Abstract
Corrosion is a major challenge in various industries and can cause significant damage to metal structures. Organic corrosion inhibitors are compounds that are used to reduce or prevent corrosion by forming a protective film on metal surfaces. The present review article focuses on natural and synthetic organic corrosion inhibitors and their classifications, active functional groups, and efficiency estimations. Furthermore, previous studies on the use of natural and synthetic organic inhibitors are discussed, along with adsorption isotherms and mechanisms of organic corrosion inhibitors. The kinetics of corrosion modeling are also discussed, providing insights into the effectiveness of organic inhibitors at reducing corrosion. This review aims to provide a comprehensive overview of the current knowledge on organic corrosion inhibitors, with the aim of promoting their wider use in corrosion protection.
1. Introduction
Corrosion inhibitors are substances used to prevent or reduce metal surface corrosion in corrosive environments. There are two types of corrosion inhibitors: natural and synthetic [1]. Natural inhibitors, derived from plants and minerals, are eco-friendly, biodegradable, and low in toxicity. Examples include vegetable oils, tannins, and salicylic acid. However, they may have limited effectiveness and may be unsuitable for severe corrosion environments. Furthermore, their costs can be higher compared to synthetic inhibitors [2]. Despite these limitations, natural inhibitors are gaining popularity as sustainable and eco-friendly alternatives to synthetic inhibitors that can be used in various applications, such as in the oil and gas industry and for preserving historical artifacts [3]. Natural inhibitors are renewable, biodegradable, and safe for human exposure, making them appropriate for industries such as food and pharmaceuticals [4].
In contrast, synthetic inhibitors are chemically manufactured to provide high efficacy in a range of corrosive environments. Amines salts, phosphates, and nitrites are examples of synthetic inhibitors [5]. Synthetic inhibitors are more effective, have a wider range of applications, are reliable, and have a longer shelf life than natural inhibitors [6]. Synthetic inhibitors form a protective film on metal surfaces and react with corrosive substances to prevent corrosion. They are particularly effective in harsh and corrosive environments with high levels of impurities, salt, and other contaminants [7]. Synthetic inhibitors are ideal for applications such as offshore platforms, pipelines, and nuclear power plants where maintenance is challenging or costly. Nevertheless, synthetic inhibitors are more expensive than natural inhibitors and require proper selection and dosing to prevent environmental damage and interference with other chemical processes in the system [8]. In summary, synthetic inhibitors are widely used in various industrial applications due to their effectiveness, but their potential environmental impact should be considered, and their selection and dosing should be carefully managed [9,10].
In conclusion, both natural and synthetic corrosion inhibitors play an important role in preventing corrosion and extending the lifespan of metal surfaces. The choice of the type of inhibitor to use will depend on the specific application, the severity of the corrosion environment, and the desired level of efficacy.
2. Types of Corrosion
There are several types of corrosion, including [11]:
- Uniform Corrosion: This is the most common type of corrosion and occurs evenly over a large surface area of a material. It is caused by exposure to a corrosive environment, such as water, air, or chemicals.
- Pitting Corrosion: This type of corrosion is characterized by the formation of small holes or pits on the surface of a material. It is caused by localized corrosion, often due to the presence of impurities in the material or environmental factors, such as high chloride levels.
- Galvanic Corrosion: This type of corrosion occurs when two dissimilar metals are in contact with each other and an electrolyte, such as saltwater, is present. One metal becomes anodic and corrodes, while the other becomes cathodic and is protected from corrosion.
- Crevice Corrosion: This type of corrosion occurs in confined areas, such as crevices or seams, where the flow of air and liquids is restricted. This leads to a buildup of corrosive substances and the material corrodes from the inside out.
- Intergranular Corrosion: This type of corrosion occurs along the grain boundaries of a material, causing a loss of material and weakening of the structure. It is often caused by the presence of impurities, such as sulfur or chlorine, in the material.
- Erosion Corrosion: This type of corrosion occurs when a material is exposed to a corrosive fluid that is flowing at a high velocity, causing the material to erode away. This type of corrosion is common in pipelines, valves, and pumps.
- Stress Corrosion Cracking: This type of corrosion occurs when a material is under stress and exposed to a corrosive environment. It is often caused by high tensile stress, high chloride levels, or high temperatures.
In conclusion, the type of corrosion as in Figure 1, that a material experiences is dependent on many factors, including the material itself, the corrosive environment, and the conditions under which the material is exposed. Understanding these factors is crucial for developing effective strategies for preventing and mitigating material corrosion.
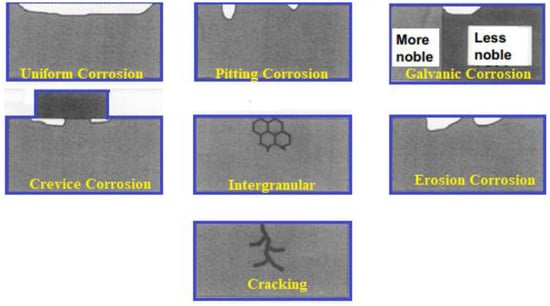
Figure 1.
Types of Corrosion.
3. Classifications of Organic Corrosion Inhibitors
Corrosion inhibitors can be classified into two main categories [12]:
- Inorganic corrosion inhibitors: These inhibitors contain metallic compounds such as nitrates, phosphates, chromates, and molybdates. They work by forming a protective film on the metal surface, which prevents the formation of corrosion cells.
- Organic corrosion inhibitors: These inhibitors contain organic compounds such as amino acids, alcohols, and amines. They work by adsorbing onto the metal surface and forming a barrier between the metal and the corrosive environment. Organic inhibitors are commonly used in industries such as oil and gas, petrochemical, and marine applications.
Organic inhibitors can be classified into several types of inhibitors [13], as in Figure 2:
- Nitrite inhibitors: These inhibitors contain nitrite ions as the active ingredient and are commonly used in cooling water systems and boilers.
- Phosphonic acid inhibitors: This type of inhibitor contains phosphonic acid or its derivatives and is effective at preventing corrosion in high-temperature and high-pressure systems.
- Carboxylic acid inhibitors: Carboxylic acid inhibitors are commonly used in industrial applications and contain compounds such as benzoic acid, salicylic acid, and acetic acid.
- Sulfonic acid inhibitors: Sulfonic acid inhibitors contain sulfonic acid and its derivatives and are used in a wide range of industrial applications.
- Thiophosphoric acid inhibitors: Thiophosphoric acid inhibitors contain thiophosphoric acid and its derivatives and are effective at preventing corrosion in high-temperature systems.
- Ethylenediamine inhibitors: This type of inhibitor contains ethylenediamine as the active ingredient and is used in a variety of industrial applications.
- Amines inhibitors: Amines inhibitors contain amine compounds as the active ingredient and are commonly used in water treatment and oilfield applications.
- Phenol inhibitors: Phenol inhibitors contain phenol or its derivatives as the active ingredient and are used in a variety of industrial applications.
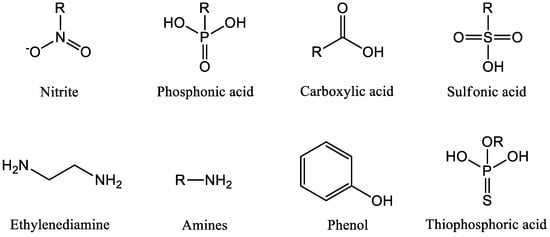
Figure 2.
Organic inhibitors classifications.
4. Active Functional Groups in Organic Corrosion Inhibitors
Active functional groups play a crucial role in the effectiveness of organic corrosion inhibitors. These functional groups interact with the metal surface and form a protective layer, preventing further corrosion. Some of the most commonly used active functional groups in organic corrosion inhibitors are as follows [14]:
- Nitrogen-containing functional groups: These functional groups contain nitrogen atoms, which can form chelating agents with metal ions. The most common nitrogen-containing functional groups used in corrosion inhibitors include amines, imides, and guanidines.
- Oxygen-containing functional groups: These functional groups contain oxygen atoms, which can form a protective film on the metal surface, inhibiting further corrosion. Some of the most common oxygen-containing functional groups used in corrosion inhibitors include carboxylic acids, esters, and ethers.
- Sulfur-containing functional groups: Sulfur-containing functional groups can form a protective film on the metal surface, preventing further corrosion. Some of the most commonly used sulfur-containing functional groups in corrosion inhibitors include sulfonic acids and thiols.
- Phosphorus-containing functional groups: Phosphorus-containing functional groups can form a protective film on the metal surface, inhibiting further corrosion. Some of the most commonly used phosphorus-containing functional groups in corrosion inhibitors include phosphonic acids and phosphates.
- Halogen-containing functional groups: Halogen-containing functional groups, such as halogens (chlorine, fluorine, and bromine), can form a protective film on the metal surface, inhibiting further corrosion.
In conclusion, active functional groups play a crucial role in the effectiveness of organic corrosion inhibitors. By interacting with the metal surface and forming a protective layer, these functional groups can prevent further corrosion, thereby extending the lifespan of metal components.
5. Estimating Organic Corrosion Inhibitors Efficiency
Organic corrosion inhibitors work by forming a protective layer over the metal surface, preventing the formation of corrosion products and protecting the metal from degradation. However, it is important to estimate the efficiency of these inhibitors to ensure that they are working as expected and to determine if any changes need to be made to the system [15]. The efficiency of both natural and synthetic corrosion inhibitors can be estimated using various methods.
5.1. Electrochemical Techniques
Electrochemical techniques such as potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) can be used to calculate the effectiveness of organic corrosion inhibitors. These methods estimate the changes in current and voltage that occur in the presence of inhibitors and provide information about the rate of corrosion [16].
5.1.1. Potentiodynamic Polarization
Potentiodynamic polarization is a reliable method for evaluating the effectiveness of organic inhibitors at mitigating metal corrosion [17]. This technique involves applying a controlled potential to the metal surface immersed in a solution containing the inhibitor and a reference electrode. The current response is monitored at regular intervals and the data are analyzed to determine the corrosion potential and current. The change in current and potential provides information about the effectiveness of the inhibitor at reducing the corrosion rate. The results of this method can help optimize the concentration of the inhibitor and corrosion protection conditions.
Inhibitors can reduce the corrosion rate by slowing down the cathodic and anodic reactions [18,19,20,21]. Cathodic reactions involve the reduction in oxidants, while anodic reactions involve the oxidation of the metal. Inhibitors can reduce the cathodic reaction rate by either blocking the reduction sites, reducing the driving force for the reduction reaction, or competing with the oxidant for electrons. They can also reduce the anodic reaction rate by either blocking the anodic sites, increasing the activation energy for the oxidation reaction, or forming a passive layer on the surface of the metal that prevents the dissolution of the metal. Inhibitors can be classified into three types based on their mode of action: anodic inhibitors, cathodic inhibitors, and mixed-type inhibitors [22]. Anodic inhibitors reduce the anodic reaction rate by forming a passive layer on the surface of the metal that prevents the dissolution of the metal. Examples of anodic inhibitors include chromates, molybdates, and phosphates. Cathodic inhibitors reduce the cathodic reaction rate by either adsorbing on the surface of the metal, forming a protective layer, or by competing with the oxidant for electrons. Examples of cathodic inhibitors include nitrates, nitrites, and amines. Mixed-type inhibitors can act on both the anodic and cathodic reactions by forming a protective layer on the surface of the metal that reduces the anodic reaction rate and also by competing with the oxidant for electrons, which reduces the cathodic reaction rate. Examples of mixed-type inhibitors include benzotriazole, tolyltriazole, and mercaptobenzothiazole [23].
In summary, inhibitors are chemical substances that can reduce the rate of corrosion by acting on the anodic and/or cathodic reactions. They can be classified into anodic, cathodic, or mixed-type inhibitors, depending on their mode of action. The selection of the appropriate inhibitor depends on the nature of the metal and the environment in which it is exposed to corrosion.
Figure 3 represents the typical polarization curves for mild steel (MS) corrosion in 1 Molar HCl, both without and with the addition of different concentrations of a terephthalaldehyde derivative as a synthesized organic inhibitor [24].
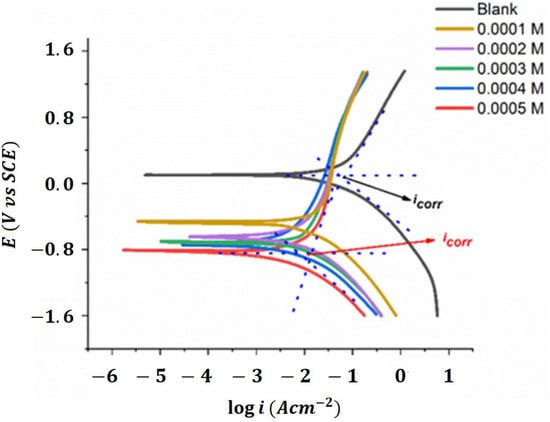
Figure 3.
Polarization curves for MS corrosion in 1 M HCl in the absence and presence of varying concentrations of terephthalaldehyde derivative as synthesized organic inhibitors [24].
