Abstract
Three sizes of copper nanoparticles (1.7 nm, 2.8 nm, 3.4 nm) were synthesized using N902 as a surface-modifying ligand and diesel as the solvent. These nanoparticles were incorporated into biodiesel at volume fractions ranging from 0.005% to 0.20%, and their impacts on the lubrication performance, combustion characteristics, and thermal behavior of biodiesel were systematically investigated. The results indicated that the addition of copper nanoparticles significantly reduced the friction coefficient and wear scar diameter. Specifically, the 1.7 nm Cu nanoparticle sample achieved the most remarkable friction-reducing and anti-wear effects, with the friction coefficient and wear scar diameter decreasing by 16.07% and 20.1%, respectively. The combustion heat value of biodiesel showed a “first increase and then decrease” trend with the increase in nanoparticle addition, with the most significant improvement observed at an addition level of 0.01%. Among the three particle sizes, the 2.8 nm Cu nanoparticle sample effectively promoted the pyrolysis of biodiesel, while the 1.7 nm Cu nanoparticle sample exhibited optimal performance in reducing the oxidation induction time (OIT) and achieving complete combustion—characterized by lower CO emissions and minimal O2 residue after combustion. Overall, the incorporation of copper nanoparticles realizes a synergistic enhancement, where lubricity improvement and combustion promotion occur concurrently, reflected by reduced OIT, lower CO emissions, and lower O2 residue.
1. Introduction
With the continuous increase in vehicle ownership, the combustion of traditional fossil fuels results in the release of large amounts of CO2 into the atmosphere, leading to serious environmental problems [1]. The development of renewable and clean alternative fuels has attracted increasing attention in the fields of energy and environmental research. Biodiesel is produced from renewable resources such as vegetable oils, animal fats, and waste oils and is generally regarded as a renewable fuel with carbon-neutral characteristics [2]. Carbon neutrality means that the CO2 emitted during biodiesel combustion is largely offset by the CO2 absorbed during plant growth [3]. Therefore, large-scale promotion of biodiesel can help mitigate the greenhouse effect and support China’s strategic goals of carbon peaking and carbon neutrality. In addition, biodiesel can be directly used in existing diesel engines without hardware modification, indicating good compatibility [4].
However, biodiesel still exhibits certain inherent limitations. Its relatively high viscosity compared with conventional diesel [5] leads to inefficient fuel atomization and incomplete combustion, which may adversely affect lubrication conditions and aggravate wear under practical operating conditions. Consequently, these issues restrict the widespread application of biodiesel in diesel engines [6]. Therefore, improving the combustion efficiency and lubrication performance of biodiesel remains an important challenge in this field.
As a novel lubricating additive, nanoparticles have been widely reported to enhance lubrication performance through various tribological mechanisms, including rolling effects, surface mending, and the formation of protective tribofilms at friction interfaces [7]. Meanwhile, numerous studies have demonstrated that the addition of nanoparticles to fuel oils can also improve combustion performance. This improvement is mainly attributed to enhanced fuel atomization induced by micro-explosion phenomena, as well as the large specific surface area and high surface energy of nanoparticles, which facilitate fuel volatilization and combustion [8].
Previous studies have demonstrated that the incorporation of nanoparticles into diesel–biodiesel blended fuels can simultaneously enhance lubrication and combustion performance. For example, TiO2 nanoparticles were reported to form lubricating nanofilms on metal surfaces, resulting in significant reductions in wear and friction coefficient [9]. Similarly, the addition of CuO-based nanoparticles was shown to improve brake thermal efficiency, reduce fuel consumption, and increase exhaust gas temperature, indicating enhanced combustion efficiency [10,11].
Among various nano additives, copper-based nanoparticles have attracted increasing attention due to their low cost, good electrical and thermal conductivity, high ductility, and excellent anti-wear properties [12]. Studies have shown that Cu nanoparticles can form low-hardness, low-modulus protective films on worn surfaces, thereby providing effective lubrication [13,14]. In addition, copper-containing nanoparticles have also been reported to improve engine combustion characteristics, reduce fuel consumption, and promote the oxidative decomposition of particulate matter, contributing to emission mitigation [8,15]. Compared with copper oxides, metallic copper nanoparticles exhibit superior potential in both combustion promotion and tribological performance. Their redox activity and electron transfer capability contribute to enhanced fuel oxidation during combustion, while their low hardness and high plasticity favor the formation of a low-shear protective film at friction interfaces, resulting in reduced friction and wear. In contrast, copper oxides, owing to their higher hardness, may act as third-body particles and increase the risk of abrasive wear under certain conditions.
In summary, although biodiesel exhibits considerable potential as a renewable and clean fuel, its insufficient lubrication and combustion performance still restrict its widespread application. Previous studies have demonstrated that nanoparticles, particularly copper-based nanoparticles, can simultaneously enhance combustion efficiency and lubrication performance. However, existing research has primarily focused on metal oxides, with limited attention paid to metallic copper nanoparticles smaller than 5 nm. Moreover, systematic investigations into the effects of copper nanoparticle size on combustion characteristics, tribological behavior, and exhaust emissions remain scarce, and the underlying size-dependent mechanisms are still not fully understood.
Based on this, ultra-small copper nanoparticles (1–4 nm) free of sulfur and phosphorus were synthesized via a one-pot method using N902 as a low-cost surface modifier and diesel as the solvent, and subsequently applied as biodiesel additives. This study systematically investigates the effects of particle size on tribological performance, combustion calorific value, thermal behavior, and exhaust emissions, aiming to provide experimental evidence and mechanistic insight into the application of copper nanoparticles as multifunctional biodiesel additives.
