Simple Summary
Sugarcane, an important cash crop in Malawi, is susceptible to numerous insect pests, and many farmers rely heavily on chemical insecticides for their control. Biopesticides containing insect pathogens are used in several countries outside Malawi; however, the occurrence and use of insect pathogens is limited in Malawi. In this study, we evaluated the natural occurrence of insect pathogenic fungi in sugarcane (Saccharum officinarum) and in soil samples from sugarcane fields in Chikwawa District, southern Malawi. Insect pathogenic fungi from soil were isolated by baiting using larvae of the greater wax moth (Galleria mellonella). Insect pathogenic fungi were also isolated from surface-sterilized sugarcane leaves, stems, and roots. We found three types of insect pathogenic fungi: Beauveria bassiana, Metarhizium spp., and Isaria spp. Beauveria bassiana and Isaria spp. were found mostly from sugarcane leaves and stems, while Metarhizium spp. was mainly found in soils. To the best of our knowledge, this is the first report of B. bassiana and Isaria spp. occurring naturally as endophytes in sugarcane. Further, it is the first report of B. bassiana, Isaria spp. and Metarhizium spp. in the soil of sugarcane fields in Africa.
Abstract
The natural occurrence of entomopathogenic fungal endophytes in sugarcane (Saccharum officinarum) and in soil samples from sugarcane fields was evaluated in Chikwawa District, southern Malawi. Fungi from soil were isolated by baiting using Galleria mellonella larva. Fungal endophytes were isolated from surface-sterilized plant tissue sections. Forty-seven isolates resembled the genus Beauveria, 9 isolates were Metarhizium, and 20 isolates were Isaria. There was no significant difference in the number and type of fungal isolates collected from soil and from plant tissue. There was, however, a significant difference in the part of the plant where fungal species were isolated, which fungal species were isolated, and the number of fungal species isolated at each location. Phylogenetic analysis of 47 Beauveria isolates based on DNA sequencing of the Bloc intergenic region indicated that these isolates all belonged to B. bassiana and aligned with sequences of B. bassiana isolates of African and Neotropical origin. The Malawian B. bassiana isolates formed a distinct clade. No larvae died from infestation by multiple fungi. To the best of our knowledge, this is the first report of B. bassiana and Isaria spp. occurring naturally as endophytes in sugarcane. Further, it is the first report of B. bassiana, Isaria spp., and Metarhizium spp. in the soil of sugarcane fields in Africa.
1. Introduction
Entomopathogenic fungi (EPF) in the order Hypocreales are known to infect and kill arthropods, occur in the soil environment, and interact with plants as endophytes [1,2,3,4,5,6]. Depending on their biology and ability to grow on artificial media, these fungi may be used in biocontrol of crop pests [6,7]. Entomopathogenic fungi can cause epizootics in populations of soil-dwelling insects and mites [8], and fungal isolates of the genera Beauveria and Isaria (Hypocreales: Cordycipitaceae) and Metarhizium (Hyporeales: Clavicipitaceae) are developed for inoculative or inundation biological control of agricultural pests [9,10,11,12,13].
The natural occurrence and diversity of entomopathogenic fungi in terrestrial ecosystems may be affected by many factors such as climate, habitat, soil properties, plant species, agricultural practices, and sampling method [10,13,14,15,16]. Klingen and Haukeland [15] suggest that the use of chemical pesticides, especially fungicides but also herbicides, may reduce the prevalence of entomopathogenic fungi in the soil. Further, Klingen et al. [13] isolated entomopathogenic fungi more frequently in soil samples from organic than from conventional arable fields. Likewise, Clifton et al. [5] reported that soils from organic soybean or maize fields had more entomopathogenic fungi than corresponding conventional fields and that the occurrence of entomopathogenic fungi was negatively affected by tillage, elevated nitrogen content of soil, and herbicide and fungicide application. Furthermore, Ramos et al. [17] recovered Beauveria bassiana more frequently in soil samples and root tissues from organic than conventional bean fields. In a review of the literature, Meyling and Eilenberg [10] reported that Metarhizium anisopliae sensu lato generally was most prevalent in soils collected from agricultural fields, compared to undisturbed areas such as hedgerows, while B. bassiana was more frequently isolated from soils from the undisturbed areas. Besides occurrence in soils, Beauveria spp. and Metarhizium spp. have been isolated as endophytes from perennial woody plants such as coffee, pine, and cocoa [1,17,18,19,20] and non-woody plants such as beans and maize [17,21,22].
Although B. bassiana and M. anisopliae are known to be effective against insect pests that infest sugarcane [20,23,24,25,26], few studies have focused on natural occurrence of entomopathogenic fungi in sugarcane cropping systems in Africa [24,27]. Ngubane et al. [27] isolated Metarhizium anisopliae, Beauveria bassiana, Beauveria brongniartii, and Lecanicillium lecanii from various insect cadavers collected from sugarcane fields in six countries in southern Africa. Goble et al. [24] characterized Beauveria spp. isolates obtained from insect, soil, and root origin from sugarcane production sites in South Africa. However, there are no published studies on the natural occurrence of entomopathogenic fungi from the Hypocreales as endophytes in sugarcane of sugarcane cropping systems from Africa. The aims of the present study were therefore to investigate whether these entomopathogenic fungi occur naturally in soils and in sugarcane tissues collected in sugarcane fields in Malawi and whether the prevalence of the fungi varies among sugarcane cropping systems.
