Simple Summary
Telenomus remus (Nixon) is an effective egg parasitoid for controlling Spodoptera frugiperda (J. E. Smith), which is a major destructive agricultural pest. Currently, this parasitoid is reared on Corcyra cephalonica (Stainton) eggs in several countries. However, previous studies carried out in China have reported that it cannot parasitize in C. cephalonica eggs. Meanwhile, those works have indicated that Spodoptera litura (Fabricius) can potentially be used as an alternative host. In order to evaluate this potential, our study compared the development and parasitism ability of T. remus on the eggs of S. frugiperda and S. litura at different temperatures in a laboratory. We found that S. litura eggs are more advantageous as an alternative host for the mass-rearing of parasitoid when compared with S. frugiperda eggs. Our results provide a more specific basis and reference for the large-scale production and low temperature storage of T. remus.
Abstract
Although Telenomus remus, a promising parasitoid of Spodoptera frugiperda, had been successfully reared on the eggs of Corcyra cephalonica in some countries, reports from China have argued that it is infeasible. Notably, studies from China have indicated that Spodoptera litura eggs could be a candidate host. Therefore, to further evaluate the potential of using S. litura eggs as hosts, we compared the development and parasitism of T. remus on the eggs of S. frugiperda and S. litura at temperatures between 20–32 °C. Our results showed that T. remus developed successfully on both host eggs at all of the tested temperatures, and the developmental duration and thermal requirements at each stage were similar between the two host species. The number of parasitized eggs was greater for S. litura than for S. frugiperda. Meanwhile, the emergence rate exceeded 86.6%, and it was significantly higher for S. litura than that for S. frugiperda, except at 29 °C. This study is the first time estimating the thermal requirements of T. remus at each stage. Moreover, we also recorded the morphological characteristics of T. remus at each stage. Our results demonstrate that S. litura eggs are more suitable than S. frugiperda eggs as an alternative host for the mass-rearing of T. remus in China. Understanding the thermal requirements and biological parameters contributes greatly to predicting the generation time and providing a reference for the mass-rearing and storage of the parasitoid.
1. Introduction
The fall armyworm Spodoptera frugiperda (J. E. Smith; Lepidoptera: Noctuidae) is a major destructive agricultural pest worldwide [1]. S. frugiperda is a polyphagous pest that can feed on 353 different plant species, including maize, rice, wheat, and soybean, and it especially prefers maize [2]. S. frugiperda poses a substantial threat to agricultural production and food security [1]. For example, if no control methods are taken, the annual maize yield losses caused by S. frugiperda are expected to reach 8.3 million to 20.6 million tonnes within the 12 main maize-producing countries of Africa [3].
As of late 2019, S. frugiperda was established in Southern China, and now persists all year round [4]. Owing to China’s diverse geography, complex crop planting structure, and abundant plant resources, controlling this pest constitutes a formidable, long-term challenge [4]. The application of insecticides is the most effective strategy for controlling S. frugiperda; however, excessive and/or inappropriate application has led to serious issues, such as evolution of resistance, elimination of beneficial insect, and environmental pollution [3,5]. To meet the need for the sustained development of agriculture in China, one ecologically friendly strategy for controlling S. frugiperda is via biological control using natural enemy insects and entomopathogens [6,7].
More than 290 natural enemy resources of S. frugiperda have been reported [8,9]. Among these agents, the egg parasitoid Telenomus remus (Nixon; Hymenoptera: Platygastridae) is one of the most commonly used effective species for controlling S. frugiperda [10,11,12]. The body length of the parasitoid adult is only 0.5–0.6 mm and usually presents as shiny black in color. T. remus lay their eggs inside the developing host, and the larva consume the nutrition of the host to satisfy its development [12]. When emerging, the parasitoid adult will chew a small hole in the host eggs and crawl out. The successful application of T. remus in biological control programs depends primarily on its high parasitism and capacity to parasitize the inner layer of insect egg masses [12]. In Brazil, for example, the inhibition of S. frugiperda egg masses by T. remus reached 54–99% in maize, cotton, and soybean fields, indicating the highly efficient control of this insect [13].
Although T. remus displays considerable potential for controlling S. frugiperda, it has proven to be challenging to find a suitable host for the mass-rearing of T. remus at an affordable cost in China [14]. Research has shown that T. remus can be reared successfully on the eggs of Corcyra cephalonica (Stainton) [15,16]. However, our preliminary experiments and other studies [14] conducted in China have indicated that T. remus cannot parasitize and develop in C. cephalonica eggs. Since the invasion of S. frugiperda in China in early 2019, researchers have reared T. remus using its natural host S. frugiperda eggs [14]. However, S. frugiperda is highly cannibalistic, making it difficult to sustain an affordable, large-scale rearing system in terms of both time and resources. Therefore, it is urgent to identify a suitable alternative host egg in China. Studies have shown that Spodoptera litura (Fabricius) eggs could potentially serve as an alternative host for the mass-rearing of T. remus [12,17]. Furthermore, S. litura larvae exhibit little or no cannibalism [18].
Understanding the relationship between development and temperature enables the prediction of the number of insect generations annually in a certain area, which is important to determine the release time of natural enemies [19]. Several reports have been published pertaining to the temperature requirements of some insects [19,20]. However, few reports have studied the temperature requirements of T. remus, from which the thermal constant (K) for complete development is 154.12 degree-days (DD) for males and 158.88 DD for females [21]. Importantly, the thermal requirements for eggs, larvae, prepupae, and pupae remain unclear for T. remus.