2. Materials and Methods
2.1. Main Reagents
The reagents used in this study included biodiesel (industrial grade, Shandong Tonglan Chemical Co., Ltd., Jinan, China), 0# diesel (industrial grade, China Petrochemical Corporation, Beijing, China), copper(II) sulfate pentahydrate (analytical grade), hydrazine hydrate (80%, analytical grade), ammonia solution (analytical grade), and N902 copper extractant. Copper(II) sulfate pentahydrate, hydrazine hydrate, and ammonia solution were supplied by Tianjin Comeo Chemical Reagent Co., Ltd. (Tianjin, China) and Luoyang Haohua Reagent Co., Ltd. (Luoyang, China), respectively.
N902 is a hydroxamic acid extractant newly developed by the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences (Shanghai, China). It appears as an amber liquid without visible impurities, with a density of 0.95–0.97 g·cm−3, a viscosity below 190 cP, and a flash point higher than 62 °C. All reagents were used as received without further purification, and distilled water was used throughout the experiments.
2.2. Preparation and Characterization of Nano Copper
Copper nanoparticles were synthesized using copper sulfate pentahydrate as the copper source, aqueous ammonia as the complexing agent and pH regulator, hydrazine hydrate as the reducing agent, diesel as the organic phase, and N902 as the surface modifier. Briefly, CuSO4·5H2O was dissolved in distilled water, and aqueous ammonia was added to form an alkaline copper–ammonia complex. Hydrazine hydrate was then introduced to initiate the reduction, leading to the nucleation of Cu nanoparticles in the aqueous phase.
Cu nanoparticles were prepared via a time-dependent chemical reduction process. First, an ammine–copper complex was formed by mixing an aqueous CuSO4 solution with ammonia under alkaline conditions. Hydrazine hydrate was then added as the reducing agent, leading to the rapid formation of Cu nanocrystal nuclei in the aqueous phase. In this stage, nuclei formed first and subsequently grew as the reaction proceeded; therefore, the reduction time played a decisive role in determining the final particle size.
In this work, the Cu nanoparticle size was tuned by precisely controlling the reduction time. Specifically, the reaction duration was recorded using a timer, and at the predetermined time point, an N902 modifier pre-dispersed in diesel was immediately introduced. The added N902 rapidly coated the Cu nanoparticles and extracted them from the aqueous phase into the diesel phase, thereby effectively terminating further particle growth and enabling controllable size regulation.
In the present synthesis system, the particle size of Cu nanoparticles is mainly governed by the reduction reaction time and the subsequent surface modification process. Experimental results show that when the particle size increases to approximately 3.4 nm, the N902 modifier can still effectively encapsulate the Cu nanoparticles and enable their stable transfer into the diesel phase. However, further prolongation of the reaction time leads to excessive growth of Cu nuclei, which exceeds the effective encapsulation capability of N902, making it difficult to obtain stably dispersed nanoparticles. Therefore, a particle size of approximately 3.4 nm can be regarded as the upper size limit achievable by this method while maintaining stable dispersion.
At a reduction time of 1 min, Cu nanoparticles with an average size of 1.7 nm were reproducibly obtained; however, further shortening of the reaction time led to poorer run-to-run reproducibility due to the limited time resolution. Based on the accessible and controllable size window, three representative sizes (1.7, 2.8, and 3.4 nm) were selected to systematically evaluate the size-dependent effects of Cu nanoparticles on fuel performance.
The as-prepared Cu nanoparticle dispersion was subsequently added to biodiesel at different volume fractions and ultrasonically treated for 30 min to ensure uniform dispersion prior to use.
The morphology, size distribution, and dispersion uniformity of the samples were examined using transmission electron microscopy (TEM, JEM-2010, JEOL, Tokyo, Japan). High-resolution transmission electron microscopy (HRTEM, JEM-2010, JEOL, Tokyo, Japan) was further employed to observe the detailed microstructure and accurately determine the particle size. The crystal structure was analyzed by X-ray diffraction (XRD, D8 Advance, Bruker, Karlsruhe, Germany), and the chemical structure was characterized using Fourier transform infrared spectroscopy (FT-IR, VERTEX 70, Bruker, Karlsruhe, Germany).
2.3. Tribological Performance Testing and Wear Scar Characterization
The lubricating performance of the fuel samples was evaluated using a four-ball friction tester (MS-10A, Xiamen Tianji Automation Co., Ltd., Xiamen, China). Although high-frequency reciprocating rigs are commonly used for fuel lubricity evaluation in standard methods, their low load and high frequency may limit their applicability under certain conditions. Therefore, alternative tribological test configurations have also been explored in previous studies [16,17,18]. The four-ball friction test provides good reproducibility and stable test conditions, making it suitable for comparative evaluation of fuel lubricating performance. In this study, the test conditions were set as follows: a load of 39.2 N, a rotational speed of 600 r·min−1, a test duration of 40 min, and a temperature of 25 °C.
During the test, the temperature was continuously monitored by the built-in sensor and regulated by the integrated heating and cooling modules, maintaining the test temperature at 25 °C to ensure repeatable conditions and reliable results.
After the friction tests, the steel balls were cleaned with petroleum ether and dried using an air blower. The wear scars on the steel balls were measured using a three-dimensional optical profilometer (3D OP, Contour GT-I30, Bruker, Karlsruhe, Germany). The morphology of the wear surfaces and the distribution of typical surface elements were further analyzed using a scanning electron microscope (SEM, JSM-5600, JEOL, Tokyo, Japan) equipped with an energy-dispersive spectroscopy (EDS) system (X-Max N, Oxford Instruments, Oxford, UK).
