Simple Summary
In recent years, Moringa oleifera leaves have been increasingly introduced into the diets of human populations that are affected by malnutrition and as a dietary supplement in Western countries. The leaves can be stocked in storage spaces that contain other herbs or food that can be infested by Pyralid moths, such as the polyphagous almond moth, rice moth, and Indian meal moth, as well as by the Geometrid Rustywave moth, which thrives on dried herbs. This paper describes laboratory tests of the susceptibility of dried and powdered leaves of M. oleifera to moths that feed on stored products. Eggs of the different species were added to dried M. oleifera leaves, powdered leaves, and an artificial diet; dried or powdered leaves were added to the latter to understand the effects on the development of the moths. The tests were carried out under the optimal temperature and relative humidity conditions for these species. The numbers of adults that emerged and the development periods were recorded. The results showed that powdered Moringa leaves were not susceptible to moth attacks, whereas dried leaves were damaged only by Rustywave moths. The explanation for why M. oleifera leaves are not susceptible to Pyralid moths and for why few Rustywave moths can complete the development from egg to adult is attributable to both nutritional deficiency and to secondary metabolites.
Abstract
The leaves of Moringa oleifera are increasingly used as a food supplement in several countries due to their nutritional composition, which is rich in protein, vitamins, and mineral salts. Foodstuffs can be damaged by several pests when stored in environments with temperatures that are favorable to insect development; therefore, the susceptibility of M. oleifera leaves to attacks of moths that feed on stored products was tested. Tests were carried out on Pyralid Cadra cautella, Corcyra cephalonica, and Plodia interpunctella, as well as Geometrid Idaea inquinata, which were reared on dried whole or powdered M. oleifera leaves, an artificial diet, or an artificial diet supplemented with dried or powdered leaves. The numbers of adults and the development periods with the different diets were recorded. M. oleifera leaves were unsuitable as a rearing medium for all of the species except I. inquinata, although only a few individuals of this species reached the adult stage. The use of an artificial diet of which one-quarter consisted of dried and powdered leaves did not affect the number of progeny or on the biological cycle, showing that the effect was due to the nutritional composition, as well as to the toxic effect. The storage of M. oleifera as powdered leaves is recommended in order to preserve the nutritional characteristics and avoid damage caused by moth larvae.
1. Introduction
Moringa oleifera Lam. is a tree that is native to South Asia and is widely grown in tropical areas. Local populations have used all of the different parts of the tree for thousands of years. The roots and flowers are used in traditional medicine, the seeds are employed for water purification, and the trees are planted to avoid soil erosion.
The leaves of M. oleifera have been introduced into the diets of several human populations to address malnutrition problems, because they contain good amounts of proteins, calcium, iron, potassium, vitamins, and β-carotene. The leaves present a very high content of fiber, antioxidant and bioactive compounds, flavonoids, and phenolic acids [1,2,3]; therefore, they are used as a food supplement in Western countries. The leaves are dried before storage and marketed whole or powdered. M. oleifera is mainly grown in South Asia, but it is also cultivated in Africa, South America, and the Caribbean. In these countries, vegetables are usually naturally air-dried in processing plants that are not completely isolated from the external environment; insects that feed on stored products can easily invade in such cases, causing huge losses by reducing the economic value and compromising the use for human nutrition. Moths that feed on stored products have already been reported to cause damage to dried herbs [4,5,6]; therefore, we chose to investigate three polyphagous moths—Cadra cautella (Walker), Corcyra cephalonica (Stainton), and Plodia interpunctella (Hübner) (Pyralidae)—and one species that feeds mainly on dried herbs—Idaea inquinata (Scopoli) (Geometridae). These Pyralid species can develop in different stored foods [7], such as cereal grains [8], flour [9], and dried fruit [10,11,12]. The damage caused by Pyralid moths is not only economically important, but it also compromises food safety, because it was demonstrated that these species produce allergens that affect human health [13,14]. The Geometrid I. inquinata is mostly found in hay lofts and in barns [15,16,17], but more recent research has shown that this species is considered as a potential pest for cereals and some of their derivatives, as well as for medicinal plants [18].
In this research, the susceptibility of dried and powdered leaves of M. oleifera to some species of moths was verified. Tests were also carried out by using an artificial diet, of which one-quarter consisted of dried and powdered leaves when rearing in the laboratory in order to determine if the effects of Moringa leaves on the development of these species were linked to their nutritional composition or to the presence of secondary components.