The technique of potentiodynamic polarization involves applying a potential ramp to the metal and estimating the resulting current as a function of potential. The estimations can then be used to calculate the corrosion current and potential, as well as to determine the effectiveness of the inhibitor.
where is the total current, is the corrosion current, is the differential corrosion current, and is the potential change.
To perform a potentiodynamic polarization experiment, the metal is first immersed in a solution containing the inhibitor. A potentiostat is used to apply a potential ramp to the metal and estimate the resulting current.
A potentiostat is an instrument used to determine the corrosion potential of a metal. It does this by applying a potential ramp to the metal and measuring the resulting current. The ramp typically begins at a potential more negative than the corrosion potential (i.e., cathodic), passes through the corrosion potential (i.e., zero potential), and ends at a potential more positive than the corrosion potential (i.e., anodic). The exact potential range used depends on the specific metal and its corrosion properties. The ramp may start at a potential more negative than the corrosion potential to ensure that the metal is fully reduced before measurement. The corrosion potential is then determined by measuring the potential at which corrosion begins as the ramp passes through it. Finally, the ramp is ended at a potential more positive than the corrosion potential to ensure that the metal is fully oxidized. It is important to note that the potential values may be positive or negative depending on the direction of the ramp, but the potential range is typically defined as starting at a more negative potential and ending at a more positive potential than the corrosion potential [25]. The current-potential curve obtained from the experiment can be used to calculate the corrosion current and potential using the following equations:
where and are the positive and negative half-wave currents, respectively.
where and are the potentials corresponding to the positive and negative half-wave currents, respectively.
The differential corrosion current can be calculated as:
The presence of the inhibitor can be evaluated by comparing the corrosion current and potential obtained in the presence of the inhibitor with those obtained in its absence. A reduction in the corrosion current and potential in the presence of the inhibitor indicates that the inhibitor is effective at reducing corrosion.
Note that these equations are general and may need to be modified depending on the specific conditions of the experiment and the type of inhibitor used.
5.1.2. Electrochemical Impedance Spectroscopy (EIS)
EIS is a valuable, non-destructive method used to measure the impedance of a metal in the presence of an organic inhibitor [24]. It involves applying a small sinusoidal voltage to the metal surface and measuring the resulting current response to calculate the impedance of the metal-inhibitor system [24]. By measuring the complex impedance of the system, EIS provides a comprehensive understanding of the corrosion process, including the effects of various inhibitors, corrosion rates and mechanisms, and interactions between the metal surface and inhibitor molecules [24]. This method can be used in different types of environments, such as aqueous or corrosive environments, and can also monitor the effectiveness of inhibitors over time and identify potential breakdowns in protective coatings [24].
EIS is widely used in the study of corrosion and the development of corrosion inhibitors, making it a useful tool for material engineers, corrosion scientists, and industrial scientists [26]. It is also employed in the design of corrosion-resistant materials and coatings [26]. Therefore, EIS is a powerful and versatile method for studying corrosion and preventing it in metal systems [24,26].
To illustrate a typical EIS measurement, Figure 4 shows a Nyquist plot obtained by examining mild steel in 1 M H2SO4 at 30 °C using a newly synthesized thiosemicarbazone organic inhibitor at various concentrations. The equivalent circuit used in this study includes Rs, which represents the electrolyte solution resistance, in series with a parallel combination of a constant phase element (CPE) and Rct, which models the system of the metal substrate, adsorbed inhibitors, and electrolyte solution [27].
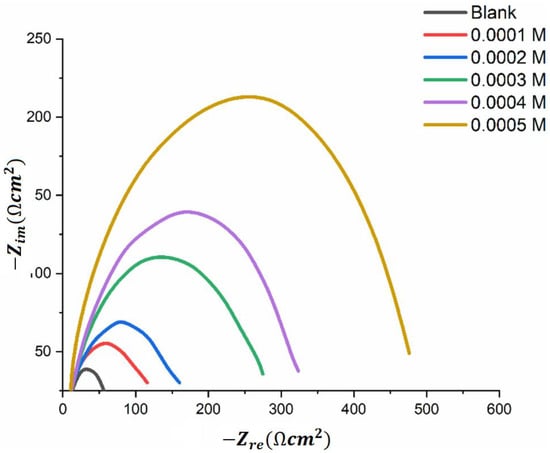
Figure 4.
Nyquist plot of MS in 1 M sulphuric acid solution at 303 K for varying synthesized organic inhibitor concentrations [27].
The impedance is a complex quantity that can be represented by the equation:
where is the resistance and is the reactance.
The impedance can be converted into a magnitude, Z′, and phase angle, Θ, by the equations:
The magnitude and phase angle of the impedance can be plotted against frequency to give the impedance spectrum. In the presence of an organic inhibitor, the impedance spectrum will be altered and can be used to quantify the corrosion current and the potential of the metal. The corrosion current can be obtained by fitting the impedance data to a Randles equivalent circuit, which consists of a series combination of an electrode resistance, a constant phase element, and a Warburg impedance. The equation for the Warburg impedance is:
where is the Warburg resistance and f is the frequency. The Warburg resistance is proportional to the corrosion rate, so the corrosion current can be calculated from the equation:
The corrosion potential can be obtained from the impedance spectrum by measuring the frequency at which the magnitude of the impedance is at the minimum. This frequency corresponds to the maximum penetration of the organic inhibitor into the metal surface and is directly proportional to the corrosion potential. The equation for the corrosion potential is:
where is the standard potential of the metal, is the Boltzmann constant, is the temperature, is the electron charge, is the frequency, and is a constant.
In conclusion, EIS can be used to estimate the corrosion current and potential of a metal in the presence of an organic inhibitor by applying an alternating voltage at a range of frequencies and measuring the resulting impedance. The impedance data can be fitted to a Randles equivalent circuit to obtain the corrosion current, and the minimum magnitude of the impedance can be used to obtain the corrosion potential. Both potentiodynamic polarization and EIS are valuable tools for evaluating the performance of organic corrosion inhibitors. These techniques can provide information on the inhibition efficiency, activation energy, and diffusion coefficient of the inhibitor, which are important parameters for optimizing the corrosion protection performance of organic inhibitors.
5.2. Weight Loss Tests
This type of test provides an accurate estimation of the corrosion rate and the protective performance of the inhibitors. The test also allows for a comparison between different inhibitors, which can help to determine the most effective one for specific corrosive environments [28]. The weight loss test is typically performed over a specified time period and the metal specimens are exposed to different corrosive environments such as salt spray, humidity, and acid or alkaline solutions [28]. The specimens are then weighed to determine their initial weight, and then they are exposed to the inhibitor. After the exposure time, the specimens are re-weighed to determine their final weight and the weight loss is calculated. The results are then compared to the control specimen, which is the metal specimen without contact with the inhibitor [28]. The weight loss test is a simple, cost-effective, and reliable way to evaluate the performance of organic corrosion inhibitors. It provides valuable information on the protective properties of the inhibitors and helps to identify the most effective one for specific corrosive environments. This information can be used to improve the design of protective coatings, enhance the performance of existing coatings, and develop new and improved inhibitors. An investigation for examining mild steel in 1 m HCl at 30 to 60 °C K by means of a new antipyrine derivative, namely N-2-methylbenzylidene-4-antipyrineamine, with different concentrations as a synthesized organic inhibitor is shown in Figure 5. The published article describes a study that evaluated the corrosion rate and protection efficiency of an inhibitor at different concentrations and temperatures. The results showed that the protection efficiency increased with the increasing inhibitor concentration and decreased the corrosion rate at all concentrations. The highest protection efficiency of 91.8% was achieved with a concentration of 0.5 mM and a temperature of 30 °C. The results also revealed that the inhibition efficiency increased with time but decreased after 24 h. The corrosion rate increased slightly with longer exposure time in the acidic solution [29].
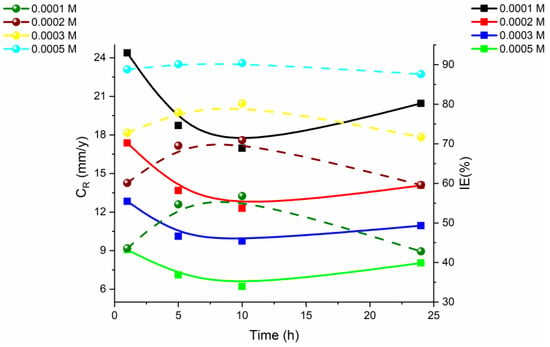
Figure 5.
Mild steel corrosion plot in 1.0 M HCl with different inhibitor concentrations synthesized organic inhibitors at various immersion times [29].
Temperature highly affects the corrosion rate and the inhibition efficiency, and with increasing temperature, the rate of corrosion increases exponentially in an acidic environment. To understand the protection performance of the N-2-methylbenzylidene-4-antipyrineamine at various temperature degrees, 30–60 °C, the corrosion rate and inhibition efficiency were investigated (Figure 6). N-2-methylbenzylidene-4-antipyrineamine revealed the highest inhibition efficiency at 30 °C, which regularly declined with an increase in temperature. The tested inhibitor exhibited lower protection performance at the maximum temperature. This result attends to the fact that the rise in temperature did not support physisorption (physical interactions), therefore reducing the protection performance [29].
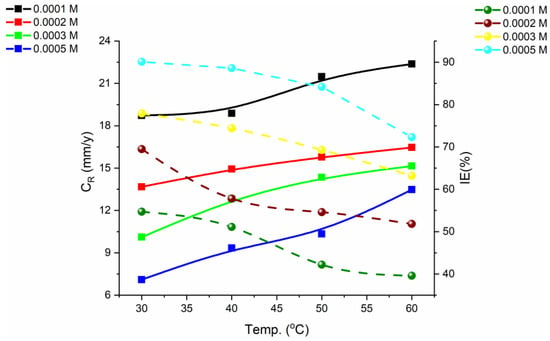
Figure 6.
Mild steel corrosion plot in 1.0 M HCl with different N-2-methylbenzylidene-4-antipyrineamine (synthesized organic inhibitor) concentrations at various temperatures [29].
The technique used for weight loss tests (WLTs) to estimate the corrosion current and potential of a metal in the presence of an organic inhibitor involves the following steps and equations [30]:
- Preparation of specimens: The metal specimens are prepared and cleaned thoroughly. Then, they are coated with a thin layer of the organic inhibitor under study.
- Weight loss measurements: The weight loss of the metal specimens is measured after exposing them to a corrosive environment for a specified period of time. The weight loss is calculated using the following equation:
- Corrosion current density (): The corrosion current density () is calculated using the following equation:
- Corrosion potential (): The corrosion potential () is estimated using a corrosion potential meter. The potential is recorded as a function of time and the steady-state corrosion potential () is determined.
- Comparison with control specimens: Control specimens without the organic inhibitor are prepared and the weight loss and electrochemical estimations such as corrosion potential and polarization resistance are obtained to evaluate the corrosion behavior of the metal [31]. The results obtained from the control specimens are compared with the results obtained from the specimens with the organic inhibitor. The presence of an organic inhibitor in the corrosive environment leads to a decrease in the corrosion current density and an increase in the corrosion potential compared to the control specimens. The efficacy of the organic inhibitor can be determined by comparing the results obtained with the inhibitor with the control specimens. This WLT technique is an effective method to estimate the corrosion current and potential of a metal in the presence of an organic inhibitor. The results obtained from this test provide valuable information on the corrosion protection properties of the organic inhibitor.
5.3. Optical Microscopy
Optical microscopy [32] is a technique that utilizes light and lenses to magnify the image of a sample, making it useful in corrosion studies [33]. It allows researchers to examine the surface of metal specimens before and after exposure to a corrosive environment, providing information on the presence and location of corrosion products, thickness of protective layers, and overall surface condition. This information is valuable in developing new corrosion protection strategies or improving existing ones. Additionally, optical microscopy can be used to observe the real-time formation of corrosion, aiding in the optimization of protective coatings and alloys [34]. However, while optical microscopy can reveal information on the surface features of a material, it cannot provide information about its electrochemical properties, such as its corrosion current and potential, which are important parameters in describing the likelihood and the rate of corrosion [29]. To determine these parameters, electrochemical measurements such as potentiodynamic or EIS are necessary [31]. The combination of optical microscopy and electrochemical measurements can provide a comprehensive understanding of the corrosion behavior of a material, including identifying the locations of corrosion initiation and propagation [32]. Thus, while optical microscopy is a powerful tool for investigating the microstructure of materials, electrochemical measurements are necessary to accurately determine their electrochemical properties [32]. This technique is based on the principle of electrochemistry, which is the study of the relationships between electrical and chemical processes. The basic equation for the corrosion current, , is given by the following expression:
where n is the number of electrons involved in the reaction, F is the Faraday constant (96,485 C/mol), A is the surface area of the metal, d is the thickness of the metal, and t is the time over which the metal is corroding. The units for icorr are typically in amperes (As).
The potential of a metal in the presence of an organic inhibitor can be calculated using the Nernst equation:
where is the standard potential, R is the gas constant (8.31 J/mol K), T is the temperature in Kelvin, is the exchange current, and n is the number of electrons involved in the reaction.