It is clear that an evaluation of the development and parasitism capacity of T. remus on the eggs of S. frugiperda or S. litura at different temperatures would be conducive to providing sufficient evidence to confirm the potential of S. litura eggs as a suitable alternative host for the mass-rearing of T. remus. The data obtained would also provide fundamental knowledge for biological control programs using T. remus for controlling S. frugiperda in China. Therefore, we compared the effect of host species, namely S. frugiperda and S. litura, on the development, survival, and parasitism of T. remus at different temperatures, and determined the developmental threshold temperature (T0), thermal constant, and correlative biological parameters of T. remus.
2. Materials and Methods
2.1. Insect Culture
T. remus was obtained from colonies kept at the maize pest laboratory at the Institute of Plant Protection, Chinese Academy of Agricultural Sciences. Parasitized egg masses of S. frugiperda were originally collected from maize fields in Jinhua, Zhejiang Province, China, in 2019. The colony of T. remus was reared on those egg masses under a climatic incubator (26 ± 1 °C, 70 ± 5% of relative humidity (RH), 14 h:10 h light (L)/dark (D)).
The S. frugiperda larvae were collected from maize fields in Kunming, Yun’nan Province, China, in 2019. The population was maintained for over 10 generations in a climatic incubator at 28 ± 1 °C, 60 ± 5% RH, 16 h:8 h L/D. The first to third instar larvae were reared together in plastic cages (34 cm × 22 cm × 4 cm) containing fresh maize leaves. To avoid cannibalism, the fourth to sixth instar larvae were separately fed an artificial diet [22] in cylindrical plastic containers (3 cm in height × 5 cm in diameter). For the purpose of mating and oviposition, the adults were reared in cylindrical wire-mesh cages (28 cm in height × 24 cm in diameter), the inner surface and the upper opening of which were covered with wax paper and wet gauze to provide an oviposition substrate and retain moisture, respectively. Adults were fed a 20% honey solution, which was replaced daily. The wax paper and gauze with egg masses were collected every morning and were used for the following experiments.
The S. litura was obtained from the Institute of Plant Protection, Jilin Academy of Agricultural Sciences, Jilin Province, China. The same aforementioned conditions and methods were used to rear this species, except that the larvae were reared in groups instead of individually (as with S. frugiperda), using a specific artificial diet [23].
2.2. Effects of Temperature and Host Species on T. remus Development
All of the trials were conducted in a climatic incubator (RXZ-500, Ningbo Jiangnan Instrument Factory, Zhejiang Province, China). A 14L:10D photoperiod was used in all of the incubators and the humidity varied between 65 and 75% RH.
Previous studies have shown that the mortality of T. remus was about 87% when reared on S. frugiperda eggs at 15 °C, and even reached 100% at 35 °C [21]. Therefore, five constant temperatures (20, 23, 26, 29, and 32 ± 1 °C) were selected to estimate the effects of temperature on the development and parasitism of T. remus reared on the eggs of S. frugiperda or S. litura in present study. Fresh egg masses of S. frugiperda or S. litura (<24 h old) were exposed to newly emerged (<12 h old) and mated T. remus adults for 1 h, after which parasitized eggs were divided equally into five groups that were kept at different temperatures for development. The egg, larvae, prepupae, and pupae stages of T. remus were observed via dissection at 4 h intervals under a stereomicroscope, and photos of T. remus at each stage were taken (SZX10, Olympus Corporation, Japan). Because the newly deposited eggs were difficult to observe, the egg and the first instar larvae were considered as a single stage, namely “egg-first instar larvae”. The parasitized eggs were checked periodically until emergence, and the duration of each stage (egg-first instar larvae, second instar larvae, prepupae, and pupae) was recorded. There were 50 replicates for each treatment.
2.3. Effects of Temperature and Host Species on the Fecundity and Lifespan of T. remus
The inner-surface wax paper for the cages, with approximately 200 fresh eggs of S. frugiperda or S. litura (<24 h old), was placed inside a transparent plastic tube (10 cm in height × 2.5 cm in diameter) containing a single newly emerged and mated female adult of T. remus (<24 h old), with subsequent incubation at various temperatures. The inner surface of the tube contained a drop of 20% honey. The egg-containing wax paper was replaced daily until the death of the female parasitoid, and then the parasitized eggs were moved into a new tube kept under the specific conditions for development until the offspring emerged. During the period in which the T. remus larvae developed, the Spodoptera caterpillars that hatched from non-parasitized eggs were removed to prevent them feeding on the parasitized eggs. Each experimental trial had at least 20 replicates. The number of parasitized eggs, emergence rate, percentage of female, and lifespan were recorded.
2.4. Statistical Analysis
The relationship between the temperature and developmental rate were estimated by linear regression using Equation (1) [24],
where Y is the developmental rate, X is the temperature, and a and b are the estimated parameters of the regression. The thermal constant and developmental threshold temperature were calculated using the parameters: K = 1/b and T0 = −a/b.