2. Materials and Methods
2.1. Description of Sugarcane Production, Location, and Sampling of Plants and Soil
Sugarcane is vegetatively cultivated using short-stem cuttings (referred to as plant cane) and from old growth (referred to as ratoon cane). In many African countries, smallholder farmers grow sugarcane for home consumption but also sell raw sugarcane in local markets [28]. These farmers grow traditional cultivars or a mixture of cultivars and intercrop the sugarcane rows with other crops such as maize and vegetables. Sugarcane is grown in seasonal wetlands of valley bottoms called “dambo” and low-lying areas called “dimba”, both of which are referred to here as “traditional” fields. Traditional farmers use a hoe for tilling the soil two or more times per year, irrigate the field as required, and apply insecticides without consideration to economic thresholds [29,30]. Commercial estates owned by foreign multinational companies also grow sugarcane for processing into sugar and other sugarcane-based products. These estates use irrigation and other cultivars that originate from both within and outside Africa. A third category of sugarcane farmers are referred to as “outgrowers”, and they grow sugarcane using the same varieties as the commercial estates either under rain-fed conditions or irrigation. Outgrowers are supposed to follow production guidelines used in commercial estates and may belong to a farmer association that provides input packages (seed, fertilizer, and herbicides) on credit or may act independently [29]. Commercial estates and outgrowers’ sugarcane fields are ploughed on average every 3.8 years. On commercial estates and at outgrowers, chemical insecticides and herbicides are applied according to economic threshold levels [29] provided by ILLOVO Malawi agronomists based at Nchalo Estate. Sugarcane is harvested green in traditional fields but is burned prior to harvesting in commercial estates and outgrowers’ fields.
In this study, field surveys were conducted from July to December 2016 in 6 locations, namely, Mitole, Maseya, Phata, Kasinthula, and Alumenda in the Chikwawa District of southern Malawi (Figure 1; Table S1). There are 3 soil associations that dominated the study area: well drained youthful soils (Cambisols, soil association A), which constitute 59%; black cracking clays (Vertisols, soil association H), comprising 22%; and Duplex soils (Luvisols, soil association C), comprising 6% [31]. The study area lies below 150 m. The climate is tropical continental with 2 seasons: the rainy season from November to April and the dry season from May to October. However, from May to July, it is relatively cool. The coldest months are June and July. The highest temperatures occur at the end of October or early November. The mean annual minimum and maximum temperatures range from 15 to 36 degrees Celsius (°C) [31]. Within each location, 2 sites were randomly selected, and a 30 × 30 m quadrat was established as a frame for 5 sampling units. In Malawi, sugarcane fields are ploughed down after an average of 3.8 harvests. Sugarcane is harvested after growing for 12–15 months. Sugarcane fields harvested 4 to 7 times, i.e., 45.6- to 105-month-old ratoon cane were sampled. The plants were sampled less than 5 months after harvest. Plants were sampled by carefully uprooting 1 plant from the center and from the 4 corners of the 30 × 30 m quadrat at each of the 2 sites per location. Collected plants (n = 60 in total) were placed in polyethylene bags and transported fresh and intact in 40 L cooler boxes to the laboratory for assessment within 24 h. Within each quadrate of the 12 sites, we collected 5 soil samples at a distance of 60 cm from the base of the collected plant and down to a 15 cm depth by the use of a garden spade. The spade was sterilized in 70% alcohol between samplings to prevent cross-contamination. Soil samples were then placed separately in 1 L polyethylene bags and transported immediately in 40 L cooler boxes to the laboratory for processing.
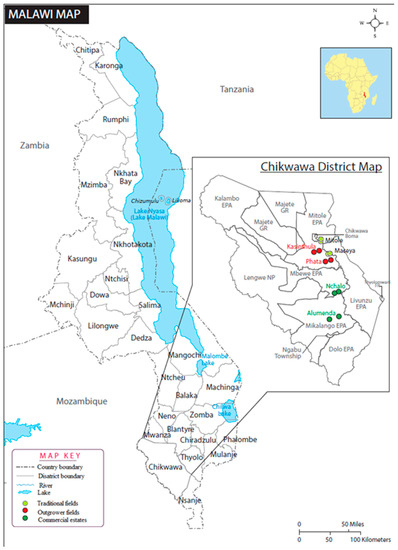
Figure 1.
Locations of the 12 sugarcane fields sampled in Chikwawa District, southern Malawi.
2.2. Isolation of Fungi
2.2.1. Isolation of Endophytic Fungi from Plant Samples
Upon arrival at the laboratory, the soil was carefully shaken off the plant roots and the roots were washed with tap water. From each sampled sugarcane, 10 cm sections of stem, leaf, and root, were cut out and surface-sterilized by immersion for 2 min in 3% sodium hypochlorite followed by 2 min in 70% ethanol and then rinsed thrice for 30 s in sterile distilled water, as described by Parsa et al. [32]. Effectiveness of the sterilization process was evaluated by plating 100 µL of the last rinse water on Sabouraud dextrose agar (SDA, Oxoid) with 1% antibiotics (0.2 g penicillin, 0.2 g chloramphenicol, and 0.2 g tetracycline dissolved in 10 mL sterile distilled water, followed by filter sterilization through a 0.2 mm filter). The absence of fungal growth from the last rinse of water indicated that sterilization was successful. The sterilized plant tissue sections were dried on sterile paper for 1 min and the edges were trimmed so that the sections measured 60 mm long. The 60 mm trimmed sections were further dissected into 5 equal size pieces and all 5 pieces were pressed onto SDA in the same Petri dish. After sealing with Parafilm, the Petri dishes were incubated in the dark for 14–21 days at 25 ± 5 °C. Fungal growth emerging from the plant tissue was reisolated onto new SDA plates to obtain pure cultures. Mycelia and conidia from pure cultures were stored on silica gel at 25 ± 5 °C and later used for morphological and molecular characterization.
