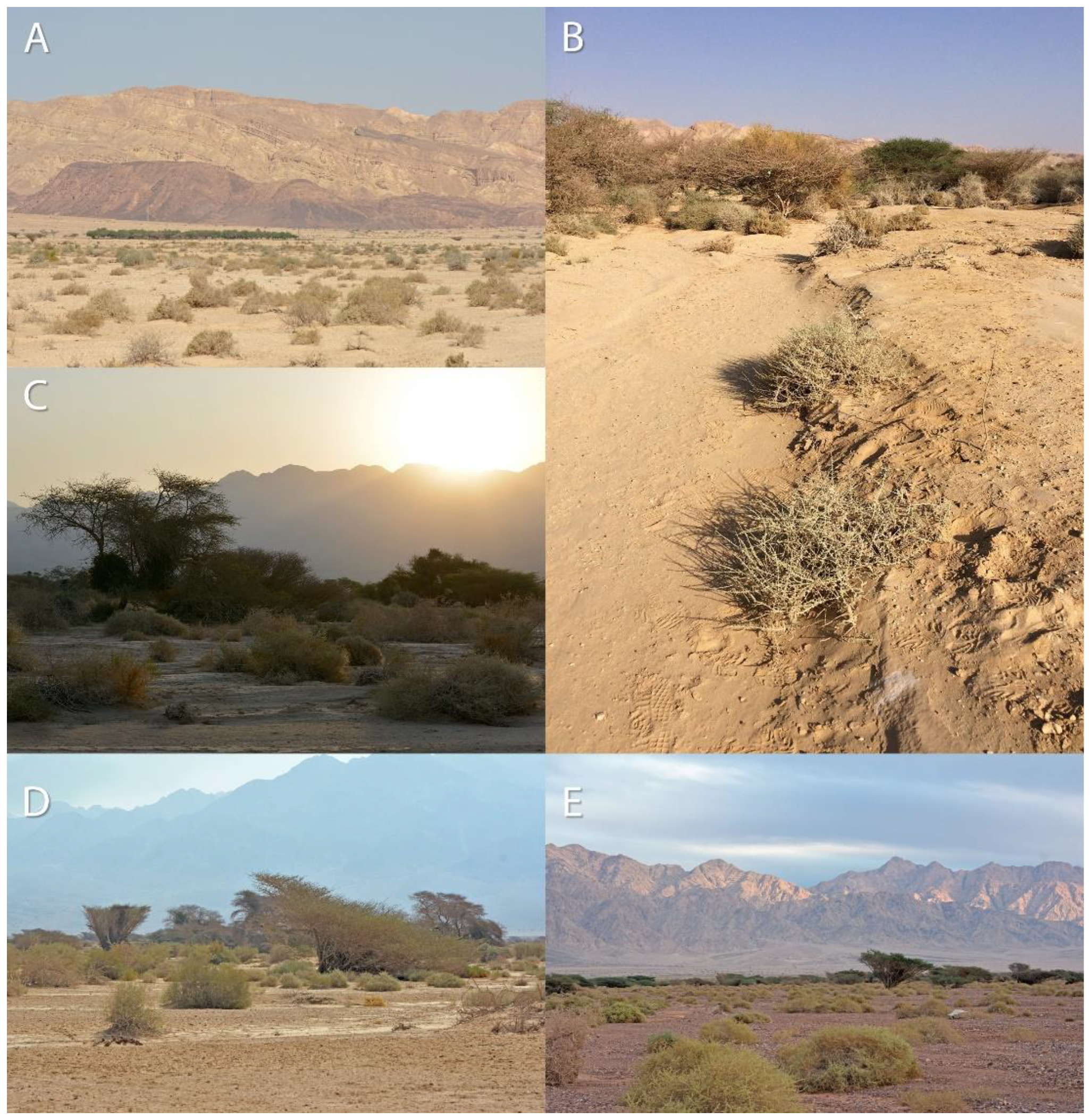
2.2. Quadrat Survey, Nearest Neighbor, and Habitat Characteristics
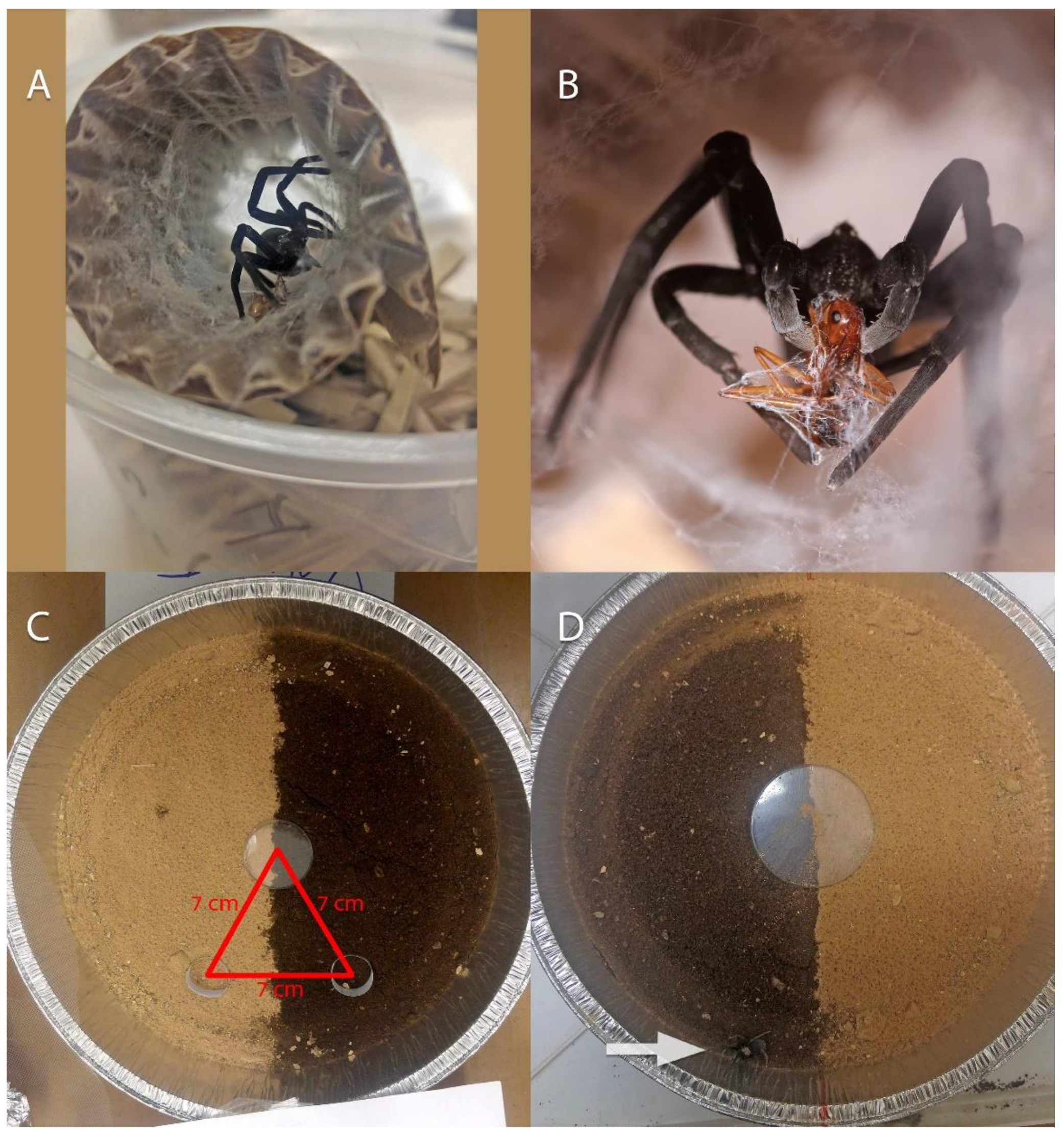
2.3. Soil Preference: Laboratory Experiments
2.3.1. Five-Days Experiment
2.3.2. 24-h Experiment
2.4. Sahastata sp. nov. Description and Natural History
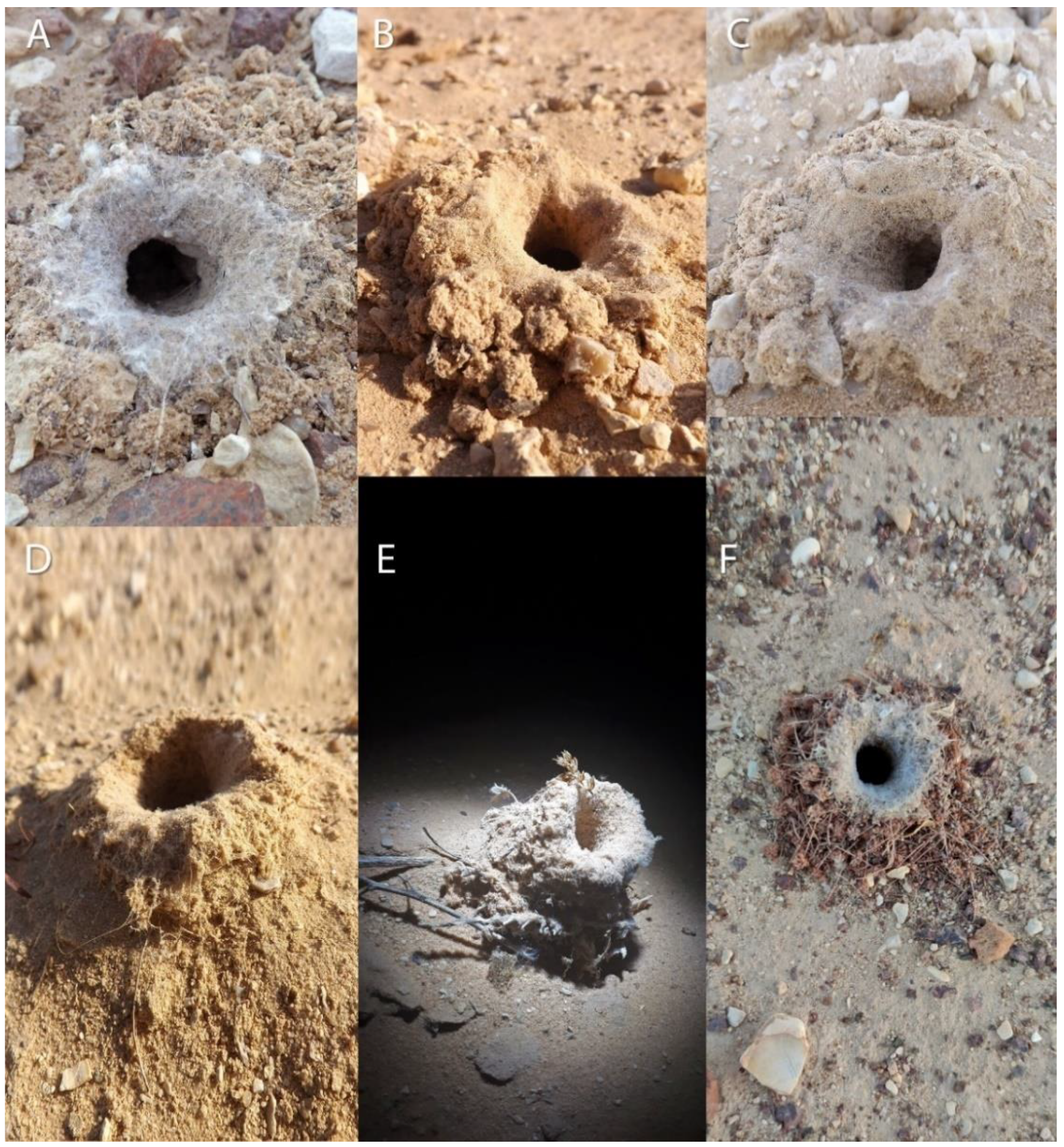
Sahastata Burrows
2.5. Statistical Analyses
2.5.1. Monitoring—Spatial Dispersion
2.5.2. Quadrat Survey, Nearest Neighbor and Habitat Characteristics
2.5.3. Soil Preference: Laboratory Experiments
3. Results
3.1. Monitoring
3.2. Quadrat Survey, Nearest Neighbor and Habitat Characteristics
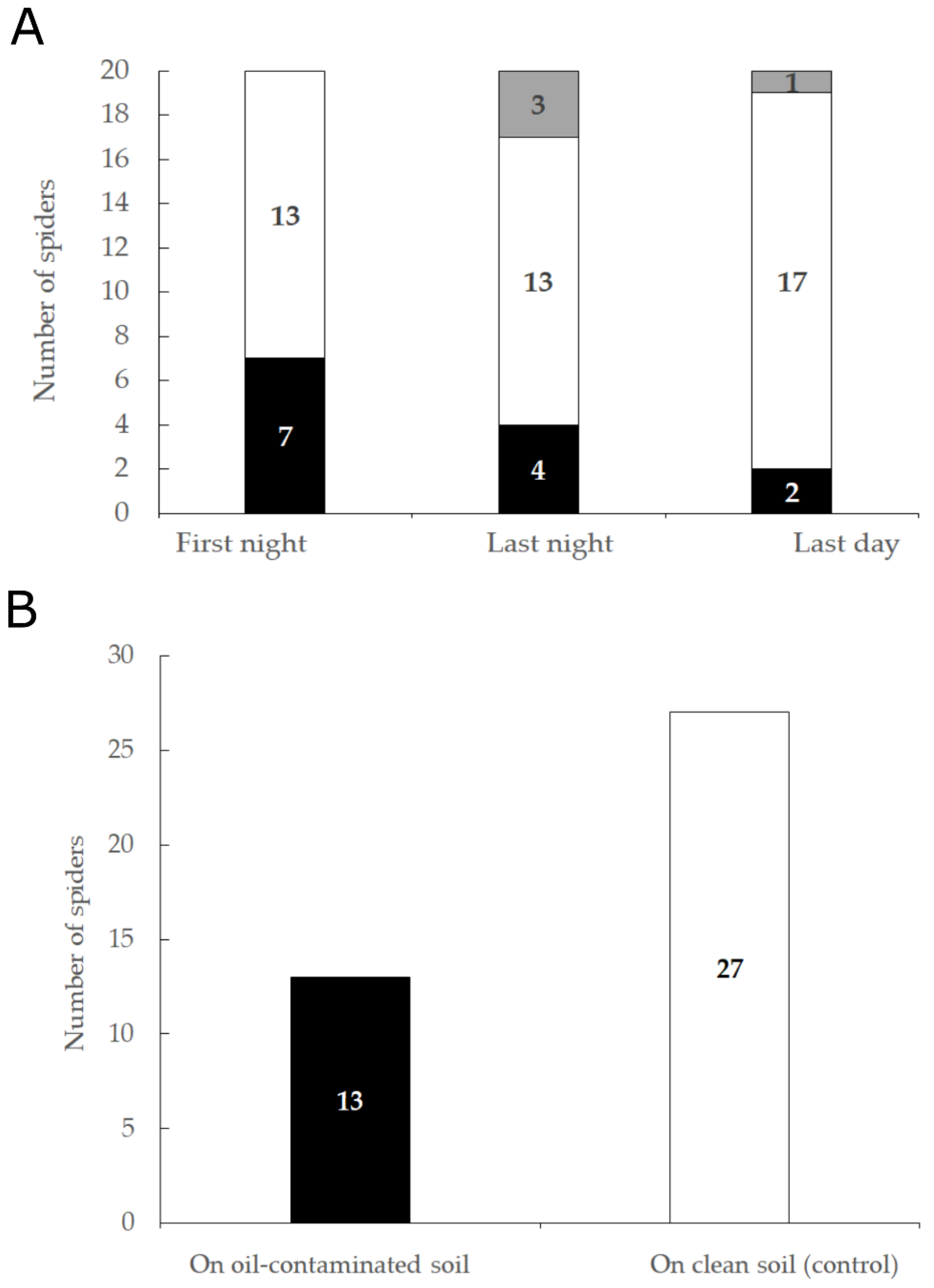
3.3. Soil Preference: Laboratory Experiments
3.3.1. Five-Days Experiment
3.3.2. 24-h Experiment
3.4. Natural History and Description
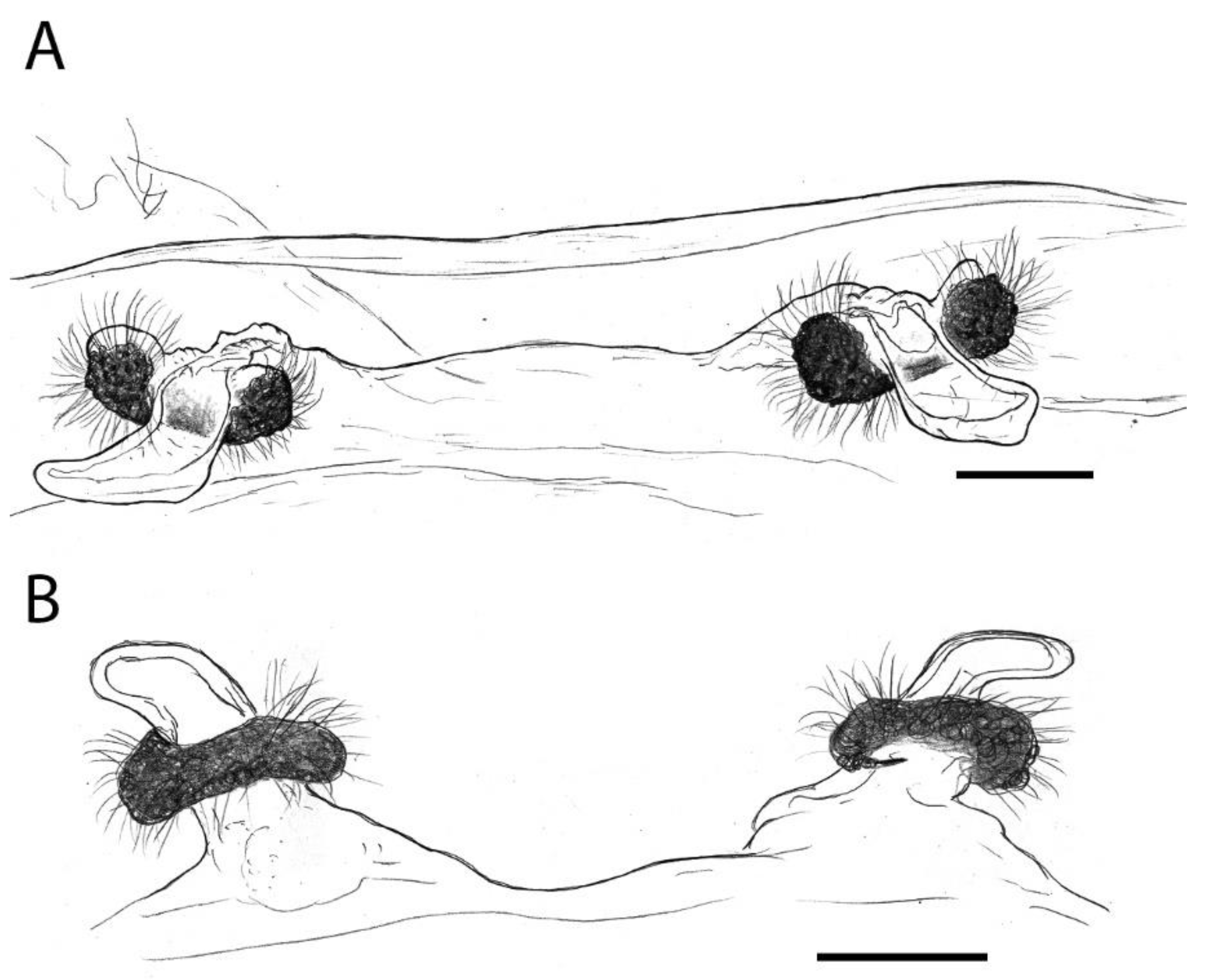
3.4.1. Taxonomy and Description
3.4.2. Natural History
3.4.3. Prey from Collected Nests
3.4.4. Burrow Use in Laboratory
3.4.5. Relationships
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Year | Season | Full Date | Number of New Burrows Found and Marked on the Monitoring Event (Number of Burrows with Spiders) | Comments |
|---|---|---|---|---|
| 2016 | Summer | 6–7 September 2016 | 3(3) | Two additional burrows were marked after this monitoring event (30 September 2016, 26 October 2016, one with a spider). |
| 2017 | Spring | 16–23 May 2017 | 4(1) | One additional burrow was marked after this monitoring event (18 June 2017, no spider observed in this burrow). |
| 2017 | Summer | 11–18 September 2017 | 25(9) | One spider collected on November 2018 for the laboratory experiments. Five additional burrows were marked before and after this monitoring event (24 July 2017, 2 August 2017, 3 September 2017, 29 October 2017, none with a spider). |
| 2018 | Spring | 14–16 May 2018 | 15(10) | Three additional burrows were marked before and after this monitoring event (23 May 2018, 29–30 May 2018, none with a spider). |
| 2018 | Summer | 25–27 September 2018 | 4(0) | Two additional burrows were marked before this monitoring event (14 August 2018, 27 August 2018, none with a spider). |
