Abstract
Cytochrome P450 (CYP) enzymes are commonly involved in drug metabolism, and genetic variation in the genes encoding CYPs are associated with variable drug response. While genotype-guided therapy has been clinically implemented in adults, these associations are less well established for pediatric patients. In order to understand the frequency of pediatric exposures to drugs with known CYP interactions, we compiled all actionable drug–CYP interactions with a high level of evidence using Clinical Pharmacogenomic Implementation Consortium (CPIC) data and surveyed 10 years of electronic health records (EHR) data for the number of children exposed to CYP-associated drugs. Subsequently, we performed a focused literature review for drugs commonly used in pediatrics, defined as more than 5000 pediatric patients exposed in the decade-long EHR cohort. There were 48 drug–CYP interactions with a high level of evidence in the CPIC database. Of those, only 10 drugs were commonly used in children (ondansetron, oxycodone, codeine, omeprazole, lansoprazole, sertraline, amitriptyline, citalopram, escitalopram, and risperidone). For these drugs, reports of the drug–CYP interaction in cohorts including children were sparse. There are adequate data for implementation of genotype-guided therapy for children for three of the 10 commonly used drugs (codeine, omeprazole and lansoprazole). For the majority of commonly used drugs with known CYP interactions, more data are required to support pharmacogenomic implementation in children.
1. Introduction
The cytochrome P450 (CYP) families of enzymes are major players for phase I metabolism of drugs. It is estimated that the CYP3A family metabolizes approximately one-third of all drugs, CYP2D6 about one-fourth of all drugs, and other CYPs over one-tenth of all drugs [1]. For many of these enzymes, there are both common and rare variants in the genes encoding the enzymes that lead to functional alterations, causing gain of function or loss of function phenotypes. These naturally occurring variants lead to differences in drug and metabolite concentrations. In instances where the therapeutic window is narrow and a critical step in the drug metabolism pathway involves a polymorphic gene, there is a potential for a drug–gene interaction (DGI). In other words, genotype may explain individual variability in response. In these instances, pre-prescription testing to determine the genotype may be a viable strategy for selecting the right drug and dose for individual patients, with the hopes of maximizing therapeutic benefit while minimizing risk of untoward effects [2,3,4,5,6,7,8,9,10,11].
Given the motivation for use of genomic information to guide prescribing and the large number of studies to date exploring potential DGIs, the Clinical Pharmacogenomic Implementation Consortium (CPIC) and Pharmacogenomics Knowledge Base (PharmGKB) resources have developed schemas for critical evaluation of evidence for drug–gene associations and clinical utility of genotype information for prescribers [12,13]. PharmGKB levels of evidence range from 1 (highest) to 4 (lowest), and are assigned based on robustness of the data in support of each specific DGI. For example, DGIs are assigned to level 1 if associations are replicated in multiple distinct cohorts with statistically significant p-values, while level 4 evidence comes from case reports or in vitro data. CPIC levels add a focus on clinical actionability and prioritization for implementation. For example, level A DGIs have a high level of supporting evidence and there is a recommendation to change prescribing based on genetic information. Given their key role in drug metabolism, CYP enzymes represent a large portion of the DGIs cataloged in these resources; genes coding for CYPs are involved in 1241 of the 7395 DGIs on PharmGKB, and 104 of the 352 on the CPIC website [14,15].
Absent from either the PharmGKB or CPIC evaluation criteria are considerations for specific patient populations. In particular, for pediatric patients, the indications for use of individual drugs are often different than in adults, side effect profiles vary by age, and ontogeny of the CYP enzymes, particularly for infants, can impact the drug–gene relationship [16]. Furthermore, compared to the body of evidence from studies in adults, there is a paucity of research supporting the use of genotype to guide prescribing in children.
Due to the potential for clinical pharmacogenomic testing and the lack of pediatric specific data, the goals of this manuscript are as follows: first, to use a single-center, longitudinal, electronic health record (EHR) cohort to determine the frequency of pediatric exposures to medications with established interactions with CYP enzymes (i.e., define the number of unique pediatric patients exposed to drugs with CPIC level A or B and PharmGKB level 1 or 2 evidence for the drug–CYP association); to review the current pediatric literature for CYP-associated drugs most frequently used in pediatric patients; and to make recommendations regarding implementation of CYP pharmacogenomics for pediatric patients.