2.2.2. Isolation of Fungi from Soil Samples
In the laboratory, the 5 soil samples per site were thoroughly mixed to produce 12 composite pooled soil samples. Soils were kept at 4 °C until processing but never for longer than 5 days. All soil samples were sieved through a 2 mm mesh sieve to remove debris. Dry soil samples were slightly moistened with sterile water while wet soils were first air-dried to remove excess water and reduce the incidence of nematodes. The widely and best-known method for selecting entomopathogenic fungi, the Galleria mellonella bait method described by Zimmermann [33], was used to isolate entomopathogenic fungi from soil samples. Before being used as baits, 4–5-week-old G. mellonella larvae were heat-conditioned as described by Woodring and Kaya [34] by immersion in 56 °C sterile water for 15 s, followed by pouring cold water on top of the larvae for 30 s and then letting the larvae rest for 1 h to recover. This was done to reduce the ability of the larvae to produce webbing while in the soil. Five live heat-conditioned G. mellonella were then added to a 350 mL plastic container with an aerated lid containing 300 g of the sifted soil sample and incubated for 14 days in the dark at 25 ± 5 °C. The plastic containers were inverted once every 2 days to promote larval movement through the soil.
Containers with soil samples were checked daily, and dead larvae were removed, surface sterilized by immersing them in 70% alcohol for 10 s, rinsed thrice in sterile water for 10 s, and left to dry on a sterile paper towel. They were then individually placed in a moist chamber and incubated for 14 days at 25 ± 5 °C. Dead larvae were observed every 2 days for fungal growth, and mycelia were isolated by placing them on SDA with 0.1% antibiotics and incubated as described above. The number of dead larvae exhibiting mycosis was recorded. Fungal growth obtained from each mycosed larva was considered an isolate. Fungal isolates were stored in silica gel until morphological and molecular characterization.
2.3. Morphological Identification of Fungi
Entomopathogenic fungi in the Hypocreales were identified morphologically to genus level according to Humber [35] by examining under a 400× phase contrast microscope.
2.4. Molecular Identification of Fungi to Species Level
The identification of entomopathogenic fungi in the Hypocreales to the species level requires molecular techniques [36,37]. Molecular analysis of fungi to species level in this study have thus far only been conducted on the 47 isolates that were morphologically identified to be in the genus Beauveria.
2.4.1. DNA Extraction, PCR Amplification, and Sequence Analysis
DNA extraction and PCR reactions were performed at NIBIO, Ås, Norway. DNA was extracted from Beauveria isolates only because Beauveria isolates are widely used in biological control programs and are effective against a wide range of arthropod pests that occur in sugarcane [9,10,11,12,13]. A few silica gel crystals from the stored fungal isolates were placed onto SDA plates (9 cm diameter) and incubated in the dark for 14 days at room temperature (21–25 °C) in the laboratory at NIBIO. Mycelia and conidia were then harvested by scraping off a small portion of the fungus using a sterile scalpel. The harvested mycelia and conidia were then ground to a fine powder using a mortar and pestle in liquid nitrogen before extracting the genomic DNA using a DNeasy Plant Mini kit (Qiagen, Germany) in accordance with the manufacturer’s instructions.
PCR amplification targeting the intergenic Bloc region for 47 Beauveria cycler was carried out using a Bio-Rad T100 Thermal. Amplification of the Bloc gene region was achieved with the primer pair B22U (5′-AGATTCGCAACGTCAACTT-3′) and B822L (5′-GTCGCAGCCAGAGCAACT-3′) [36]. PCR targeting the Bloc regions was performed. The reaction volume of 50 µL contained 1.5 µL Mm MgCL2, 1× PCR buffer, 4 µ 200 µM deoxynucleotides (dNTPs), 1 µL of each primer (10 µM), 0.1 µL 0.5U Platinum Taq DNA polymerase, and 3 µL genomic DNA. Cycling conditions for Bloc gene regions were as follows: 5 min at 95 °C denaturation followed by a touch-down protocol with 30 s denaturation at 95 °C, 30 s at 70–60 °C (reducing annealing temperature by 1 °C per cycle), and 1 min at 72 °C. An additional 30 cycles were performed including 30 s at 95 °C, 30 s annealing at 60 °C, and 1 min at 72 °C, followed by a final extension of 5 min at 72 °C.
The PCR products were visualized by gel electrophoresis—1.0% agarose gel with TBE (45 mM Tris base, 45 mM boric acid, 1 mM Ethylenediaminetetraacetic acid (EDTA) (pH 8.0)). Staining of bands with ethidium bromide (Thermo Fisher Scientific, New York, NY, USA) was performed to help with visualization of the amplified DNA through a GelDoc EQ gel imaging system equipped with PDQuest 2-D analysis software (Bio-Rad Laboratories, Califonia, USA). The size of the PCR products was determined through comparison to a 100 bp DNA ladder (New England Biolabs, Hertfordshire, UK). PCR products were diluted (where necessary) in nuclease-free water to acquire the right concentration (10–50 ng-µL) recommended for sequencing. PCR products were sent to GATC Biotech (Germany) for sequencing. Sanger sequencing was performed by GATC Biotech (Germany) using the B22U/B822L primer pair.
2.4.2. Phylogenetic Analysis
The sequences obtained from the Bloc gene analysis were traced, edited, and assembled using CLC Main workbench 7. Nucleotide sequence consensus sequences were aligned using ClustalW in BioEdit 7.2.5 [38,39]. Published sequences for Beauveria [24,26,36] were included in the phylogenetic analysis. Intraspecific divergence was calculated using Mega6 [40]. Preliminary neighbor-joining (NJ) and maximum likelihood (ML) trees were generated for the aligned sequences using Mega6 [40]. Both NJ and ML trees were based on the Kimura 2-parameter model K2P [40]. Using the model selection option in Mega6, we found that the Kimura 2-parameter with discreet Gamma distribution (K2 + G) was the best-fit model for our dataset on the basis of the lowest Bayesian information criterion (BIC) value. We used the best-fit model to generate ML analysis using 1000 bootstrap replications. A reference sequence of Beauveria malawiensis was included to root the phylogenetic tree.