Y = a + b × X
The effects of the temperature, host egg species, and the interactions among these factors on developmental duration, number of parasitized eggs, emergence rate, percentage of females, and lifespan were analyzed by two-way ANOVA. The differences among the five different temperatures (20, 23, 26, 29, and 32 °C) were compared using Tukey’s honest significant difference (HSD) tests at a 0.05 level. The differences between the two host egg species (S. frugiperda and S. litura) were compared using an independent-samples t-test. The percentage data were arcsine square-root-transformed to homogenize the variances prior to analysis, whereas the data for female lifespan was log10-transformed to fit a normal distribution. All of the statistical analyses were performed using SPSS version 19.0 (IBM Crop., Chicago, IL, USA) for Windows. The figures were created using GraphPad Prism for Windows (version 8.0) (Graphpad Software, Inc., San Diego, CA, USA).
3. Results
3.1. Development of T. remus Reared on Eggs of S. frugiperda or S. litura
The typical morphological characters regarding the development of T. remus reared on S. frugiperda eggs at 26 °C are shown in Figure 1, and the duration from egg to adulthood of T. remus was ~10 days.
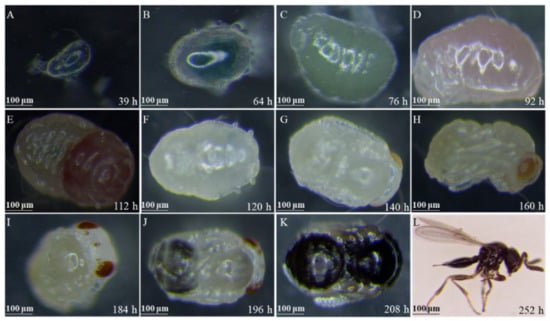
Figure 1.
Morphological characters of T. remus at the first instar larvae (A,B), second instar larvae (C), prepupae (D,E), pupae (F–K), and adult (L).
The results showed that T. remus was able to develop successfully on the eggs of S. frugiperda and S. litura at all of the temperatures we tested. However, the developmental duration of T. remus at each stage lengthened with the decrease in temperature. The temperature and host species have a significant effect on the duration of the egg-first instar larvae. However, the interaction between the temperature and host species had no effect on the duration (Table 1). At 32 °C, the duration of the egg-first instar larvae was significantly longer for the S. frugiperda eggs than the S. litura eggs (t = 3.301; df = 98; p = 0.001) (Table 2). The duration of both the second instar larvae and prepupae were influenced by the temperature, but not by the host species or their interactions (Table 1). There was no obvious difference in the prepupae duration between 29 °C and 32 °C (Table 2). The temperature, host species, and the interaction between these two factors significantly influenced the developmental duration of both the pupae and generation (Table 1). At 20 °C (pupae: t = −5.589; df = 98; p < 0.0001; generation: t = −3.526; df = 98; p = 0.0001), 26 °C (pupae: t = 5.300; df = 98; p < 0.0001; generation: t = 5.534; df = 98; p = 0.0001), 29 °C (pupae: t = −16.081; df = 98; p < 0.0001; generation: t = −14.973; df = 98; p < 0.0001), and 32 °C (pupae: t = 5.411; df = 98; p < 0.0001; generation: t = 9.276; df = 98; p < 0.0001), the duration of both the pupae and generation obviously differed between the host species; however, at 23 °C, there was no difference between the host species (Table 2). For each host species, there was an approximately three-fold difference in generation time between 20 °C and 32 °C (Table 2).

Table 1.
Results from a two-way ANOVA analysis on the effects of the temperature, host species, and their interactions on the development and biological characteristics of T. remus.

Table 2.
Developmental duration of each of the stages of T. remus on the eggs of S. frugiperda or S. litura at constant temperatures.
3.2. Thermal Requirements of T. remus Reared on the Eggs of S. frugiperda or S. litura
The values K and T0, as well as the regression equations of developmental rate for each developmental stage are shown in Figure 2. The T0 and K of each stage were not significantly different between the host species. The second instar larvae and prepupae presented the lowest T0 and K, respectively. The K of the parasitoids was similar when reared on the eggs of S. frugiperda or S. litura, with 140.0 DD above a threshold of 13.0 °C and 136.0 DD above a threshold of 13.4 °C, respectively. All of the regression equations showed a coefficient of correlation (R2) higher than 0.900, demonstrating that the model provided a satisfactory fit for the relationship between the developmental rate and temperature (Figure 2).
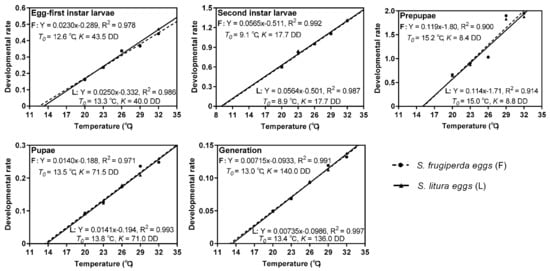
Figure 2.
Developmental rate of each stage of T. remus reared on eggs of S. frugiperda or S. litura at different temperatures.
3.3. Biological Characteristics of T. remus Reared on Eggs of S. frugiperda or S. litura
The oviposition rhythm of T. remus varied with the temperature and host species, and daily parasitism decreased gradually over the female lifespan. The parasitism capacity was maximal on the first day at all of the tested temperatures (Figure 3). For S. frugiperda, the number of parasitized eggs on the first day was 29.3, 43.5, 51, 37.9, and 36.8 at 20, 23, 26, 29, and 32 °C, respectively, and the corresponding values for the S. litura eggs were 36.5, 61.9, 64.5, 59.6, and 53.9, respectively, each of which was greater than that for S. frugiperda. For the S. frugiperda eggs, the time to reach 80% parasitism was 8, 5, 5, 5, and 4 days at the five temperatures, and the corresponding times were 11, 8, 5, 4, and 6 days, respectively, for the S. litura eggs (Figure 3).