2.4. Calorific Value Test
The calorific value of the fuel under oxygen-deficient conditions was tested using an automatic calorimeter (9000B, Shanghai Laihu Metal Products Co., Ltd., Shanghai, China). Literature reports indicate that the calorific value of fuels under oxygen deficiency can be determined by adjusting the oxygen charging pressure [19]. At an altitude of 3000 m, the calculated oxygen charging pressure is approximately 0.60 MPa. This study was conducted at an altitude of about 70 m, with the corresponding oxygen charging pressure of the bomb calorimeter being 0.89 MPa. The calorific values of biodiesel blended with nano copper at varying concentrations and particle sizes were measured in the experiments.
2.5. Thermal Analysis (TGA/DSC) Testing
2.5.1. Thermogravimetric Analysis (TG/DTG)
To investigate the effect of nano-copper on the thermal decomposition behavior of biodiesel, tests were conducted under a nitrogen atmosphere using a thermogravimetric analyzer (TGA/DSC, TGA/DSC3+, METTLER TOLEDO, Zurich, Switzerland). Considering that an excessively high heating rate would cause the fuel to enter a higher temperature range before sufficient volatilization or decomposition, thermal lag in mass loss and pyrolysis, a heating rate of 10 °C/min was adopted in this study to ensure the accuracy and repeatability of the tests. The activation energy of the fuel at different pyrolysis conversion degree was calculated by the Starink method, as reported in reference [20].
where α is the conversion degree, β is the heating rate, and E is the activation energy. B, C, and D are constants, and the results are most accurate when B = 1.92 and D = 1.0008.
2.5.2. Programmed Heating Differential Scanning Calorimetry (PDSC)
The thermal oxidation performance of the fuel was characterized using a high-pressure differential scanning calorimeter (HP-DSC, DSC204 HP, NETZSCH, Selb, Germany) [21]. The experimental conditions were as follows: oxygen atmosphere, pressure of 2 bar, flow rate of 50 mL/min, and heating rate of 10 °C/min. The initial oxidation temperature (OOT) of the fuel was determined from the heating curve. The oxidation induction time (OIT) was obtained via isothermal testing at 70 °C and 25 bar.
2.6. Exhaust Gas Composition Analysis
Exhaust gas was collected in gas sampling bags and passed through a drying tube packed with silica gel and molecular sieves at an inlet flow rate of approximately 5 mL/min to remove water vapor. The product composition was quantitatively analyzed using a gas chromatograph (GC, 7890B, Agilent Technologies, Inc., Santa Clara, CA, USA) equipped with a flame ionization detector (FID) and a thermal conductivity detector (TCD). A Shincarbon column was used with argon (Ar) as the carrier gas, and the column temperature was maintained at 90 °C. The relative peak areas of N2, O2, and CO were detected and compared with those of neat biodiesel.
3. Results and Discussion
3.1. Structural Characterization of Nano-Copper Particles
Figure 1 presents the TEM images of N902-modified Cu nanoparticles. Owing to the ultra-small particle size and low contrast of metallic Cu nanoparticles, individual particles could only be observed under under-focused imaging conditions. In this case, direct statistical analysis of particle size from conventional TEM images is not reliable, as the apparent particle size tends to be overestimated under under-focused conditions.
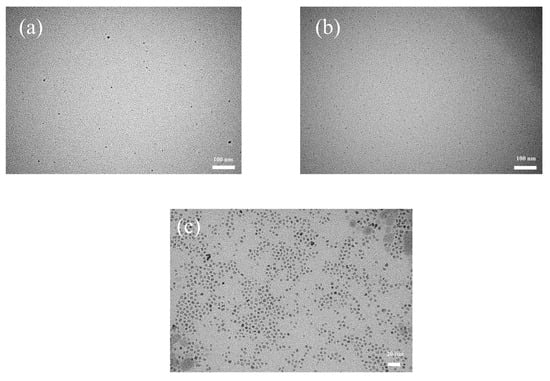
Figure 1.
Transmission electron microscopy (TEM) images of Cu nanoparticles with different diameters: (a) 1.7 nm, (b) 2.8 nm, (c) 3.4 nm.
Nevertheless, the TEM images clearly indicate that the Cu nanoparticles are uniformly distributed on the TEM grid without obvious agglomeration, demonstrating good dispersion and size uniformity, which provides a prerequisite for subsequent size analysis. On this basis, high-resolution TEM (HRTEM) was further employed to precisely measure the particle size of individual Cu nanocrystals with clearly resolved lattice fringes in selected local regions.
Uncertainty in the particle-size measurement mainly originates from two factors. First, the field of view in HRTEM is limited, resulting in a relatively small number of particles available for statistics. Second, TEM specimen preparation (dilution, ultrasonic dispersion, and drop-casting onto Cu grids) may lead to local variations in particle coverage, introducing additional variability. Considering the good dispersion and narrow size distribution observed in conventional TEM, the HRTEM-measured crystallite sizes from well-resolved individual particles are taken as representative for the samples.
Figure 2 shows the HRTEM images of nano-copper modified with N902. It can be seen that three types of copper nanoparticles with different particle sizes were synthesized. Among them, the copper nanoparticles with a particle size of approximately 3.4 nm are the most uniformly dispersed and exhibit the best nucleation performance. The introduction of N902 effectively inhibits the excessive growth of copper nanoparticles, resulting in a more concentrated particle size distribution. Meanwhile, the other end of the N902 molecule contains a long alkyl chain with good lipophilicity; the organic modification layer formed on the copper surface can reduce the particle surface energy and inhibit agglomeration, thereby improving the dispersion stability of Cu in biodiesel.