2. Results
2.1. CYP-Associated Drugs with High Levels of Evidence
The CPIC drug–gene pairs table included a total of 352 DGIs, representing 223 unique drugs and 127 unique genes. Filtering for CYP genes and a high level of CPIC and PharmGKB evidence resulted in 48 actionable drug-CYP pairs, including 41 unique drug names (Figure 1).
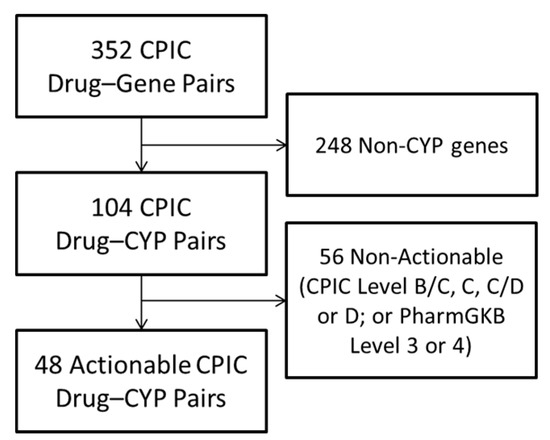
Figure 1.
Selection of Actionable Drug–Gene Pairs. Of the 352 Drug–gene pairs categorized on the Clinical Pharmacogenomics Implementation Consortium (CPIC) website, 104 represented genes encoding Cytochrome P450 (CYP) enzymes, and 48 (representing 41 unique drugs) were deemed actionable, defined as having CPIC Level A or B and PharmGKB level 1 or 2 evidence. DGI—Drug–gene interaction; PharmGKB—Pharmacogenomics Knowledgebase.
2.2. Pediatric Exposures to 41 CYP-Associated Drugs
Using the Synthetic Derivative (SD), the Vanderbilt University Medical Center de-identified database of EHR data, we performed a search for the number of unique individuals exposed to each of the 41 CYP-associated drugs when age <18 years and identified subsets of CYP-associated drugs that are used commonly, moderately and rarely in pediatric patients (Table 1). Over 5000 children were exposed to the drugs over the decade-long time period of the query for 10 of the CYP-associated drugs (ondansetron, oxycodone, codeine, omeprazole, lansoprazole, sertraline, amitriptyline, citalopram, risperidone and escitalopram). The age distribution at the time of first exposure varied by drug, with some drugs (e.g., methadone) predominately used in infants, others in early childhood (e.g., ondansetron), and others in adolescence (e.g., sertraline). The race and ethnicity of the exposed patients, obtained from administrative data, approximated that of the patient population as a whole, except for drugs with specific indications that are disproportionately present by race. For example, the antiviral drugs nevirapine and efavirenz, used to treat HIV, had higher percentages of Black/African American patients, consistent with higher rates of HIV infection seen in these children, although the majority of children exposed to these drugs were of white or unknown race [17].

Table 1.
Number of Pediatric Patients Exposed to each CYP-Associated Drug in a 10-year Period.
2.3. Review of the Literature Supporting Drug–CYP Interactions for Drugs Commonly Used in Children
We performed a review of the literature, searching for primary data sources with pediatric research participants for each of the 10 CYP-associated drugs most frequently used in children. A summary of the literature reviewed is provided in Table 2. In all, 38 unique relevant manuscripts that involved pediatric participants were included; two manuscripts described multiple DGIs, and data relevant for each are included in the table. The number of manuscripts supporting each CYP–drug interaction varied from 0 (for ondansetron and sertraline) to 15 (for risperidone). The number of individuals comprising the cohort for each study also varied, from case reports of one to three individuals (six such reports for codeine and one for amitriptyline) to cohorts of 19 to 830 children. The median number of study participants across all studies was 72 individuals (interquartile range (20–120) individuals), and for cohort studies was 84 (38–133) individuals. Despite the small cohort size, the majority (20/30) of cohort studies reported at least one significant association of a CYP to a pharmacokinetic or pharmacodynamic outcome.