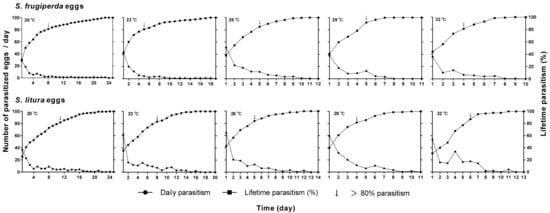
Figure 3.
Cumulative and daily parasitism of T. remus reared on the eggs of S. frugiperda and S. litura at different temperatures.
There was an effect from the temperature and host species, as well as the interaction between these two factors, on the number of parasitized eggs (Table 1). For S. frugiperda, the total number of parasitized eggs differed significantly among the various tested temperatures (F = 16.118; df = 4, 151; p < 0.0001), with the highest parasitism ability occurring at 26 °C (133.4 eggs); however, for S. litura, the parasitism was similar among the various temperatures (from 136.1 eggs at 20 °C to 164.2 eggs at 32 °C). The capacity of T. remus to parasitize the eggs of S. litura was significantly greater than for the S. frugiperda eggs at 20 °C (t = −5.664; df = 52; p < 0.0001), 23 °C (t = −7.115; df = 50; p < 0.0001), 29 °C (t = −6.015; df = 62; p < 0.0001), and 32 °C (t = −7.486; df = 50.973; p < 0.0001; Figure 4A).
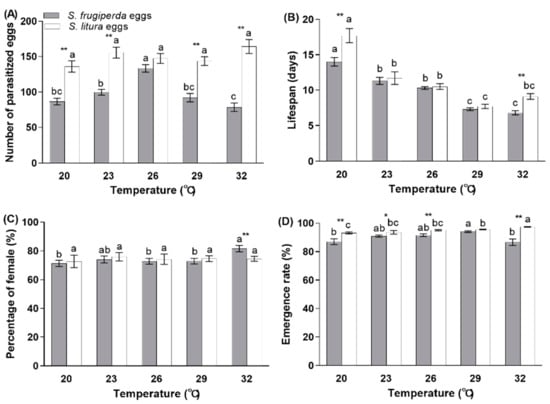
Figure 4.
The number of parasitized eggs (A), female lifespan (B), percentage of females (C), and emergence rate (D) for T. remus reared on eggs of S. frugiperda or S. litura at different temperatures. Different lowercase letters indicate a statistically significant difference among different temperatures (Tukey’s test, p < 0.05). Asterisks indicate a significant difference between two host species (t-test, * p < 0.05; ** p< 0.01).
Both the temperature and host species significantly impacted the lifespan of the parental females, as well as the interaction between the temperature and host species (Table 1). The lifespan of the female adult differed between host species only at 20 °C (t = −2.690; df = 52; p = 0.01) and 32 °C (t = −3.203; df = 60; p = 0.002). The females that developed on S. litura eggs lived longer than those on S. frugiperda eggs at 20 and 32 °C, but the lifespans were similar between the two host species within the range of 23–29 °C. The female lifespan decreased continuously from 20 °C to 32 °C, with a range from 14 to 6.8 days (F = 50.893; df = 4, 151; p < 0.0001) for S. frugiperda eggs and from 17.7 to 7.7 days (F = 25.679; df = 4, 123; p < 0.0001) for S. litura eggs, respectively (Figure 4B).
There was no effect from the temperature and host species, as well as the temperature and host species interaction, on the number of female offspring (Table 1). The percentage of female progeny only differed between the host species at 32 °C (t = 2.816; df = 60; p = 0.007), with a higher offspring sex ratio for S. frugiperda eggs. For S. frugiperda eggs (F = 3.970; df = 4, 151; p = 0.004), the temperature had a significant effect, with fewer female offspring at 20, 26, and 29 °C compared with 32 °C. For the S. litura eggs, the percentage of females was similar at different temperatures (Figure 4C).
The temperature and host species, as well as the temperature and host species interaction, had a significant effect on the adult emergence (Table 1). The emergence rate of T. remus was higher for S. litura eggs than for S. frugiperda eggs at 20 °C (t = −2.838; df = 45.573; p = 0.007), 23 °C (t = −2.526; df = 50; p = 0.015), 26 °C (t = −3.700; df = 47.277; p = 0.001), and 32 °C (t = −6.211; df = 35.188; p < 0.0001), but not at 29 °C. For the S. frugiperda eggs (F = 5.197; df = 4, 151; p = 0.001), the emergence rate was the highest at 29 °C (94%); this value was less at 20 °C (87%) and 32 °C (86.6%). For the S. litura eggs (F = 13.099; df = 4, 123; p < 0.0001), the emergence rate was higher than 93.1% for all of the tested temperatures, with the highest rate at 32 °C (Figure 4D).