2. Materials and Methods
2.1. Insect Rearing
Stock rearing of Cadra cautella, Corcyra cephalonica, Plodia interpunctella, and Idaea inquinata has been maintained for 10 years at DeFENS, Università degli Studi di Milano “La Statale”. The insects were reared in Petri dishes (Ø 15 cm), placed in a growth chamber (Piardi mod. CFT600) at 26 ± 1 °C with 60 ± 5% RH, and a photoperiod of 16:8 (light–dark).
C. cautella, C. cephalonica, and P. interpunctella were reared on an artificial diet consisting of glycerol, honey, cornmeal, bran, wheat meal, wheat germ, and yeast [19]. The artificial diet of I. inquinata comprised the same ingredients, but with a higher bran percentage [20]. Proximate analyses were performed on 50 g of the artificial diets of I. inquinata and Pyralidae to determine their nutritional value (two replicates, expressed as means ± S.D.). Different methods were used: the fiber content was analyzed according to the method of Prosky et al. [21]; carbohydrates were determined with the method of Rocklin and Pohl [22]; and the methods of the Association of Analytical Communities and the American Association for Clinical Chemistry were performed to measure proteins [23], fats [24], and ashes [25].
2.2. Egg Collection
For each species, newly emerged adults were placed in a glass jar (1.7 L), which was closed with tulle that was fixed with a plastic band, and the jar was turned upside down and placed on an open Petri dish. The bottom was covered with black paper. After 24 h, the eggs were collected for the tests.
2.3. Tests
For each species—namely, Cadra cautella, Corcyra cephalonica, Plodia interpunctella, and Idaea inquinata—one hundred eggs were reared on 10 g of the following six media: artificial diet, dried Moringa oleifera leaves, powdered M. oleifera leaves, ½ dried M. oleifera leaves + ½ powdered leaves, ¼ dried M. oleifera leaves + ¾ artificial diet, ¼ powdered M. oleifera leaves + ¾ artificial diet. M. oleifera was cultivated and dried in Haiti; dried leaves were powdered in our laboratory.
The rearing media were placed in PVC containers (height: 5 cm, diameter: 7 cm). A hole that was 2 cm in diameter was added to the lids of the containers and closed with a wire net to allow gas exchange. Before the tests, the PVC containers with the six media plates were maintained in the testing conditions for a week. One hundred eggs that were laid in 24 h were added. For each species and each rearing medium, five replicates were carried out. The testing conditions were kept at 26 ± 1 °C with 60 ± 5% RH and a photoperiod of 16:8 (light–dark). After 20 days, the replicates were checked daily. The adults that emerged were counted and removed, and the development time was recorded.
To assess the normality distribution of data, a Kolmogorov–Smirnov test was carried out, and the normally distributed data were subjected to one-way ANOVA and the least significant difference (LSD) test (IBM SPSS Statistics 26).
3. Results
Adults of the Cadra cautella, Corcyra cephalonica, and Plodia interpunctella groups were not observed in the tests on the dried or powdered Moringa oleifera leaves (Table 1). Only a few individuals of Idaea inquinata developed on dried leaves of M. oleifera; none developed on the powdered leaves. All the test data were normally distributed.

Table 1.
Mean numbers (±S.E.) of adults and the development times (days ± S.E.) of Cadra cautella (Walker), Corcyra cephalonica (Stainton), Plodia interpunctella (Hübner), and Idaea inquinata (Scopoli), from 100 eggs reared on the artificial diet (AD), dried Moringa oleifera leaves (DML), and powdered Moringa oleifera leaves (PML) at 26 ± 1 °C with 60 ± 5% RH and a photoperiod of 16:8 (light–dark). Means followed by different letters are significantly different (LSD test).
The number of adults of C. cautella (Table 1, Figure 1) reared on ¼ dried or powdered leaves and ¾ of the artificial diet was significantly lower than the number of adults reared on the artificial diet, but the development time was not influenced (one-way ANOVA: Adults F 2, 12 = 155.730, p < 0.05; time F 2, 12 = 1.334, P = 0.3 n.s.). The number of adults and the development time of C. cephalonica (Table 1, Figure 2) were not influenced by the artificial diet with one-quarter of dried or powdered leaves (one-way ANOVA: adults F 2, 12 = 0.947, P = 0.415 n.s.; time F 2, 12 = 3.295, P = 0.072 n.s.). In the tests on P. interpunctella (Table 1, Figure 3), the addition of powdered leaves to the artificial diet at a proportion of one-quarter did not influence the number of adults, but caused a significant increase in the development time, whereas the addition of dried leaves resulted in fewer adults emerging and a shorter development period (one-way ANOVA: adults F 2, 12 = 2.458, P = 0.127 n.s.; time F 2, 12 = 5.203, p < 0.05).