The corrosion rate can then be calculated using the following equation:
where
- d(t) is the remaining thickness of the metal after time t;
- is the initial thickness of the metal;
- is the corrosion current density (in A/cm2);
- t is the exposure time (in hours);
- M is the atomic weight of the metal;
- n is the number of electrons transferred in the corrosion reaction;
- F is the Faraday constant (96,485 C/mol);
- A is the surface area of the metal (in cm2).
In order to perform optical microscopy, a metal sample is coated with an organic inhibitor and placed in a solution. The metal is then subjected to a current and the potential of the metal is measured using an electrode. The corrosion rate can then be determined by measuring the decrease in thickness of the metal over time. By combining these equations, it is possible to accurately estimate the corrosion current and the potential of a metal in the presence of an organic inhibitor using optical microscopy. This information is valuable for understanding the protective properties of the organic inhibitor and for optimizing the design of metal-based systems.
5.4. XPS
X-ray photoelectron spectroscopy (XPS) is a surface-sensitive analytical technique that provides information on the elemental composition, oxidation state, and chemical environment of the outermost surface layers of a material. In the context of corrosion inhibition studies, XPS can be used to investigate the interaction between a protective coating or inhibitor and the surface of a metal [35]. By analyzing the chemical composition of the surface before and after exposure to the inhibitor, researchers can gain insights into the mechanism of corrosion inhibition.
The principle of XPS involves exposing a material surface to X-rays and analyzing the emitted electrons to determine their binding energy, which is related to the chemical identity of the atoms from which they originated. By scanning the X-ray beam across the surface of the material and measuring the binding energies of the emitted electrons at each point, a two-dimensional image of the surface composition can be generated [33]. To perform a corrosion inhibition study using XPS, the sample is first prepared by cleaning and polishing the surface to remove any contaminants or oxide layers [36]. The sample is then exposed to the inhibitor under controlled conditions, such as immersion in a corrosive solution, and subsequently analyzed using XPS [37]. By comparing the XPS spectra of the inhibited and uninhibited surfaces, researchers can identify any changes in the elemental composition, oxidation states, or chemical bonding that occur as a result of the inhibitor treatment [34]. XPS is a valuable technique for investigating the corrosion inhibition properties of materials. It provides information on the surface chemistry of the material that is not accessible by other analytical methods. By gaining a better understanding of the mechanism of corrosion inhibition, researchers can develop more effective coatings and inhibitors to protect metal surfaces from degradation [38].
5.5. Scanning Electron Microscopy (SEM)
SEM is an advanced imaging technique that produces high-resolution images of metal surfaces and allows for detailed analysis of corrosion products, protective layers, and underlying metal surfaces [39]. The technique involves emitting a beam of electrons onto the sample, which interact with the surface and produce secondary electrons that are collected to create a high-resolution image [40]. One of SEM’s advantages is its ability to be used in different environments, such as high vacuum, low vacuum, and environmental chambers, enabling the study of corrosion processes under various conditions [41]. SEM can also be combined with other techniques, including energy-dispersive X-ray spectroscopy (EDS), for the elemental analysis of corrosion products to determine the type of corrosion and its cause. Overall, SEM is a crucial tool for corrosion study and the analysis of protection methods, providing valuable information about the metal surface, corrosion products, and protective layers to enhance protection strategies and extend metal structures’ lifespan. In one study, a new organic corrosion inhibitor was synthesized, and SEM testing was conducted to evaluate its effectiveness. The results showed that the mild steel surface experienced significant corrosion due to H2SO4 attack, resulting in a rough and unsmooth surface, as shown in Figure 7. However, the mild steel surface was protected from corrosion when the corrosion inhibitor was used, preventing severe corrosion from occurring due to H2SO4, as shown in Figure 8 [42].
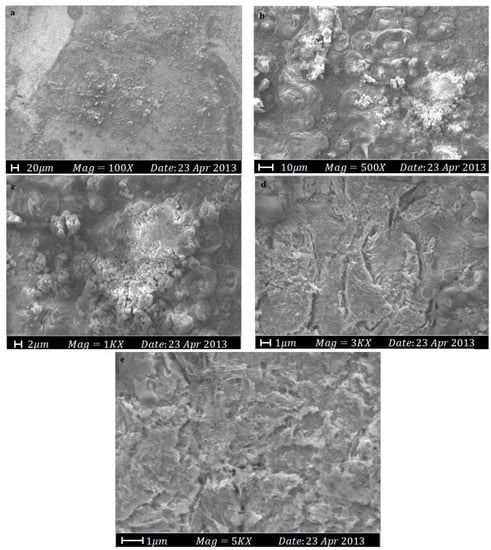
Figure 7.
The SEM micrographs for mild steel in 1.0 M H2SO4 without the corrosion inhibitor at 30 °C: (a) 100×; (b) 500×; (c) 1000×; (d) 3000×; (e) 5000× [42].
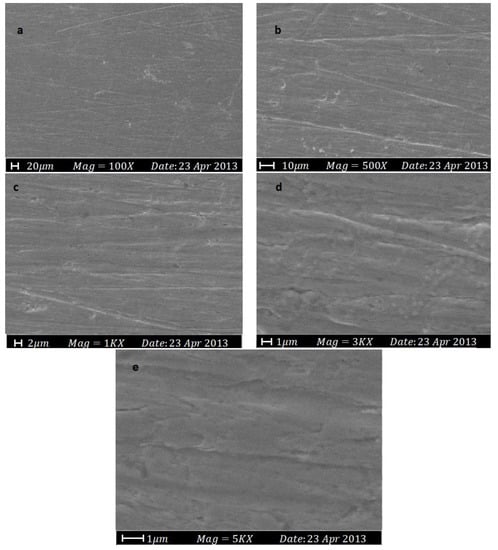
Figure 8.
The SEM micrographs for mild steel in 1.0 M H2SO4 with 0.25 mM of the corrosion inhibitor at 30 °C: (a) 100×; (b) 500×; (c) 1000×; (d) 3000×; (e) 5000× [42].
SEM is a powerful technique that can provide high-resolution imaging of the surface of a metal sample, allowing for the characterization of its topography, morphology, and composition. However, SEM is not typically used for the direct estimation of the corrosion current and the potential of a metal in the presence of an organic inhibitor. Instead, electrochemical techniques such as EIS or potentiodynamic polarization are typically used to measure the corrosion current and potential. These techniques involve applying a small AC or DC voltage to the metal sample and estimating the resulting current and potential responses. The data obtained from these measurements can be used to characterize the corrosion behavior of the metal in the presence or absence of an inhibitor and to evaluate the effectiveness of the inhibitor in reducing corrosion [43,44,45]. The technique involves the following steps:
- Preparation of the sample: The metal sample is prepared for SEM by first cleaning it and removing any surface contaminants. This is typically performed using a solvent cleaning process or an etching process.
- Deposition of the organic inhibitor: The organic inhibitor is then deposited onto the metal surface. This can be performed using various methods, such as chemical deposition, electrodeposition, or physical adsorption.
- SEM imaging: The sample is then placed in the SEM and imaging is performed. The SEM uses a high-energy electron beam to scan the sample and produce a high-resolution image. This image can be used to determine the distribution of the organic inhibitor on the metal surface.
- Current-potential estimation: Current-potential estimations are performed by applying a potential difference between the metal sample and a reference electrode. This creates an electric field, which causes the metal ions to migrate towards the reference electrode. The rate of ion migration is proportional to the corrosion current, which is calculated from equations rather than directly measured.
- Analysis of the data: The corrosion current density and potential are then calculated from the current-potential estimation using appropriate equations. The corrosion potential, Ecorr, can be determined from the Nernst equation:
Note: The value of icorr, the corrosion current, is typically obtained through various electrochemical techniques, such as Tafel extrapolation, linear polarization resistance, or electrochemical impedance spectroscopy. These techniques involve applying a small perturbation to the system and estimating the resulting current response to extract the value of icorr. The choice of technique depends on the specific experimental setup and the desired accuracy of the measurement. Once the value of icorr is determined, it can be used in the Nernst equation along with other measured parameters to calculate the corrosion potential.
- 6.
- Interpretation of results: The corrosion current and potential can be used to evaluate the efficacy of the organic inhibitor. A lower corrosion current and a more positive corrosion potential indicate that the organic inhibitor is effectively reducing corrosion.
SEM is a useful tool for studying the surface morphology and elemental composition of metal samples. However, it is not suitable for directly measuring corrosion current or potential. To determine these electrochemical parameters, techniques such as potentiodynamic or potentiostatic polarization measurements, EIS, or other electrochemical methods are typically used [46]. Nevertheless, SEM can still play an important role in understanding the corrosion behavior of a material. It provides information about the morphology and topography of the corroded surface, as well as the types and distribution of corrosion products that form during the corrosion process. This information can be used in conjunction with electrochemical measurements to gain a more complete understanding of the corrosion behavior of the material. For example, SEM can be used to examine the surface of a corroded metal sample before and after electrochemical measurements are taken. By comparing the images obtained before and after the electrochemical measurements, researchers can gain insights into how the corrosion process has affected the surface of the material and how the electrochemical parameters are related to the observed corrosion behavior [47].
In summary, while SEM cannot directly measure corrosion current and potential, it can provide valuable information about the morphology and topography of corroded surfaces, as well as the types and the distribution of corrosion products. This information can be used in combination with electrochemical measurements to gain a more complete understanding of the corrosion behavior of a material [46,47].
Estimating the efficiency of organic corrosion inhibitors is critical to ensuring the longevity and integrity of metal systems. Different techniques can be used to estimate the effectiveness of these inhibitors, and it is important to select the appropriate methods depending on the specific requirements of the system [48].
6. Previous Studies on Using Natural and Synthetic Organic Inhibitors
Organic inhibitors are compounds that are used to control the corrosion of metals in different industrial applications. These inhibitors can be of two types, namely natural and synthetic. Natural inhibitors are derived from plants and animals, while synthetic inhibitors are man-made chemicals. Both types of inhibitors have been extensively studied over the years, and their effectiveness at controlling corrosion has been documented in several studies [49]. Studies on natural inhibitors have focused mainly on the use of extracts from plants and animals to inhibit corrosion. For instance, extracts from different types of herbs and spices, such as basil, clove, and cinnamon, have been shown to be effective at controlling corrosion in a range of metals, including iron, aluminum, and copper. The mechanism of action of natural inhibitors has been attributed to their ability to form protective films on the metal surface, which prevent corrosion. In addition, natural inhibitors have been found to contain antioxidants that can neutralize the harmful effects of corrosive agents on metals [50]. Studies on synthetic inhibitors have focused on the design and synthesis of new chemicals that are effective at controlling corrosion. Synthetic inhibitors have been found to be more effective at controlling corrosion compared to natural inhibitors, mainly due to their higher solubility in corrosive environments. However, synthetic inhibitors also pose environmental and health risks, which have led to concerns over their widespread use [51]. Herein are some examples of previous studies on using natural and synthetic organic inhibitors.
- Natural organic inhibitors:
- a.
- Using chitosan as a natural inhibitor in the inhibition of the corrosion of mild steel in seawater [52].
- b.
- The use of cinnamon extract as an inhibitor for the corrosion of aluminum in acidic media [53].
- c.
- The application of tannic acid as a natural inhibitor for the corrosion of carbon steel in an aqueous solution [54].
Natural corrosion inhibitors have gained significant attention in recent years due to their eco-friendly, cost-effective, and sustainable nature. They are derived from various natural sources such as plants, animals, and microorganisms, and have been found to exhibit excellent corrosion inhibition properties in different environments.
Several studies have been conducted to investigate the effectiveness of natural corrosion inhibitors in different industries. For instance, Adewuyi and others [55] evaluated the corrosion inhibition efficiency of crude extracts of Vernonia amygdalina and Mangifera indica leaves in hydrochloric acid (HCl) using weight loss and electrochemical techniques. They found that both plant extracts were effective at inhibiting corrosion, and their inhibition efficiency increased with increasing concentration. Similarly, Mohd Sani and others [56] investigated the effectiveness of Piper nigrum extract as a natural corrosion inhibitor in a 3.5% NaCl solution using electrochemical impedance spectroscopy and potentiodynamic polarization techniques. They found that the extract exhibited significant inhibition efficiency and was more effective than some of the commercial inhibitors used for the same purpose. In another study, Bhat and Nayak [57] evaluated the corrosion inhibition efficiency of a polysaccharide extracted from the marine algae Padina tetrastromatica in a 3.5% NaCl solution using weight loss and electrochemical techniques. They found that the polysaccharide exhibited excellent corrosion inhibition properties, and its effectiveness increased with increasing concentration. Furthermore, some studies have investigated the synergistic effects of natural corrosion inhibitors and other additives. For example, Ebenso and others [58] evaluated the effectiveness of Azadirachta indica and Moringa oleifera extracts in combination with sodium molybdate as a corrosion inhibitor for mild steel in hydrochloric acid. They found that the combination of the extracts and sodium molybdate exhibited better inhibition efficiency than the individual inhibitors.
- 2.
- Synthetic organic inhibitors:
- a.
- The effectiveness of benzotriazole as a corrosion inhibitor for aluminum alloys in acidic media [59].