4. Discussion
Regarding parasitoid developmental duration of each stage, the results obtained in this study indicated that the developmental duration of T. remus on the eggs of S. frugiperda and S. litura is similar at any given temperature, which is similar to the results found by Pomari et al. [25], who studied the egg-to-adult period of T. remus reared on the eggs of S. frugiperda, S. albula (Walker), S. cosmioides (Walker), and S. eridania (Cramer) over a temperature range of 19–34 °C, who reported a similar developmental time for T. remus among the four host eggs. Generally, the parasitoid biological parameters differ depending on the host species [20]. These different traits of host egg species, including the size, surface, and chemical cues of the host egg chorion structure, affect the host suitability for the parasitoid [26,27]. The adaptability of the parasitoid to different host species may be affected by differences in their coevolutionary relationships with each species [28]. The eggs of S. frugiperda are 0.4 mm in diameter and 0.42 mm in length [26], whereas S. litura eggs are 0.6 mm in diameter [29]. The parasitoid usually showed better development and adaptation on the more suitable host eggs. Consistent with this, the parasitism capacity of T. remus was greater for the S. litura eggs because of their bigger egg size, which may provide more nutrients and thus affect the parental parasitism and progeny fitness of the parasitoid [30]. Theoretically, an abundant availability of nutrients might permit more than one parasitoid larva to complete development and emergence. However, the phenomenon of a single progeny parasitoid per host eggs, observed in this and other studies [12], might be due to the consequence of parasitoid larva competition for nutrients when two or more parasitoid eggs are laid inside one host egg [12]. Furthermore, the host egg quality has previously been pointed out as a major factor affecting the sex ratio of the parasitoid, particularly for Trichogrammatidae [28]. This might be due to the ability of the parental parasitoid to distinguish the quality of host egg before laying male or female eggs [28]. The offspring represented a similar percentage of females between the eggs of S. frugiperda and S. litura, indicating a similar quality for the two host species.
Consistent with previous studies [25,31], the development and biological characteristics of T. remus were highly affected by temperature. Therefore, exploring the relationship between temperature and biological performance is of great significance for a more comprehensive understanding of the ecology and management of insects [32]. The results from the present study demonstrate that when lengthening the developmental duration, egg-first instar larvae, second instar larvae, prepupae, pupae, and generation, inversely follow a decrease in temperature. Behavioral plasticity may explain the effects of temperature on insect development [33]. Specifically, a high temperature increases the metabolic rate, resulting in a shortened developmental time, which is prolonged at low temperatures [34]. T. remus could survive and complete development in the temperature range of 20–30 °C. However, a high mortality was apparent at 34 °C, and at 35 °C, no T. remus adult emergence occurred, which showed that the upper limit of temperature for this species may be between 34 °C and 35 °C [21,25].
Temperature is a key factor governing both the metabolic rate and lipid consumption of parasitoids. There is generally an inverse relationship for both factors [35]. Adult fecundity and lifespan are largely dependent on the amount of body lipids that have accumulated during the larval stage, and adult feeding on honey or nectar does not increase the lipid reserves [36]. Thus, temperature may impact the distribution of lipids, which may further affect the balance between lifespan and fecundity. Generally, the oviposition peak of T. remus occurs in the first 24 h, regardless of environmental conditions, parasitoid population, or host species, and parasitism gradually decreases thereafter [35,37]. The egg parasitoids of the genus Trichogramma display the same characteristics, which might imply that the females need to search an appropriate host promptly on which to lay their eggs after emergence [30,38]. Overall, it is must be considered whether the parasitism activity is concentrated during a certain timeframe, namely, soon after emergence or continuously throughout adulthood, which may affect the performance of parasitoid in the field [31,35].
It was reported previously that T. remus presents poor parasitism at extreme temperatures [31]. The decreased fecundity of female parasitoids at high temperatures is a consequence of a reduced lifespan [39], which implies that temperature optimization is vital for the build-up of a large-scale population. In addition, eggs lose water faster at a high temperature, which may explain the observed decrease in the parasitism capacity [31]. The influence of temperature on the lifespan of female parasitoids was inversely related, as reported by Bueno et al. and Pomari et al. [31,35], probably because both the metabolic activity and energy expenditure decreased at lower temperatures [31,40]. Thus, to ensure the high control efficiency of pests, parasitoids may have to be released with greater frequency in warm regions because of the reduction in adult lifespan [31,35]. A low emergence rate may directly reduce the biological control performance, regardless of the parasitism capacity [37]. Our results show that this T. remus strain could be reared at temperatures ranging 20–32 °C, without negatively affecting the emergence rate, which may reflect the powerful adaptive capacity of T. remus over a wide temperature range. As female parasitoids could directly suppress populations of the target pest, a high female to male ratio may elevate the control efficiency of the parasitoid in biological control programs [21]. Our results reveal that the percentage of females was not clearly impacted by the temperature, and it was in agreement with previous reports [21,25].
Information regarding the thermal requirements of insect development will greatly influence mass-rearing programs [41]. The developmental threshold temperature and thermal constant for T. remus reared on eggs of S. frugiperda in our results (13.0 °C and 140.0 DD) differed from that of a study from Brazil, which reported that the corresponding values for T. remus reared on S. frugiperda were 14.9 °C and 125.6 DD, respectively [25]. These differences might correspond to the different geographic latitudes the parasitoid specimens were collected. Generally, insects originating from temperate regions have a lower development threshold, so they require more degree-days to complete development [42]. The developmental threshold temperature obtained from the current study was lower than that reported by Pomari et al. [25], which might be attributable to the higher geographic latitude of our population, which has a better tolerance to low temperatures. In this study, the slight differences in the thermal requirements of T. remus between the two host species tested might be due to the quality and amount of nutrients in the host egg, as well as the adaption of T. remus to specific eggs. Although the number of annual generations was predicted based on the thermal requirements [21], different field conditions need to be considered when developing a biological control program in certain regions. Moreover, information about the thermal requirements also provides a reference for the low-temperature storage of parasitoids.