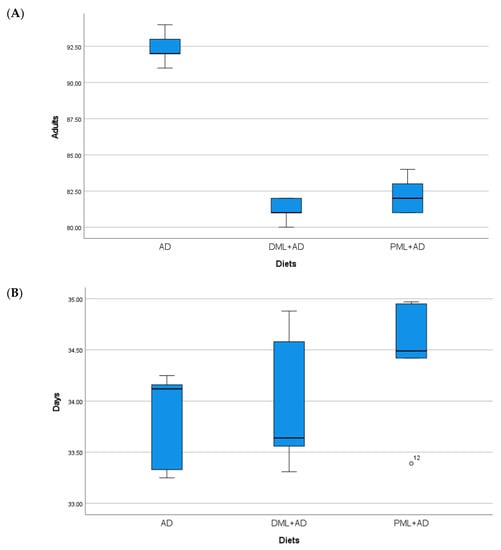
Figure 1.
(A) Mean number (±S.E.) of adults; (B), mean development times (days ± S.E.) of Cadra cautella (Walker), from 100 eggs reared on artificial diet (AD), ¼ dried Moringa oleifera leaves + ¾ artificial diet, ¼ (DML+AD), powdered M. oleifera leaves + ¾ artificial diet (PML+AD), at 26 ± 1 °C with 60 ± 5% RH and a photoperiod of 16:8 (light–dark).
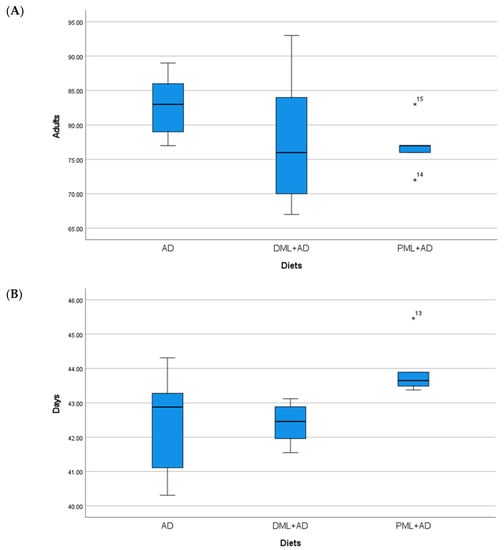
Figure 2.
(A) Mean number (±S.E.) of adults; (B) mean development times (days ± S.E.) of Corcyra cephalonica (Stainton), from 100 eggs reared on artificial diet (AD), ¼ dried Moringa oleifera leaves + ¾ artificial diet, ¼ (DML+AD), powdered M. oleifera leaves + ¾ artificial diet (PML+AD), at 26 ± 1 °C with 60 ± 5% RH and a photoperiod of 16:8 (light–dark).
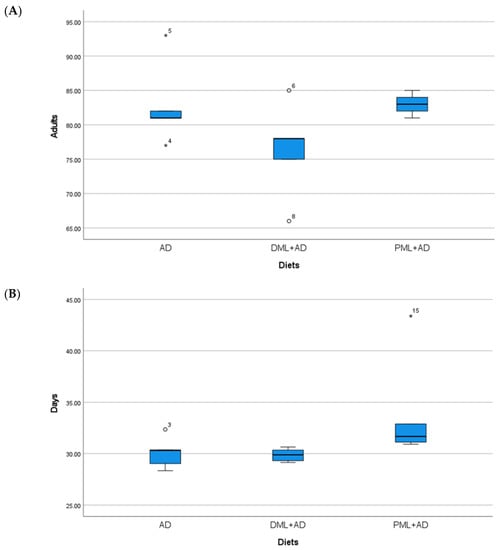
Figure 3.