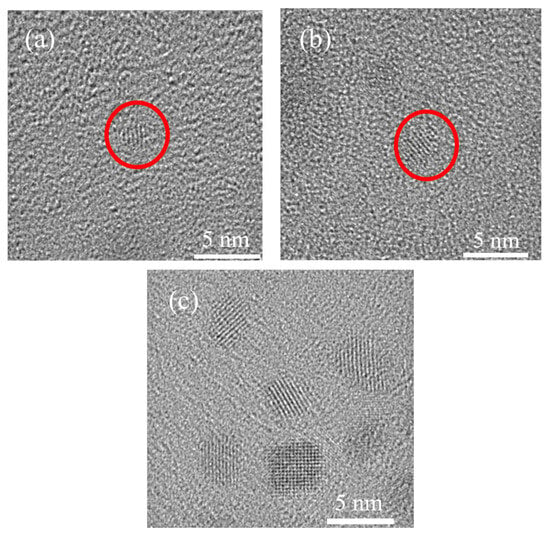
Figure 2.
High-resolution transmission electron microscopy (HRTEM) images of Cu nanoparticles with different diameters: (a) 1.7 nm, (b) 2.8 nm, (c) 3.4 nm. The red circles indicate individual nanocrystals with visible lattice fringes.
Figure 3 shows the XRD patterns of copper nanoparticles. It can be observed that within the measured range, the samples exhibit obvious broadening of diffraction peaks with a bulging band-like shape, indicating that the prepared copper nanoparticles have a small particle size and exhibit low crystallinity.
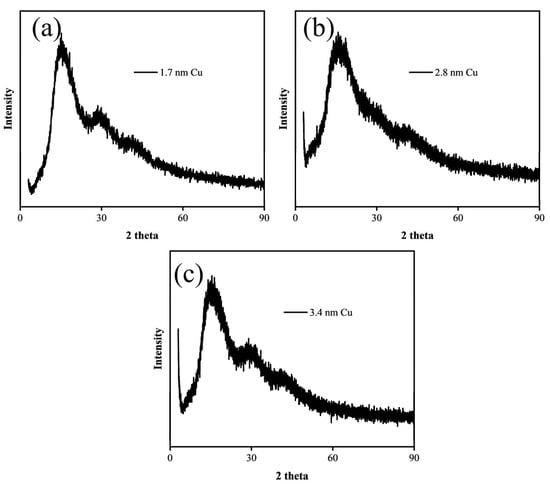
Figure 3.
X-ray diffraction (XRD) patterns of Cu nanoparticles with different diameters: (a) 1.7 nm, (b) 2.8 nm, (c) 3.4 nm.
Figure 4 shows the FTIR spectra of pure N902 and N 902-modified copper nanoparticles. It can be observed that the closely spaced double peaks of the carbonyl group (C=O) in the range of 1750–1700 cm−1 merged into a single peak after modification (from 1716.6 cm−1 and its shoulder peak to 1728.1 cm−1). Meanwhile, the C=N absorption peak shifted from 1624 cm−1 to 1620 cm−1, the N-O absorption peak shifted from 995 cm−1 to 955 cm−1, the C-O absorption peak shifted from 1265 cm−1 to 1283 cm−1, and the broad O-H band was significantly weakened. The FTIR results indicate that N902 molecules form coordination bonds with the copper surface through the N and O atoms of the oxime group and the O atom of the phenolic hydroxyl group, suggesting effective surface coordination and anchoring.
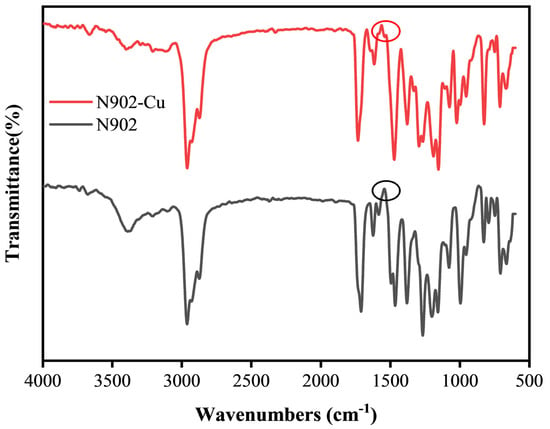
Figure 4.
Fourier transform infrared (FTIR) spectra of Cu nanoparticles and N902. The circles indicate the stretching vibration peaks of the carbonyl group (C=O), illustrating the peak shift and merger due to surface coordination.
Figure 5 shows the optical photographs of copper nanoparticles with three different particle sizes dispersed in biodiesel at an addition level of 0.2%. After standing for 15 days, the system still maintained good uniformity, which further confirms that N902 modification significantly improved the dispersion stability of nano-copper in biodiesel.
Figure 5.
Optical photographs of the mixed fuel: (a) after 20 min of ultrasonication, (b) after 15 days of standing.
3.2. Tribological Properties
Tribological performance of biodiesel containing copper nanoparticles with different particle sizes was evaluated via four-ball friction tester (Xiamen Tianji Automation Co., Ltd., MS-10A, Xiamen, China), with results presented in Figure 6 and Figure 7. Compared to neat biodiesel, the addition of nano-copper reduced both the friction coefficient and wear scar diameter, with a clear particle-size dependence:
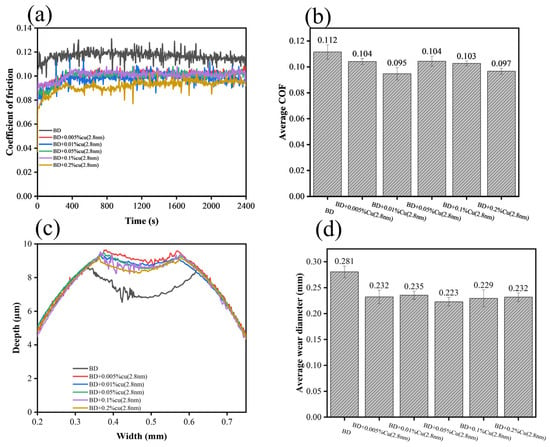
Figure 6.