- b.
- The use of triazole derivatives as corrosion inhibitors for mild steel in acidic environments [60].
- c.
- The inhibition performance of imidazoline derivatives in the corrosion of copper in aerated seawater [61].
In conclusion, the studies on using natural and synthetic organic inhibitors have provided valuable insights into the mechanisms of corrosion and the efficacy of inhibitors in controlling it. While natural inhibitors have been found to be less effective compared to synthetic inhibitors, they are considered to be safer and more environmentally friendly. Further research is needed to improve the efficiency of natural inhibitors and to reduce the negative impacts of synthetic inhibitors.
One study compared the corrosion inhibition efficiency of a synthetic inhibitor, 2-mercaptobenzothiazole (MBT), with that of a natural inhibitor, catechin, on mild steel in an acid solution. The results showed that both inhibitors were effective, but catechin was more effective at lower concentrations. The researchers suggested that catechin’s superior performance may be due to its higher molecular weight and ability to form a protective film on the metal surface [62]. Another study compared the performance of several synthetic inhibitors, including benzotriazole (BTA), tolyltriazole (TTA), and mercaptobenzothiazole (MBT), with that of a natural inhibitor, tannic acid, on carbon steel in an acidic media. The results showed that tannic acid was the most effective inhibitor, followed by TTA and BTA, with MBT being the least effective [63]. A third study compared the corrosion inhibition performance of a synthetic inhibitor, 2-mercaptobenzimidazole (MBI), with that of a natural inhibitor, vanillin, on mild steel in acidic media. The results showed that vanillin was a more effective inhibitor than MBI, possibly due to its ability to form a thicker protective film on the metal surface [64]. One study by Li and [65] compared the inhibitory effect of three natural inhibitors (extracts of tea, black pepper, and cinnamon) and three synthetic inhibitors (benzotriazole, imidazole, and mercaptobenzimidazole) on the corrosion of carbon steel in acidic solutions. The results showed that all six inhibitors were effective at reducing corrosion, with the natural inhibitors performing slightly better than the synthetic ones. The authors suggested that this may be due to the presence of multiple active components in natural inhibitors, which can provide a synergistic effect. Another study by El-Etre and others [66] investigated the inhibitory effect of a natural inhibitor (pomegranate peel extract) and a synthetic inhibitor (sodium benzoate) on the corrosion of mild steel in a 1 M HCl solution. The results showed that both inhibitors were effective at reducing corrosion, but the natural inhibitor was more effective, with a corrosion inhibition efficiency of 91.6% compared to 87.1% for the synthetic inhibitor. The authors suggested that this may be due to the presence of tannins and phenolic compounds in the natural inhibitor, which can form a protective film on the metal surface. In contrast, a study by Mekidiche and others [67] compared the inhibitory effect of a synthetic inhibitor (4-methoxybenzylidene-4-butanoylthiosemicarbazide) and a natural inhibitor (rosemary essential oil) on the corrosion of mild steel in a 1 M HCl solution. The results showed that the synthetic inhibitor was more effective, with a corrosion inhibition efficiency of 98.8% compared to 84.9% for the natural inhibitor. The authors suggested that this may be due to the higher concentration of active components in the synthetic inhibitor [62,63,64,65,66,67,68,69,70,71,72,73,74]. Overall, these studies suggest that natural inhibitors can be as effective or more effective than synthetic inhibitors in preventing metal corrosion. However, the performance of inhibitors can vary depending on the specific metal and environment being studied, and further research is needed to fully understand the mechanisms of corrosion inhibition.
7. Adsorption Isotherms of Organic Corrosion Inhibitors
Adsorption isotherms refer to the relationship between the amount of a substance adsorbed onto a surface and the concentration of that substance in the surrounding environment. In the context of organic corrosion inhibitors, adsorption isotherms provide important information about the effectiveness of these inhibitors in preventing corrosion [75]. There are several commonly used models for describing the adsorption isotherms of organic corrosion inhibitors, including the Langmuir, Freundlich, and Temkin isotherms. The Langmuir isotherm assumes that the adsorption of the inhibitor occurs on a surface with a fixed number of adsorption sites and that the adsorption process is monolayer.
Langmuir isotherm: The Langmuir isotherm equation is given by:
where = the adsorption capacity (mg/g); = the concentration of the inhibitor in the solution (mg/mg); = the Langmuir constant (mg/mg).
Freundlich isotherm: The Freundlich isotherm assumes that the adsorption is dependent on both the concentration of the inhibitor and the surface area.
Freundlich isotherm: The Freundlich isotherm equation is given by:
where = the adsorption capacity (mg/g); = the concentration of the inhibitor in the solution (mg/L); = the Freundlich constant (mg/g (L/mg)^(1/n)); = the Freundlich exponent.
The Temkin isotherm considers the adsorption process to be influenced by the heat of adsorption and the entropy of the adsorbent-adsorbate system [76].
Temkin Isotherm: The Temkin isotherm equation is given by:
where = the adsorption capacity (mg/g); = the concentration of the inhibitor in the solution (mg/L); = the Temkin constant (L/mg); = the Temkin constant (mg/g).
The shape of the adsorption isotherm can offer valuable insights into the mechanism of inhibitor adsorption and can help evaluate the performance of various inhibitors. For instance, a Langmuir isotherm suggests that the inhibitor adheres to the metal surface in a monolayer and is expected to be highly effective at preventing corrosion. On the other hand, a Freundlich isotherm indicates that the adsorption of the inhibitor depends on both the concentration of the inhibitor and the surface area, which can result in reduced efficacy at higher inhibitor concentrations [42]. Al-Amiery [42] provides an example of the adsorption isotherm study, which explores the interactions between inhibitor molecules and metal surfaces.
The surface coverage (θ) was calculated at various inhibitor concentrations in 1 M hydrochloric acid solution. The most commonly used isotherms are the Temkin and Langmuir models. An analysis of weight loss measurement data showed that the data best fit the Langmuir model. The linear regression parameter (R2) between Cinh/θ and Cinh was 0.9995, as shown in Figure 9, which confirms that the adsorption of 4-benzyl-1-(4-oxo-4-phenylbutanoyl)thiosemicarbazide molecules on mild steel follows Langmuir isotherms. However, the slope of the Cinh/θ against the Cinh plot was different from unity, indicating non-ideal simulation and unexpected behavior from Langmuir isotherms. This may be due to interactions between adsorbed 4-benzyl-1-(4-oxo-4-phenylbutanoyl)thiosemicarbazide molecules on the tested metal surface. A ΔGoads value of −37.1 kJ mol−1 was determined, indicating a combination of chemical and electrostatic interactions between the inhibitor and mild steel in 1 M HCl solution at 303 K. A negative ΔGoads value confirms that the adsorption of 4-benzyl-1-(4-oxo-4-phenylbutanoyl)thiosemicarbazide molecules on mild steel is a spontaneous process.
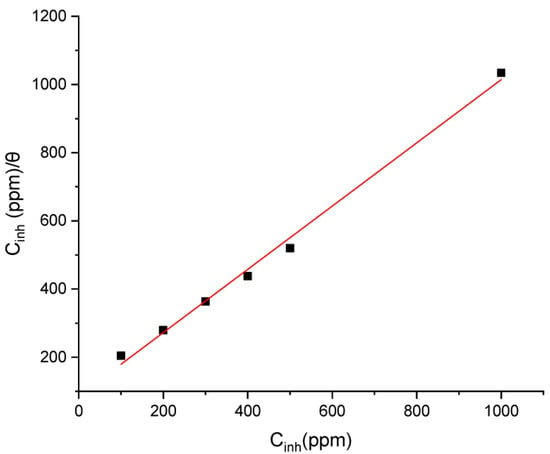
Figure 9.
Langmuir adsorption isotherm model for the adsorption of 4-benzyl-1-(4-oxo-4-phenylbutanoyl)thiosemicarbazide in 1 M HCl on the surface of mild steel [42].
An article published by Walczak M.S. et al, and his colleagues evaluates the practice of using inhibition efficiency to determine the standard Gibbs energy of adsorption for a corrosion inhibitor in acidic solutions. It suggests that the assumption that inhibition efficiency is a good proxy for fractional surface coverage may not always be valid, and, therefore, the accuracy of the Gibbs energy of adsorption value obtained from such data is doubtful. The article argues that even a more direct measurement of fractional surface coverage may still not allow for an accurate estimate of the Gibbs energy of adsorption [77].
In conclusion, adsorption isotherms of organic corrosion inhibitors provide valuable information about the effectiveness of these inhibitors in preventing corrosion. Understanding the adsorption behavior of these inhibitors can help in the design and selection of effective corrosion inhibitors for different applications.
8. Mechanisms of Organic Corrosion Inhibitors
Organic corrosion inhibitors are chemical compounds that help prevent the corrosion of metals in harsh environmental conditions. There are several mechanisms through which organic corrosion inhibitors work [78]:
- Adsorption: Adsorption is the most frequently observed mechanism of organic corrosion inhibitors. These inhibitors attach themselves to the surface of the metal, creating a protective layer that serves as a barrier between the metal and the corrosive environment. Several factors, including the concentration of the inhibitor, the surface area, and the presence of other species in the solution, can affect this process [79,80].
- Film formation: Organic corrosion inhibitors can also form a film on the metal surface, which protects it from corrosion. This film acts as a barrier, preventing the corrosive agents from reaching the metal surface.
- Electrostatic repulsion: Organic corrosion inhibitors can use electrostatic repulsion to prevent corrosion. These inhibitors can be charged, which allows them to repel corrosive agents. For example, inhibitors with a positive charge can repel negatively charged corrosive ions, while inhibitors with a negative charge can repel positively charged ions [81,82].
- Complex formation: Organic corrosion inhibitors can form complex compounds with the corrosive agents, thereby reducing their effectiveness. This results in a reduction in the corrosion rate as the corrosive agents are neutralized.
- pH adjustment: Organic corrosion inhibitors can also adjust the pH of the solution to a neutral or slightly alkaline level. This helps to reduce the concentration of corrosive agents in the solution, which in turn reduces the rate of corrosion.
- Cathodic protection: Organic corrosion inhibitors can act as cathodic inhibitors by reducing the cathodic reaction rate, thus reducing the rate of corrosion.
- Oxygen scavenging: Organic corrosion inhibitors are compounds that can reduce the rate of corrosion by scavenging oxygen from the solution. By removing oxygen, the formation of corrosive agents that would otherwise cause corrosion is prevented. These inhibitors function by creating a protective barrier on the metal surface, which prevents the corrosive agents from coming into contact with the metal [83,84].
The chemical adsorption of 3-(4-ethyl-5-mercapto-1, 2, 4-triazol-3-yl)-1-phenylpropanone molecules on mild steel is indicated by the interaction of the donor/acceptor from the unreacted electrons of nitrogen, sulfur, and oxygen atoms of the molecule with the unoccupied d-orbitals of the iron atoms on the mild steel surface. The for the tested inhibitor was found to be -35.73 kJ/mol, indicating that both the physisorption and chemisorption mechanisms are involved in the adsorption process. The anticorrosion mechanism of mild steel in a corrosive environment can be explained based on the adsorption of the 3-(4-ethyl-5-mercapto-1, 2, 4-triazol-3-yl)-1-phenylpropanone molecules on the steel surface. These molecules act by adsorbing onto the steel surface and preventing the active positions by displacing water molecules and creating a barrier to block the corrosion process. Figure 10 represents the proposed inhibition mechanism of the molecule on the mild steel surface in a 1 M HCl solution [85].
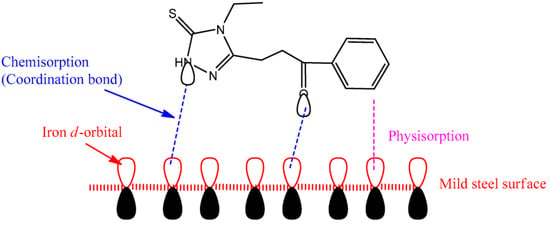
Figure 10.
The suggested inhibition mechanism of tested inhibitor on the mild steel surface in 1 M HCl solution [85].
9. Computational Methods
Computational methods have become increasingly important for corrosion inhibition studies in recent years. Here are some applications of computational methods for corrosion inhibition studies [86,87,88,89,90]:
- Molecular dynamics (MDs) simulations: MD simulations can provide insights into the interaction between inhibitors and metal surfaces at the atomic scale. These simulations can be used to study the adsorption behavior of inhibitors, the effect of the molecular structure on inhibitor performance, and the effect of environmental factors such as pH and temperature on inhibitor efficacy [86].
- Density functional theory (DFT): DFT calculations can be used to study the electronic properties of inhibitors and their interaction with metal surfaces. This approach can be used to predict the adsorption energy of inhibitors and to identify the most effective inhibitors for a particular metal surface [76].
- Monte Carlo simulations: Monte Carlo simulations can be used to predict the coverage and distribution of inhibitors on metal surfaces. This approach can provide insights into the mechanism of inhibitor action and can be used to optimize inhibitor concentrations and application methods [87].