| 2018 | Autumn | 26–28 November 2018 | 31(18) | Seven spiders collected for the laboratory experiments. |
| 2019 | Winter | 2 January 2019 | 6(2) | Two spiders collected for the laboratory experiments. |
| 2019 | Spring | 21–27 May2019 | 10(5) | One additional burrow was marked before this monitoring event (15 April 2019, a spider observed in this burrow). |
| 2019 | Summer | 28–29 July 2019 | 6(5) | Nine additional burrows were marked after this monitoring event (7–8 August 2019, 10–11 September 2019, 28 September 2019, 6–7 December 2019, four with a spider). |
| 2020 | Summer | 19 August 2020 | 7(3) | Five additional burrows were marked before this monitoring event (28–30 July 2020, four with a spider). |
Appendix B
| Spider Field Code | Coordinates | Plot | Cephalothorax Length (mm) | Maximum Burrow Length (cm) | Trial | Laboratory 24-h Experiment Starting Location |
|---|---|---|---|---|---|---|
| S9 | 29.67854° N, 34.99977° E | Between 2014C3 to 2014C4 | 4.44 | 2 | 1 | Oil-contaminated soil side of arena |
| 2 | Clean soil side of arena (control) | |||||
| 3 | Oil-contaminated soil side of arena | |||||
| 4 | Clean soil side of arena (control) | |||||
| S116 | 29.66962° N, 34.99362° E | 1975C1 | 4.21 | 3 | 1 | Clean soil side of arena (control) |
| 2 | Oil-contaminated soil side of arena | |||||
| 3 | Clean soil side of arena (control) | |||||
| 4 | Oil-contaminated soil side of arena | |||||
| S123 | 29.66586° N, 34.99758° E | 1975C3 | 5.54 | 1.6 | 1 | Oil-contaminated soil side of arena |
| 2 | Clean soil side of arena (control) | |||||
| 3 | Oil-contaminated soil side of arena | |||||
| 4 | Clean soil side of arena (control) | |||||
| S124 | 29.66639° N, 34.99721° E | 1975C3 | 4.98 | 1.6 | 1 | Clean soil side of arena (control) |
| 2 | Oil-contaminated soil side of arena | |||||
| 3 | Clean soil side of arena (control) | |||||
| 4 | Oil-contaminated soil side of arena | |||||
| S125 | 29.66767° N, 34.99843° E | 1975T3 (but not on oil) | 4.77 | 1.8 | 1 | Oil-contaminated soil side of arena |
| 2 | Clean soil side of arena (control) | |||||
| 3 | Oil-contaminated soil side of arena | |||||
| 4 | Clean soil side of arena (control) | |||||