(A) Mean number (±S.E.) of adults; (B) mean development times (days ± S.E.) of Plodia interpunctella (Hübner), from 100 eggs reared on artificial diet (AD), ¼ dried Moringa oleifera leaves + ¾ artificial diet, ¼ (DML+AD), powdered M. oleifera leaves + ¾ artificial diet (PML+AD), at 26 ± 1 °C with 60 ± 5% RH and a photoperiod of 16:8 (light–dark).
The numbers of adults and the development times of I. inquinata (Table 1, Figure 4) reared on the artificial diet alone and with the addition of dried leaves were not significantly different (one-way ANOVA: Adults F 4, 20 = 2346.23, p < 0.05; time F 4, 20 = 11.695, p < 0.05). The addition of powdered leaves to the artificial diet caused a decrease in the number of adults, but not in the development time. Fewer adults and a longer development time were observed when using dried leaves alone and when using the mixture with powdered leaves of M. oleifera.
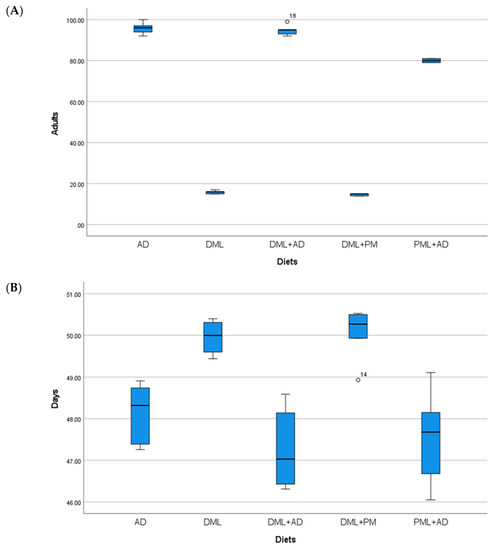
Figure 4.
(A) Mean number (±S.E.) of adults; (B) mean development times (days ± S.E.) of Idaea inquinata (Scopoli), from 100 eggs reared on artificial diet (AD), dried Moringa oleifera leaves (DML), powdered M. oleifera leaves (PML), ½ dried M. oleifera leaves + ½ powdered leaves (DML+PM), ¼ dried M. oleifera leaves + ¾ artificial diet (DML+AD), ¼ powdered M. oleifera leaves + ¾ artificial diet (PML+AD), at 26 ± 1 °C with 60 ± 5% RH and a photoperiod of 16:8 (light–dark).
The main nutrients of the artificial diets are summarized in Table 2. In the case of the main nutrients of M. oleifera, we refer to Leone et al. [26], considering the values of Moringa from Haiti. The highest protein and lipid contents were recorded in M. oleifera (20.8% and 7.0%, respectively), and the lowest were recorded in the artificial Pyralid diet (8.6% and 2.0%, respectively). The starch content was higher in the artificial diets of Pyralid and I. inquinata (29.3% and 22.0%, respectively), and it was lower in the M. oleifera leaves (13.7%). The total fiber was high in M. oleifera (37.6%) and in the artificial I. inquinata diet (27.6); it was low (9.7%) in the artificial Pyralid diet. The moisture content was 12.4 (±0.09) in the Pyralid diet, 12.8 (±0.01) in the I. inquinata diet, and 8.8 (±0.1) in dried Moringa leaves.

Table 2.
Nutritional characterization of the artificial diets of Idaea inquinata and of the Pyralid moths (mean ± S.D.).
4. Discussion
Dried and powdered Moringa oleifera leaves were unsuitable as rearing media for Cadra cautella, Corcyra cephalonica, and Plodia interpunctella. Only a few Idaea inquinata specimens developed on the dried leaves. M. oleifera leaves lack essential nutrients for the postembryonic development of the Pyralid moths. When comparing artificial diets and the M. oleifera leaves, it should be noted that they have different nutritional compositions, because M. oleifera leaves have higher contents of protein, lipids, and ash, and lower starch and moisture contents compared to the other diets. The artificial diets also contained glycerol, which contributes some nutritive factors, increases the diet water content, and is considered a booster for P. interpunctella larval growth [27].
Powdered M. oleifera leaves were unsuitable as a rearing medium for I. inquinata, as has also been observed for different flours [18]; in this case, the cause was the fine particle size, rather than the nutritional composition.