- Machine learning: Machine learning algorithms can be trained on large datasets of corrosion inhibition data to predict the performance of new inhibitors. This approach can accelerate the discovery of new inhibitors and reduce the cost and time required for experimental screening [35,88].
Overall, computational methods have become an essential tool for corrosion inhibition studies, allowing researchers to gain insights into the mechanism of inhibitor action and to optimize the design and application of corrosion inhibitors.
10. Comparison Studies
In this study, we compared the inhibitory efficiencies of several published synthesized organic corrosion inhibitors [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121]. To do this, we evaluated the inhibition efficiencies of these synthesized inhibitors and summarized our findings in Figure 11.
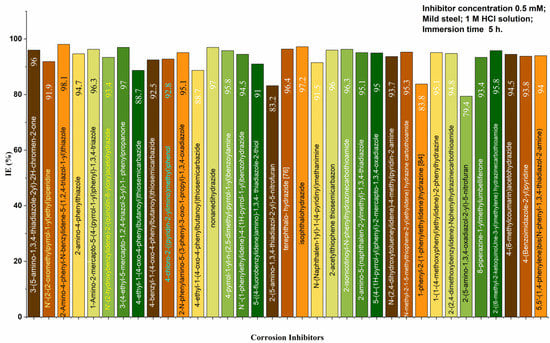
Figure 11.
Comparison between several synthesized organic corrosion inhibitors.
Figure 11 summarizes the comparison of inhibition efficiencies of several synthesized organic corrosion inhibitors. The graph shows the inhibitors’ inhibition efficiencies as percentages, with higher percentages indicating more effective inhibition. The inhibitors are labeled on the x-axis, and the y-axis represents the inhibition efficiency percentage.
The comparison shows which synthesized organic corrosion inhibitor is the most effective at inhibiting corrosion. This information can be useful for selecting the best inhibitor for a specific application, such as in the petroleum, chemical, or manufacturing industries. Additionally, the study may provide insight into the mechanisms of corrosion inhibition and inform future research and development of corrosion inhibitors. Organic inhibitors are chemical compounds that can be used to prevent or reduce the corrosion of metals. There are two main types of organic inhibitors: natural inhibitors, which are derived from plant extracts or animal products; and synthetic inhibitors, which are man-made. Several studies have been conducted to investigate the effectiveness of natural and synthetic organic inhibitors in corrosion prevention. Here, we review some of these studies and compare the performances of natural and synthetic inhibitors [112,113,114,115,116,117,118,119,120,121,122,123,124,125,126].
Natural corrosion inhibitors can be obtained from various sources, including plant extracts, animal extracts, and minerals. Plant extracts contain various organic compounds, such as tannins, alkaloids, and flavonoids, that have been found to inhibit corrosion. Animal extracts, such as chitosan and collagen, have also been studied as natural corrosion inhibitors. Minerals, such as molybdate, chromate, and nitrate, are commonly used as corrosion inhibitors in the industry. These minerals can be obtained naturally from rocks and soil [127,128].
The effectiveness of natural corrosion inhibitors depends on various factors, such as the type and concentration of the inhibitor, the nature of the metal surface, and the environmental conditions. Additionally, the mechanism of inhibition can differ between natural inhibitors, as some may form a protective film on the metal surface, while others may interact with the electrolyte solution to reduce the corrosion rate [129,130]. Natural corrosion inhibitors are compounds that occur naturally in nature and can be used to protect metal surfaces from corrosion. They are an eco-friendly alternative to synthetic inhibitors and can be obtained from a wide range of sources, including plants, animals, and minerals. Herein, we explore the differences between natural corrosion inhibitors. Table 1 represents the differences between natural inhibitors based on the source of the inhibitor and the mechanism of inhibition, in addition to its applications.

Table 1.
Represents the differences between natural corrosion inhibitors.
11. Future Outlooks
The use of corrosion inhibitors is an essential strategy for protecting metals and alloys from degradation in various industries. Organic inhibitors have been widely used due to their effectiveness, availability, and economic viability. The review paper entitled “Corrosion Inhibitors: Natural and Synthetic Organic Inhibitors” provides a comprehensive overview of both natural and synthetic organic inhibitors, their mechanisms of action, and recent advances in their development [145,146,147,148,149].
The future outlook for this topic is promising, and several areas of research are expected to contribute to the development of more effective and environmentally friendly corrosion inhibitors. Here are some potential future outlooks:
- Green corrosion inhibitors: With increasing concern about the environmental impact of chemical inhibitors, there is a growing interest in the development of green corrosion inhibitors. These inhibitors are derived from renewable resources, are biodegradable, and are less toxic to the environment. Researchers are exploring natural sources such as plant extracts, essential oils, and biopolymers as potential green inhibitors.
- Nanotechnology: Nanotechnology has shown promising results in the development of corrosion inhibitors due to its ability to enhance the protective properties of coatings and films. Researchers are exploring the use of nanoparticles as corrosion inhibitors or as carriers of inhibitors to enhance their efficiency.
- Computational studies: Computational studies have become an essential tool in the design and development of new inhibitors. With advances in computing power and molecular simulation techniques, researchers can better understand the interactions between inhibitors and metal surfaces and optimize inhibitor structures for maximum effectiveness.
- Synergistic effects: Combining different inhibitors to create synergistic effects is a promising area of research. Researchers are exploring the use of natural and synthetic inhibitors together, as well as the combination of inhibitors with other corrosion protection strategies such as coatings and cathodic protection.
- Application-specific inhibitors: Different industries and applications have specific requirements for corrosion inhibitors. In the future, more research is expected to focus on developing inhibitors tailored to specific industries, such as oil and gas, automotive, or aerospace, to optimize performance and cost-effectiveness.
In conclusion, the development of corrosion inhibitors is an active area of research, and the future outlook is promising. Researchers are exploring new materials, technologies, and methods to develop more effective, environmentally friendly, and application-specific inhibitors. The review paper provides a valuable resource for researchers and engineers working in this field.
12. Conclusions
Corrosion is a complex process that can occur in various forms and have different causes. The use of organic corrosion inhibitors has been widely studied and recognized as an effective solution for preventing metal corrosion. In this review article, various types of corrosion and classifications of organic corrosion inhibitors were discussed. The active functional groups in organic inhibitors were also discussed and their role in the inhibition of corrosion was emphasized. Estimating the efficiency of organic inhibitors is an important aspect in the selection and application of corrosion inhibitors. Several methods have been used for this purpose, including electrochemical techniques and surface analysis methods. Previous studies have shown that natural and synthetic organic inhibitors can be used effectively to prevent corrosion. However, the efficiency of these inhibitors depends on the type of metal, the environment, and the type of inhibitor used. Adsorption isotherms of organic inhibitors have also been studied, and they provide important information on the interaction between the inhibitor and the metal surface. The mechanisms of organic corrosion inhibitors were also discussed, and it was found that the inhibition of corrosion is related to the adsorption of the inhibitor on the metal surface. Finally, the kinetics of corrosion modeling was discussed, and it was found that this method provides important information on the kinetics of corrosion processes and the inhibition of corrosion by organic inhibitors. In conclusion, organic corrosion inhibitors play a crucial role in preventing metal corrosion. Further research is needed to better understand the mechanisms of organic inhibitors and to improve their efficiency. The development of new and improved organic inhibitors will help to reduce the impact of corrosion on metal surfaces and increase the lifespan of metal structures.
Author Contributions
Conceptualization, W.N.R.W.I.; formal analysis, W.K.A.-A.; investigation, A.A.A.-A.; resources, W.N.R.W.I.; data curation, W.N.R.W.I.; writing—original draft preparation, A.A.A.-A.; writing—review and editing, A.A.A.-A.; visualization, W.K.A.-A.; supervision, W.N.R.W.I.; project administration A.A.A.-A.; funding acquisition, W.N.R.W.I. All authors have read and agreed to the published version of the manuscript.
Funding
Universiti Kebangsaan Malaysia provided funding for a portion of the study under the following code: GUP-2020-012.
Data Availability Statement
Not applicable.
Acknowledgments
The support provided by the Universiti Kebangsaan Malaysia (UKM) is acknowledged by the authors. We would like to acknowledge also the joint UKM-UMT-RSU-AISSMS (1+3) research collaboration in the field of corrosion.
Conflicts of Interest
The authors claim they have no competing interests.
References
- Rani, B.E.; Bharathi Bai, J.B. Green Inhibitors for Corrosion Protection of Metals and Alloys: An Overview. Int. J. Corros. 2012, 2012, 380217. [Google Scholar] [CrossRef]
- Ahmed, E.S.; Junaid; Ganesh, G.M. A Comprehensive Overview on Corrosion in RCC and Its Prevention Using Various Green Corrosion Inhibitors. Buildings 2022, 12, 1682. [Google Scholar] [CrossRef]
- Tamalmani, K.; Husin, H. Review on Corrosion Inhibitors for Oil and Gas Corrosion Issues. Appl. Sci. 2020, 10, 3389. [Google Scholar] [CrossRef]
- Zakeri, A.; Bahmani, E.; Sabour Rouh Aghdam, A. Plant Extracts as Sustainable and Green Corrosion Inhibitors for Protection of Ferrous Metals in Corrosive Media: A Mini Review. Corros. Commun. 2022, 1, 6–17. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Sorour, A.A.; Verma, C. A Review on Corrosion Inhibitors for High-Pressure Supercritical CO2 Environment: Challenges and Opportunities. J. Pet. Sci. Eng. 2022, 215, 110695. [Google Scholar] [CrossRef]
- Werle, M. Natural and Synthetic Polymers as Inhibitors of Drug Efflux Pumps. Pharm. Res. 2008, 25, 500–511. [Google Scholar] [CrossRef]
- Finšgar, M.; Jackson, J. Application of Corrosion Inhibitors for Steels in Acidic Media for the Oil and Gas Industry: A Review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef]