| S126 | 29.66946° N, 34.99886° E | Near 1975C4 | 4.52 | 1.8 | 1 | Oil-contaminated soil side of arena |
| 2 | Clean soil side of arena (control) | |||||
| 3 | Oil-contaminated soil side of arena | |||||
| 4 | Clean soil side of arena (control) | |||||
| S127 | 29.67462° N, 35.00213° E | Between 2014C1 to 2014C2 | 4.62 | 1.3 | 1 | Clean soil side of arena (control) |
| 2 | Oil-contaminated soil side of arena | |||||
| 3 | Clean soil side of arena (control) | |||||
| 4 | Oil-contaminated soil side of arena | |||||
| S128 | 29.67516° N, 35.00169° E | Near 2014C2 | 4.92 | 1.2 | 1 | Oil-contaminated soil side of arena |
| 2 | Clean soil side of arena (control) | |||||
| 3 | Oil-contaminated soil side of arena | |||||
| 4 | Clean soil side of arena (control) | |||||
| S129 | 29.67949° N, 34.99946° E | Between 2014C3 to 2014C4 | 5.28 | 1.6 | 1 | Clean soil side of arena (control) |
| 2 | Oil-contaminated soil side of arena | |||||
| 3 | Clean soil side of arena (control) | |||||
| 4 | Oil-contaminated soil side of arena | |||||
| S130 | 29.68094° N, 34.99762° E | 2014C4 | 4.87 | 1.6 | 1 | Clean soil side of arena (control) |
| 2 | Oil-contaminated soil side of arena | |||||
| 3 | Clean soil side of arena (control) | |||||
| 4 | Oil-contaminated soil side of arena |
References
- Ward, D. The Biology of Deserts, 2nd ed.; The Biology of Habitats Series; Oxford University Press: Oxford, UK, 2016; ISBN 978-0-19-873276-1. [Google Scholar]
- Sharon, D. The spottiness of rainfall in a desert area. J. Hydrol. 1972, 17, 161–175. [Google Scholar] [CrossRef]
- Lovich, J.E. Anthropogenic Degradation of the Southern California Desert Ecosystem and Prospects for Natural Recovery and Restoration. Environ. Manag. 1999, 24, 309–326. [Google Scholar] [CrossRef]
- Guo, Q. Slow Recovery in Desert Perennial Vegetation Following Prolonged Human Disturbance. J. Veg. Sci. 2004, 15, 757–762. [Google Scholar] [CrossRef]
- Shulov, A. On some Israeli scorpions. Dapim Refuiim. Folia Med. 1962, 21, 657–660. [Google Scholar]
- Shulov, A.; Levy, G.; Efrati, P.; Zlotkin, E.; Miranda, F.; Rochat, H. Venoms of Buthinae. In Arthropod Venoms; Handbook of Experimental Pharmacology/Handbuch der Experimentellen Pharmakologie; Bettini, S., Ed.; Springer: Berlin, Heidelberg, 1978; pp. 309–369. ISBN 978-3-642-45501-8. [Google Scholar]