The effect on the moths’ development can be ascribed to the nutritional composition, although it must be considered that the powdered leaves of M. oleifera exert insecticidal and repellent activities on the larvae and adults of Trogoderma granarium [28,29]. The leaves contain catechol, tannins, gallic tannins, steroids, triterpenoids, flavonoids, saponins, anthraquinones, and alkaloids [30], and secondary metabolite, such as glucosinolates and isothiocyanates. It has been demonstrated that tannins and saponins exert toxic action on insects [31,32,33,34,35]. In our experiments, the numbers of adults and the development periods of Corcyra cephalonica and Idaea inquinata were not affected by the addition of M. oleifera leaves to the artificial diet in a proportion of one-quarter; therefore, the number of leaves was insufficient for the secondary metabolites to exert their toxic action.
Concerning the behavior of the larvae on the different diets, we observed that the first instar larvae of C. cautella, C. cephalonica, and P. interpunctella reared on dried or powdered moringa leaves wandered until they starved. In a double-choice test with 50 eggs in the center of a Petri dish—dried M. oleifera leaves on one side and the artificial diet on the other side—we also observed that newly hatched first instar larvae were attracted by and fed only on the artificial diet (authors’ observations).
I. inquinata feed on some dried herbs [18]; when the dried M. oleifera leaves were the rearing medium, 15% of the I. inquinata eggs developed into adults. The secondary metabolites of M. oleifera likely interfered with larval development, as was observed in Camellia sinensis and Vitis vinifera, which are characterized by their high tannin contents and were unsuitable as rearing media for I. inquinata. Tannins act on ß-glucosidase and esterase [35], act as an antifeedant in Leptinotarsa decemlineata [36], and cause a delay in larval development when added to the diet of Lepidoptera larvae [37].
Dried herbs in warehouses can be infested by several pests [38,39,40], including C. cautella and P. interpunctella; these species were not able to develop on dried and powdered M. oleifera leaves. M. oleifera leaves can be safely stored as far as Pyralid moths are concerned, but the dried leaves can be infested by I. inquinata. This species can compromise the use of the leaves with its exuviae and excrements, especially if the Moringa leaves are stored with other herbs that can be infested by this species. The results obtained in this research show that in order to preserve the nutritional characteristics and avoid damage, the storage of powdered leaves is recommended. Although M. oleifera leaves are hardly susceptible to pest infestation, it is recommended to carefully check dried herbs, monitor warehouses carefully, avoid debris accumulation, and protect windows with wire-nets.
Author Contributions
The authors equally contributed to the research and the manuscript. Both authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maida, A.; Rarooq, A.; Razyia, N.; Umer, R.; Kazi, T.G.; Nadeem, M. Mineral composition of Moringa oleifera leaves and pods from different regions of Punjab, Pakistan. Asian J. Plant Sci. 2005, 4, 417–421. [Google Scholar]
- Jongrungruangchok, S.; Bunrathep, S.; Songsak, T. Nutrients and minerals content of eleven different samples of Moringa oleifera cultivated in Thailand. J. Health Res. 2018, 24, 123–127. [Google Scholar]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Moringa oleifera seeds and oil: Characteristics and uses for human health. Int. J. Mol. Sci. 2016, 17, 2141. [Google Scholar] [CrossRef] [Green Version]
- Heykal, A.; El Wahab, A.E.A.; Asran, A.M. Biological studies on Plodia interpunctella Hbn. on certain medical and aromatical dried plants (Lepidoptera: Phycitidae). Agric. Res. Rev. 1978, 56, 147–149. [Google Scholar]
- Wahab, A.E.A.; El Halfawy, M.A.; Heykal, A.; Asran, A.M. Biology of Anagasta kuehniella Zell. on certain medical and aromatical dried plants (Lepidoptera; Phycitidae). Agric. Res. Rev. 1978, 56, 143–146. [Google Scholar]
