Abstract
Biobased greases are derived from renewable resources, are considered more environmentally friendly, and offer comparable performance to petroleum-based greases. In this study, lubricating greases from frying cooking oils were prepared, thus valorizing waste in order to obtain sustainable and environmentally friendly products. Twelve batches (500 g each) were produced from sunflower and palm frying oils, with 20% by weight calcium/lithium stearate soaps prepared in situ and filled with 15 wt.% cellulose or lignin sulfate. The greases were rheologically characterized. Their consistency was assessed by the penetration test performed before and after working the greases. Dropping point determinations offered information about the stability at higher temperatures, and oil bleeding tests were performed. The average values of the friction coefficient (COF), the contact resistance, and the wear scar diameter were measured through mechanical tests. The greases prove to be comparable to those obtained from mineral oils, with good rheological properties, soft consistency, and good antiwearing behavior, e.g., in open or total-loss lubricating systems, like in open gears and certain food processing machinery; they are thermally stable andprone touse in low-loading working mechanisms.
1. Introduction
For some material goods production, waste cooking oils represent a more environmentally friendly alternative to mineral oils. First, vegetable oils are raw materials for a sustainable economy, unlike mineral oils obtained from crude oil, which is increasingly difficult to find, commanding higher and higher prices. Secondly, vegetable oils and the products obtained from them are more easily biodegradable than mineral oils and have minimal toxicity [1,2], while keeping the good characteristics of mineral lubricant oils [3], so in case of accidental pollution, they are less dangerous than petroleum products. It is even more important to use raw materials from the second generation, such as waste vegetable oils, thus avoiding the allocation of agricultural areas, namely for the cultivation of plants not designated for food.
The need to introduce vegetable oils to replace mineral lubricants (oils and lubricant greases) emerged during the crises caused by the two world wars and the 1973 oil crisis [4]. After a period of unconcern, in the first decade of this century, an increasing interest in replacing mineral oils with bio-lubricants was manifested, especially for those obtained from vegetable oils [5,6,7,8]. The research focused on their rheological properties and increased some of their performances, such as poor oxidation stability, poor performance at low temperatures, or high production costs [6,7]. These disadvantages can be mitigated by chemical modifications, thermal treatment, or additives [9,10].
Like mineral oils, the main function of grease is to provide lubrication to machine components by reducing friction and wear. Lubricating greases are used in not-easy-access mechanisms or when these mechanisms work intermittently and are inactive for a long period of time [4,11]. The soap molecules are dispersed in the oil and arrange themselves in a three-dimensional structure (lattice), entrapping the oil in its voids and preventing the oil’s free flow. So, the grease presents itself as a consistent semi-fluid material.
Classical lubricating greases are produced from mineral oil, thickening soap, and possibly solid additives. Usually, the thickening soap is added to the base oil or prepared in situ from a saturated or unsaturated fatty acid reacting with a lithium, calcium, or natrium base. The preparation from vegetable oils is generally made in the same manner by replacing the mineral oil with the vegetable one.
The manufacturing process is performed either in batches, using open or closed reactors, or in a slow and continuous process;its productivity islimited by the water evaporating rate, which must be removed from the system.
Besides the raw materials, the preparation mode influences decisively the quality of the obtained grease. A traditional mode for the preparation of the grease with natrium soap is describedby Salahuddin and Majed in 2016 [12]: the vegetable oil is mixed from the beginning with all the other materials in a proportion corresponding to the productionof grease with a soap content between 5% and 25 wt.%; the mixture is stirredfor 125 min at 125 °C; this time span is necessary in order to perfect the saponification reaction and to remove the water formed in the reaction; then the product is cooled down slowly (during 48 h). In many cases, the traditional methods use water-free or bound compounds (e.g., monohydrated lithium hydroxide) when starting the saponification reaction, keeping in view that water contributes to a better dispersion of the soap in the oil. However, from a given moment, the reaction can be inhibited by the formation of a soap layer at the surface of larger hydroxide particles.
The use of an aqueous hydroxide solution to obtain first-generation greases had such major shortcomings [13] as a large amount of dispersion to be processed, high mixing energy required, the need to remove large amounts of water at the end of the reaction, and a slow and expensive process.
In order to obtain a polar oil more compatible with the thickener, Grignou et al. [14] considered the epoxidation of a part of the vegetable base oil with hydrogen peroxide in a concentration of 30–70% in an acid medium of formic acid, then obtaining a mixture of 8% epoxidized oil +90% base oil +2% lithium hydroxide, followed by saponification with 12-hydroxystearic acid in stoichiometric proportion to the lithium base. Replacing stearic acid with 12-hydroxystearic acid is beneficial because a soap with hydroxyl groups in the molecule is prepared, thus forming hydrogen bonds between the soap fibers with an effect on the grease consistency. Moreover, after the saponification of the epoxidized oil with 12-hydroxystearic acid, the resulting glycerol does not allow the formation of large soap crystals, and this improves the dropping point from 190 °C to 210 °C compared to the unmodified base oil.
Also, lubricating greases can be produced by the alcoholysis of vegetable oil in alkaline catalysis, followed by saponification [15]. By using the correct proportions of oil, alcohol, and base, the desired grease composition can be obtained.
Instead of soaps, other thickeners can be synthesized in situ, such as polyesters resulting from the condensation of dicarboxylic acids (succinic, sebacic, etc.) with diols (1–2 propanediol, 1–4 butanediol) [16]. Oleogels with polyurea are popular thickeners [17] with better colloidal stability, structural resistance, and antiwear performance; the studies on gel formation from a polyester-based thickener poly(hexane dodecanoate) and three different base oils (a synthetic polyolefin, a mineral oil, and castor oil) show a high stability degree of the gel formed following the interaction of polyester with castor oil [18]. More recently, ionic liquids have been added to the greases produced with lithium and calcium [19,20]. LDH (layered double hydroxide) oleogels intercalated with organic acids, inspired by traditional greases, but preparing the so-called “prepacked salts”can replace grease soaps [21].