2.5. Data Analysis
Preliminary data exploration indicated that the data (frequencies of occurrence of soil and plant samples positive for Beauveria spp., Isaria spp., and Metarhizium spp. collected from soil and sugarcane plants) did not follow a normal distribution. Hence, frequency data were analyzed using non-parametric tests, one-sample option. Chi-squared (Χ2) tests and cross-tabulations were also computed to find relationships between location, farm type, and occurrence of EPF. Pairwise comparison test was carried out to compare the distribution of EPF across farm type. All statistical analyses were carried out in SPSS version 22 (IBM Statistics Software, IBM, New York, NY, USA).
3. Results
3.1. Morphological Identification and Frequency of Occurrence
A total of 60 sugarcane plant samples and 60 G. mellonella larvae were used to bait the 12 soil samples evaluated in this study. On the basis of morphological features, we identified 76 fungal isolates. Of these, 47 isolates resembled Beauveria, 9 isolates were Metarhizium, and 20 isolates were Isaria (Table 1). Location had a significant effect on the type of fungal species isolated (Χ² = 19.462, d.f. = 11, p = 0.035) (Table 1). Pairwise comparison indicated that distribution of EPF was the same across the category of farm type (p = 0.111). Beauveria spp. was present at all the locations sampled. There were significant differences in the part of a plant part where fungal species were isolated (Χ² = 18.750, d.f. = 2, p < 0.001), which fungal species were isolated (Χ² = 14.683, d.f. = 2, p = 0.001), and the number of fungal species isolated (Χ² = 18.750, d.f. = 2, p < 0.001). More Beauveria spp. were recovered from leaf tissue than in stem and root tissue.

Table 1.
Number of Beauveria spp., Metarhizium spp., and Isaria spp. obtained from soils (n = 60) and as endophytes of sugarcane (Saccharum officinarum) tissues (root, n = 60; stem, n = 60; leaf, n = 60) from two commercial estates (Alumenda and Nchalo), two outgrowers fields (Kasinthula and Phata), and two traditional fields (Maseya and Mitole) in the Chikwawa District, southern Malawi.
3.2. Phylogenetic Analysis
Sequences for the 47 Beauveria isolates were generated. A highly similar sequences match search done using Basic Local Alignment Search Tool (BLAST) on the National Center for Biotechnology Information (NCBI) database indicated that the Beauveria isolates were B. bassiana. The additional 18 bloc sequences of Beauveria were downloaded from the GenBank for phylogenetic placement of the sequences. The alignment contained 845 positions. After eliminating gaps and missing data, we resulted in 727 nucleotide positions included in the final dataset. ML analysis based on the Bloc produced trees with similar topologies with well-resolved clusters representing isolates of five different species of Beauveria (Figure 2). All the 47 Beauveria isolates sequenced in this study had similar sequences and formed their own unique clade with high branch support, which clustered within B. bassiana clade (Figure 2), specifically the clade containing reference sequences from Africa (Cameroon, Côte d’Ivoire, Kenya, Togo) and the Neotropics (Brazil, Colombia, Costa Rica, Mexico, Nicaragua) denoted as AFNEO_1 by Rehner et al. [36].
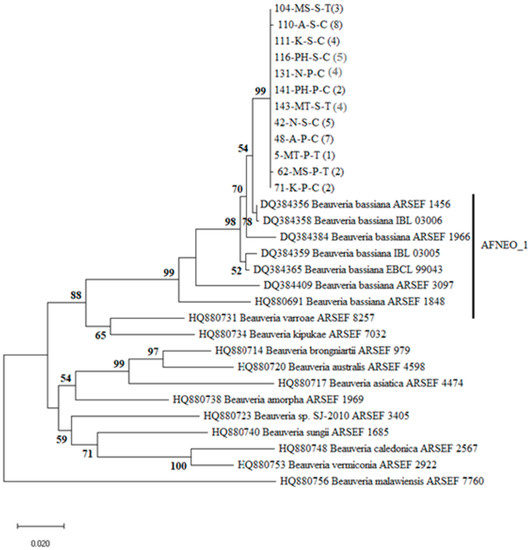
Figure 2.
Phylogenetic tree of Beauveria indicating the position of Malawian isolates collected from sugarcane fields within the worldwide Beauveria genetic structure. The tree was inferred by the maximum likelihood (ML) method based on the Kimura 2-parameter model with a discrete Gamma distribution (K2 + G) using the Kimura 2-parameter method of intergenic Bloc region of 47 Malawian and 18 reference sequences from GenBank (given with their associated accession number). For the isolates from Malawi, the code after the isolate number denotes substrate: S = soil, P = plant; location: MS = Maseya, MT = Mitole, N = Nchalo, P = Phata, K = Kasinthula, A = Alumenda; in brackets = number of isolates. Branch support was measured through 1000 bootstrap repetitions.
4. Discussion
To the best of our knowledge, this is the first report of B. bassiana and Isaria spp. occurring naturally as endophytes in sugarcane. B. bassiana and Isaria spp. isolated from sterilized sugarcane plant tissue represent naturally colonized plant parts, which can have been inoculated from fungal propagules in the soil or from infected insect hosts. This expected route of entry is supported by a recent study demonstrating the ability of B. bassiana to experimentally establish as an endophyte of sugarcane [41]. The incidence of B. bassiana in leaf tissue in our study could also have been a result of aerial deposition of B. bassiana propagules [10,42]. In addition, insects have the ability to transport B. bassiana to plant surfaces [43]. As an endophyte, B. bassiana has been isolated from plant tissues of common bean [17], coffee and cocoa plants [1,11,19], faba beans [44], maize [21], and pine needles [18]. Isaria spp. (formerly Paecilomyces) [32] have been reported as endophytes in rice (Paecilomyces sp.) [45], mangrove (Paecilomyces varioti) [46], banana (Paecilomyces sp.) [9], and coffee plants (Paecilomyces cf. fumosoroseus, P. cf. javanicus) [47]. Endophytism between entomopathogenic fungi such as B. bassiana and Metarhizium (not all Isaria spp. are pathogenic to insects) and plants is considered to be detrimental to insect pests [48,49,50]. The negative impact on insect pests may be direct by infection, induction of secondary metabolites (terpenoids) involved in plant defense against herbivory, or synthesis of herbivore-induced plant volatiles (HIPVs) and kairomones that can be used by parasitoids in locating their insect hosts [9,24,48,49,50].