Host preference is a key factor influencing the mass-rearing and application of parasitoids. Although parasitoid rearing on the factitious host is one of the essential steps in a biological control program, continuous rearing on the same host may affect its efficiency against the target pest after being released into the field [43]. These changes in host preference or parasitism are probably due to preimaginal conditioning occurring during parasitoid larval development [43]. Based on a previous study, the ability of parasitoids to distinguish hosts may be reduced or even lost after successive rearing on an alternative host [43]. However, according to Queiroz et al. [44], there was no obvious difference in the parasitism or host preference of T. remus on S. frugiperda eggs whether they were reared on S. frugiperda or C. cephalonica eggs. Although our results indicated that the S. litura eggs could be an alternative host of T. remus, in order to ensure the control efficiency against S. frugiperda in the field, it is necessary to further evaluate the host preference and parasitism when T. remus was successively reared on the S. litura eggs for several generations. Furthermore, if T. remus reared on S. litura eggs are released in a field where S. litura and S. frugiperda outbreaks concurrently, they may be controlled simultaneously. However, as S. litura is also an important agricultural pest, special attention should be paid so as to avoid bringing S. litura into the field when releasing parasitoids. In the application process, one of the effective strategies to improve the field practice of biological control programs is through the combined use of different biological control agents [6]. Investigations have revealed that predators are the most important species for controlling S. frugiperda in central Mexico, such as mite of the genus Balaustium, Doru taeniatum Dohrn and Hippodamia convergens Guérin-Meneville, and their control efficiency reached 63% [45]. Therefore, if different agents are used in combination, such as the combined use of parasitoids with predators, the control efficiency may be greatly improved.
5. Conclusions
Our work proves that T. remus performed better on S. litura eggs in parasitism, as well as their suitability of progeny, compared with S. frugiperda eggs under all of the tested temperatures. Moreover, because there is little or no cannibalism, S. litura larvae can be reared in groups at a lower cost than S. frugiperda, suggesting that S. litura eggs might be more suitable as an alternative host for mass-rearing T. remus in China. This information on the biological characteristics and thermal requirements of T. remus provides important references for the large-scale reproduction and application of the parasitoid in biological control programs.
Author Contributions
Conceptualization, W.C. and L.Z.; methodology, W.C., J.M., and L.Z.; formal analysis, W.C.; writing—original draft preparation, W.C.; writing—review and editing, W.C., Y.L., and L.Z.; funding acquisition, M.W. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2017YFD0201000, 2019YFD0300104), the Major projects of State Tobacco Monopoly Administration of China (110202001032(LS-01)), and the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-ZDRW202108).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article.
Acknowledgments
We are very grateful to Zhenying Wang, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, for the provided with the experimental materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- CABI. Spodoptera Frugiperda (Fall Armyworm). Available online: https://www.cabi.org/isc/datasheet/29810#94987198-9f50-4173-8bbd-30bd93840e73?tdsourcetag=s_pcqq_aiomsg (accessed on 1 September 2020).
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J.; et al. Fall armyworm: Impacts and implications for Africa. Outlooks Pest Manag. 2017, 28, 196–201. [Google Scholar] [CrossRef]
- Wu, K.M. Management strategies of fall armyworm (Spodoptera frugiperda) in China. Plant Prot. 2020, 46, 1–5. [Google Scholar] [CrossRef]
- Okuma, D.M.; Bernardi, D.; Horikoshi, R.J.; Bernardi, O.; Silva, A.P.; Omoto, C. Inheritance and fitness costs of Spodoptera frugiperda (Lepidoptera: Noctuidae) resistance to spinosad in Brazil. Pest Manag. Sci. 2017, 78, 1441–1448. [Google Scholar] [CrossRef]
- Guo, J.F.; Wu, S.Y.; Zhang, F.; Huang, C.L.; He, K.L.; Babendreier, D.; Wang, Z.Y. Prospects for microbial control of the fall armyworm Spodoptera frugiperda: A review. BioControl 2020, 65, 647–662. [Google Scholar] [CrossRef]
- Shylesha, A.N.; Jalali, S.K.; Gupta, A.; Varshney, R.; Venkatesan, T.; Shetty, P.; Ojha, R.; Ganiger, P.C.; Navik, O.; Subaharan, K.; et al. Studies on new invasive pest Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) and its natural enemies. J. Biol. Control 2018, 32, 145–151. [Google Scholar] [CrossRef]
- Chen, W.B.; Li, Y.Y.; Wang, M.Q.; Liu, C.X.; Mao, J.J.; Chen, H.Y.; Zhang, L.S. Natural enemy insect resources of the fall armyworm Spodoptera frugiperda, their application status, and existing problems and suggestions. Chin. J. Biol. Control 2019, 34, 658–673. [Google Scholar] [CrossRef]