Tribological performance of 2.8 nm Cu at different concentrations: (a) friction coefficient curves, (b) average friction coefficient, (c) 2D wear profiles, (d) average wear scar diameter.
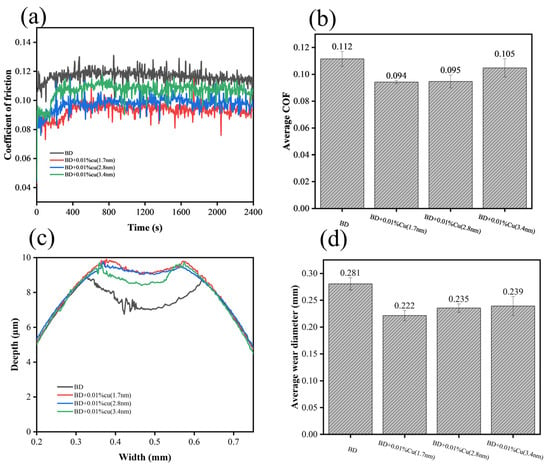
Figure 7.
Tribological performance of Cu nanoparticles with three particle sizes at their optimal combustion-promoting concentrations: (a) friction coefficient curves, (b) average friction coefficient, (c) 2D wear profiles, (d) average wear scar diameter.
The 1.7 nm sample exhibited the optimal performance, with a 16.07% reduction in friction coefficient and a 20.1% decrease in wear scar diameter;
The 2.8 nm sample followed closely, showing a 15.17% reduction in friction coefficient and a 16.4% decrease in wear scar diameter;
The 3.4 nm sample had limited improvement, with a friction reduction of less than 7% and an anti-wear improvement of less than 15%.
Notably, the friction coefficient showed little variation within the addition concentration range of 0.005–0.20%, indicating that the tribological performance was relatively insensitive to concentration under the present test conditions. SEM images and 3D surface morphology maps of wear scars and energy-dispersive spectroscopy (EDS) results are displayed in Figure 8 and Figure 9, respectively. In Figure 8, the parallel grooves on the worn surface indicate abrasive wear, whereas the circled regions correspond to localized adhesive wear accompanied by spalling (delamination). Observations revealed that the wear scars became smoother with fewer grooves after adding nano-copper. However, no typical continuous tribofilm was observed in the SEM images, and EDS analysis showed no obvious Cu enrichment on the wear surface.
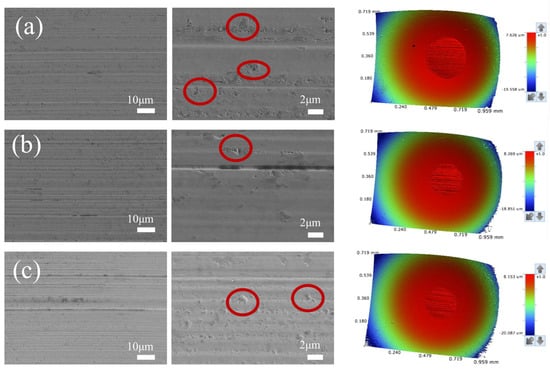
Figure 8.
Scanning electron microscopy (SEM) and three-dimensional (3D) surface morphology images of the wear scars for the 2.8 nm Cu sample at different concentrations: (a) biodiesel, (b) 0.01% Cu, (c) 0.2% Cu.
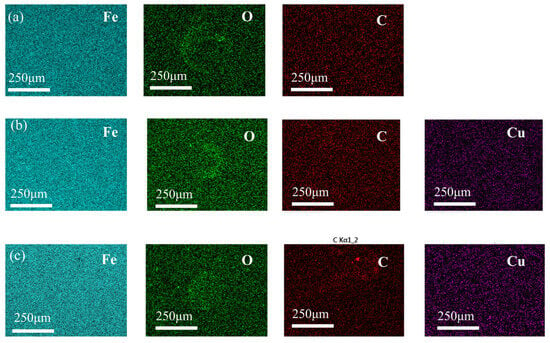
Figure 9.
Energy-dispersive X-ray spectroscopy (EDS) images of 2.8 nm Cu at different concentrations: (a) biodiesel, (b) 0.01% Cu, (c) 0.2% Cu. The elemental maps are color-coded as follows: Iron (Fe) in cyan, Oxygen (O) in green, Carbon (C) in red, and Copper (Cu) in purple.
These results suggest that the friction-reducing mechanism of nano-copper differs from that of traditional sulfur- or phosphorus-containing extreme pressure anti-wear additives. Its main action mechanisms may include: (1) Third-body effect: Nanoparticles enter the friction interface and act as “rolling or filling media,” alleviating direct contact between rough peaks; (2) Nano-polishing effect: Under shear force, the particles may repair grooves and pits, rendering the surface smoother; (3) Wettability improvement: The surface-modified groups of nano-copper are expected to enhance the wettability of biodiesel on metal surfaces by increasing interfacial affinity, thereby contributing to improved liquid film stability during lubrication.
In summary, the improved lubrication performance of nano-copper is attributed to the synergistic effect of physical mechanisms and possible wettability enhancement, rather than the protective effect of a chemical film.