Table 2.
Evidence for CYP–drug Interactions in Pediatrics.
2.3.1. Ondansetron
Ondansetron is a 5-hydroxytryptamine type 3 (5-HT3) receptor antagonist used in adults and children to prevent nausea and vomiting in the setting of chemotherapy, radiation and surgery. The mechanism of action includes binding of the drug to central and peripheral 5-HT3 receptors to prevent serotonin-meditated emetogenic signaling. Intravenous ondansetron is approved for use in patients six months of age and older for nausea and vomiting due to emetogenic cancer chemotherapy and for patients aged one month and older for postoperative nausea and vomiting [56]. The oral ondansetron drug label includes dosing information for ages ≥4 years [57]. Ondansetron is also used off-label in the treatment of nausea and vomiting in a variety of settings including gastroenteritis in patients over one month of age.
The metabolism of ondansetron involves CYP3A4, CYP1A2 and CYP2D6, as well as glucuronide conjugation to inactive metabolites. Multiple studies have linked variability in CYP2D6 function to the pharmacokinetics of ondansetron and drug response in adults (reviewed in [58]); CYP2D6 ultra-rapid metabolizers have lower ondansetron exposure and less efficacy. Therefore, the CPIC guideline recommends alternate antiemetics that are not CYP2D6 substrates (e.g., granisetron) for individuals who are known CYP2D6 ultra-rapid metabolizers. Of note, tropisetron is not a good choice as an alternative agent, as it is also predominately metabolized by CYP2D6. There are no data from studies including pediatric patient populations to support or refute the ondansetron–CYP interaction in children. CYP2D6 function increases rapidly in the first month after birth, thus it is expected that CYP2D6 genetic variation will similarly affect ondansetron response in children as adults, alternative age-specific metabolic pathways notwithstanding [58,59].
2.3.2. Oxycodone and Codeine
Oxycodone and codeine are opioid analgesics used to relieve pain when alternative non-opioid treatment options are inadequate. Codeine is a naturally occurring methylated morphine compound that is metabolized by CYP2D6 to morphine, which then binds to μ-opioid receptors to effect pain relief. Oxycodone is a semisynthetic opioid, and although the parent compound can bind to μ-opioid receptors, the O-demethylated CYP2D6 metabolite of oxycodone binds with much higher affinity and potency. Single-ingredient codeine and all oxycodone-containing products are approved by the US Food and Drug Administration (FDA) only for use in adults [60,61]. Codeine with acetaminophen was approved by the FDA in patients over three years of age [62]. However, the FDA announced in April 2017 that due to safety concerns, the drug label for all codeine-containing products must include a Contraindication (the FDA’s strongest warning), stating that codeine should not be used to treat pain or cough in children younger than 12 years, a new Warning that codeine should not be used in adolescents 12 to 18 years of age who are obese or have obstructive sleep apnea or severe lung disease, and a strengthened Warning to mothers that breastfeeding is not recommended when taking codeine. The codeine label also includes black box warnings stating that respiratory depression and death have occurred in children who received codeine following tonsillectomy and/or adenoidectomy, and that CYP2D6 inhibitors may impact drug response. Both codeine and oxycodone have been widely used in pediatric patients for analgesia.