This is the first report of the natural occurrence of Beauveria bassiana in soil from sugarcane fields in Malawi. The frequency of occurrence of Beauveria spp. differed among locations. Several previous studies indicate that convectional agricultural practices such as application of synthetic pesticides decrease the natural incidence and diversity of the Hypocreales [14,16,17,51]. However, farm type did not significantly affect the distribution of EPF across locations. Goble et al. [52] and Meyling et al. [53] also found no difference in the occurrence of B. bassiana and M. anisopliae in organically and conventionally managed citrus orchards in South Africa and white cabbage and onion fields in Denmark, respectively. On, the contrary, in Cuba, the overall frequency of occurrence of Beauveria isolates in conventional bean fields was significantly lower than that in organic fields [17]. All the sugarcane fields sampled were subjected to conventional tillage, i.e., inverting and loosening of soil after harvest. This practice is more frequent in traditional fields (multiple times in a year) compared to commercial estates and outgrowers’ fields (performed once every 3.8 years). Some studies have negatively associated abundance of entomopathogenic fungi in agricultural fields with conventional tillage [5,21,54]. In the sugarcane fields studied, insecticides were applied [29]. Insecticides reduce insect populations in a field and possibly the endophytic inoculum in the plant due to fewer fungal-infected hosts. This may be important for the dissemination of entomopathogenic fungal inoculum between the soil and the phyllosphere [14,55,56,57,58]. However, several other factors that are known to influence the natural occurrence and diversity of entomopathogenic fungi in agroecosystems could also have an impact on the present results [5,14,15,16,59,60,61,62], although these factors were not considered in the present study.
Our phylogenetic analysis placed all the 47 Malawian Beauveria spp. isolates within the same B. bassiana clade, which is closely related to B. bassiana of other countries in Africa (Cameroon, Côte d’Ivoire, Kenya, and Togo) and Neotropical regions (Brazil, Colombia, Costa Rica, Mexico, and Nicaragua) referred to as AFNEO_1 [36]. The close relationship among the B. bassiana isolates from the sugarcane fields characterized in the present study indicate that they all represent a single B. bassiana population distributed among the six locations in Chikwawa district in southern Malawi. Our results are similar to those of Ramos et al. [17], who found limited diversity among B. bassiana isolates from common bean fields in Cuba distributed in samples of soil and plant material. These two B. bassiana population studies are in contrast to reports from Europe, where two separate clades of B. bassiana were found infecting pollen beetles in Switzerland [59]. In another study conducted in Denmark, several clades of B. bassiana coexisted in the soil of the hedgerow bordering an agriculture field [60]. The reason for the seemingly limited diversity within B. bassiana populations from agricultural systems in East Africa and the Caribbean compared to Europe could be related to the tropical climate or limited dispersal mechanisms within these regions. It has previously been reported that B. bassiana is effective when applied against arthropod pests that infest sugarcane [20,23,24,25] and the naturally occurring fungi may contribute to the regulation of pest populations [63,64,65,66,67].
5. Conclusions
The present study is the first to report of B. bassiana and Isaria spp. as naturally occurring endophytic fungi in sugarcane. Further, the results suggest that B. bassiana and Isaria spp. constitute a naturally occurring reservoir of entomopathogenic fungi in soils and crop tissues of conventionally and traditionally grown sugarcane. The limited molecular diversity among the B. bassiana isolates in Malawi suggests that they comprise a single population with low gene flow and with the ability to both infect insects and colonize plant tissues. Future studies should focus on determining the effect of B. bassiana as endophytes of sugarcane against insect pest populations.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4450/12/2/160/s1: Table S1. Study site description.
Author Contributions
Conceptualization, T.K.D. and I.K.; methodology, T.K.D., I.K., and N.V.M.; validation, T.K.D., I.K., N.V.M., and R.M.; formal analysis, T.K.D.; investigation, T.K.D.; writing—original draft preparation, T.K.D.; writing—review and editing, T.K.D.; supervision, I.K., N.V.M., and R.M.; funding acquisition, T.K.D. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by Capacity Building for Climate Change Adaptation in Malawi (CABMACC) project number 1207026003 and Nowergian Institute for Bioeconomy Research (NIBIO). CABMACC is a collaborative project supported by the Norwegian Government and the Government of the Republic of Malawi implemented by International Environment and Development Studies (Noragric) of the Norwegian University of Life Sciences (NMBU) and Lilongwe University of Agriculture and Natural Resources, (LUANAR), Malawi.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Sequences for the Malawian Beauveria bassiana are available on NCBI GenBank. GenBank accession numbers MW570829-MW570841.