3.3. Combustion Calorific Value and Thermal Analysis
3.3.1. Combustion Calorific Value
The calorific values measured under oxygen-deficient conditions are presented in Figure 10. Copper nanoparticles of three distinct particle sizes increased the measured calorific value of biodiesel, with the calorific values first increasing and then decreasing as the addition concentration rose. At an addition level of 0.01%, the calorific value improvement was most pronounced: the 1.7 nm sample showed the largest increment, indicating it was most effective in promoting complete combustion, followed by the 2.8 nm sample; the 3.4 nm sample exhibited a relatively weaker promotional effect. As the addition concentration continued to increase, the calorific value declined instead. This trend suggests that the enhancement effect of nano-Cu on combustion is not linear with dosage and that an optimal concentration exists. At higher loadings, the effective contribution of nano-Cu to the combustion process may reach a saturation regime, in which additional nanoparticles no longer provide further enhancement. The detailed mechanisms responsible for this behavior are likely related to the complex and dynamic nature of the combustion process and require further investigation.
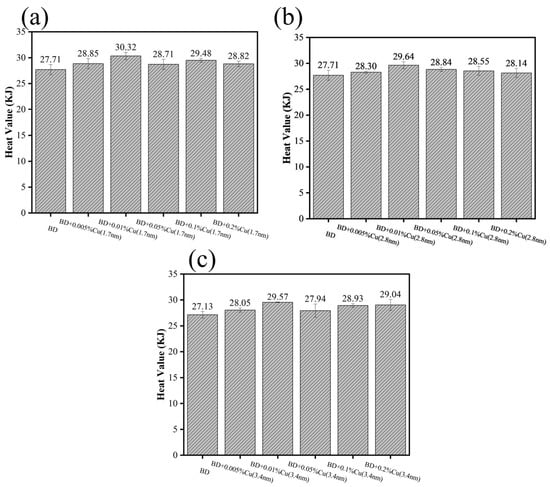
Figure 10.
Combustion enhancement performance of Cu nanoparticles: (a) 1.7 nm, (b) 2.8 nm, (c) 3.4 nm.
3.3.2. Results of Thermogravimetric Analysis (TG/DTG)
Analytical results of thermogravimetric (TG) curves, derivative thermogravimetric (DTG) curves, and activation energy-conversion degree(E-α) curves are presented in Figure 11. Under a nitrogen atmosphere, the 2.8 nm copper nanoparticle sample exhibited a significant reduction in activation energy, demonstrating a promoting pyrolysis activation effect. In contrast, the thermal decomposition temperatures of the 1.7 nm and 3.4 nm samples were delayed. Within the main pyrolysis range (conversion rate α ≈ 0.1–0.9), calculations via the Starink method showed that only the 2.8 nm sample had an activation energy lower than that of neat biodiesel, while the activation energies of the 1.7 nm and 3.4 nm samples were comparable to or even higher than the neat counterpart. This indicates that under an inert atmosphere, the 2.8 nm nano-copper facilitated the main pyrolysis process of biodiesel, whereas the 1.7 nm and 3.4 nm nano-copper exerted a slight inhibitory effect on pyrolysis.
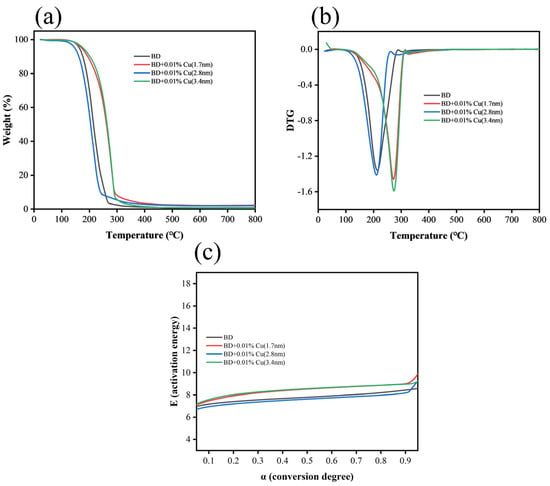
Figure 11.
Thermogravimetric analysis (TGA) results of biodiesel and Cu nanoparticle-doped mixed fuels (1.7 nm, 2.8 nm, 3.4 nm): (a) Thermogravimetry (TG) curves, (b) Derivative thermogravimetry (DTG) curves, (c) E-α kinetic curves.
3.3.3. Results of Programmed Heating Differential Scanning Calorimetry (PDSC)
Figure 12 presents the PDSC results under an oxygen atmosphere. The 2.8 nm sample exhibited the lowest initial oxidation temperature (OOT), and its oxidation induction time (OIT) shortened markedly, indicating accelerated oxidation initiation. Although the 1.7 nm sample showed a slightly higher OOT than the 2.8 nm counterpart, its OIT shortened even further, suggesting a faster overall oxidation response under the isothermal condition. In contrast, the 3.4 nm sample showed a delayed OOT and a weaker effect on oxidation behavior, indicating a limited influence on the oxidative reactivity of biodiesel under the present conditions.
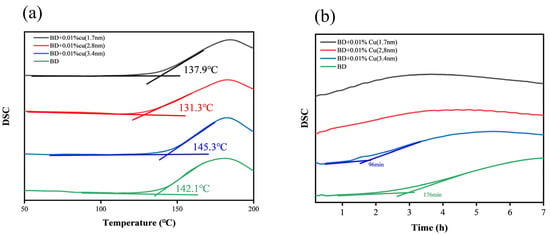
Figure 12.
Pressure differential scanning calorimetry (PDSC) results of biodiesel and mixed fuel: (a) Onset oxidation temperature (OOT) curves, (b) Oxidation induction time (OIT) curves.