As a prodrug that requires CYP2D6 metabolism to the active compound, codeine is a prototype for drug–CYP interaction. Individuals who lack CYP2D6 function (poor metabolizers) are unable to convert codeine to morphine, and thus have no therapeutic effect. Conversely, CYP2D6 ultra-rapid metabolizers, who have more than two functional copies of the CYP2D6 gene, are able to generate excess morphine and are at risk for toxicity, including respiratory depression and death. Although much of high-quality evidence for the CYP–codeine interaction comes from adults (reviewed in [63,64]) there is ample evidence from studies including pediatric patients to demonstrate the clinical impact of CYP2D6 genotype in children. There are several case reports of infant mortality and respiratory failure after exposure to codeine either through breast milk or for analgesia after tonsillectomy/adenoidectomy. In many of these cases, the infants or children (and/or their mother in the cases of exposure through breast milk) were found to be CYP2D6 ultra-rapid metabolizers [21,22,24,25,26,29]. Furthermore, cohort and case-control studies exploring the pharmacokinetics and pharmacodynamics of codeine in children have confirmed higher morphine levels and risk for adverse events in children with ultra-rapid CYP2D6 function [19,20,23,27,28,30]. There is one report of clinical implementation of CYP2D6 genotyping for pediatric patients with sickle cell disease in order to provide genotype-guided therapy; clinical genetic testing coupled with decision support resulted in no patients with ultra-rapid or poor metabolizer genotypes being prescribed codeine [31]. There are strong data supporting the impact of CYP2D6 on codeine response in children. The FDA contraindication for use in children precludes any use of codeine in patients under 12 years of age and some adolescents. For patients who are ≥12 years of age, if codeine therapy is considered, CYP2D6 metabolizer status should be determined in order to prevent inefficacy and toxicity.
The evidence for the impact of CYP2D6 variation on oxycodone response is less robust. Although differences in the pharmacokinetics of oxycodone based on CYP2D6 metabolizer status are evident, there are contradictory data regarding the differences in analgesia or toxicity (reviewed in [63,64]). There is one report of the impact of CYP2D6 metabolizer status on oxycodone pharmacokinetics in adolescents, where results were consistent with adult pharmacokinetic studies [18]. Although this study confirmed that CYP2D6 normal metabolizers had higher oxymorphone exposure than poor or intermediate metabolizers, more data are needed to support the oxycodone– CYP interaction before genotype-guided oxycodone dosing is clinically implemented for children.
2.3.3. Omeprazole and Lansoprazole
Proton pump inhibitors (PPIs) are one of the highest-selling prescription medication classes, both in the United States and globally, with growing popularity among pediatric practitioners [65]. The mechanism of action targets the gastric cells, covalently binding to and irreversibly inactivating the proton (acid) pump, thereby suppressing acid secretion. While the primary indication for PPI use has historically been gastroesophageal reflux disease (GERD), PPIs are routinely prescribed for a variety of upper intestinal tract conditions and are increasingly utilized in chronic respiratory diseases as well [66,67]. Despite their broad use, the only FDA-approved indications for PPI use in children are short-term treatment of symptomatic GERD and treatment of eosinophilic esophagitis [68,69,70,71,72,73]. Furthermore, of the five available PPI formulations approved for pediatric use, esomeprazole is the only drug approved by the FDA for use in children less than one year of age. Thus, the use of omeprazole and lansoprazole, the two most commonly prescribed PPIs for the treatment of GERD in infants, is off-label and outside of the treatment guidelines [74].
PPIs are metabolized primarily by the liver microsomal enzyme CYP2C19 and to a lesser extent CYP3A4. Genetic variations in the CYP2C19 gene give rise to metabolizer phenotypes with varying degrees of PPI clearance. Individuals with loss of function alleles are termed poor metabolizers and experience reduced drug clearance relative to the normal metabolizer phenotype [75]. Conversely, ultra-rapid metabolizers have gain of function alleles which confer increased rates of clearance and reduced drug exposure compared to normal metabolizers [76]. These pharmacokinetic associations have been demonstrated repeatedly in the adult population. For example, drug exposure as represented by the area under the plasma concentration versus time curve (AUC) for omeprazole, lansoprazole, and pantoprazole is 4- to 15-fold higher in poor metabolizers than in normal metabolizers [77]. Additionally, poor metabolizers exposed to omeprazole have higher (less acidic) intra-gastric pH than normal and intermediate metabolizers, supporting the role CYP2C19 genetic polymorphisms play in PPI efficacy [78].