- Chen, W.B.; Li, Y.Y.; Wang, M.Q.; Liu, C.X.; Mao, J.J.; Chen, H.Y.; Zhang, L.S. Entomopathogen resources of the fall armyworm Spodoptera frugiperda, and their application status. Plant Prot. 2019, 45, 1–9. [Google Scholar] [CrossRef]
- Salazar-Mendoza, P.; Rodriguez-Saona, C.; Fernandes, O.A. Release density, dispersal capacity, and optimal rearing conditions for Telenomus remus, an egg parasitoid of Spodoptera frugiperda, in maize. Biocontrol Sci. Technol. 2020, 30, 1040–1059. [Google Scholar] [CrossRef]
- Kenis, M.; du Plessis, H.; Van den Berg, J.; Ba, M.N.; Goergen, G.; Kwadjo, K.E.; Baoua, I.; Tefera, T.; Buddie, A.; Cafà, G.; et al. Telenomus remus, a candidate parasitoid for the biological control of Spodoptera frugiperda in Africa, is already present on the continent. Insects 2019, 10, 92. [Google Scholar] [CrossRef]
- Cave, R.D. Biology, ecology and use in pest management of Telenomus remus. Biocontrol News Inf. 2000, 21, 21–26. [Google Scholar]
- Pomari, A.F.; Bueno, A.F.; Bueno, R.C.O.F.; Junior, M.; de Oliceriras, A.; Fonseca, A.C.P.F. Releasing number of Telenomus remus (Nixon) (Hymenoptera: Platygastridae) against Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) in corn, cotton and soybean. Ciência Rural. 2013, 43, 377–382. [Google Scholar] [CrossRef]
- Huo, L.X.; Zhou, J.C.; Ning, S.F.; Zhao, Q.; Zhang, L.X.; Zhang, Z.T.; Zhang, L.S.; Dong, H. Biological characteristics of Telenomus remus against Spodoptera frugiperda and Spodoptera litura eggs. Plant Prot. 2019, 45, 60–64. [Google Scholar] [CrossRef]
- Queiroz, A.P.; Bueno, A.F.; Pomari-Fernandes, A.; Grande, M.L.M.; Bortolotto, O.C.; Silva, D.M. Quality control of Telenomus remus (Hymenoptera: Platygastridae) reared on the factitious host Corcyra cephalonica (Lepidoptera: Pyralidae) for successive generations. Bull. Entomol. Res. 2017, 107, 791–798. [Google Scholar] [CrossRef]
- Queiroz, A.P.; Bueno, A.F.; Pomari-Fernandes, A.; Grande, M.L.M.; Bortolotto, O.C.; Silva, D.M. Low temperature storage of Telenomus remus (Nixon) (Hymenoptera: Platygastridae) and its factitious host Corcyra cephalonica (Stainton) (Lepidoptera: Pyralidae). Neotrop. Entomol. 2017, 46, 182–192. [Google Scholar] [CrossRef]
- Jalali, S.K.; Venkatesan, T.; Murthy, K.S.; Biswas, S.R.; Lalitha, Y. Influence of temperature and host density on functional response of Telenomus remus Nixon, an egg parasitoid of Spodoptera litura Fabricius. Entomon-Trivandrum 2005, 30, 193–199. [Google Scholar]
- Zhao, S.Y.; Luo, Q.M.; Sun, X.X.; Yang, X.M.; Jiang, Y.Y.; Wu, K.M. Comparison of morphological and biological characteristics between Spodoptera frugiperda and Spodoptera litura. Chin. Plant Prot. 2019, 39, 26–35. [Google Scholar]
- Iranipour, S.; Bonab, Z.N.; Michaud, J.P. Thermal requirements of Trissolcus grandis (Hymenoptera: Scelionidae), an egg parasitoid of sunn pest. Eur. J. Entomol. 2010, 107, 47–53. [Google Scholar] [CrossRef]
- Hougardy, E.; Hogg, B.N.; Wang, X.G.; Daane, K.M. Comparison of thermal performances of two Asian larval parasitoids of Drosophila suzukii. Biol. Control. 2019, 136, 10400. [Google Scholar] [CrossRef]
- Bueno, R.C.O.F.; Carneiro, T.R.; Pratissoli, D.; Bueno, A.F.; Fernandes, O.A. Biology and thermal requirements of Telenomus remus reared on fall armyworm Spodoptera frugiperda eggs. Cienc. Rural. 2008, 38, 1–6. [Google Scholar] [CrossRef]
- Greene, G.L.; Leppla, N.C.; Dickerson, W.A. Velvetbean caterpillar: A rearing procedure and artificial medium. J. Econ. Entomol. 1976, 69, 487–488. [Google Scholar] [CrossRef]
- Chen, Q.J.; Li, G.H.; Pang, Y. A simple artificial diet for mass rearing of some noctuid species. Entomol. Knowl. 2000, 37, 325–327. [Google Scholar]
- Campbell, A.; Frazer, B.D.; Gilbert, N.; Mackauer, M. Temperature requirements of some aphids and their parasites. J. Appl. Ecol. 1974, 11, 431–438. [Google Scholar] [CrossRef]
- Pomari, A.F.; Bueno, A.F.; Bueno, R.C.O.F.; Junior, A.O.M. Biological characteristics and thermal requirements of the biological control agent Telenomus remus (Hymenoptera: Platygastridae) reared on eggs of different species of the Genus Spodoptera (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 2012, 105, 73–81. [Google Scholar] [CrossRef]
- Pinto, J.R.L.; Fernandes, O.A. Parasitism capacity of Telenomus remus and Trichogramma pretiosum on eggs of moth pests of peanut. Bull. Insectol. 2020, 73, 71–78. [Google Scholar]
- Pak, G.A.; Buis, H.C.E.M.; Heck, I.C.C.; Hermans, M.L.G. Behavioural variations among strains of Trichogramma spp. Host-age selection. Entomol. Exp. Appl. 1986, 40, 247–258. [Google Scholar] [CrossRef]
- Bueno, R.C.O.F.; Parra, J.R.P.; Bueno, A.F. Biological characteristics and thermal requirements of a Brazilian strain of the parasitoid Trichogramma pretiosum reared on eggs of Pseudoplusia includens and Anticarsia gemmatalis. Biol. Control. 2009, 51, 355–361. [Google Scholar] [CrossRef]
- CABI. Spodoptera litura (Taro Caterpillar). Available online: https://www.cabi.org/isc/datasheet/44520 (accessed on 11 December 2020).