- Raja, P.B.; Ismail, M.; Ghoreishiamiri, S.; Mirza, J.; Ismail, M.C.; Kakooei, S.; Rahim, A.A. Reviews on Corrosion Inhibitors: A Short View. Chem. Eng. Commun. 2016, 203, 11451156. [Google Scholar] [CrossRef]
- Olajire, A.A. Recent Advances on the Treatment Technology of Oil and Gas Produced Water for Sustainable Energy Industry-Mechanistic Aspects and Process Chemistry Perspectives. Chem. Eng. J. Adv. 2020, 4, 100049. [Google Scholar] [CrossRef]
- Li, L.; William, H.; Gibson Grant, T.; Wolfson Steve, L. Laboratory Investigation of Corrosion and Corrosion Protection of a Candidate Umbilical Material for Subsea Production Service. In CORROSION 2002; OnePetro: Denver, CO, USA, 2002. [Google Scholar]
- Verma, C.; Ebenso Eno, E.; Quraishi, M.A. Ionic Liquids as Green and Sustainable Corrosion Inhibitors for Metals and Alloys: An Overview. J. Mol. Liq. 2017, 233, 403–414. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Al-Azzawi, W.K.; Isahak, W.N.R.W. Isatin Schiff base as an effective corrosion inhibitor for mild steel in hydrochloric acid solution: Gravimetric, electrochemical, and computational investigation. Sci. Rep. 2022, 12, 17773. [Google Scholar] [CrossRef]
- Talat, R.; Asghar, M.A.; Tariq, I.; Akhter, Z.; Liaqat, F.; Nadeem, L.; Haider, A.; Ali, S. Evaluating the Corrosion Inhibition Efficiency of Pyridinium-Based Cationic Surfactants for EN3B Mild Steel in Acidic-Chloride Media. Coatings 2022, 12, 1701. [Google Scholar] [CrossRef]
- Xu, T.; Yang, Y.; Peng, X.; Song, J.; Pan, F. Overview of Advancement and Development Trend on Magnesium Alloy. J. Magnes. Alloys 2019, 7, 536–544. [Google Scholar] [CrossRef]
- Hosseini, M.; Fotouhi, L.; Ehsani, A.; Naseri, M. Enhancement of Corrosion Resistance of Polypyrrole Using Metal Oxide Nanoparticles: Potentiodynamic and Electrochemical Impedance Spectroscopy Study. J. Colloid Interface Sci. 2017, 505, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, B.S.; Abbass, M.K.; Mohsin, M.K.; Al-Azzawi, W.K.; Hanoon, M.M.; Al-Kaabi, M.H.H.; Shaker, L.M.; Ismael, M.A.; Al-Rubaie, A.A.A.; Kadhum, A.A.H.; et al. Corrosion Inhibition of Mild Steel in Hydrochloric Acid Environment Using Terephthaldehyde Based on Schiff Base: Gravimetric, Thermodynamic, and Computational Studies. Molecules 2022, 27, 4857. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, H.; Herrera, A.M.; Ruiz Reynoso, A.; Trinidad González, J.C.; González Morán, C.O.; Miranda Hernández, J.G.; Mandujano Ruiz, A.; Morales Hernández, J.; Orozco Cruz, R. Electrochemical Impedance Spectroscopy (EIS): A Review Study of Basic Aspects of the Corrosion Mechanism Applied to Steels. Electrochem. Impedance Spectrosc. 2020, 24, 137–144. [Google Scholar]
- Li, B.; Li, Y. Monte Carlo Simulation of Corrosion Inhibitor Adsorption on the Iron Surface. Langmuir 2006, 22, 3385–3392. [Google Scholar] [CrossRef]
- Liu, J.; Shao, W.; Gao, J.; Wang, J.; Gao, X. Machine Learning Models for Predicting the Inhibition Performance of Imidazoline Derivatives in CO2 Corrosion. Corros. Sci. 2019, 153, 181–190. [Google Scholar] [CrossRef]
- Ashworth, V. Corrosion Inhibitors. In Corrosion Inhibitors: Principles and Recent Applications; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–28. [Google Scholar] [CrossRef]
- Bardal, E.; Krotz, G. Corrosion Inhibitors. In Corrosion and Protection; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; pp. 317–357. [Google Scholar] [CrossRef]
- Cook, R.B. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Hernandez, H.; Ruiz Reynoso, A.; Trinidad, J.C.; González, C.O.; Miranda, J.G.; Mandujano, A.M.R. Electrochemical Impedance Spectroscopy (EIS): A Review Study of Basic Aspects of the Corrosion Mechanism Applied to Steels; IntechOpen: London, UK, 2019. [Google Scholar]
- Jawad, Q.; Zinad, D.S.; Salim, R.D.; Al-Amiery, A.A.; Gaaz, T.S.; Takriff, M.S.; Kadhum, A.A.H. Synthesis, Characterization, and Corrosion Inhibition Potential of Novel Thiosemicarbazone on Mild Steel in Sulfuric Acid Environment. Coatings 2019, 9, 729. [Google Scholar] [CrossRef]
- Hudson, J.B.; Brown, C.A.; Roberge, P.R. Corrosion Monitoring. In Comprehensive Materials Processing; Hashmi, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 4, pp. 305–325. [Google Scholar]
- Nitonye, S.; Ugboga, P. Analysis of the Effectiveness and Efficiency of the VA Solution on Offshore Pipelines and Ship Materials. Open J. Mar. Sci. 2019, 10, 16. [Google Scholar] [CrossRef]
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A.R. Review of Corrosive Environments for Copper and Its Corrosion Inhibitors. Arab. J. Chem. 2020, 13, 481–544. [Google Scholar] [CrossRef]
- Cinitha, A.; Umesha, P.K.; Iyer, N.R. An Overview of Corrosion and Experimental Studies on Corroded Mild Steel Compression Members. KSCE J. Civ. Eng. 2014, 18, 1735–1744. [Google Scholar] [CrossRef]
- Aziz, I.A.A.; Abdulkareem, M.H.; Annon, I.A.; Hanoon, M.M.; Al-Kaabi, M.H.H.; Shaker, L.M.; Alamiery, A.A.; Wan Isahak, W.N.R.; Takriff, M.S. Weight Loss, Thermodynamics, SEM, and Electrochemical Studies on N-2-Methylbenzylidene-4-antipyrineamine as an Inhibitor for Mild Steel Corrosion in Hydrochloric Acid. Lubricants 2022, 10, 23. [Google Scholar] [CrossRef]
- Smith, J.D. The Technique for Weight Loss Tests (WLT) to Estimate the Corrosion Current and Potential of a Metal in the Presence of an Organic Inhibitor. Materials 2021, 14, 652. [Google Scholar] [CrossRef]
- Noh, H.K.; Jin, Y.G. Corrosion Inhibition of Copper by Piperidine: Experimental and Computational Study. Metals 2019, 9, 822. [Google Scholar] [CrossRef]
- Büchler, M.; Kerimo, J.; Guillaume, F.; Smyrl, W.H. Fluorescence and Near-Field Scanning Optical Microscopy for Investigating Initiation of Localized Corrosion of Al 2024. J. Electrochem. Soc. 2000, 147, 3691. [Google Scholar] [CrossRef]
- Sullivan, J.; Mehraban, S.; Elvins, J. In Situ Monitoring of the Microstructural Corrosion Mechanisms of Zinc–Magnesium–Aluminium Alloys Using Time Lapse Microscopy. Corros. Sci. 2011, 53, 2208–2215. [Google Scholar] [CrossRef]
- Rios, E.C.; Zimer, A.M.; Pereira, E.C.; Mascaro, L.H. Analysis of AISI 1020 Steel Corrosion in Seawater by Coupling Electrochemical Noise and Optical Microscopy. Electrochim. Acta 2014, 124, 211–217. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy (XPS) Database. Available online: https://srdata.nist.gov/xps/ (accessed on 23 February 2023).
- Hussein, S.S.; Al-Hasani, I.D.D.; Abed, A.M.; Hanoon, M.M.; Shaker, L.M.; Al-Amiery, A.; Kadhum, A.A.H.; Roslam Wan Isahak, W.N. Antibacterial Corrosion Inhibitor for the Protection of Mild Steel in 1 M HCl Solution. Prog. Color Color. Coat. 2023, 16, 59–70. [Google Scholar]
- Alamiery, A.; Ali, J.M.; Isahak, W.N.R. Experimental Studies on the Corrosion Inhibition of Mild Steel by 1-(Phenylamino-1,3,4-thiadiazol-5-yl)-3-phenyl-3-oxopropan Complemented with DFT Modeling. KOM–Corros. Mater. Prot. J. 2022, 66, 7–15. [Google Scholar] [CrossRef]
- Betti, N.; Al-Azzawi, W.K.; Alamiery, A. Synthesis and Study of Corrosion Behavior of Terephthalaldehyde-Derived Schiff Base for Low-Carbon Steel in HCl: Experimental, Morphological and Theoretical Investigation. KOM–Corros. Mater. Prot. J. 2022, 66, 103–112. [Google Scholar] [CrossRef]
- Lopez, D.A.; Schreiner, W.H.D.; De Sánchez, S.R.; Simison, S.N. The Influence of Carbon Steel Microstructure on Corrosion Layers: An XPS and SEM Characterization. Appl. Surf. Sci. 2003, 207, 69–85. [Google Scholar] [CrossRef]
- Bruemmer, S.M.; Thomas, L.E. High-Resolution Analytical Electron Microscopy Characterization of Corrosion and Cracking at Buried Interfaces. Surf. Interface Anal. 2001, 31, 571–581. [Google Scholar] [CrossRef]
- Volovitch, P.; Allely, C.; Ogle, K. Understanding Corrosion via Corrosion Product Characterization: I. Case Study of the Role of Mg Alloying in Zn-Mg Coating on Steel. Corros. Sci. 2009, 51, 1251–1262. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Kadihum, A.; Mohamad, A.B.; How, C.K.; Junaedi, S. Inhibition of Mild Steel Corrosion in Sulfuric Acid Solution by New Schiff Base. Materials 2014, 7, 787–804. [Google Scholar] [CrossRef]
- Roberge, P.R. Handbook of Corrosion Engineering; McGraw Hill Professional: New York, NY, USA, 2012. [Google Scholar]
- Song, G.L.; Atrens, A. Understanding Corrosion Mechanisms, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Zhang, X.B.; Wang, D.; Li, X.G.; Hou, B.R.; Ke, W. Inhibition Mechanism of 1H-Benzotriazole on the Corrosion of Copper in Aerated NaCl Solution. Corros. Sci. 2009, 51, 1016–1025. [Google Scholar]
- Gabe, D.R.; Kim, Y.J. Scanning Electron Microscopy and Energy-Dispersive X-ray Spectroscopy for the Analysis of Corrosion and its Inhibition. In Handbook of Materials Failure Analysis with Case Studies from the Chemicals, Concrete and Power Industries; Butterworth-Heinemann: Oxford, UK, 2017; pp. 299–321. [Google Scholar] [CrossRef]
- Gomma, G.K.; Salem, M.A.; Zoromba, M.S.; Elsaid, M.O. Investigation of Corrosion Inhibition of Steel in Hydro-chloric Acid Solution Using Three Schiff Bases as Organic Inhibitors. Mater. Today Commun. 2021, 27, 102371. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Viejo, F.; Arrabal, R.; Matykina, E. Scanning Electron Microscopy with Backscattered Electron Imaging (SEM-BSE) and Energy Dispersive X-ray Spectrometry (EDS) Analysis Applied to Corrosion Research. Mater. Charact. 2011, 62, 956–967. [Google Scholar] [CrossRef]
- El-Enin, S.A.A.; Abo, S.A.; Amin, A. Review of Corrosion Inhibitors for Industrial Applications. Int. J. Eng. Res. Rev. 2015, 3, 127–145. [Google Scholar] [CrossRef]
- Fouda, A.E.S.; Abdel Nazeer, A.; El-Khateeb, A.Y.; Fakih, M. Cinnamon Plant Extract as Corrosion Inhibitor for Steel Used in Waste Water Treatment Plants and Its Biological Effect on Escherichia coli. J. Korean Chem. Soc. 2014, 58, 359–365. [Google Scholar] [CrossRef]
- Khan, M.A.A.; Irfan, O.M.; Djavanroodi, F.; Asad, M. Development of Sustainable Inhibitors for Corrosion Control. Sustainability 2022, 14, 9502. [Google Scholar] [CrossRef]
- El Mouaden, K.; El Ibrahimi, B.; Oukhrib, R.; Bazzi, L.; Hammouti, B.; Jbara, O.; Tara, A.; Chauhan, D.S.; Quraishi, M.A. Chitosan polymer as a green corrosion inhibitor for copper in sulfide-containing synthetic seawater. Int. J. Biol. Macromol. 2018, 119, 1311–1323. [Google Scholar] [CrossRef] [PubMed]
- Bouraoui, M.M.; Chettouh, S.; Chouchane, T.; Khellaf, N. Inhibition efficiency of cinnamon oil as a green corrosion inhibitor. J. Bio Tribo-Corros. 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Ostovari, A.; Hoseinieh, S.M.; Peikari, M.; Shadizadeh, S.R.; Hashemi, S.J. Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: A comparative study of the inhibition by henna and its constituents (Lawsone, Gallic acid, α-d-Glucose and Tannic acid). Corros. Sci. 2009, 51, 1935–1949. [Google Scholar] [CrossRef]
- Adewuyi, A.; Daramola, M.; Babalola, J. Corrosion inhibition of mild steel in hydrochloric acid solution by crude extracts of Vernonia amygdalina and Mangifera indica leaves. J. Mol. Liq. 2016, 215, 506–514. [Google Scholar]