Acknowledgments
Sunday Ekesi, Fathiya Khamis, Sevgan Subramanian, Solveig Haukeland, and Jane Kimemia at the Arthropod Pathology Unit at the International Centre of Insect Physiology and Ecology, Kenya, are thanked for training within the field of invertebrate pathology. Thanks to Wilson Msuku at Lilongwe University of Agriculture and Natural Resources, Bunda Campus, Lilongwe, and Misheck Soko of Bvumbwe Agricultural Research Station in Thyolo for help with morphological analysis and Monika Skogen for technical assistance in the molecular work at the Norwegian Institute of Bioeconomy Research.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Vega, F.E.; Posada, F.; Aime, M.C.; Pava-Ripoll, M.; Infante, F.; Rehner, S.A. Entomopathogenic fungal endophytes. Biol. Control 2008, 46, 72–82. [Google Scholar] [CrossRef]
- Gurulingappa, P.; Sword, G.A.; Murdoch, G.; McGee, P.A. Colonization of crop plants by entomopathogenic fungi and their effects on two insect pests when in planta. Biological 2010, 55, 34–41. [Google Scholar]
- Reay, S.D.; Brownbridge, M.; Gicquel, B.; Cummings, N.J.; Nelson, T.L. Isolation and characterization of endophytic Beauveria spp. (Ascomycota: Hypocreales) from Pinus radiata in New Zealand forests. Biol. Control 2010, 54, 52–60. [Google Scholar] [CrossRef]
- Fisher, J.J.; Rehner, S.A.; Bruck, D.J. Diversity of rhizosphere associated entomopathogenic fungi of perennial herbs, shrubs and coniferous trees. J. Invertebr. Pathol. 2011, 106, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Clifton, E.H.; Jaronski, S.T.; Hodgson, E.W.; Gassmann, A.J. Abundance of soil-borne entomopathogenic fungi in organic and conventional fields in the Midwestern USA with an emphasis on the effect of herbicides and fungicides on fungal persistence. PLoS ONE 2015, 10, e0133613. [Google Scholar] [CrossRef] [PubMed]
- Lacey, L.A.; Solter, L.F. Initial Handling and Diagnosis of Diseased Invertebrates. In Manual of the Techniques in Invertebrate Pathology, 2nd ed.; Lacey, L.A., Ed.; Academic Press: London, UK, 2012; pp. 1–13. [Google Scholar]
- Onwley, B.H.; Gwinn, K.D.; Vega, F.E. Endophytic entomopathogenic fungi with activity against plant pathogens: Ecology and evolution. BioControl 2010, 55, 113–128. [Google Scholar]
- Pell, J.; Eilenberg, J.; Hajek, A.E.; Steinkraus, D.C. Biology, ecology and pest management potential of Entomophthorales. In Fungi as Biocontrol Agents: Progress, Problems and Potential; Butt, T.M., Jackson, C.W., Magan, N., Eds.; CABI: Wallingford, CT, USA, 2001; pp. 71–153. [Google Scholar]
- Akello, J.; Dubois, T.; Gold, C.S.; Coyne, D.; Nakavuma, J.; Paparu, P. Beauveria bassiana (Balsamo) Vuillemin as an endophyte in tissue culture banana (Musa spp.). J. Invertebr. Pathol. 2008, 96, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Meyling, N.V.; Eilenberg, J. Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: Potential for conservation biological control. Biol. Control 2007, 43, 145–155. [Google Scholar] [CrossRef]
- Posada, F.; Aime, M.C.; Peterson, S.W.; Rehner, S.A.; Vega, F.E. Inoculation of coffee plants with the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales). Mycol. Res. 2007, 111, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Cory, J.S.; Ericsson, J.D. Fungal entomopathogens in a tritrophic context. In The Ecology of Fungal Entomopathogens; Roy, H.E., Vega, F.E., Chandler, D., Goettel, M.S., Chandler, D., Pell, J.K., Wajnberg, E., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 75–88. [Google Scholar]
- Bruck, D.J. Entomopathogenic fungi in the rhizosphere. Biocontrol 2010, 55, 103–112. [Google Scholar] [CrossRef]
- Klingen, I.; Eilenberg, J.; Meadow, R. Effects of farming system, field margins and bait insect on the occurrence of entomopathogenic fungi in soils. Agric. Ecosyst. Environ. 2002, 91, 191–198. [Google Scholar] [CrossRef]
- Klingen, I.; Haukeland, S. The soil as a reservoir for natural enemies of pest insects and mites with emphasis on fungi and nematodes. In An Ecological and Societal Approach to Biological Control; Eilenberg, J., Hokkanen, H.M.T., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 145–211. [Google Scholar]
- Quesada-Moraga, E.; Navas-Cortés, J.A.; Maranhao, E.A.A.; Ortiz-Urquiza, A.; Santiago-Alvarez, C. Factors affecting the occurrence and distribution of entomopathogenic fungi in natural and cultivated soils. Mycol. Res. 2007, 111, 947–966. [Google Scholar] [CrossRef] [PubMed]
- Ramos, Y.; Portal, O.; Lysøe, E.; Meyling, N.V.; Klingen, I. Diversity and abundance of Beauveria bassiana in soils, stink bugs and plant tissues of common bean from organic and conventional fields. J. Invertebr. Pathol. 2017, 150, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Ganley, R.J.; Newcombe, G. Fungal endophytes in seeds and needles of Pinus monticola. Mycol. Res. 2006, 110, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Posada, F.; Vega, F.E. Establishment of the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales) as an endophyte in cocoa seedlings (Theobroma cacao). Mycologia 2005, 97, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Cherry, A.J.; Banito, A.; Djegui, D.; Lomer, C. Suppression of the stem borer Sesamia calamistis (Lepidoptera: Noctuidae) in maize following seed dressing, topical application and stem injection with African isolates of Beauveria bassiana. Int. J. Pest Manag. 2004, 50, 67–73. [Google Scholar] [CrossRef]