Several pediatric studies have also validated the PPI and CYP2C19 drug–gene interaction, with pantoprazole and lansoprazole being the two most investigated drugs. Children identified as poor metabolizers treated with therapeutic doses of pantoprazole or lansoprazole have significantly higher AUCs, delayed clearance, and longer drug half-life than normal metabolizers [32,33,79,80,81]. In addition to these pharmacokinetic parameters, clinical outcomes of efficacy and adverse events have also correlated with metabolizer phenotypes, particularly for lansoprazole [33,34,35]. While some data fail to support a gene–dose relationship for omeprazole [32], a recent study demonstrated increased acid exposure (decreased PPI efficacy) in ultra-rapid metabolizers as compared to normal, intermediate, and poor metabolizers, collectively [36]. While more studies are needed to further characterize the impact of CYP2C19 polymorphisms on PPI therapy, there are sufficient data to support pharmacogenomic implementation in pediatric patients.
2.3.4. Sertraline
Sertraline is an antidepressant in the Selective Serotonin Reuptake Inhibitor (SSRI) class. The mechanism of action for SSRIs is to prevent the reuptake of serotonin by presynaptic receptors, which in turn increases the amount of serotonin available to bind to the postsynaptic receptors. Sertraline is FDA-approved to treat major depressive disorder, obsessive–compulsive disorder, panic disorder, post-traumatic stress disorder, social anxiety disorder and premenstrual dysphoric disorder in adults and obsessive–compulsive disorder in children 6 years old and older [82]. The FDA label for SSRIs includes a black box warning due to an increased risk of suicidal thoughts and behavior in pediatric and young adult patients. Sertraline is commonly used off-label for childhood depression with close monitoring for suicidal thoughts.
Sertraline is metabolized to desmethylsertraline by several cytochrome P450 enzymes, including CYP2B6, CYP2C19, CYP2C9, CYP2D6 and CYP3A4. Sertraline is also a moderate inhibitor of CYP2D6. Decreased CYP2C19 function (due to concomitant inhibitor or poor metabolizer genotype), leads to increased plasma levels of the drug [83,84]. However, the results from studies of the impact of specific CYP gene variants to treatment outcomes are not consistent [85,86,87,88]. Despite this limited evidence, both CPIC and the Pharmacogenetics Working Group of the Royal Dutch Pharmacists recommend a 50% dose reduction of sertraline in CYP2C19 poor metabolizers [89]. There are no pediatric studies evaluating genetic variants or metabolizer phenotypes of CYP2B6, CYP2C19, CYP2C9, CYP2D6 and CYP3A4 with treatment outcomes. More research needs to be done in this area before routine pharmacogenetic testing can be recommended to optimize sertraline dosing in children.
2.3.5. Amitriptyline
Amitriptyline is a tertiary amine tricyclic antidepressant (TCA). The mechanism of action of TCAs includes inhibition of reuptake of both serotonin and norepinephrine. Amitriptyline is FDA-approved for the treatment of depression in adults and adolescents over 12 years of age, with a black box warning due to the increase in suicidality for young patients [90]. Off-label uses in children include migraine prophylaxis, as an adjunctive therapy for chronic neuropathic pain, and for symptomatic management of pain-predominant functional gastrointestinal disorders.
Amitriptyline is metabolized by CYP2C19 to pharmacologically active secondary amines, which have more predominant noradrenergic effects than the parent compound. Both amitriptyline and the secondary amine metabolites are metabolized by CYP2D6 to inactive compounds. Variation in CYP2D6 function is hypothesized to impact drug clearance, while CYP2C19 variability contributes to individual differences in response by affecting the balance of noradrenergic and serotonergic effects. In adults being treated for depression, dose reduction or alternate therapy is recommended by CPIC if the patient is known to be a CYP2C19 or CYP2D6 poor metabolizer [91]. Alternate therapy is also recommended for CYP2C19 or CYP2D6 ultra-rapid metabolizers due to the risks for inefficacy or side effects. Our literature search revealed one case report of a 6-year-old female who survived chronic overdose of amitriptyline (10-fold typical dosing administered nightly for one month); genotyping in this individual revealed normal metabolizer status for both CYP2D6 and CYP2C19, which may have prevented a more disastrous outcome for the patient [37]. Whether or not the genotype-guided dosing recommendations for treating depression in adults are appropriate for other indications and/or pediatric patients has not been rigorously assessed.