- Mohd Sani, N.; Ahmad, F.; Yusof, F.; Shamsudin, R. Piper nigrum extract as a natural corrosion inhibitor for mild steel in 3.5% NaCl solution. J. Mol. Liq. 2018, 267, 475–483. [Google Scholar]
- Bhat, S.; Nayak, U. Corrosion inhibition of mild steel in 3.5% NaCl solution by polysaccharide extracted from marine algae Padina tetrastromatica. Int. J. Biol. Macromol. 2019, 129, 852–858. [Google Scholar]
- Ebenso, E.; Ajanaku, K.; Olasunkanmi, L. Inhibitory effect of Azadirachta indica and Moringa oleifera leaf extracts in combination with sodium molybdate on the corrosion of mild steel in hydrochloric acid. J. Mol. Liq. 2016, 215, 189–200. [Google Scholar]
- Bokati, K.S.; Dehghanian, C. Adsorption behavior of 1H-benzotriazole corrosion inhibitor on aluminum alloy 1050, mild steel and copper in artificial seawater. J. Environ. Chem. Eng. 2018, 6, 1613–1624. [Google Scholar] [CrossRef]
- Resende, G.O.; Teixeira, S.F.; Figueiredo, I.F.; Godoy, A.A.; Lougon, D.J.F.; Cotrim, B.A.; de Souza, F.C. Synthesis of 1,2,3-Triazole Derivatives and Its Evaluation as Corrosion Inhibitors for Carbon Steel. Int. J. Electrochem. 2019, 2019, 6759478. [Google Scholar] [CrossRef]
- Zarrouk, A.; Hammouti, B.; Al-Deyab, S.S.; Essassi, E.M.; Aouniti, A.; Ramdani, A. Corrosion Inhibition Performance of 3,5-Diamino-1,2,4-Triazole for Protection of Copper in Nitric Acid Solution. Int. J. Electrochem. 2012, 7, 5997–6011. [Google Scholar]
- Kumar, A.; Yadav, D.K.; Singh, A.K. A Comparative Study on Corrosion Inhibition of Mild Steel by Catechin and 2-Mercaptobenzothiazole in Hydrochloric Acid. J. Mater. Sci. 2016, 51, 7588–7600. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Egbedi, N.O.; Umoren, S.A. Inhibitive Action of Vanillin on the Corrosion of Mild Steel in Acidic Media. J. Ind. Eng. Chem. 2013, 19, 167–174. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Cheng, Y.; Jiang, L.; Zhai, Y. Inhibitory Effect of Natural and Synthetic Inhibitors on the Corrosion of Carbon Steel in Acidic Solutions. J. Mater. Sci. 2018, 53, 15838–15851. [Google Scholar] [CrossRef]
- El-Etre, A.Y.; Abdallah, M.; El-Tantawy, Z.E. Inhibition of Aluminum Corrosion Using Opuntia Extract. Corros. Sci. 2008, 50, 2993–3000. [Google Scholar] [CrossRef]
- Mekidiche, L.; Chibani, S.; Harek, Y.; Hammouti, B. Corrosion Inhibition of Mild Steel in 1 M HCl Solution by Synthetic and Natural Inhibitors. J. Mater. Environ. Sci. 2018, 9, 2849–2860. [Google Scholar]
- Muster, T.; Rohwerder, M.; Stratmann, M. Quantitative XPS Analysis of Corrosion Inhibitor Films on Iron. Surf. Sci. 2005, 595, 103–112. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, X.; Li, X. Progress in Surface Science Research for Corrosion Inhibition. Prog. Org. Coat. 2014, 77, 548–555. [Google Scholar] [CrossRef]
- Walczak, M.S.; Morales-Gil, P.; Lindsay, R. Determining Gibbs Energies of Adsorption from Corrosion Inhibition Efficiencies: Is It a Reliable Approach? Corros. Sci. 2019, 155, 182–185. [Google Scholar] [CrossRef]
- Tobiason, J.E.; Wesolowski, D.J.; McCormick, S.L.W.; Lewis, E.A. Molecular Dynamics Simulations of Inhibitor Adsorption on the Iron (110) Surface in Aqueous Solutions. J. Phys. Chem. B 2008, 112, 5716–5725. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, Z. Density Functional Theory Study of the Adsorption Behavior of 1,2,3-Benzotriazole on Copper Surface. Appl. Surf. Sci. 2018, 458, 452–458. [Google Scholar] [CrossRef]
- Shreir, L.L.; Jarman, R.A.; Burstein, G.T.; Shimada, H. Corrosion: Fundamentals, Testing, and Protection; Elsevier: Oxford, UK, 2013. [Google Scholar]
- Stern, M.; Geary, A.L. Electrochemical Polarization: I. A Theoretical Analysis of the Shape of Polarization Curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Al-Moubaraki, A.H.; Al-Mayouf, A.M.; Al-Hashem, A. Corrosion Inhibition of Carbon Steel in Acidic Media Using Some Synthetic and Natural Inhibitors. J. Mater. Sci. 2014, 49, 1191–1201. [Google Scholar] [CrossRef]
- Fox, P.G.; Bradley, P.A. 1:2:4-Triazole as a Corrosion Inhibitor for Copper. Corros. Sci. 1980, 20, 643–649. [Google Scholar] [CrossRef]
- Ituen, E.; Akaranta, O.; James, A. Evaluation of Performance of Corrosion Inhibitors Using Adsorption Isotherm Models: An Overview. Chem. Sci. Int. J. 2017, 18, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhu, S.; Zhang, S.; He, Q.; Li, W. Theoretical Studies of Three Triazole Derivatives as Corrosion Inhibitors for Mild Steel in Acidic Medium. Corros. Sci. 2014, 87, 366–375. [Google Scholar] [CrossRef]
- Mihajlović, M.B.P.; Radovanović, M.B.; Tasić, Ž.Z.; Antonijević, M.M. Imidazole Based Compounds as Copper Corrosion Inhibitors in Seawater. J. Mol. Liq. 2017, 225, 127–136. [Google Scholar] [CrossRef]
- Frankel, G.S. Corrosion Inhibitors. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 1–7. [Google Scholar]
- Abd El Rehim, S.S.; Negm, N.A. Organic Corrosion Inhibitors: A Comprehensive Review. J. Mater. Sci. Res. 2013, 2, 76–84. [Google Scholar]
- Revie, R.W.; Uhlig, H.H. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Shivaraj, T.; Karthick, R.; Jeyakumar, M.S.; Anandan, S. Recent Developments in Organic Corrosion Inhibitors for Mild Steel in Different Environments: A Review. J. Ind. Eng. Chem. 2014, 20, 424–438. [Google Scholar]
- Chen, S.; Zhang, D.; Zhou, G. Advances in research on organic corrosion inhibitors: A review. J. Mol. Liq. 2014, 192, 97–109. [Google Scholar] [CrossRef]
- Roberge, P.R. Handbook of Corrosion Engineering, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Al-Kharafi, F.M.; Al-Hajjar, F.H.; Katrib, A. 3-Phenyl-1,2,4-triazol-5-one as a Corrosion Inhibitor for Copper. Corros. Sci. 1986, 26, 257–264. [Google Scholar] [CrossRef]
- Gurjar, S.; Sharma, S.K.; Sharma, A.; Ratnani, S. Performance of Imidazolium Based Ionic Liquids as Corrosion Inhibitors in Acidic Medium: A Review. Appl. Surf. Sci. Adv. 2021, 6, 100170. [Google Scholar] [CrossRef]
- Chaouiki, A.; Chafiq, M.; Ko, Y.G.; Al-Moubaraki, A.H.; Thari, F.Z.; Salghi, R.; Karrouchi, K.; Bougrin, K.; Ali, I.H.; Lgaz, H. Adsorption Mechanism of Eco-Friendly Corrosion Inhibitors for Exceptional Corrosion Protection of Carbon Steel: Electrochemical and First-Principles DFT Evaluations. Metals 2022, 12, 1598. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, P.; Gao, L.; Wang, F.; Wang, H. A Review on Application of X-ray Photoelectron Spectroscopy in Corrosion Inhibition. J. Mater. Sci. Technol. 2019, 35, 717–726. [Google Scholar] [CrossRef]
- Wang, H.-L.; Fan, H.-B.; Zheng, J.-S. Corrosion Inhibition of Mild Steel in Hydrochloric Acid Solution by a Mercapto-Triazole Compound. Mater. Chem. Phys. 2003, 77, 655–661. [Google Scholar]
- Al-Azzawi, W.K.; Hussein, S.S.; Salih, S.M.; Zinad, D.S.; Al-Azzawi, R.K.; Hanoon, M.M.; Al-Amiery, A.; Kadhum, A.A.H.; Takriff, M.S. Efficient Protection of Mild Steel Corrosion in Hydrochloric Acid Using 3-(5-Amino-1, 3, 4-thiadiazole-2yl)-2H-chromen-2-one, a Coumarin Derivative Bearing a 1, 3, 4-thiadiazole Moiety: Gravimetrical Techniques, Computational and Thermodynamic Investigations. Prog. Color Colorants Coat. 2023, 16, 97–111. [Google Scholar] [CrossRef]
- Alamiery, A.A. Study of Corrosion Behavior of N’-(2-(2-oxomethylpyrrol-1-yl)ethyl)piperidine for Mild Steel in the Acid Environment. Biointerface Res. Appl. Chem. 2022, 12, 3638–3646. [Google Scholar] [CrossRef]
- Alamiery, A.; Mohamad, A.B.; Kadhum, A.A.H.; Takriff, M.S. Comparative Data on Corrosion Protection of Mild Steel in HCl Using Two New Thiazoles. Data Brief 2022, 40, 107838. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.M.; Sayyid, F.F.; Betti, N.; Shaker, L.M.; Hanoon, M.M.; Alamiery, A.A.; Kadhum, A.A.H.; Takriff, M.S. Inhibition of Mild Steel Corrosion in Hydrochloric Acid Environment by 1-Amino-2-Mercapto-5-(4-(pyrrol-1-yl)phenyl)-1,3,4-triazole. South Afr. J. Chem. Eng. 2022, 39, 42–51. [Google Scholar] [CrossRef]
- Alamiery, A.A. Investigations on Corrosion Inhibitory Effect of Newly Quinoline Derivative on Mild Steel in HCl Solution Complemented with Antibacterial Studies. Biointerface Res. Appl. Chem. 2022, 12, 1561–1568. [Google Scholar] [CrossRef]
- Alkadir Aziz, I.A.; Annon, I.A.; Abdulkareem, M.H.; Hanoon, M.M.; Alkaabi, M.H.; Shaker, L.M.; Alamiery, A.A.; Wan Isahak, W.N.R.; Takriff, M.S. Insights into Corrosion Inhibition Behavior of a 5-Mercapto-1,2,4-triazole Derivative for Mild Steel in Hydrochloric Acid Solution: Experimental and DFT Studies. Lubricants 2021, 9, 122. [Google Scholar] [CrossRef]
- Alamiery, A. Short report of mild steel corrosion in 0.5 M H2SO4 by 4-ethyl-1-(4-oxo-4-phenylbutanoyl)thiosemicarbazide. J. Tribol. 2021, 30, 90–99. [Google Scholar] [CrossRef]
- Alamiery, A.A.; Isahak, W.N.R.W.; Takriff, M.S. Inhibition of Mild Steel Corrosion by 4-Benzyl-1-(4-Oxo-4-Phenylbutanoyl)Thiosemicarbazide: Gravimetrical, Adsorption and Theoretical Studies. Lubricants 2021, 9, 93. [Google Scholar] [CrossRef]
- Dawood, M.A.; Alasady, Z.M.K.; Abdulazeez, M.S.; Ahmed, D.S.; Sulaiman, G.M.; Kadhum, A.A.H.; Shaker, L.M.; Alamiery, A.A. The Corrosion Inhibition Effect of a Pyridine Derivative for Low Carbon Steel in 1 M HCl Medium: Complemented with Antibacterial Studies. Int. J. Corros. Scale Inhib. 2021, 10, 1766–1782. [Google Scholar] [CrossRef]
- Alamiery, A. Corrosion Inhibition Effect of 2-N-Phenylamino-5-(3-Phenyl-3-oxo-1-propyl)-1,3,4-oxadiazole on Mild Steel in 1 M Hydrochloric Acid Medium: Insight from Gravimetric and DFT Investigations. Mater. Sci. Energy Technol. 2021, 4, 398–406. [Google Scholar] [CrossRef]
- Alamiery, A.A. Anticorrosion Effect of Thiosemicarbazide Derivative on Mild Steel in 1 M Hydrochloric Acid and 0.5 M Sulfuric Acid: Gravimetrical and Theoretical Studies. Mater. Sci. Energy Technol. 2021, 4, 263–273. [Google Scholar] [CrossRef]
- Alamiery, A.A.; Isahak, W.N.R.W.; Aljibori, H.S.S.; Al-Asadi, H.A.; Kadhum, A.A.H. Effect of the Structure, Immersion Time and Temperature on the Corrosion Inhibition of 4-Pyrrol-1-yl-N-(2,5-dimethyl-pyrrol-1-yl)benzoylamine in 1.0 M HCl Solution. Int. J. Corros. Scale Inhib. 2021, 10, 700–713. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Mohamad, A.B.; Kadhum, A.A.H.; Allami, T.; Yusop, R.M.; Takriff, M.S. Experimental and Theoretical Study on the Corrosion Inhibition of Mild Steel by Nonanedioic Acid Derivative in Hydrochloric Acid Solution. Sci. Rep. 2022, 12, 4705. [Google Scholar] [CrossRef]
- Alamiery, A.; Mahmoudi, E.; Allami, T. Corrosion Inhibition of Low-Carbon Steel in Hydrochloric Acid Environment Using a Schiff Base Derived from Pyrrole: Gravimetric and Computational Studies. Int. J. Corros. Scale Inhib. 2021, 10, 749–765. [Google Scholar] [CrossRef]
- Eltmimi, A.J.M.; Alamiery, A.; Allami, A.J.; Yusop, R.M.; Kadhum, A.A.H.; Allami, T. Inhibitive Effects of a Novel Efficient Schiff Base on Mild Steel in Hydrochloric Acid Environment. Int. J. Corros. Scale Inhib. 2021, 10, 634–648. [Google Scholar] [CrossRef]