- Bing, L.A.; Lewis, L.C. Occurrence of entomopathogen Beauveria bassiana (Balsamo) Vuillemin in different tillage regimes in Zea mays L. and virulence towards Ostrinia nubilalis (Hubner). Agric. Ecosyst. Environ. 1993, 45, 147–156. [Google Scholar] [CrossRef]
- Parsa, S.; Garcia-Lemos, A.M.; Castillo, K.; Ortiz, V.; Lopez-Lavalle, L.A.B.; Braum, J.; Vega, F.E. Fungal endophytes in germinated seeds of the common bean, Phaseolus vulgaris. Fungal Biol. 2016, 120, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Tefera, T.; Pringle, K.L. Mortality and maize leaf consumption of Chilo partellus (Lepidoptera: Pyralidae) larvae treated with Beauveria bassiana and Metarhizium anisopliae. Int. J. Pest Manag. 2004, 50, 29–34. [Google Scholar] [CrossRef]
- Goble, T.A.; Costet, L.; Robene, I.; Nibouche, S.; Rutherford, R.S.; Conlong, D.E.; Hill, M.P. Beauveria brongniartii on white grubs attacking sugarcane in South Africa. J. Invertebr. Pathol. 2012, 111, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Gao, Y.; Zhang, Y.; Wang, E.; Xu, X.; Lei, Z. An entomopathogenic strain of Beauveria bassiana against Frankliniella occidentalis with no detrimental effect on the predatory mite Neoseiulus barkeri: Evidence from laboratory bioassay and scanning electron microscopic observation. PLoS ONE 2014, 9, e84732. [Google Scholar] [CrossRef] [PubMed]
- Kernasa, N.; Uraichuen, S.; Kamata, N. Phylogenetic variation of the green muscadine fungus, Metarhizium anisopliae (Metchnikoff) Sorokin and its virulence to larvae of the sugarcane longhorn stem borer, Dorysthenes buqueti Guerin (Coleoptera: Cerambycidae). Agric. Nat. Resour. 2016, 50, 427–431. [Google Scholar] [CrossRef]
- Ngubane, N.P.; Hatting, J.L.; Truter, M. Entomopathogens associated with African and Mauritian Scarabaeidea affecting sugarcane. Proc. S. Afr. Sugar Technol. Assoc. 2012, 85, 114–117. [Google Scholar]
- Baucum, L.; Rice, R.W.; Muralles, L. Backyard Sugarcane. Sugarcane Handbook. University of Florida. Publication #SS-AGR-253. 2009. Available online: http://edis.ifas.ufl.edu/sc052 (accessed on 17 February 2018).
- Donga, T.K.; Eklo, O.M. Environmental load of pesticides used in conventional sugarcane production in Malawi. Crop Prot. 2018, 108, 71–77. [Google Scholar] [CrossRef]
- Orr, A.; Ritchie, J.M. Learning from failure: Smallholder farming systems and IPM in Malawi. Agric. Syst. 2004, 79, 31–54. [Google Scholar] [CrossRef]
- Meyer, J.H.; Heathman, W.Z. Report of Further Outcomes from the Reconnaissance Soil Suitability Survey of Five Estates in the Nchalo Sugarcane Supply Area; Jan Meyer Soil Fertility Consultants: Cape Town, South Africa, 2015; 61p. [Google Scholar]
- Parsa, S.; Ortiz, V.; Vega, F.E. Establishing entomopathogenic fungi as endophytes: Towards endophytic biological control. J. Vis. Exp. 2013, 74, e50360. [Google Scholar] [CrossRef]
- Zimmermann, G. The ‘Galleria bait method’ for detection of entomopathogenic fungi. J. Appl. Entomol. 1986, 102, 213–215. [Google Scholar] [CrossRef]
- Woodring, J.L.; Kaya, H.K. Steinermematid and Heterorhabditid Nematodes: A Handbook of Biology and Techniques Southern Cooperative Series Bulletin; Arkansas Agicultural Experiment Station: Fayetteville, AR, USA, 1988; Volume 331, pp. 1–17. [Google Scholar]
- Humber, R.A. Identification of entomopathogenic fungi. In Manual of the Techniques in Invertebrate Pathology, 2nd ed.; Lacey, L.A., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 154–182. [Google Scholar]
- Rehner, S.A.; Posada, F.; Buckley, E.P.; Infante, F.; Castillo, A.; Vega, F.E. Phylogenetic origins of African and Neotropical Beauveria bassiana s.l. pathogens of the coffee berry borer, Hypothenemus hampei. J. Invert. Pathol. 2006, 93, 11–21. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Clifton, E.H.; Hajek, A.E.; Jenkins, N.E.; Roush, R.T.; Rost, J.P.; Biddinger, D.J. Applications of Beauveria bassiana (Hypocreales: Cordycipitaceae) to control populations of spotted lanternfly (Hemiptera: Fulgoridae), in semi-natural landscapes and on grapevines. Environ. Entomol. 2020, 49, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Donga, T.K.; Vega, E.; Klingen, I. Establishment of the fungal entomopathogen Beauveria bassiana as an endophyte in sugarcane, Saccharum officinarum. Fungal Ecol. 2018, 35, 70–77. [Google Scholar] [CrossRef]
- Hajek, A.E. Ecology of terrestrial fungal entomopathogens. Adv. Microb. Ecol. 1997, 15, 193–249. [Google Scholar]
- Bruck, D.J.; Lewis, L.C. Carpophilus freemani (Coleoptera: Nitidulidae) as a vector of Beauveria bassiana. J. Invertebr. Pathol. 2002, 80, 188–190. [Google Scholar] [CrossRef]
- Akutse, K.S.; Maniania, N.K.; Fiaboe, K.K.M.; van Den Berg, J.; Ekesi, S. Endophytic colonization of Vicia faba and Phaseolus vulgaris (Fabaceae) by fungal pathogens and their effects on the life history parameters of Liriomyza huidobrensis (Diptera: Agromyzidae). Fungal Ecol. 2013, 6, 293–301. [Google Scholar] [CrossRef]
- Tian, X.L.; Cao, L.X.; Tan, H.M.; Zeng, Q.G.; Jia, Y.Y.; Han, W.Q.; Zhou, S.N. Study on the communities of endophytic fungi and endophytic Actinomycetes from rice and their antipathogenic activities in vitro. World J. Microbiol. Biotechnol. 2004, 20, 303–309. [Google Scholar] [CrossRef]