2.3.6. Citalopram and Escitalopram
Citalopram and escitalopram are SSRIs widely used for the treatment of depression, anxiety disorders, and obsessive–compulsive disorder. Citalopram is a racemic mixture of R- and S-enantiomers, while escitalopram contains only the pharmacologically active S-enantiomer. Citalopram is approved for the treatment of depression in adults, and escitalopram is approved for the treatment of anxiety in adults and depression in patients over 12 years of age [92,93]. As with sertraline, both drug labels include a black box warning due to increased risk for suicidal thinking and behavior in young patients. Both drugs are frequently used off-label in children with depression, anxiety, obsessive–compulsive disorder, autism, and/or pervasive developmental disorders [38,89,94].
Both drugs are primarily metabolized by CYP2C19 and to a lesser extent by CYP2D6 and CYP3A4 [95]. CPIC guidelines recommend a 50% dose reduction or alternative drug not metabolized by CYP2C19 for poor metabolizers, as CYP2C19 poor metabolizers have higher plasma concentrations and this may increase the probability of side effects [89]. In pediatric patients, CYP2C19 genotyping was conducted in a study investigating citalopram and metabolite concentrations at steady state in 19 adolescents, two-thirds of whom were >18 years of age; individuals with CYP2D6 and/or CYP2C19 variants were not significantly different from those without variants, although numbers were small (n = 3 CYP2D6*4 heterozygotes, n = 2 CYP2D6 duplication, n = 3 CYP2C19*2 heterozygotes) [38]. An additional study of 83 adolescent and adult patients treated with citalopram or escitalopram demonstrated that CYP2C19*2 carriers had higher drug concentration to dose ratios, indicating impaired drug metabolism [39]. An investigation of the clinical endpoint of improvement in irritability among 89 pediatric and adult patients with autism spectrum disorder treated with escitalopram found no difference by CYP2C19 metabolizer status, although a secondary analysis demonstrated slower rate of change in dose over time for CYP2C19 ultra-rapid metabolizers [40]. Based on these mixed results from small studies, pharmacogenomic-guided dose titration for citalopram and escitalopram is not yet well supported in children.
2.3.7. Risperidone
Risperidone, a serotonin-dopamine antagonist, is one of the atypical antipsychotic drugs used for treating schizophrenia, bipolar mania, autism and other impulsive or aggressive behaviors mostly in adults [96]. Risperidone, like other atypical antipsychotic drugs, has affinity for dopamine (D2), serotonin (5-HT2A), alpha adrenergic (α-1 and α-2), and histamine (H1) receptors. The mechanism of action of risperidone is not fully understood but current theories focus mainly on its inhibitory effects on D2 and 5-HT2A receptors [97,98]. The drug is metabolized in the liver primarily by CYP2D6 and to a lesser extent by CYP3A4 via hydroxylation to 9-hydroxyrisperidone, an equipotent metabolite [96,99]. CYP2D6 variation is known to impact this metabolic transformation [100]. Risperidone is FDA-approved for use in children 5–16 years of age to treat irritability associated with autism, children 10–17 years to treat mania and mixed state due to bipolar disorder, and children 13–17 years to treat schizophrenia [101]. The drug is also increasingly used off-label for conditions including developmental and disruptive disorders, depression, obsessive–compulsive disorder, post-traumatic stress disorder, personality disorder, attention deficit-hyperactivity disorder, and Tourette’s syndrome [102].