- Abella, S. Post-fire plant recovery in the Mojave and Sonoran Deserts of western North America. J. Arid. Environ. 2009, 73, 699–707. [Google Scholar] [CrossRef]
- Marín-García, D.C.; Adams, R.H.; Hernández-Barajas, R. Effect of crude petroleum on water repellency in a clayey alluvial soil. Int. J. Environ. Sci. Technol. 2016, 13, 55–64. [Google Scholar] [CrossRef]
- Girsowicz, R.; Koryachenko, O.; Sherman, C.; Mayzlish-Gati, E.; Doniger, T.; Steinberger, Y. Impact of Oil-Spill Contamination on a Soil Bacterial Community: A 40-Year History of Rehabilitation in the Arava Valley. Soil Sediment Contam. Int. J. 2018, 27, 175–185. [Google Scholar] [CrossRef]
- Gordon, G.; Stavi, I.; Shavit, U.; Rosenzweig, R. Oil spill effects on soil hydrophobicity and related properties in a hyper-arid region. Geoderma 2018, 312, 114–120. [Google Scholar] [CrossRef]
- Nothers, M.; Segev, N.; Kreyling, J.; Hjazin, A.; Groner, E. Desert Vegetation Forty Years after an Oil Spill. J. Environ. Qual. 2017, 46, 568–575. [Google Scholar] [CrossRef]
- Golan, S.; Faraj, T.; Rahamim, E.; Zemach, H.; Lifshitz, D.; Singer, A.; Bar, D.; Carmeli, D.; Steinberger, Y.; Sherman, C.; et al. The Effect of Petroleum Hydrocarbons on Seed Germination, Development and Survival of Wild and Cultivated Plants in Extreme Desert Soil. Int. J. Agric. Environ. Res. 2016, 2, 1743–1767. [Google Scholar]
- Pelta, R.; Carmon, N.; Ben-Dor, E. A Machine Learning Approach to Detect Crude Oil Contamination in a Real Scenario Using Hyperspectral Remote Sensing. Int. J. Appl. Earth Obs. Geoinf. 2019, 82, 101901. [Google Scholar] [CrossRef]
- Banet, G.; Turaani, A.K.; Farber, R.; Armoza-Zvuloni, R.; Rotem, N.; Stavi, I.; Cahan, R. The Effects of Biostimulation and Bioaugmentation on Crude Oil Biodegradation in Two Adjacent Terrestrial Oil Spills of Different Age, in a Hyper-Arid Region. J. Environ. Manag. 2021, 286, 112248. [Google Scholar] [CrossRef]
- Stavi, I.; Rosenzweig, R. Tillage Effect on Hydrophobicity and Hydrological Properties of Oil-Contaminated Sediments in a Hyper-Arid Region. Arid. Land Res. Manag. 2020, 34, 26–35. [Google Scholar] [CrossRef]