Biopolymers are attractive as additives due to their abundance in nature and because of the various functional groups in their molecular chain adhering to the metallic surface and forming protective layers. However, they are considered challenging because of their hydrophilic moieties, which are incompatible with the hydrocarbon chain of the baseoil [3]. However, by selecting the appropriate combinations, it is possible to overcome this disadvantage through the synergistic interactions of ingredients. For example, Sexena and co-workers [3] added Gum Accacia and Guar Gum to a grease prepared with soybean oil and organoclay, and the synergistic effect was reflected in an increase inantiwear characteristics by 22% and frictional response improved by 42% [22].
Cellulose and lignin are abundant natural polymers meeting the requirements of an environmentally friendly additive: available, renewable, nontoxic, and biodegradable. However, native cellulose and lignin tend to agglomerate due to their different polarities in the base oil, so they can be used in small concentrations as additives; moreover, they can be used as the main thickeners after chemical treatment to make them compatible with the oil [23].
Highly crystalline nanocellulose has recently been studied as an additive to pristine lithium greases [24] and has been found to increase consistency and greatly improve the mending and protection of metal surfaces in mechanisms. The nanocellulose is expensive and implies a high processing cost to remove water from the grease (the nanocellulose being delivered and used in an aqueous suspension). Thus, it is worth finding new fabrication methods with inexpensive materials.
In order to obtain micrometer-sized fibers, the lignin can be treated by various spinning methods, such as melt, solution, or electrospinning [25]. These methods not only change the fiber size but also their morphology and mechanical properties.
Cellulose and lignin added to the grease certainly improve its consistency and can also improve mechanical stability and high-temperature performance. Having hydrophilic properties, the fibers can absorb and retain moisture, thus contributing to the resistance of the grease to water washout.
A group of researchers from the Ovidius University of Constanta (Romania) prepared greases from fresh vegetable oils (olive, corn, and palm), thickened with calcium soap, and filled with microcrystalline cellulose and lignin fibers. Based on a literature review and on the encouraging results previously obtained, they decided to continue their research using waste cooking oil, lithium/calcium stearate prepared in situ, and vegetable additives for valorizing waste and enhancing the sustainability of lubricating grease production.
The products previously obtained in small batches of 100 g had several characteristics similar to those of greases obtained from mineral oil [26]. The addition of lignin or cellulose had a beneficial effect on the rheological properties and consistency, especially when the concentration of additives was higher. This study continues the research work in larger batches (500 g), keeping in view the production at a larger scale without affecting the quality of the products.
2. Materials and Methods
2.1. Materials
In order to synthesize the greases, waste oils resulting from the food-frying process were procured from restaurants. These were some of the most common in the region: sunflower oil and palm oil waste.
The deterioration of vegetable oils during the frying process is caused by heat, moisture, and oxygen presence; the oil undergoes thermolytic, hydrolytic, and oxidation reactions, thus modifying the composition of the original material.
The sunflower oil was taken as a whole, since from the waste palm oil, only the oleic part (the liquid upper layer) of the waste was collected for the manufacture of the greases. The oils were prepared in the laboratory by removing gross particulates through a metallic sieve and then filtering through filter paper for finer particle separation. Their quality was constant during the experiment because they procured a large quantity of oils from the beginning of the experiment. The main characteristics of the oils are shown in Table 1.

Table 1.
The main characteristics of the waste vegetable oils used for the preparation of greases.
As shown in the table above, the vegetable oils are comparable in terms of density at 20 °C, while the viscosity of the palm oil at 20 °C is significantly higher than that of the sunflower oil. This observation is also valid for fresh oils. The flash points are still high, even if the oils used underwent slight decomposition during the frying process [32]. The viscosity indices calculated from the viscosity values at 40 °C and 100 °C are very good for un-additivated oils: 202.3 for sunflower oil and 180.8 for palm oil, indicating good lubricating properties of the base oils at high temperatures. This has a great impact because, during the working process, some of the oil “bleeds” and is found in the lower parts of the mechanism, ensuring their good lubrication. The iodine number, which measures the susceptibility to oxidation due to the large number of double bonds, is lower in palm oil, whose molecules have a more saturated nature. During the frying process, the iodine number decreases due to the oxidation of double bonds [33]. FAO and WHO recommend the following standards for the iodine value offresh edible oils: 50–55 g I2/100 g oil in the case of palm oil and 110–143 g I2/100 g for sunflower oil [34]. Our analyses have shown that the iodine value for the sunflower oil decreased during frying; in the case of palm oil, it should be taken into consideration that we used only the oleic part separated in the superior layer in the container, which hasa more unsaturated character than the whole oil, so the iodine value of 60 g I2/100 g is explainable.
The density versus temperature and kinematic viscosity versus temperature curves (Figure 1) were plotted with the same Anton Parr SMV 3000 apparatus. Curves (a) and (c) are for the sunflower oil, and (b) and (d) are for the palm oil, respectively. Both density and viscosity decrease with temperature. These curves show how the properties are kept at acceptable values when the temperature increases. They are also important for the calculations linked to the running of the mechanisms; for calculation purposes, the equations describing the variation in the properties as a function of temperature can be useful.

Figure 1.
Correlations between density versus temperature and kinematic viscosity vs. temperature for base oils. Legend: (a,c) curves for the sunflower oil; (b,d) curves for the palm oil.
For the insitu preparation of soaps, a cheap available fatty acid was used, i.e., stearic acid, even though most recipes use 12-hydroxystearic acid, with the advantage mentioned in the Introduction. The use of solid bases with a minimum concentration of water was consideredin order to perform a rapid process and minimize energy consumption. For the lithium soap preparation, anhydrous lithium hydroxide was used since, for the calcium soap, a paste of slaked lime with 47.7 wt.% water content was the least wet material available. The lithium hydroxide was purchased from Merck, and the slaked lime was supplied by Carpat Var SRL.