- Bai, B.; Luck, R.F.; Forster, L.; Stephens, B.; Janssen, J.A.M. The effect of host size on quality attributes of the egg parasitoid, Trichogramma pretiosum. Entomol. Exp. Appl. 1992, 64, 37–48. [Google Scholar] [CrossRef]
- Bueno, R.C.O.F.; Carneiro, T.R.; Bueno, A.F.; Pratissoli, D.; Fernandes, O.A.; Vieira, S.S. Parasitism capacity of Telenomus remus Nixon (Hymenoptera: Scelionidae) on Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) eggs. Braz. Arch. Biol. Techn. 2010, 53, 133–139. [Google Scholar] [CrossRef]
- Kang, L.; Chen, B.; Wei, J.N.; Liu, T.X. Roles of thermal adaptation and chemical ecology in Liriomyza distribution and control. Annu. Rev. Entomol. 2009, 54, 127–145. [Google Scholar] [CrossRef]
- Sunday, J.M.; Bates, A.E.; Kearney, M.R.; Colwell, R.K.; Dulvy, N.K.; Longino, J.T.; Huey, R.B. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl. Acad. Sci. USA 2014, 111, 5610–5615. [Google Scholar] [CrossRef]
- Krechemer, F.S.; Foerster, L.A. Tuta absoluta (Lepidoptera: Gelechiidae): Thermal requirements and effect of temperature on development, survival, reproduction and longevity. Eur. J. Entomol. 2015, 112, 658–663. [Google Scholar] [CrossRef]
- Pomari, A.F.; Bueno, A.F.; Bueno, R.C.O.F.; Menezes, A.O. Telenomus remus Nixon egg parasitization of three species of Spodoptera under different temperatures. Neotrop. Entomol. 2013, 42, 399–406. [Google Scholar] [CrossRef]
- Visser, B.; Ellers, J. Lack of lipogenesis in parasitoids: A review of physiological mechanisms and evolutionary implications. J. Insect. Physiol. 2008, 54, 1315–1322. [Google Scholar] [CrossRef]
- Bueno, R.C.O.F.; Bueno, A.F.; Xavier, M.F.C.; Carvalho, M.M. Telenomus remus (Hymenoptera: Platygastridae) parasitism on eggs of Anticarsia gemmatalis (Lepidoptera: Eribidae) compared with its natural host Spodoptera frugiperda (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 2014, 107, 799–808. [Google Scholar] [CrossRef]
- Pak, G.A.; Oatman, E.R. Biology of Trichogramma brevicapillum. Entomol. Exp. Appl. 1982, 32, 61–67. [Google Scholar] [CrossRef]
- Bari, M.N.; Jahan, M.; Islam, K.S. Effects of temperature on the life table parameters of Trichogramma zahiri (Hymenoptera: Trichogrammatidae), an egg parasitoid of Dicladispa armigera (Chrysomelidae: Coleoptera). Environ. Entomol. 2015, 44, 368–378. [Google Scholar] [CrossRef]
- Gerling, D. The developmental biology of Telenomus remus Nixon (Hym., Scelionidae). Bull. Entomol. Res. 1972, 61, 385–388. [Google Scholar] [CrossRef]
- Chia, S.Y.; Tanga, C.M.; Khamis, F.M.; Mohamed, S.A.; Salifu, D.; Sevgan, S.; Fiaboe, K.K.M.; Niassy, S.; van Loon, J.J.; Dicke, M.; et al. Threshold temperatures and thermal requirements of black soldier fly Hermetia illucens: Implications for mass production. PLoS ONE 2018, 13, e206097. [Google Scholar] [CrossRef]
- Honek, A. Geographical variation in thermal requirements for insect development. Eur. J. Entomol. 1996, 93, 303–312. [Google Scholar]
- Corbet, S.A. Insect chemosensory responses: A chemical legacy hypothesis. Ecol. Entomol. 1985, 10, 143–153. [Google Scholar] [CrossRef]
- Queiroz, A.P.; Bueno, A.F.; Pomari-Fernande, A.; Bortolotto, O.C.; Mikami, A.Y.; Olive, L. Influence of host preference, mating, and release density on the parasitism of Telenomus remus (Nixon) (Hymenoptera, Platygastridae). Rev. Bras. Entomol. 2017, 61, 86–90. [Google Scholar] [CrossRef]
- Jaraleño-Teniente, J.; Lomeli-Flores, J.R.; Rodríguez-Leyva, E.; Bujanos-Muñiz, R.; Rodríguez-Rodríguez, S.E. Egg parasitoids survey of Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) in maize and sorghum in central Mexico. Insects 2020, 11, 157. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).