- Ferrante, M.; Dangol, A.; Didi-Cohen, S.; Winters, G.; Tzin, V.; Segoli, M. Oil Pollution Affects the Central Metabolism of Keystone Vachellia (Acacia) Trees. Sustainability 2021, 13, 6660. [Google Scholar] [CrossRef]
- Tran, T.H.; Mayzlish Gati, E.; Eshel, A.; Winters, G. Germination, Physiological and Biochemical Responses of Acacia Seedlings (Acacia Raddiana and Acacia Tortilis) to Petroleum Contaminated Soils. Environ. Pollut. 2018, 234, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Möller, D.M.; Möller, G.M.; Lubin, Y.; Segoli, M. Seed Predation on Oil-Polluted and Unpolluted Vachellia (Acacia) Trees in a Hyper-Arid Desert Ecosystem. Insects 2020, 11, 665. [Google Scholar] [CrossRef]
- Möller, D.M.; Ferrante, M.; Möller, G.M.; Rozenberg, T.; Segoli, M. The Impact of Terrestrial Oil Pollution on Parasitoid Wasps Associated With Vachellia (Fabales: Fabaceae) Trees in a Desert Ecosystem, Israel. Environ. Entomol. 2020, 49, 1355–1362. [Google Scholar] [CrossRef]
- Polis, G.A.; Yamashita, T. The Ecology and Importance of Predaceous Arthropods in Desert Communities. In The Ecology of Desert Communities; Polis, G.A., Ed.; U. Arizona Press: Tucson, AZ, USA, 1991; pp. 180–222. [Google Scholar]
- Henschel, J. The Biology of Leucorchestris Arenicola (Araneae: Heteropodidae), a Burrowing Spider of the Namib Dunes. In Namib Ecoloy: 25 Years of Namib Research; Transvaal Museum Monograph: Pretoria, South Africa, 1990; pp. 115–127. [Google Scholar]
- Lubin, Y.; Ferrante, M.; Musli, I.; Lövei, G.L. Diversity of Ground-Active Spiders in Negev Desert Habitats, Israel. J. Arid. Environ. 2020, 183, 104252. [Google Scholar] [CrossRef]
- Shanas, U.; Galyun, Y.A.; Alshamlih, M.; Cnaani, J.; Guscio, D.; Khoury, F.; Mittler, S.; Nassar, K.; Shapira, I.; Simon, D.; et al. Landscape and a Political Border Determine Desert Arthropods Distribution. J. Arid. Environ. 2011, 75, 284–289. [Google Scholar] [CrossRef]
- Whitehouse, M.E.A.; Shochat, E.; Shachak, M.; Lubin, Y. The Influence of Scale and Patchiness on Spider Diversity in a Semi-Arid Environment. Ecography 2002, 25, 395–404. [Google Scholar] [CrossRef]