In order to have an environmentally friendly solution, additives of vegetal origin were chosen, microcrystalline cellulose and lignin, taking into account that cellulose was reported in other studies as beneficial for the grease performance [35,36,37] and expecting that lignin, a cross-linked type of polymer, be similar, if not better. Microcrystalline cellulose 102 was purchased from DFE PHARMA GMBH&Co., Goch, Germany, with a molecular weight of 160.255 g/mol and the molecular formula (C6H10O5)n/2, where n = 229. Calcium lignosulfonate in powder form with purity >93% was purchased from Carl Roth GmbH+Co. KG—Karlsruhe, Germany. The sulfate form of the lignin was preferred to the Kraft lignin used in previous studies due toits better compatibility with the greases.
2.2. The Preparation Procedure
The base greases were prepared in 500 g batches; then 15 wt.% of filler was added, cellulose or lignin; thus, 4 simple greases were obtained: one with 20 wt.% lithium soap in sunflower oil, one with 20 wt.% calcium soap in sunflower oil, one with 20 wt.% lithium soap in palm oil, and one with 20 wt.% calcium soap in palm oil.The other 8 filled greases were obtained by adding 88.23 g cellulose or lignin in order to obtain 588.23 g grease with a 15 wt.% additive.
The base grease was obtained from 400 g waste oil plus stearic acid and solid base (considered dry matter) in stoichiometric proportion to yield 100 g soap. The procedure was the following:
- -
- The waste frying oil is introduced into an open agitator vessel.
- -
- It is heated up to 65 °C (0.4 °C/min), then stearic acid is added, and the heating continues until it dissolves in the reaction mass.
- -
- At 80 °C, the solution is cleared, and the base is added gradually for 15 min while stirring continuously.
- -
- The heating and stirring continue up to 95–100 °C for the calcium hydroxide or 85 °C for the lithium hydroxide, respectively, maintaining this temperature for the finalization of the saponification reaction, a moment visualized by the change in texture ofthe grease (it becomes consistent and homogenous).
- -
- If applicable, the cellulose/lignin is introduced, and the stirring continues for 30 min; then, the heating turns off, and the grease is allowed to cool down naturally.
2.3. Characterization Methods for Greases
The optical microscopyshowed the morphology of greases in order to compare different greases or to make the difference—in terms of aspect—between “unworked greases” (freshly prepared) and “worked greases” (at the end of the mechanical test). For this purpose, the researchers used a digital Bresser 5201000 microscope (Explore Scientific, Rhede, Germany) with an LCD MICRO screen, 5 megapixels, and a magnifying capacity of ×5, ×125, and ×500.
The rheological tests were performed with the rotative rheometer Anton Parr, MCR 72, with a plate of 50 mm in diameter and a gap of 1 mm between plates. The rheometer measures the variation in the shear of stress () [Pa] versus the shear rate [s−1], the variation in dynamic viscosity (η) [mPa] versus the shear rate ; it also calculates the hysteresis area of plastic fluids. It serves to detail the rheological characterization, and, due to the acquisition of all data in an Excel file, it further allows mathematical modeling.
The cone penetrometer APA-OA, made by Cangzou Oubeiruike Instrument and Equipment Co. Ltd. (Cangzhou City, China), served to determine the consistency of greases in accordance with ASTM D217-02 [38]. The penetration values were determined at 25 °C, both for unworked and worked greases. The greases were “worked” in a mixing vessel by applying 10,000 strokes (200 rotations per minute) for 50 min. This analysis is called “prolonged work penetration”.
The characterization method used in order to determine grease consistency at rising temperatures, specific to running mechanisms, is represented by the measurement of the dropping point. This is a static method that measures the temperature at which the first drop of oil separates; thus, its relevance for grease thermal stability during mechanism operation is limited. However, this is a standardized method for grease stability characterization [39].
The oil separation capacity was tested via the bleeding test on the SKF Ma Pro [40]. A fixed volume of grease is placed on a piece of absorbent paper and kept at 60 °C in the oven for two hours. Part of the base oil is released, creating a greasy stain whose diameter was measured. Measurements were made both for unworked and worked greases. Based on these data, the researchers estimated the change in the oil separation capacity ofthe grease after running the mechanism as follows:
where represent the area of the stain for the unworked (fresh) grease and for the worked (used) grease, respectively; are their corresponding diameters; is the relative difference in the wet areas, offering a measure of change in oil separation capacity before and after working the grease.
2.4. The Mechanical Tests
2.4.1. The High-Frequency Reciprocating Rig (HFRR) Test
The high-frequency reciprocating rig test (HFRR) is a test that involves rubbing a steel ball (AISI-E 52100/535A99 (PCS Instruments, London, UK), roughness Ra = 0.050 μm, hardness RC 58–66) against a steel disk (AISI-E 52100/535A99 with 10 mm diameter, roughness Ra = 0.020 μm, hardness RC 76–79) under the test conditions of the chosen standard. The analyses were performed according to [41] using a PCS Instruments D985 apparatus. Tests ran under the following conditions: 60 °C, 200 μm stroke, 50 Hz frequency, 400 g load, for 60 min.
2.4.2. Standard Test Method for Wear Preventive Characteristics of Lubricating Grease (Four-Ball Method)—[42]
The test involves rotating a steel ball at a set rotational speed according to the test standard against three fixed steel balls that have been covered by the lubricant tested. Before each test, all the materials used are thoroughly cleaned with hexane. The three balls are loaded into the sample recipient and fixed using a steel ring, after which they are covered with the sample. The fourth ball is placed into a holder, which is inserted and fixed into the apparatus, allowing it to rotate at the required rotational speed. The test took place under a 392 N load for 60 min and 1200 rotations per minute. After the test, the wear scars on the three fixed balls are measured, and the average is calculated.
3. Results and Discussion
3.1. The Microscopic Aspect of Greases
Optical microscopy was used to observe the “working” effects of the vigorous mixing (200 rotations per minute for 50 min) on the morphology of greases. It is exemplified in Figure 2, on the grease madeup of sunflower oil, containing 20 wt.% lithium stearate without filler (a) and with 15 wt.% cellulose (b) or 15 wt.% lignin (c) before “working” it, and the same samples after submitting them to 200 rotations per minute for 50 min (d, e, and f).