- Stampini, M. Development observations of Plodia interpunctella (Hbn.) (Lepidoptera Pyralidae) on some dried medicinal plants. Boll. Zool. Agr. Bachic. 2009, 41, 41–50. [Google Scholar]
- Jagadish, P.S.; Nirmala, P.; Rashmi, M.A.; Neelu, N. Biology of rice moth Corcyra cephalonica Stainton on foxtail millet. Karnataka J. Agric. Sci. 2009, 22, 674–675. [Google Scholar]
- Soderstrom, E.; Hinsch, R.; Bongers, A.; Brandl, D.; Hoogendorn, H. Detecting adult Phycitinae (Lepidoptera: Pyralidae) infesta-tions in a raisin-marketing channel. J. Econ. Entomol. 1987, 80, 1229–1232. [Google Scholar] [CrossRef]
- Sinha, R.N.; Watters, F.L. Insect Pests of Flour Mills, Grain Elevators, and Feed Mills and Their Control; Agriculture Canada Publication: Ottawa, ON, Canada, 1985; pp. 1–290. [Google Scholar]
- Cox, P.D. The suitability of dried fruits, almonds and carobs for the development of Ephestia figulilella Gregson, E. calidella (Guenee) and E. cautella (Walker) (Lepidoptera: Phycitidae). J. Stored Prod. Res. 1975, 11, 229–233. [Google Scholar] [CrossRef]
- Na, J.H.; Ryoo, M.I. The influence of temperature on development of Plodia interpunctella (Lepidoptera: Pyralidae) on dried vegetable commodities. J. Stored Prod. Res. 2000, 36, 125–129. [Google Scholar] [CrossRef]
- Burks, C.S.; Johnson, J.A. Biology, behavior, and ecology of stored fruit and nut insects. In Stored Product Protection; Hagstrum, D.W., Phillips, T.W., Cuperus, G., Eds.; Kansas State University: Manhattan, KA, USA, 2012; pp. 21–32. [Google Scholar]
- Binder, M.; Mahler, V.; Hayek, B.; Sperr, W.R.; Scholler, M.; Prozell, S.; Wiedermann, G.; Valent, P.; Valenta, R.; Duchêne, M. Molecular and immunological characterization of arginine kinase from the Indianmeal moth, Plodia interpunctella, a novel cross-reactive invertebrate pan-allergen. J. Immunol. 2001, 167, 5470–5477. [Google Scholar] [CrossRef] [Green Version]
- Hubert, J.; Stejskal, V.; Athanassiou, C.G.; Throne, J.E. Health hazards associated with arthropod infestation of stored products. Ann. Rev. Entomol. 2018, 63, 553–573. [Google Scholar] [CrossRef]
- South, R. The Moth of the British Isles; Second Series; Warne: London, UK; New York, NY, USA, 1961; pp. 1–379. [Google Scholar]
- Koch, M. Wir Bestimmen Schmetterlinge; Neumann-Neudamm: Melsunghen, Germany, 1984; pp. 1–792. [Google Scholar]
- Skinner, B. Moth of the British Isles; Viking, Penguin Books: Harmondsworth, UK, 1984; pp. 1–267. [Google Scholar]
- Locatelli, D.P.; Di Egidio, V.; Stampini, M. Observation of the development of Idaea inquinata (Scop.) (Lepidoptera Geometridae) on medicinal plants and other food substrates. Boll. Zool. Agr. Bachic. Ser II 2005, 37, 123–132. [Google Scholar]
- Locatelli, D.P.; Savoldelli, S.; Girgenti, P.; Lucchini, G.A.; Limonta, L. Can environmental dust from silo area allow the development of stored product insects? J. Stored Prod. Res. 2017, 71, 41–46. [Google Scholar] [CrossRef]
- Limonta, L.; Stampini, M.; Locatelli, D.P. Development of rusty wave Idaea inquinata at constant temperatures, relative humidities and photoperiod. Bull. Insectology 2010, 63, 171–174. [Google Scholar]
- Prosky, L.; Asp, N.G.; Schweizer, T.F.; DeVries, J.W.; Furda, I. Determination of insoluble, soluble, and total dietary fiber in foods and food products: Interlaboratory study. JAOAC 1988, 71, 1017–1023. [Google Scholar] [CrossRef]
- Rocklin, R.D.; Pohl, C.A. Determination of Carbohydrates by Anion Exchange Chromatography with Pulsed Amperometric Detection. J. Liq. Chromatogr. 1983, 6, 1577–1590. [Google Scholar] [CrossRef]
- Anonymous. AOAC 34.01.05 n.925.31, Nitrogen in eggs. In Official Methods of Analysis of AOAC International, 16th ed.; Cunniff, P., Ed.; Publisher AOAC International: Gaithersburg, MD, USA, 1995; Ch. 34; p. 2. [Google Scholar]
- Anonymous. AOAC 31.04.02 n. 963.15, Fat in cacao products. In Official Methods of Analysis of AOAC International, 16th ed.; Cunniff, P., Ed.; AOAC International: Gaithersburg, MD, USA, 1996; Ch. 31; p. 10. [Google Scholar]
- AACC 08-01.01. AACC Approved Method of Analysis, 11th ed. Available online: http://methods.aaccnet.org/summaries/08-01-01.aspx (accessed on 10 May 2021).