- Ananda, K.; Sridhar, K.R. Diversity of endophytic fungi in the roots of mangrove species on the west coast of India. Can. J. Microbiol. 2002, 48, 871–887.8. [Google Scholar] [CrossRef] [PubMed]
- Vega, F.E.; Simpkins, A.; Aime, M.C.; Posada, F.; Peterson, S.W.; Rehner, S.A.; Infante, F.; Castillo, A.; Arnold, A.E. Fungal endophyte diversity in coffee plants from Colombia, Hawai’i, Mexico and Puerto Rico. Fungal Ecol. 2010, 3, 122–138. [Google Scholar] [CrossRef]
- Gange, A.C.; Koricheva, J.; Currie, A.F.; Jaber, L.R.; Vidal, S. Meta-analysis of the role of entomopathogenic and unspecialized fungal endophytes as plant bodyguards. New Phytol. 2019. [Google Scholar] [CrossRef]
- Vega, F.E. The use of fungal entomopathogens as endophytes in insects: A review. Mycologia 2018, 110, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Iwanicki, N.S.; Pereira, A.A.; Botelho, A.B.R.Z.; Rezende, J.M.; de Moral, R.A.; Zucchi, M.I.; Delalibera, I., Jr. Monitoring of the field application of Metarhizium anisopliae in Brazil revealed high molecular diversity of Metarhizium spp in insects, soil and sugarcane roots. Sci. Rep. 2019, 9, 4443. [Google Scholar] [CrossRef] [PubMed]
- Tkaczuk, C.; Król, A.; Majchrowska-Safaryan, A.; Nicewicz, Ł. The occurrence of entomopathogenic fungi in soils from fields cultivated in a conventional and organic system. J. Ecol. Eng. 2014, 15, 137–144. [Google Scholar]
- Goble, T.A.; Dames, J.F.; Hill, M.P.; Moore, S.D. The effects of farming system, habitat type and bait type on the isolation of entomopathogenic fungi from citrus soils in the Eastern Cape province, South Africa. BioControl 2010, 55, 399–412. [Google Scholar] [CrossRef]
- Meyling, N.V.; Thorup-Kristensen, K.; Eilenberg, J. Below-and aboveground abundance and distribution of fungal entomopathogens in experimental conventional and organic cropping systems. Biol. Control 2011, 59, 180–186. [Google Scholar] [CrossRef]
- Oliveira, I.; Pereira, J.A.; Quesada-Moraga, E.; Lino-Neto, T.; Bento, A.; Baptista, P. Effect of soil tillage on natural occurrence of fungal entomopathogens associated to Prays oleae Bern. Sci. Hortic. 2013, 159, 190–196. [Google Scholar] [CrossRef]
- Arnold, A.E.; Herre, E.A. Canopy cover and leaf age affect colonization by tropical fungal endophytes: Ecological pattern and process in Theobroma cacao (Malvaceae). Mycologia 2003, 95, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Bon, H.; Huat, J.; Parrot, L.; Sinzogan, A.; Martin, T.; Malézieux, E.; Vayssières, J. Pesticide risks from fruit and vegetable pest management by small farmers in sub-Saharan Africa. A review. Agron. Sustain. Dev. 2014, 34, 723–736. [Google Scholar] [CrossRef]
- De Snoo, G.R. Unsprayed margins: Effects on environment, biodiversity and agricultural practice. Landsc. Urban Plan. 1999, 46, 151–160. [Google Scholar] [CrossRef]
- Geiger, F.; Bengtsson, J.; Berendse, F.; Weisser, W.W.; Emmerson, M.; Morales, M.B.; Inchausti, P. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Meyling, N.V.; Pilz, C.; Keller, S.; Widmer, F.; Enkerli, J. Diversity of Beauveria spp. isolates from pollen beetles Meligethes aeneus in Switzerland. J. Invertebr. Pathol. 2012, 109, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Meyling, N.V.; Lübeck, M.; Buckley, E.P.; Eilenberg, J.; Rehner, S.A. Community composition, host range and genetic structure of the fungal entomopathogen Beauveria in adjoining agricultural and seminatural habitats. Mol. Ecol. 2009, 18, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- López-González, R.C.; Gómez-Cornelio, S.; de la Rosa-García, S.C.; Garrido, E.; Oropeza- Mariano, O.; Heil, M.; Partida-Martínez, L.P. The age of lima bean leaves influences the richness and diversity of the endophytic fungal community, but not the antagonistic effect of endophytes against Colletotrichum lindemuthianum. Fungal Ecol. 2017, 26, 1–10. [Google Scholar] [CrossRef]
- Sanchez-Azofeifa, A.; Oki, Y.; Fernandes, G.W.; Ball, R.A.; Gamon, J. Relationships between endophyte diversity and leaf optical properties. Trees 2012, 26, 291–299. [Google Scholar] [CrossRef]
- Lin, Y.; Qasim, M.; Hussain, M.; Akutse, K.S.; Avery, P.B.; Dash, C.K.; Wang, L. The herbivore-induced plant volatiles methyl salicylate and menthol positively affect growth and pathogenicity of entomopathogenic fungi. Sci. Rep. 2017, 7, 40494. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Hussain, M.; Avery, P.B.; Qasim, M.; Fang, D.; Wang, L. Volatiles from plants induced by multiple aphid attacks promote conidial performance of Lecanicillium lecanii. PLoS ONE 2016, 11, e0151844. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, G.; Ownley, B.H.; Augé, R.M.; Toler, H.; Dee, M.; Vu, A.; Köllner, T.G.; Chen, F. Colonization by arbuscular mycorrhizal and endophytic fungi enhanced terpene production in tomato plants and their defense against a herbivorous insect. Symbiosis 2015, 65, 65–74. [Google Scholar] [CrossRef]
- Thompson, S.R.; Brandenburg, R.L. Tunneling responses of mole crickets (Orthoptera: Gryllotalpidae) to the entomopathogenic fungus, Beauveria bassiana. Environ. Entomol. 2005, 34, 140–147. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).