Figure 2.
The microscopic aspect of the greases prepared from sunflower oil containing 20 wt.% lithium stearate before (a–c) and after (d–f) being worked (×500). Legend: (a,d)—without filler; (b,e)—with 15 wt.% cellulose; (c,f)—with 15 wt.% lignin.
As seen in Figure 2a, the unworked simple grease shows a net of soap (white), a lot of small oil-dispersed drops, and a few larger drops, up to 20 μm diameter, since the same grease work contains a multitude of more uniform-sized drops (an average diameter of 6 μm) as an effect of the vigorous stirring. The cellulose particles are visible in Figure 2b, in dark gray, interwoven with the soap net, and a few dispersed oil drops are also seen, since in the worked grease (Figure 2e), the cellulose appears as balls with 30–70 μm in diameter, and smaller drops of oil tend to adsorb on the cellulose. In Figure 2c, the lignin particles appear as black balls with a larger size (up to 100 μm), and oil drops appear adsorbed on the lignin with yellowish reflections. In the worked grease (Figure 2f), we captured smaller lignin balls (up to 35 μm) and very small oil drops dispersed among the soap net and lignin balls. So, the morphology of the greases shows that cellulose and lignin seem to be compatible with the base grease, and the vigorous stirring has an effect on the shredding of the filler particles and a decrease in oil drop size, with an effect on the emulsion’s stability.
3.2. The Rheology of Prepared Greases
The rheological study consisted ofdrawing the rheological curves (τ vs. and η vs. ) at 20 °C, 30 °C, 40 °C, 50 °C, and 60 °C, determining the rheological parameters and from the Bingham model, as well as highlighting the values of dynamic viscosity in two reference points, i.e., = 0.1 s−1 and = 100 s−1; everything was automated and performed by the plate-to-plate Anton Parr rheometer. As an example, the results are shown in Figure 3 (τ vs. ) and Figure 4 (η vs. ) for the grease prepared with 20 wt.% calcium soap and filled with 15 wt.% lignin, but all the curves are shown in Figures S1 and S2 (Supplementary Materials). The viscosity at = 0.1 s−1 and = 100 s−1, the parameters of the Bingham model plus the hysteresis area are presented in Table S1 (Supplementary Materials).

Figure 3.
Rheological curves τ versus for the grease prepared with 20 wt.% Calcium soap and filled with 15 wt.% lignin (Sample #6). Legend:  at 20 °C;
at 20 °C;  at 30 °C;
at 30 °C;  at 40 °C;
at 40 °C;  at 50 °C;
at 50 °C;  at 60 °C.
at 60 °C.
 at 20 °C;
at 20 °C;  at 30 °C;
at 30 °C;  at 40 °C;
at 40 °C;  at 50 °C;
at 50 °C;  at 60 °C.
at 60 °C.

Figure 4.
Rheological curves η versus for the grease prepared with 20 wt.% Calcium soap and filled with 15 wt.% lignin (Sample #6).
The curves τ versus have the aspect of non-Newtonian viscoplastic fluids, with an initial shear stress τ0 and hysteresis, its area narrowing down astemperature increases, as observed in Figure 3 and Figure S1. It must be mentioned that the determination at 20 °C was repeated, and the second determination was taken into account, allowing the grease to be churned before the analysis. Otherwise, the hysteresis area would have been too large at 20 °C compared to 30 °C, which does not correspond to reality.
Corroborating the aspect of rheological curves from Figure S1 (Supplementary Materials) with the data in Table S1 (Supplementary Materials), one can observe that the hysteresis area at 20 °C, 30 °C, and 40 °C is larger at filled greases, compared tothe grease without an additive; then, at 50 °C and 60 °C, the simple grease has a hysteresis area that is larger than the filled ones, thus indicating a more pronounced viscoplastic nature of the simple grease at higher temperature; moreover, at 60 °C, some greases have negative values for the hysteresis area, which is typical of rheopectic fluids, similar to dilatant fluids, whose characteristics are dependent on time. This is an exception observed in greases filled with lignin, indicating that lignin may be less compatible with the base grease than cellulose.
As a rule, viscosity values decrease when increasing temperature and shear rate, as seen in Figure 4 and Figure S2 (Supplementary Materials), and the curves at 50 °C and 60 °C almost overlap. The aspect of the rheological curves η vs. makes one think of a law-power-governed process, which was demonstrated by the statistical processing of the experimental data in Excel; the correlation between the dynamic viscosity and the shear rate at different temperatures resulted, and the equations are presented in Table S2 (Supplementary Materials).
The correlation coefficients between 0.9534 and 1.0000 prove that the power law applies in each case with accuracy. In general, the constant in front of the variable x decreases when the temperature increases, showing that viscosity decreases with temperature. The exponent also decreases with temperature, in general, showing that at higher temperatures, the variation in viscosity with shear rate is smaller.
By comparing the viscosity values at 20 °C of the greases prepared in 100 g batches [26] with those prepared in the present study, in batches of 500 g, close values were observed both for the simple greases (ranging from 1.1 to 3.0 Pa·s for the small batches and 1.2–3.03 Pa·s for the larger ones, respectively) and forfilled greases (ranging from 2.2 to 7.0 Pa·s for the small batches and 2.24–5.46 Pa·s for the larger ones, respectively). This indicates a controlled preparation process, both ona small scale and a larger one.
3.3. The Consistency of Greases
The consistency of greases can be assessed on the one hand, based on the rheological data: the value of the initial shear stress, τ0, and by comparing the viscosity values at the same temperature and shear rate conditions; on the other hand, the penetration values (in 10−1 mm or dmm), determined with the cone penetrometer, represent a standardized method for evaluating the consistency. There are two grease conditions that undergo this assessment: the fresh grease (the unworked grease) and the grease that underwent a “working” process, namely after the grease was subjected to efforts mimicking those in the lubricated mechanism (the worked grease). In Table 2, the two sets of penetration values determined by ASTM 217-02 [38] are presented.