- Leone, A.; Fiorillo, G.; Criscuoli, F.; Ravasenghi, S.; Santagostini, L.; Fico, G.; Spadafranca, A.; Battezzati, A.; Schiraldi, A.; Pozzi, F.; et al. Nutritional characterization and phenolic profiling of Moringa oleifera leaves grown in Chad, Sahrawi Refugee Camps, and Haiti. Int. J. Mol. Sci. 2015, 16, 18923–18937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silhacek, D.; Murphy, C. Moisture content in a wheat germ diet and its effect on the growth of Plodia interpunctella (Hubner). J. Stored Prod. Res. 2008, 44, 36–40. [Google Scholar] [CrossRef]
- Ashfaq, M.; Shahzad, M.A.; Basra Ashfaq, U. Moringa: A miracle plant for agro-forestry. J. Agri. Sci. 2012, 8, 115–122. [Google Scholar]
- Musa, A.K. Influence of plant powders on infestation by adults and larvae of Khapra Beetle, Trogoderma granarium Everts (Coleoptera: Dermestidae) in stored groundnut. AJBAS 2013, 7, 427–432. [Google Scholar]
- Kasolo, J.N.; Bimenya, G.S.; Ojok, L.; Ochieng, J.; Ogwa-Okeng, J.W. Phytochemical and uses of Moringa oleifera leaves in Ugandan rural communities. J. Med. Plants Res. 2010, 4, 753–757. [Google Scholar]
- Moreira, X.; Galman, A.; Francisco, M.; Castagneyrol, B.; Abdala-Roberts, L. Host plant frequency and secondary metabolites are concurrently associated with insect herbivory in a dominant riparian tree. Biol. Lett. 2018, 14, 20180281. [Google Scholar] [CrossRef] [Green Version]
- Su, Q.; Zhou, Z.; Zhang, J.; Shi, C.; Zhang, G.; Jin, Z.; Wang, W.; Li, C. Effect of plant secondary metabolites on common cutworm, Spodoptera litura (Lepidoptera: Noctuidae). Entomol. Res. 2018, 48, 18–26. [Google Scholar] [CrossRef]
- Motevalli-Haghi, S.F.; Fathi, M.; Ebrahimzadeh, M.A.; Eslami, S.; Karamie, M.; Eslamifar, M.; Dehghan, O. Evaluation of Phytochemical, total phenolic and flavonoid content, antioxidant activities and repelling property of Sambucus ebulus. JMPB 2020, 1, 97–105. [Google Scholar]
- Qasim, M.; Islam, W.; Ashraf, J.H.; Ali, I.; Wang, L. Saponins in Insect Pest Control. In Co-Evolution of Secondary Metabolites; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 898–924. [Google Scholar]
- Juntheikki, M.R.; Julkunene-Tiitto, R. Inibition of beta-glucosidase and esterase by tannins from Betula, Salix and Pinus species. J. Chem. Ecol. 2000, 26, 1151–1165. [Google Scholar] [CrossRef]
- Pospisil, J. The response of Leptinotarsa decemlineata (Coleoptera) to tannin as an antifeedant. Acta Entomol. Bohemoslov. 1982, 6, 429–434. [Google Scholar]
- Manuwoto, S.; Scriber, J.M. Effects of hydrolysable and condensed tannin on growth and development of two species of polyphagous Lepidoptera: Spodoptera eridania and Callosomia promethean. Oecology 1986, 69, 225–230. [Google Scholar] [CrossRef]
- El-Halfawy, M.A. Entomofauna of dried medical and aromatical plants with a short note on their occurrence and populations. Agric. Res. Rev. 1977, 55, 103–160. [Google Scholar]
- Kalinovič, I.; Rozman, V. Infestation of stored medicinal plants and herbal tea by insects and mites. Plant Prot. Sci. 2000, 36, 21–22. [Google Scholar] [CrossRef]
- Arbogast, R.T.; Kendra, P.E.; Mankin, R.W.; McDonald, R.C. Insect infestation of a botanicals warehouse in north-central Florida. J. Stored Prod. Res. 2002, 38, 349–363. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).