- Cloudsley-Thompson, J.L. Desert Adaptations in Spiders. J. Arid. Environ. 1983, 6, 307–317. [Google Scholar] [CrossRef]
- Gavish-Regev, E.; Armiach, I.; Levy, T.; Uzan, A.; Majer, M.; Salman, I.; Segev, N.; Lubin, Y. Burrow Placement of Sahastata Nigra (Simon, 1897), and Their Potential as Indicators of Habitat Recovery in a Hyper-Arid Desert. In Proceedings of the 31st European Congress of Arachnology, Vác, Hungary, 8–13 July 2018; p. 55. [Google Scholar]
- World Spider Catalog Version 22.5. Available online: http://wsc.nmbe.ch (accessed on 21 July 2021).
- Magalhaes, I.L.F.; Stockmann, M.; Marusik, Y.M.; Zonstein, S.L. On Sahastata (Araneae: Filistatidae): Complementary Description of the Generotype and Two New Species from Oman and Morocco. Zootaxa 2020, 4899, 215–246. [Google Scholar] [CrossRef]
- Wiens, J.A.; Parker, K.R. Analyzing the Effects of Accidental Environmental Impacts: Approaches and Assumptions. Ecol. Appl. 1995, 5, 1069–1083. [Google Scholar] [CrossRef]
- Goldreich, Y.; Karni, O. Climate and Precipitation Regime in the Arava Valley, Israel. Srael J. Earth Sci. 2001, 50, 53–60. [Google Scholar]
- Amit, R.; Zilberman, E.; Porat, N.; Enzel, Y. Relief Inversion in the Avrona Playa as Evidence of Large-Magnitude Historical Earthquakes, Southern Arava Valley, Dead Sea Rift. Quat. Res. 1999, 52, 76–91. [Google Scholar] [CrossRef]
- Spatial Networks; Fulcrum Inc.: Little Canada, MN, USA, 2021.
- Marusik, Y.M.; Koponen, S.; Zonstein, S. A Revalidation and Redescription of Sahastata infuscata, with Notes on S. Nigra (Araneae: Filistatidae). Arachnology 2017, 17, 309–311. [Google Scholar] [CrossRef]
- Magalhaes, I.L.F. Spreadsheets to Expedite Taxonomic Publications by Automatic Generation of Morphological Descriptions and Specimen Lists. Zootaxa 2019, 4624, 147–150. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecological Methodology, 2nd ed.; Benjamin/Cummings: Menlo Park, CA, USA, 1999; ISBN 978-0-321-02173-1. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Arganda-Carreras, I.; Kaynig, V.; Rueden, C.; Eliceiri, K.W.; Schindelin, J.; Cardona, A.; Sebastian Seung, H. Trainable Weka Segmentation: A Machine Learning Tool for Microscopy Pixel Classification. Bioinformatics 2017, 33, 2424–2426. [Google Scholar] [CrossRef] [PubMed]