Table 2.
Penetration values at 25 °C for unworked and worked greases.
The freshly prepared greases (i.e., the unworked ones) had 3–6 NLGI grades, so they can be described as firm (grade 3), very firm (grade 4), hard (grade 5), or very hard (grade 6); thus, they are characterized as very good for storage before use or for being kept inside the lubricated mechanism in long downtimes.
After the submission to 10,000 strokes, mimicked by mixing at 200 rotations per minute for 50 min, the greases softened to grade 0 (very soft) or 1 (soft); these grades are recommendable for mild conditions, namely for lighter loads. All greases filled with cellulose or lignin were characterized by grade 1, and the simple ones—by grade 0, except the simple grease based on sunflower oil with lithium stearate, which was characterized by grade 1. Comparatively, classical greases, based on mineral oils and lithium soap, were characterized by grades 1–2; thus, some of the greases prepared here were similar, namely those with grade 1. Greases characterized by grades 0–1 are solid, unlike the lower categories 00 and 000, which are semi-fluid or fluid greases, respectively.
By comparing the penetration test results with those obtained by Kumar and co-authors [43] for a grease containing 20% lithium 12-hydroxystearate soap and additivated with 4% hexagonal boron nitride (hBN) nanoparticles, they are quite different. Our unworked greases filled with biopolymers were more consistent (penetration between 170 and 204 dmm) than those with hBN particles of various sizes (penetrations between 399 and 407 dmm). Also, the corresponding worked greaseswere 315–353 dmm for biopolymer-filled greases compared with 400–405 dmm for hBN additivated greases. Both types of grease increase in consistency when additivated/filled. It is notable that hDN-additivated greases have a smaller difference in consistency between the worked and unworked greases, indicating their better stability in operation.
3.4. Grease Stability at High Temperature
The dropping point test shows the temperature at which the first drop of oil separates and drops when placed in a device with standard dimensions [38]; this constitutes an indication of the grease stability at higher temperatures, specific to the operating conditions in the lubricated mechanism.
In Table 3, the values of the dropping point are presented for the greases prepared here.

Table 3.
The dropping points of the prepared greases.
The dropping points were included in the interval 87.5–104.5 °C, which indicates a rather low service temperature specific to the mechanisms working under lighter loads. The simple greases had a lower dropping point, while the filled ones hadbetter thermal stability. However, this characteristic is not defined for the quality of the grease since the separation of the oil is, to some extent, beneficial for the lubrication of the mechanism. Grease softening was observed during the operation of the lubricated mechanism due to the so-called “churning” inside the mechanism; the grease heats up because of friction. It softens, and the oil separation begins after a certain operation period (“bleeding”). It drains into the lower parts of the mechanism (“leakage”), thus ensuring the lubrication of the lower parts [40], the oil itself having good lubricant qualities; occasionally, refilling the mechanism with some grease is necessary in order to ensure the lubrication of the upper parts of the mechanism.
3.5. The Variation inthe Oil Separation Capacity
The way in which the mechanism operation is affected by the separation of the oil from the grease can be measured by the SFK Ma Pro bleeding test [40], which provides a supplementary indication ofthe grease stability at moderate temperatures. The diameters of the oil stains (i.e., the traces left by the same quantity of grease) were measured on absorbent paper at 60 °C before and after “working” it. The procedure and calculations are presented in Section 2. The results are presented in Table 4.

Table 4.
The change in oil separation capacity (the SFK Ma Pro bleeding test) after working the greases at 200 rotations per minute for 50 min.
As expected, the worked greases have a higher separation oil capacity than the fresh ones. This tendency manifests itself more strongly in greases with cellulose/lignin compared to those with only soap thickener in the same category of greases (based on the same vegetable oil with the same soap). The palm oil-based greases release less oil than the sunflower oil-based ones, proving better cohesion with the lignin. In addition, one can observe that palm oil-based greases have weaker cohesion with the added cellulose compared to sunflower oil-based greases. This can be explained by the difference in the composition of oils and, hence, by the difference in their polarity, which triggers different compatibility with the added material.
3.6. The Mechanical Tests
The twelve prepared samples were first subjected to a high-frequency reciprocating rig test (HFRR analysis) as a preliminary rheological study. The average values of the contact resistance (grease film,%) resulted. The coefficient of friction (COF) was calculated by the software. The wear scar is observed by optical microscopy, and the diameter is measured on two axes; the average of these measurements isthe result. An example showing the recording of the preliminary rheological study for sample #5 is given in Figure 5, and an image of the correspondent wear scar is presented in Figure 6.
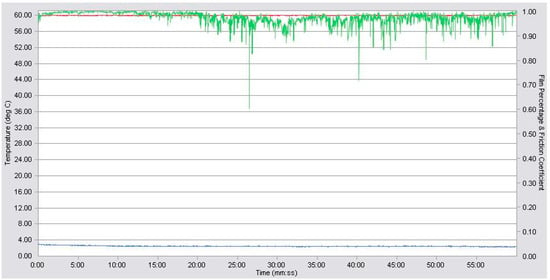
Figure 5.
Variation in film percentage (green line), friction coefficient (blue line), and temperature (red line) during the preliminary rheological test for sample #5 (grease with 20% calcium stearate and 15% cellulose).
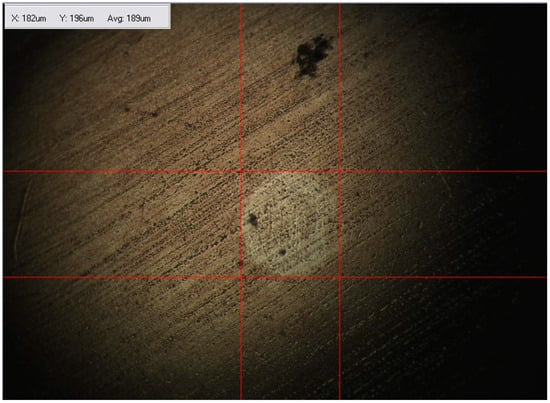
Figure 6.