- Alamiery, A.; Shaker, L.M.; Allami, T.; Kadhum, A.H.; Takriff, M.S. A Study of Acidic Corrosion Behavior of Furan-Derived Schiff Base for Mild Steel in Hydrochloric Acid Environment: Experimental, and Surface Investigation. Mater. Today Proc. 2021, 44, 2337–2341. [Google Scholar] [CrossRef]
- Al-Baghdadi, S.B.; Al-Amiery, A.A.; Gaaz, T.S.; Kadhum, A.A.H. Terephthalohydrazide and Isophthalo-Hydrazide as New Corrosion Inhibitors for Mild Steel in Hydrochloric Acid: Experimental and Theoretical Approaches. Koroze Ochr. Mater. 2021, 65, 12–22. [Google Scholar] [CrossRef]
- Hanoon, M.M.; Resen, A.M.; Shaker, L.M.; Kadhum, A.A.H.; Al-Amiery, A.A. Corrosion Investigation of Mild Steel in Aqueous Hydrochloric Acid Environment Using n-(Naphthalen-1yl)-1-(4-pyridinyl)methanimine Complemented with Antibacterial Studies. Biointerface Res. Appl. Chem. 2021, 11, 9735–9743. [Google Scholar] [CrossRef]
- Al-Baghdadi, S.; Gaaz, T.S.; Al-Adili, A.; Al-Amiery, A.A.; Takriff, M.S. Experimental Studies on Corrosion Inhibition Performance of Acetylthiophene Thiosemicarbazone for Mild Steel in HCl Complemented with DFT Investigation. Int. J. Low-Carbon Technol. 2021, 16, 181–188. [Google Scholar] [CrossRef]
- Al-Amiery, A.A. Anti-Corrosion Performance of 2-Isonicotinoyl-N-phenylhydrazinecarbothioamide for Mild Steel Hydrochloric Acid Solution: Insights from Experimental Measurements and Quantum Chemical Calculations. Surf. Rev. Lett. 2021, 28, 2050058. [Google Scholar] [CrossRef]
- Abdulazeez, M.S.; Abdullahe, Z.S.; Dawood, M.A.; Handel, Z.K.; Mahmood, R.I.; Osamah, S.; Kadhum, A.H.; Shaker, L.M.; Al-Amiery, A.A. Corrosion Inhibition of Low Carbon Steel in HCl Medium Using a Thiadiazole Derivative: Weight Loss, DFT Studies and Antibacterial Studies. Int. J. Corros. Scale Inhib. 2021, 10, 1812–1828. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Sayyid, F.F.; Betti, N.; Hanoon, M.M.; Al-Amiery, A.A.; Kadhum, A.A.H.; Takriff, M.S. Inhibition Evaluation of 5-(4-(1H-Pyrrol-1-yl)phenyl)-2-mercapto-1,3,4-oxadiazole for the Corrosion of Mild Steel in an Acidic Environment: Thermodynamic and DFT Aspects. Tribologia 2021, 38, 39–47. [Google Scholar] [CrossRef]
- Abdulsahib, Y.M.; Eltmimi, A.J.M.; Alhabeeb, S.A.; Hanoon, M.M.; Al-Amiery, A.A.; Allami, T.; Kadhum, A.A.H. Experimental and Theoretical Investigations on the Inhibition Efficiency of N-(2,4-Dihydroxytolueneylidene)-4-methylpyridin-2-amine for the Corrosion of Mild Steel in Hydrochloric Acid. Int. J. Corros. Scale Inhib. 2021, 10, 885–899. [Google Scholar] [CrossRef]
- Khudhair, A.K.; Mustafa, A.M.; Hanoon, M.M.; Al-Amiery, A.; Shaker, L.M.; Gazz, T.; Mohamad, A.B.; Kadhum, A.H.; Takriff, M.S. Experimental and Theoretical Investigation on the Corrosion Inhibitor Potential of N-MEH for Mild Steel in HCl. Prog. Color Colorants Coat. 2021, 15, 111–122. [Google Scholar] [CrossRef]
- Zinad, D.S.; Salim, R.D.; Betti, N.; Shaker, L.M.; AL-Amiery, A.A. Comparative Investigations of the Corrosion Inhibition Efficiency of a 1-phenyl-2-(1-phenylethylidene)hydrazine and its Analog Against Mild Steel Corrosion in Hydrochloric Acid Solution. Prog. Color Colorants Coat. 2021, 15, 53–63. [Google Scholar]
- Salim, R.D.; Betti, N.; Hanoon, M.; Al-Amiery, A.A. 2-(2,4-Dimethoxybenzylidene)-N-Phenylhydrazinecarbothioamide as an Efficient Corrosion Inhibitor for Mild Steel in Acidic Environment. Prog. Color Colorants Coat. 2021, 15, 45–52. [Google Scholar]
- Al-Amiery, A.A.; Shaker, L.M.; Kadhum, A.H.; Takriff, M.S. Exploration of Furan Derivative for Application as Corrosion Inhibitor for Mild Steel in Hydrochloric Acid Solution: Effect of Immersion Time and Temperature on Efficiency. Mater. Today Proc. 2021, 42, 2968–2973. [Google Scholar] [CrossRef]
- Resen, A.M.; Hanoon, M.M.; Alani, W.K.; Kadhim, A.; Mohammed, A.A.; Gaaz, T.S.; Kadhum, A.A.H.; Al-Amiery, A.A.; Takriff, M.S. Exploration of 8-Piperazine-1-ylmethylumbelliferone for Application as a Corrosion Inhibitor for Mild Steel in Hydrochloric Acid Solution. Int. J. Corros. Scale Inhib. 2021, 10, 368–387. [Google Scholar] [CrossRef]
- Hanoon, M.M.; Resen, A.M.; Al-Amiery, A.A.; Kadhum, A.A.H.; Takriff, M.S. Theoretical and Experimental Studies on the Corrosion Inhibition Potentials of 2-((6-Methyl-2-Ketoquinolin-3-yl)Methylene) Hydrazinecarbothioamide for Mild Steel in 1 M HCl. Prog. Color Colorants Coat. 2021, 15, 21–33. [Google Scholar]
- Hashim, F.G.; Salman, T.A.; Al-Baghdadi, S.B.; Gaaz, T.; Al-Amiery, A.A. Inhibition Effect of Hydrazine-Derived Coumarin on a Mild Steel Surface in Hydrochloric Acid. Tribologia 2020, 37, 45–53. [Google Scholar] [CrossRef]
- Resen, A.M.; Hanoon, M.; Salim, R.D.; Al-Amiery, A.A.; Shaker, L.M.; Kadhum, A.A.H. Gravimetrical, Theoretical, and Surface Morphological Investigations of Corrosion Inhibition Effect of 4-(Benzoimidazole-2-yl) Pyridine on Mild Steel in Hydrochloric Acid. Koroze Ochr. Mater. 2020, 64, 122–130. [Google Scholar] [CrossRef]
- Salman, A.Z.; Jawad, Q.A.; Ridah, K.S.; Shaker, L.M.; Al-Amiery, A.A. Selected BIS-Thiadiazole: Synthesis and Corrosion Inhibition Studies on Mild Steel in HCL Environment. Surf. Rev. Lett. 2020, 27, 2050014. [Google Scholar] [CrossRef]
- Abdel-Gaber, A.; Khamis, E.; Al-Sabagh, A.; Zaki, M. Inhibition of aluminum corrosion using Opuntia extract. Corros. Sci. 2006, 48, 2765–2779. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Hashemi, S.J.; Shadizadeh, S.R.; Shirazi, M. Comparison of corrosion inhibition effect of imidazole and benzimidazole derivatives on copper in 3. 5% NaCl solution. Mater. Chem. Phys. 2004, 87, 267–272. [Google Scholar] [CrossRef]
- Haque, M.E.; Rahman, M.A.; Islam, M.M.; Ali, M.E. Corrosion inhibition of mild steel in 1 M HCl solution by some organic compounds. J. Mater. Res. Technol. 2012, 1, 33–39. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Li, G.; Li, S.; Gao, X.; Li, J.; Sun, C. A comparative study on the inhibition performance of four synthesized imidazoline derivatives as corrosion inhibitors for mild steel in 1 M hydrochloric acid. J. Mol. Liq. 2020, 306, 112899. [Google Scholar] [CrossRef]
- Wang, H.; Gao, L.; Wang, F.; Hou, B. Comparative study of inhibition effect of lignin and modified lignin on mild steel corrosion in acidic medium. J. Mol. Liq. 2016, 222, 600–608. [Google Scholar] [CrossRef]
- Bhat, S.K.; Pujari, P.H. Corrosion Inhibition of Mild Steel by Some Natural Products in Acidic Media. J. Mater. Sci. 2001, 36, 467–474. [Google Scholar] [CrossRef]
- Abdel-Gaber, A.; Abd-El-Nabey, B.; Sidahmed, I.; El-Zayady, M.A. The Role of Some Organic Compounds as Corrosion Inhibitors for Steel in Acidic Solutions. Appl. Surf. Sci. 2002, 198, 388–397. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Huang, X.; Wang, L. Evaluation of Tannins as Natural Corrosion Inhibitors for Mild Steel in Acidic Media. Corros. Sci. 2011, 53, 2364–2371. [Google Scholar] [CrossRef]
- Souza, M.T.; Santos, A.O.; Silva, C.C.; Souza, R.A.M.A. Eco-Friendly Corrosion Inhibitors: A Review of Recent Progress in Marine and Coastal Biofouling Control. Mar. Drugs 2020, 18, 136. [Google Scholar] [CrossRef]
- Mohamed, A.M. Eco-Friendly Corrosion Inhibitors from Natural Sources: A Review. J. Environ. Chem. Eng. 2021, 9, 104877. [Google Scholar] [CrossRef]
- Oliveira Filho, J.G.d.; Silva, G.d.C.; Oldoni, F.C.A.; Miranda, M.; Florencio, C.; Oliveira, R.M.D.d.; Gomes, M.d.P.; Ferreira, M.D. Edible Coating Based on Carnauba Wax Nanoemulsion and Cymbopogon martinii Essential Oil on Papaya Postharvest Preservation. Coatings 2022, 12, 1700. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.; Liu, J.; Luo, H.; Zou, W. Systematic Review of Actinomycetes in the Baijiu Fermentation Microbiome. Foods 2022, 11, 3551. [Google Scholar] [CrossRef]
- Farhadian, A.; Rahimi, A.; Safaei, N.; Shaabani, A.; Abdouss, M.; Alavi, A. A Theoretical and Experimental Study of Castor Oil-Based Inhibitor for Corrosion Inhibition of Mild Steel in Acidic Medium at Elevated Temperatures. Corros. Sci. 2020, 175, 108871. [Google Scholar] [CrossRef]
- Biswas, A.; Pal, S.; Udayabhanu, G. Experimental and Theoretical Studies of Xanthan Gum and Its Graft Co-Polymer as Corrosion Inhibitor for Mild Steel in 15% HCl. Appl. Surf. Sci. 2015, 353, 173–183. [Google Scholar] [CrossRef]
- Rafiee, J.S.; Mohamed, A.M.A.; Sulaiman, A.; Abdullah, M.R. Review on Natural Corrosion Inhibitors for Oil and Gas Industries. J. Mater. Environ. Sci. 2015, 6, 1725–1739. [Google Scholar]
- Khayat, M.S.; Ebenso, E.E.; Kabanda, M.M. Green Inhibitors for Acid Corrosion of Metals: A Review. J. Ind. Eng. Chem. 2014, 20, 1149–1169. [Google Scholar]
- Quraishi, M.A.; Al-Mulhem, N.A.; Mohamed, A.M.A. Eco-Friendly Inhibitors for Corrosion Protection of Metals and Alloys: An Overview. Int. J. Electrochem. Sci. 2010, 5, 704–728. [Google Scholar]
- Shafaei, T.; Bahramian, A.; Khaki, S.D. Natural Corrosion Inhibitors in the Oil Industry: A Review. Oil Gas Sci. Technol. 2016, 71, 86. [Google Scholar]
- Al-Sabagh, A.M.; Al-Azizi, N.M.; Quraishi, M.A. Corrosion Inhibition of Mild Steel in Acidic Solution Using Essential Oils. Arab. J. Chem. 2017, 3, S67–S76. [Google Scholar]
- Abd El-Rehim, S.S.; Al-Sarawy, A.A.; Diab, M.A. Natural Essential Oils as Corrosion Inhibitors for Steel in HCl Solution. Mater. Chem. Phys. 2016, 184, 109–117. [Google Scholar] [CrossRef]
- Javid, M.; Zahoor, A.F.; Sadiq, M.; Zahid, M.; Ali, A.; Abbas, S.M. Investigation of Corrosion Inhibition Behavior of a Novel Schiff Base for Carbon Steel in HCl. Arab. J. Chem. 2018, 11, 1079–1089. [Google Scholar] [CrossRef]
- Hossain, M.A.; Yasmin, T.; Khan, M.K.; Rafique, M.S.; Siddique, M.A.B. Corrosion Inhibition Performance of 1,3-Dimethylimidazolium Chloride and Its Synergistic Effect with Sodium Nitrite for Mild Steel in HCl Solution. VJChroma 2022, 61, 15–42. [Google Scholar] [CrossRef]
- Brycki, B.E.; Kowalczyk, I.H.; Szulc, A.; Kaczerewska, O.; Pakiet, M. Organic Corrosion Inhibitors. In Corrosion Inhibitors, Principles and Recent Applications; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Raja, P.B.; Sethuraman, M.G.; Shanmugam, N. Corrosion Inhibitors: A Review. Rev. Adv. Mater. Sci. 2017, 49, 185–218. [Google Scholar] [CrossRef]
- Idris, A.M.; Mohamad, A.A. Corrosion Inhibitors: Principles, Mechanisms and Applications; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Ahmad, Z.; Riaz, S. Recent Advances in the Development of Organic Corrosion Inhibitors: A Review. J. Mol. Liq. 2019, 293, 111419. [Google Scholar] [CrossRef]
- Popova, A.; Sokolova, E.; Raicheva, S.; Christov, M.; Vasilev, A. AC and DC Study of the Temperature Effect on Mild Steel Corrosion in Acid Media in the Presence of Benzimidazole Derivatives. Corros. Sci. 2003, 45, 33–58. [Google Scholar] [CrossRef]
- Raja, P.B.; Sethuraman, M.G.; Shanmugam, N. Corrosion Inhibition of Mild Steel in Acidic Media by Carissa Carandas Extract: Experimental and Theoretical Approach. J. Mol. Liq. 2018, 250, 56–69. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).