- Benoit, P.L.G. Synopsis Des Filistatidae Africains (Araneae). Ann. Del. Mus. Civ. D Stor. Nat. Giacomo Doria 1968, 77, 92–102. [Google Scholar]
- Patel, B.H. Studies on Indian Filistatid Spiders (Araneae: Arachnida). J. Bombay Nat. Hist. Soc. 1978, 75, 183–189. [Google Scholar]
- Brignoli, P.M. Contribution à La Connaissance Des Filistatidae Paléarctiques (Araneae). Rev. Arachnol. 1982, 4, 65–75. [Google Scholar]
- Marusik, Y.M.; Zamani, A.; Mirshamsi, O. Three New Species of Mygalomorph and Filistatid Spiders from Iran (Araneae, Cyrtaucheniidae, Nemesiidae and Filistatidae). ZK 2014, 463, 1–10. [Google Scholar] [CrossRef]
- Marusik, Y.; Zamani, A. The Spider Family Filistatidae (Araneae) in Iran. ZK 2015, 516, 123–135. [Google Scholar] [CrossRef]
- Marusik, Y.M.; Zamani, A. A New Species of Sahastata (Aranei, Filistatidae) from Southern Iran. Vestn. Zool. 2016, 50, 267–270. [Google Scholar] [CrossRef]
- Zonstein, S.; Marusik, Y.M. A Revision of the Spider Genus Filistata (Araneae: Filistatidae). Arachnology 2019, 18, 53. [Google Scholar] [CrossRef]
- Machałowski, T.; Wysokowski, M.; Petrenko, I.; Fursov, A.; Rahimi-Nasrabadi, M.; Amro, M.M.; Meissner, H.; Joseph, Y.; Fazilov, B.; Ehrlich, H.; et al. Naturally Pre-Designed Biomaterials: Spider Molting Cuticle as a Functional Crude Oil Sorbent. J. Environ. Manag. 2020, 261, 110218. [Google Scholar] [CrossRef]
- Segev, N. The Influence of Oil Spills in ‘Avrona Nature Reserve on the Distribution and Behavior of Reptiles and Insects. Ph.D. Thesis, Ben Gurion University of the Negev, Midreshet Ben-Gurion, Be’er Sheva, Israel, 2021. [Google Scholar]
- Bam, W.; Hooper-Bui, L.M.; Strecker, R.M.; Adhikari, P.L.; Overton, E.B. Coupled Effects of Oil Spill and Hurricane on Saltmarsh Terrestrial Arthropods. PLoS ONE 2018, 13, e0194941. [Google Scholar] [CrossRef]
- Al-Hashem, M. Monitoring Population Abundance of the Sand Lizard Acanthodactylus Scutellatus and Their Ant Prey in Oil Polluted Soils at Kuwait’s Greater Al-Burgan Oil Field. Pak. J. Biol. Sci. 2009, 12, 1425–1429. [Google Scholar] [CrossRef]