As a major substrate of CYP2D6, risperidone has a potential for drug–CYP interaction. CYP2D6 variants may contribute to an increased risk of adverse events associated with risperidone therapy. CYP2D6 poor metabolizers have prolonged drug exposure and therapeutic effect and may be at risk for toxicity [103]. In contrast, CYP2D6 ultra-rapid metabolizers, who have duplicate or multiple functional copies of the CYP2D6 gene, may be at risk for therapeutic failure. There are few studies in adults supporting CYP–risperidone interaction with respect to pharmacokinetics, efficacy, and side effects [104,105,106,107,108,109], whereas others have failed to find risperidone–CYP associations [110]. Some studies have also demonstrated the clinical impact of CYP2D6 genotype in children. In a small study involving children with autism, those who were CYP2D6 poor metabolizers had higher drug concentration and exhibited adverse effects including hyperprolactinemia and tardive dyskinesia, while ultra-rapid metabolizers exhibited no adverse effect [47]; however, the differences in drug response were not statistically significant. A cohort study evaluating the pharmacokinetics and pharmacodynamics of risperidone in children with pervasive developmental disorder did not demonstrate any clinically significant adverse effect of hyperprolactinemia after 8 weeks of risperidone therapy [41]. In the same study, serum prolactin level after 8 weeks of therapy was positively correlated with risperidone dosage, number of functional CYP2D6 genes, and serum 9-hydroxyrisperidone, but not with risperidone plasma levels. In another study, children who were CYP2D6 ultra-rapid metabolizers exhibited less weight gain while on risperidone therapy compared to normal metabolizers; however, poor metabolizers showed similar effect to the normal metabolizers [43]. Other studies evaluating the effects of major and minor CYP2D6 inhibitors, rather than CYP2D6 genotype, on the metabolism of risperidone in children demonstrated a much stronger association of CYP2D6 function to plasma levels of risperidone than with its major metabolite [111]. The Dutch Pharmacogenetics Working Group has recently changed its dosing recommendations of risperidone to “no action is required” for CYP2D6 poor metabolizers. The evidence for the impact of CYP2D6 variability on the efficacy and adverse effects of risperidone is insufficient to support changes in prescribing based on genotype at this time.
3. Discussion
Beginning with the compendium of drug–gene interactions from CPIC, we identified 48 actionable drug–CYP pairs, representing 41 distinct drugs, with high-quality evidence. Of those, the majority of drugs are rarely used in pediatric patients; only 10 of the 41 drugs had over 5000 individual pediatric patients exposed over a 10-year period at this tertiary children’s medical center. For these 10 drugs with a high level of evidence for clinical use of pharmacogenomic information in adults and over 500 pediatric patients exposed per year, we surveyed the literature to determine what data support implementation of genome-guided prescribing in children. Despite relatively high use of these medications in children, few drugs have robust evidence for the drug–CYP interaction in children: codeine (for which the FDA has now issued a contraindication against use in children under 12 years of age) and the PPIs, including omeprazole, lansoprazole and pantoprazole. For the remaining drugs, there are little data or conflicting data for pediatric patients.
Pharmacogenomic associations discovered in adults may be applied to pediatric patients in some circumstances. Specifically, when the gene is expected to have the same functional impact in children as adults and the indication and side effect profile for the drug are the same for children as adults, guidelines such as CPIC can be used in pediatrics, as suggested for ondansetron. It is important to pay heed to how children and adolescents differ from adults [112]. There are several issues that may preclude the extrapolation of drug–gene interaction from adults to children. One important issue is the effect of gene ontogeny, or the developmental regulation of gene expression. Many CYP enzymes are not expressed, or expressed at very low levels, in the neonatal period, with increases to the equivalent of adult levels of expression over weeks to years [113]. However, expression of CYP2D6 and CYP2C19, the genes with the most potential clinical relevance at this time, reach levels equivalent to adults during infancy and then are stable through childhood and adolescence. Thus, the issue of ontogeny is most important for drugs given in the neonatal period, such as PPIs. Less is known about the unique CYP enzymes that are up-regulated in infancy, childhood and adolescence. In addition to the issue of ontogeny, many drugs are used in children for different indications than in adults (e.g., non-steroidal anti-inflammatory drugs to close patent ductus arteriosus in neonates). The drug–CYP interactions defined from adult studies may or may not be relevant to the clinical use of the drug for these pediatric indications. Pediatric patients may also have a unique side effect profile (e.g., adolescents and young adults are at increased risk for suicidal ideation with antidepressant therapy). These age-specific side effects may also have age-specific drug–gene interactions that modify the risk of their occurrence. For all these reasons, it is important to study pharmacogenomic associations in children prior to clinical implementation, unless there are robust data to indicate that ontogeny is not an issue, the indication for drug use is the same in adults and children, and the side effect profile is the same in adults and children. Indeed, the same logic can be applied across special populations, whether they are defined by age, ancestry, comorbid conditions, or any other stratifying factor.