Notably, as shown in Figure 12b, the OIT could be clearly determined for neat biodiesel and for biodiesel containing 3.4 nm Cu nanoparticles. In contrast, for biodiesel containing 1.7 nm or 2.8 nm Cu nanoparticles, the oxidation proceeded too rapidly under the isothermal condition, and a distinct induction period could not be reliably resolved. Nevertheless, the time corresponding to the maximum of the exothermic peak on the DSC curve (time-to-peak) can still be used as a comparative indicator of oxidation reactivity, showing that the 1.7 nm sample reached the peak earlier than the 2.8 nm sample, i.e., it promoted oxidation more strongly.
3.4. Results of Exhaust Gas Composition Analysis
The results of gas composition analysis are presented in Figure 13. Compared with neat biodiesel:
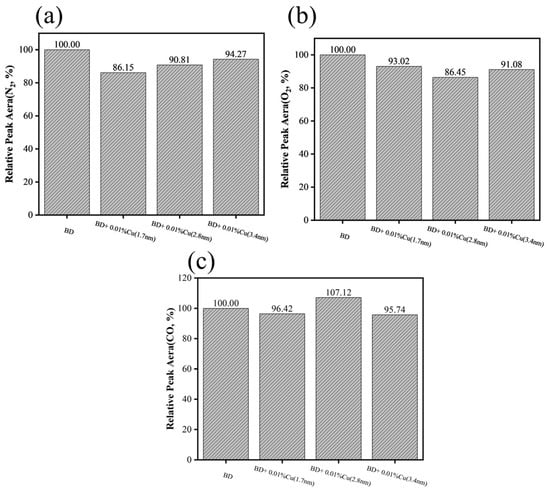
Figure 13.
Exhaust gas composition analysis of biodiesel and mixed fuel: (a) N2, (b) O2, (c) CO.
For the 1.7 nm sample: CO content decreased by 3.58%, residual O2 content decreased by 13.55%, and the relative peak area of N2 decreased by 9.19%;
For the 2.8 nm sample: residual O2 content was the lowest, but CO content increased by 7.12%, suggesting less complete oxidation during the late stage of combustion;
For the 3.4 nm sample: no significant changes were observed in these three components.
These results suggest that the 1.7 nm sample exhibited the best overall combustion performance, whereas the 2.8 nm sample showed stronger early-stage activation but a tendency toward incomplete late-stage oxidation. The particle size of copper nanoparticles not only affects lubrication performance but also influences the combustion process by altering the activation energy and pyrolysis behavior as reflected by the TG/PDSC analyses.
4. Conclusions
- (1)
- The introduction of Cu nanoparticles significantly improved the tribological performance of biodiesel. The 1.7 nm sample showed the greatest reduction in friction coefficient and wear scar diameter, followed by the 2.8 nm sample, while the 3.4 nm sample exhibited relatively weaker effects. Within the additive concentration range of 0.005–0.20%, the tribological performance was relatively insensitive to concentration but highly sensitive to particle size.
- (2)
- The calorific value measured under oxygen-deficient conditions showed a “first increase, then decrease” trend with increasing Cu-nanoparticle concentration, with the most pronounced improvement at 0.01%. The 1.7 nm sample produced the largest increase in calorific value, followed by the 2.8 nm sample, whereas the 3.4 nm sample showed a limited effect.
- (3)
- Thermal analysis indicated that under a nitrogen atmosphere, the 2.8 nm sample promoted biodiesel pyrolysis by reducing the activation energy and advancing the initial thermal decomposition behavior, while the decomposition temperatures of the 1.7 nm and 3.4 nm samples were delayed. Under an oxygen atmosphere, the 2.8 nm sample exhibited the lowest OOT, whereas the 1.7 nm sample showed the most pronounced shortening of OIT, and the 3.4 nm sample demonstrated the weakest effect.
- (4)
- Exhaust gas composition analysis suggested that the 1.7 nm sample exhibited the best overall oxidation performance, with reduced residual O2 and decreased CO content. The 2.8 nm sample consumed the most oxygen but generated more CO, while the 3.4 nm sample showed no significant changes.
- (5)
- Based on the comprehensive results of tribology, calorific value, thermal analysis, and exhaust gas composition, Cu nanoparticles exhibited a distinct particle-size effect in the biodiesel fuel system: the 2.8 nm sample mainly promoted activation in the initial stage, the 1.7 nm sample provided the most balanced improvement in frictional properties and overall combustion performance, while the 3.4 nm sample showed relatively limited effects. These findings provide experimental evidence for the optimized design and engineering application of low-cost, multifunctional Cu-nanoparticle biodiesel additives. However, their performance under actual engine operating conditions still requires further validation.