- Henschel, J.R.; Lubin, Y.D. A Test of Habitat Selection at Two Spatial Scales in a Sitand-Wait Predator: A Web Spider in the Namib Desert Dunes. J. Anim. Ecol. 1997, 66, 401. [Google Scholar] [CrossRef]
- Conti, E.; Costa, G.; Liberatori, G.; Vannuccini, M.L.; Protano, G.; Nannoni, F.; Corsi, I. Ariadna Spiders as Bioindicator of Heavy Elements Contamination in the Central Namib Desert. Ecol. Indic. 2018, 95, 663–672. [Google Scholar] [CrossRef]
- Hansson, S.V.; Høye, T.T.; Bach, L.; Mielec, C.; Mosbech, A.; Søndergaard, J. Spiders as Biomonitors of Metal Pollution at Arctic Mine Sites: The Case of the Black Angel Pb-Zn-Mine, Maarmorilik, West Greenland. Ecol. Indic. 2019, 106, 105489. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavish-Regev, E.; Armiach Steinpress, I.; Salman, I.N.A.; Segev, N.; Uzan, A.; Byun, Y.; Levy, T.; Aharon, S.; Zvik, Y.; Shtuhin, R.; et al. Five-Year Monitoring of a Desert Burrow-Dwelling Spider Following an Environmental Disaster Indicates Long-Term Impacts. Insects 2022, 13, 101. https://doi.org/10.3390/insects13010101
Gavish-Regev E, Armiach Steinpress I, Salman INA, Segev N, Uzan A, Byun Y, Levy T, Aharon S, Zvik Y, Shtuhin R, et al. Five-Year Monitoring of a Desert Burrow-Dwelling Spider Following an Environmental Disaster Indicates Long-Term Impacts. Insects. 2022; 13(1):101. https://doi.org/10.3390/insects13010101
Chicago/Turabian StyleGavish-Regev, Efrat, Igor Armiach Steinpress, Ibrahim N. A. Salman, Nitzan Segev, Assaf Uzan, Yebin Byun, Tanya Levy, Shlomi Aharon, Yoram Zvik, Raisa Shtuhin, and et al. 2022. "Five-Year Monitoring of a Desert Burrow-Dwelling Spider Following an Environmental Disaster Indicates Long-Term Impacts" Insects 13, no. 1: 101. https://doi.org/10.3390/insects13010101
APA StyleGavish-Regev, E., Armiach Steinpress, I., Salman, I. N. A., Segev, N., Uzan, A., Byun, Y., Levy, T., Aharon, S., Zvik, Y., Shtuhin, R., Shapira, Y., Majer, M., Ganem, Z., Zonstein, S., Magalhaes, I. L. F., & Lubin, Y. (2022). Five-Year Monitoring of a Desert Burrow-Dwelling Spider Following an Environmental Disaster Indicates Long-Term Impacts. Insects, 13(1), 101. https://doi.org/10.3390/insects13010101