The measurement of the wear spot on the disc after the HFRR test for sample #5 (grease with 20% calcium stearate and 15% cellulose).
The results of the HFRR analysis for all 12 samples are shown in Table 5.

Table 5.
The mean values of the mechanical properties of the samples.
The friction coefficients (Avg COF) vary from 0.030 to 0.062, depending on the lubricating properties of the prepared greases, all the values being satisfactory by comparing with similar data (between 0.03 and 0.06) for commercial greases with mineral base oil and Li and Ca soaps as thickeners [44].
The contact resistance of the film (Avg Film) differs a lot from one sample to another and indicates the soft consistency of some prepared materials. In general, sunflower oil-based greases form more resistant films than those based on palm oil, except for the grease filled with cellulose (sample 11), which is as resistant as the previous ones (samples 1–6).
The wear scar diameter (WSD) falls within the range of 168.5–250 μm, except for the simple sunflower oil-based greases with 20% calcium soap, which had a smaller wear scar, i.e., 63 µm. All the WSD values are low, indicating good lubrification at 60 °C. In general, the sunflower oil-based greases have smaller WSD values, but sample 11, made with palm oil, fits the sunflower oil-based grease behavior. Corroborated by its good film resistance, sample 11 (palm oil with 20% calcium stearate, and 15% cellulose) demonstrates a good quality lubricant.
Tribological performances were compared with other results from the literature, even though the tests were performed in different conditions. Closer conditions were used by Wang et al. [45]: 5 N load and test temperature 25 °C for paraffin oil-based commercial greases thickened with lithium 12-hydroxystearate; their consistency was measured as penetration for the unworked grease and revealedsoft greases: 230, 250, 270, and 300 dmm, respectively. Our greases were more consistent, with penetration between 171 and 240 dmm. The wear spots of our greases were smaller (160.5–250 μm, at 60 °C) than those of Wu et al. [46] (445–720 μm). Polyurea commercial grease with comparable consistency (230, 250, and 300 dmm), tested in work [45], got even bigger wear spots (867–874 μm).
For the best-performing samples in the previous test (samples 4 and 5), the 4-ball friction test was performed, which is a harsher test (load 392 N, 60 min duration, 1200 rotations per minute), according to ASTM D2266-01 Standard Test Method for Antiwear Properties of Lubricating Grease (Four-Ball Method) [42], at 75 °C on a Seta Stanhope 19800-7T (Stanhope-Seta, Chertsey, Surrey, UK). The test was performed in triplicate, and three out of the four test balls were cleaned and placed under a microscope in order to determine the average WSD value; the results are shown in Table 6.

Table 6.
Wear scar diameters, according to [42], for the sunflower oil-based greases with 20 wt.% calcium soap, simple (sample 4) and filled with 15 wt.% cellulose (sample 5).
Sample 5 was found to be more fluid at the test temperature but retained a liquid film that provided a smaller wear footprint. Sample 4 is more consistent, and it is possible that metal particles appeared because of friction, which further contributed to the increase in wear scar diameters. In this case, the addition of cellulose seems to be beneficial for lubrication in harsher conditions, thus confirming the findings of Li et al. [24].
Lin et al. [47] findings for a commercial Li soap grease additivated with 0.03–0.3% graphene nanoparticles disclosed wear scar diameters of 350–440 μm in the same running conditions since our results (average 504.75–584.4 μm) show a poorer antiwear resistance. But biopolymer-filled greases prepared in our study show comparable results with those of complex greases prepared by Kumar et al. with hBN [43] having a WSD between 535.4 μm and 655.9 μm.
4. Conclusions
Efforts are being made by scientists to manufacture environmentally friendly greases and ensure their sustainability. In this study, lubricating greases were prepared and characterized using waste oils from food frying processes and natural polymers (cellulose and lignin sulfate). Following other studies on the preparation of greases from vegetable oils, the present work has proposed some elements of novelty: batches of 500 g were prepared in view of scaling up production and waste vegetable oils, for which no similar studies have been carried out so far.
The preparation process is simple and takes place in mild conditions at 85–100 °C and at atmospheric pressure, provided that heating steps and the order of ingredient addition are respected and carefully controlled in order to obtain quality products.
Solid bases with water content as low as possible are used, thus reducing the energy required for the removal of water by evaporation.
Viscoplastic fluids, like the lubricating greases described in the literature, were obtained. The addition of cellulose or lignin increased grease viscosity. The viscosity variation with the shear rate follows the power law at different temperatures in the range of 30–60 °C. The Bingham model is applicable to the rheological curves, and the model parameters were determined.
The consistency of fresh greases, as measured by the penetration test, was equal to or better in this study compared to greases prepared in smaller batches [26]. It decreases after working the greases, which is normal, becoming soft greases, prone to use in lower-load mechanisms, and comparable to commercial ones.
Grease stability, measured as dropping points around 100 °C, and the variation in the oil separation capacity, measured by the SFK Ma Pro bleeding test, confirm that the greases are “soft”.
The mechanical tests were carried out on the twelve grease samples obtained in the laboratory and revealed that our greases are comparable to commercial soft greases. The best results were obtained for the samples based on sunflower oil and Ca 20% by weight soap, filled or not with cellulose.
In conclusion, the greases prepared in the present study can be used in industrial applications, according to their quality, in mechanisms with lower loads.
The research will continue with efforts to improve the quality of waste oils by reducing the number of double bonds in molecules and increasing their polarity, possibly through epoxidation, and by performing some changes in the preparation procedure so that the products can be compared with the best lubricating greases obtained from mineral oils.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/lubricants12060197/s1. Figure S1: Rheological diagrams τ versus for the prepared lubricating greases; Figure S2: Rheological diagrams η versus for the prepared lubricating greases, Table S1: The rheological analysis of all prepared greases at 20 °C, 30 °C, 40 °C, 50 °C, and 60 °C; Table S2: The correlation η = f () for all the prepared greases at 20 °C, 30 °C, 40 °C, 50 °C, and 60 °C.