Author Contributions
H.S. and S.L., conceptualization, methodology, and writing—original draft preparation; S.F. and C.Z., investigation and data curation; G.Y., writing—review and editing; Y.Z. and S.Z., resources, writing—review and editing, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge the financial support provided by the National Key Research Development Plan (grant 2023YFB3812104).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (S.Z.) upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Oyekunle, D.T.; Gendy, E.A.; Barasa, M.; Oyekunle, D.O.; Oni, B.; Tiong, S.K. Review on utilization of rubber seed oil for biodiesel production: Oil extraction, biodiesel conversion, merits, and challenges. Clean. Eng. Technol. 2024, 21, 100773. [Google Scholar] [CrossRef]
- Devarajan, Y.; Munuswamy, D.B.; Mahalingam, A. Investigation on behavior of diesel engine performance, emission, and combustion characteristics using nano-additive in neat biodiesel. Heat Mass Transf. 2019, 55, 1641–1650. [Google Scholar] [CrossRef]
- Suresh, M.; Jawahar, C.P.; Richard, A. A review on biodiesel production, combustion, performance, and emission characteristics of non-edible oils in variable compression ratio diesel engine using biodiesel and its blends. Renew. Sustain. Energy Rev. 2018, 92, 38–49. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Das, L.M. Biodiesel Development and Characterization for Use as a Fuel in Compression Ignition Engines. J. Eng. Gas Turbines Power 2001, 123, 440–447. [Google Scholar] [CrossRef]
- Tuan Hoang, A.; Xuan Le, M.; Nižetić, S.; Huang, Z.; Ağbulut, Ü.; Veza, I.; Said, Z.; Le, A.T.; Tran, V.D.; Nguyen, X.P. Understanding behaviors of compression ignition engine running on metal nanoparticle additives-included fuels: A control comparison between biodiesel and diesel fuel. Fuel 2022, 326, 124981. [Google Scholar] [CrossRef]
- Hoang, A.T. Prediction of the density and viscosity of biodiesel and the influence of biodiesel properties on a diesel engine fuel supply system. J. Mar. Eng. Technol. 2021, 20, 299–311. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Han, Z.; Sinyukov, A.; Clearfield, A.; Liang, H. Amphiphilic Zirconium Phosphate Nanoparticles as Tribo-Catalytic Additives of Multi-Performance Lubricants. J. Tribol. 2022, 144, 071901. [Google Scholar] [CrossRef]
- Gavhane, R.S.; Kate, A.M.; Pawar, A.; Soudagar, M.E.M.; Fayaz, H. Effect of Soybean biodiesel and Copper coated Zinc oxide Nanoparticles on Enhancement of Diesel Engine Characteristics. Energy Sources Part A Recovery Util. Environ. Eff. 2025, 47, 3547–3565. [Google Scholar]
- Mujtaba, M.A.; Muk Cho, H.; Masjuki, H.H.; Kalam, M.A.; Farooq, M.; Soudagar, M.E.M.; Gul, M.; Ahmed, W.; Afzal, A.; Bashir, S.; et al. Effect of alcoholic and nano-particles additives on tribological properties of diesel–palm–sesame–biodiesel blends. Energy Rep. 2021, 7, 1162–1171. [Google Scholar] [CrossRef]
- Kalaimurugan, K.; Karthikeyan, S.; Periyasamy, M.; Mahendran, G.; Dharmaprabhakaran, T. Experimental studies on the influence of copper oxide nanoparticle on biodiesel-diesel fuel blend in CI engine. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 8997–9012. [Google Scholar]
- Perumal, V.; Ilangkumaran, M. The influence of copper oxide nano particle added pongamia methyl ester biodiesel on the performance, combustion and emission of a diesel engine. Fuel 2018, 232, 791–802. [Google Scholar] [CrossRef]
- Cao, L.; Luo, B.; Gao, H.; Miao, M.; Wang, T.; Deng, Y. Structure induced wide range wettability: Controlled surface of micro-nano/nano structured copper films for enhanced interface. J. Mater. Sci. Technol. 2021, 84, 147–158. [Google Scholar] [CrossRef]
- Yu, H.-l.; Xu, Y.; Shi, P.-j.; Xu, B.-s.; Wang, X.-l.; Liu, Q. Tribological properties and lubricating mechanisms of Cu nanoparticles in lubricant. Trans. Nonferrous Met. Soc. China 2008, 18, 636–641. [Google Scholar] [CrossRef]
- Singh, N.; Singh, Y.; Sharma, A.; Singla, A. Effect of addition of copper nanoparticles on the tribological behavior of macadamia oil at different sliding speeds. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 2917–2928. [Google Scholar] [CrossRef]
- Guo, J.W.; Wang, Q.; Meng, Z. Exhaust emissions of diesel engines with nano-copper additives. Appl. Nanosci. 2020, 10, 1045–1052. [Google Scholar]
- Asif, T.; Rizwan, T.; Jahangir, S.; Noor, F.; Qamar, A.; Shahbaz, M.A.; Abbas, M.M.; Riaz, F. A Comparative Study of Litchi-chinensis Biodiesel–Diesel Blends With Alcoholic and Nanoparticle Additives on Engine Performance, Emissions, and Coefficient of Friction. Energy Sci. Eng. 2025, 13, 5154–5171. [Google Scholar] [CrossRef]
- Sundus, F.; Fazal, M.A.; Masjuki, H.H. Effect of anti-oxidants on the lubricity of B30 biodiesel–diesel blend. Lubr. Sci. 2017, 29, 3–15. [Google Scholar] [CrossRef]
- Awang, M.S.N.; Zulkifli, N.W.M.; Abbas, M.M.; Zulkifli, S.A.; Kalam, M.A.; Yusoff, M.N.A.M.; Daud, W.M.A.W.; Ahmad, M.H. Effect of diesel-palm biodiesel fuel with plastic pyrolysis oil and waste cooking biodiesel on tribological characteristics of lubricating oil. Alex. Eng. J. 2022, 61, 7221–7231. [Google Scholar] [CrossRef]
- Shiferaw, Y.; Tedla, A.; Mellese, C.; Mengistu, A.; Debay, B.; Selamawi, Y.; Merene, E.; Awol, N. Conversion of coffee residue waste and Eucalyptus globulus leaf extract into an alternative solid fuel. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 780–786. [Google Scholar]
- Starink, M.J. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Dunn, R.O. Effect of antioxidants on the oxidative stability of methyl soyate (biodiesel). Fuel Process. Technol. 2005, 86, 1071–1085. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