Author Contributions
Conceptualization, O.V.S. and A.E.S.; methodology, C.A.V. and A.E.S.; validation, A.E.S., J.N. and C.I.K.; formal analysis, C.A.V. and S.O.; investigation, O.V.S., C.D.-V., J.N., S.O. and C.I.K.; data curation, A.E.S. and J.N.; writing—original draft preparation, O.V.S. and A.E.S.; writing—review and editing, C.I.K. and C.D.-V.; supervision, C.I.K.; project administration, O.V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abu-Elella, R.; Ossman, M.; Farouq, R.; Abd-Elfatah, M. Used motor oil treatment: Turning waste oil into valuable products. Int. J. Chem. Biochem. Sci. 2015, 7, 57–67. [Google Scholar]
- Battersby, N.S. Biodegradable lubricants: What does “biodegradable”really mean? J. Synth. Lubr. 2005, 22, 3–18. [Google Scholar] [CrossRef]
- Saxena, A.; Kumar, D.; Tandon, N. Development of lubricious environmentally friendly greases using synergistic natural resources: A potential alternative to mineral oil-based greases. J. Clean. Prod. 2022, 380, 135047. [Google Scholar] [CrossRef]
- Honary, L.A.T.; Richter, E. Chapter 7—Biobased lubricants technology. In Biobased Lubricants and Greases: Technology and Products, 1st ed.; John Wiley&Sons, Ltd.: Chichester, UK, 2011; Volume 9, pp. 91–101. [Google Scholar] [CrossRef]
- Dinda, S.; Patwardhan, A.V.; Goyd, V.V.; Pradhan, N.C. Epoxidation of cotton seed oil by aqueous hydrogen peroxide catalyzed by liquid inorganic acids. Bioresour. Technol. 2008, 99, 3737–3744. [Google Scholar] [CrossRef] [PubMed]
- Erhan, S.Z.; Asadauskas, S. Lubricant base stocks from vegetable oils. Ind. Crop. Prod. 2000, 11, 277–282. [Google Scholar] [CrossRef]
- Erhan, S.Z.; Sharma, B.K.; Perez, J.M. Oxidation and low temperature stability of vegetable oil-based lubricants. Ind. Crop. Prod. 2006, 24, 292–299. [Google Scholar] [CrossRef]
- Fox, N.J.; Stachowiak, G.W. Vegetable oil-based lubricants—A review of oxidation. Tribol. Int. 2007, 40, 1035–1046. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.Z.; Perez, J.M. Tribological studies of thermally and chemically modified vegetable oils for use as environmentally friendly lubricants. Wear 2004, 257, 359–367. [Google Scholar] [CrossRef]
- Jayadas, N.H.; PrabhakaranNair, K. Coconut oil as base oil for industrial lubricants—Evaluation and modification of thermal, oxidative and low temperature properties. Tribol. Int. 2006, 39, 873–878. [Google Scholar] [CrossRef]
- Wright, J. Grease basics. Machinery Lubrication. 2008, Volume 5. Available online: https://www.machinerylubrication.com/Read/1352/grease-basics (accessed on 30 April 2024).
- Salahuddin, A.; Majeed, B. Production of lubricant grease from used oil. Abstracts of the 17th European Congresson Biotechnology. New Biotechnol. 2016, 33, S93. [Google Scholar] [CrossRef]
- Nolan, S.J.; Zeitz, J.B. Anhydrous lithium hydroxide dispersion: A new and Efficient way to makes impleand complex lithiumgreases. NLGI Spokesm. Noiembrie 2007, 71, 17–25. [Google Scholar]
- Grignou, H.; Hoang, L.C.; Moigner, L. Procédé de fabrication d’une graisse lubrifiante àpartir d’huile vegetale et graisse obtenue. Patent European EP 1621601 A1, 1 February 2006. [Google Scholar]
- Dwivedi, M.C.; Sapre, S. Total vegetable-oil based greases prepared from castor oil. J. Synt. Lubr. 2002, 19, 229–241. [Google Scholar] [CrossRef]
- Vafaei, S.; Jopen, M.; Jacobs, G.; König, F.; Weberskirch, R. Synthesis and tribological behavior ofbio-based lubrication greaseswith bio-based polyester thickener systems. J. Clean. Prod. 2022, 364, 132659. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Gao, X.; Cheng, Y. Rheological and tribological properties of polyurea greases containing additives ofMoDDPandPB. Tribol. Int. 2023, 180, 108291. [Google Scholar] [CrossRef]
- Jopen, M.; Paulus, M.; Sternemann, C.; Degen, P.; Weberskirch, R. Comparative Studies on the Organo gel Formation of a Polyester in Three Different Base Oils by X-ray Analysis, Rheology and Infrared Spectroscopy. Gels 2023, 9, 696. [Google Scholar] [CrossRef] [PubMed]
- GarcíaTuero, A.; Bartolomé, M.; Gonçalves, D.; Viesca, J.L.; Fernández-González, A.; Seabra, J.H.O.; HernándezBattez, A. Phosphonium-based ionic liquids as additives in calcium/lithium greases. J. Mol. Liq. 2021, 338, 116697. [Google Scholar] [CrossRef]
- Bartolomé, M.; Gonçalves, D.; García Tuero, A.; Gonzalez, R.; HernandezBattez, A.; Seabra, J.H.O. Greases additised withphosphonium-based ionic liquids—Part I: Rheology, lubricant film thickness and Stribeck curves. Tribol. Int. 2021, 156, 106851. [Google Scholar] [CrossRef]
- Tian, C.; Xu, H.; Dong, J. A novel layered double hydroxides oleogel lubricant: Inspired by conventional greases. Tribol. Int. 2023, 187, 108746. [Google Scholar] [CrossRef]
- Lodhi, A.P.S.; Kumar, D. Natural ingredients based environmental friendly metal working fluid with superior lubricity. Colloids Surf. APhysicochem. Eng. Asp. 2021, 613, 126071. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Gorbacheva, S.N.; Yadykova, A.Y. Rheology and tribology of nanocellulose-based biodegradable greases: Wear and friction protection mechanisms of cellulose microfibrils. Tribol. Int. 2023, 178 Pt B, 108080. [Google Scholar] [CrossRef]
- Li, J.; Lin, N.; Du, C.; Ge, Y.; Amann, T.; Feng, H.; Yuan, C.; Li, K. Tribological behavior of cellulose nanocrystal as an eco-friendly additive in lithium-based greases. Carbohydr. Polym. 2022, 290, 119478. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bai, J.; Innocent, M.T.; Wang, Q.; Xiang, H.; Tang, J.; Zhu, M. Lignin-based carbonfibers: Formation, modification and potential applications. Green Energy Environ. 2022, 7, 578–605. [Google Scholar] [CrossRef]
- Săpunaru, O.V.; Sterpu, A.E.; Vodounon, C.A.; Osman, S.; Koncsag, C.I. Rheology of new lubricating greases made from renewable materials. Ovidius Univ. Ann. Chem. 2023, 34, 91–98. [Google Scholar] [CrossRef]
- ASTM D4052-22; Standard Test Method For Density, Relative Density, And API Gravity of Liquids By Digital Density Meter. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D7042-21a; Standard Test Method For Dynamic Viscosity And Density of Liquids By Stabinger Viscometer (And the Calculation of Kinematic Viscosity). ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D2270-10; Standard Practice for Calculating Viscosity Index from Kinematic Viscosity at 40 °C and 100 °C. ASTM International: West Conshohocken, PA, USA, 2016.
- SR 5489:2008; Liquid Petroleum Products. Determination of Flash Points in Marcusson Open Vessels. Available online: https://magazin.asro.ro/ro/standard/162837 (accessed on 30 April 2024).
- ISO 3961:2018; Animal and Vegetable Fats and Oil. Determination of Iodine Value. Available online: https://www.iso.org/standard/71868.html (accessed on 30 April 2024).
- Maher, K.D.; Bressler, D.C. Pyrolysis of triglyceride materials for the production of renewable fuels and chemicals. Bioresour. Technol. 2007, 98, 2351–2368. [Google Scholar] [CrossRef]
- Chebet, J.; Kinianjui, T.; Cheplogoi, P.K. Impact of frying iodine value of vegetable oils before and after deep frying in different types of food in Kenya. J. Sci. Innov. Res. 2016, 5, 193–196. [Google Scholar] [CrossRef]
- Codex Alimentarus Commission. JointFAO/WHO Food Strandards Programme, Report of the Fourteenth Session of the CODEX Committee on Fats and Oils, London-UK, Rome-Italy. 1995. Available online: https://www.fao.org/input/download/report/312/al95_17e.pdf (accessed on 30 April 2024).
- García-Zapateiro, L.A.; Valencia, C.; Franco, J.M. Formulation of lubricating greases from renewable base stocks and thickener agents: A rheological approach. Ind. Crop. Prod. 2014, 54, 115–121. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Núñez, N.; Valencia, C.; Franco, J.M.; Díaz, M.J. Formulation of new biodegradable lubricating greases using ethylated cellulose pulp as thickener agent. J. Ind. Eng. Chem. 2011, 17, 818–823. [Google Scholar] [CrossRef]
- Núñez, N.; Martín-Alfonso, J.E.; Valencia, C.; Sanchez, M.C.; Franco, J.M. Rheology of new green lubricating grease formulations containing cellulose pulp and its methylated derivative as thickener agents. Ind. Crop. Prod. 2012, 37, 500–507. [Google Scholar] [CrossRef]
- ASTM D217-02; Standard Test Methods for Cone Penetration of Lubricating Grease. ASTM International: West Conshohocken, PA, USA, 2002.
- ASTM D566-02; Standard Test Method for Dropping Point of Lubricating Grease. ASTM International: West Conshohocken, PA, USA, 2002.
- SathwikChatra, K.R.; Osara, J.A.; Lugt, P.M. Impact of grease churning on grease leakage, oil bleeding and grease rheology. Tribol. Int. 2022, 176, 107926. [Google Scholar] [CrossRef]
- ASTM D6079-22; Standard Test Method for Evaluating Lubricity of Diesel Fuels by the High-Frequency Reciprocating Rig (HFRR). ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM D2266-01; Standard Test Method for Wear Preventive Characteristics of Lubricating Grease (Four-Ball Method). ASTM International: West Conshohocken, PA, USA, 2015.
- Kumar, N.; Saini, V.; Bijwe, J. Dependency of Lithium Complex Grease on the Size of hBN Particles for Enhanced Performance. Tribol. Lett. 2023, 71, 20. [Google Scholar] [CrossRef]
- DeLaurentis, N.; Kadiric, A.; Lugt, P.; Cann, P. The influence of bearing grease composition on friction in rolling/sliding concentrated contacts. Tribol. Int. 2016, 94, 624–632. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Lin, J.; Gao, X. Rheological and Tribological Properties of Lithium Grease and Polyurea Grease with Different Consistencies. Coatings 2022, 12, 527. [Google Scholar] [CrossRef]
- Wu, C.; Li, S.; Chen, Y.; Yao, L.; Li, X.; Ni, J. Tribological properties of chemical composite and physical mixture of ZnO and SiO2 nanoparticles as grease additives. Applied Surface Science 2023, 612, 155932. [Google Scholar] [CrossRef]
- Lin, B.; Rustamov, I.; Li, Z.; Luo, J.; Wan, X. Graphene-Reinforced Lithium Grease for Antifriction and Antiwear. ACS Appl. Nano Mater. 2020, 3, 10508–10521. Available online: https://pubs.acs.org/action/showCitFormats?doi=10.1021/acsanm.0c02461&ref=pdf (accessed on 30 April 2024). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).