Several themes are apparent from our focused literature review. First is that for each of the drug–CYP associations, there are a small number of studies available that include pediatric participants, despite the fact that we focused on the most commonly used medications. Second, each study generally included a small number of participants. This is particularly problematic for studies reporting negative findings, as the study may not have sufficient power to detect a clinically meaningful difference between groups, especially if the genotype of interest is infrequently found in the population. Third, a wide variety of primary outcomes were used, from drug levels to biomarkers to measures of drug efficacy or adverse events. This may also contribute to apparently contradictory results across studies. There was also a wide variety with respect to interrogation of the gene of interest. For example, some studies genotyped selected SNPs in CYP2D6 and searched for association, while others genotyped many SNPs across the entire gene, as well as assessing for deletion and duplication. While it may be appropriate to focus genotyping efforts on the most common variants in the specific population being studied, false negative results may also stem from incomplete interrogation of the gene of interest. Thus, in addition to a paucity of studies in pediatric patients, there is a lack of high-quality, rigorous studies from which to draw conclusions.
Our approach to this topic has several limitations. We began our analysis with the compendium of CPIC drug–gene interactions, which is not a complete assessment of all drugs and all pharmacogenomic observations. There are several drug–gene interactions that are well-established and relevant in the care of pediatric patients, such as thiopurine drugs and TPMT, that are not included in CYP-focused review [114,115]. There are also several CYP-associated drugs with pediatric evidence that fell below our threshold for focused literature review, including atomoxetine, which has pediatric evidence for CYP2D6-guided therapy, and warfarin, for which there are pediatric-specific dosing calculators using CYP2C9 and VKORC1 genotypes [116,117]. We also used data from a single tertiary referral children’s hospital (which includes both inpatient orders and outpatient prescriptions) in order to assess for rates of exposure in a 10-year period. These data are subject to local practice patterns and regional trends. Our use of data from the last 10 years may also affect results, as new drugs may be under-represented, and there may be medications where use is on the decline (e.g., codeine). Our definition of exposure required only a single mention of a drug name with a dose, route or frequency, which may have false positives, and our EHR data may not be complete, particularly as many patients receive some of their care outside this institution. Also, while EHR data are robust for determining medication start dates, discontinuation dates are difficult to discern, and thus we have not calculated length of exposure and cannot comment on whether pediatric patients were treated briefly or chronically with these drugs. Despite these limitations, the data likely represent a reasonable approximation for the current state of the field of pharmacogenomics and pediatric exposures to drugs with actionable drug–CYP interactions.
Based on our analysis, it is apparent that there is much work to do in the field of pediatric pharmacogenomics. For most drugs with known interactions with CYP enzymes, data must be collected in a robust fashion to determine the veracity of those associations in children. There are a few drugs with robust evidence, namely codeine and PPIs. The former is now contraindicated in children under 12 and is likely to be used less and less in pediatric patients. PPIs are very frequently used, and efforts can move forward towards implementation of CYP2C19-guided therapy. It may be appropriate to extrapolate from adult data to non-neonatal pediatric patients for the ondansetron–CYP2D6 interaction. For the remaining drug–CYP interactions, further work must be completed prior to implementation. Given the fact that many drugs with known interactions are commonly used in children, these high-use drugs provide a reasonable starting point.
4. Materials and Methods
4.1. Identification of CYP-Associated Drugs with High Levels of Evidence
In order to generate a list of candidate drugs to assess in the pediatric population, all gene–drug pairs from the CPIC website were downloaded on 14 August 2017 [12,15]. The downloaded table included CPIC and PharmGKB level of evidence for each gene–drug pair. The complete list of all gene–drugs pairs was filtered to include only those with CYP in the gene name. The list was then further filtered to include only those with PharmGKB level 1A, 1B, 2A or 2B evidence and CPIC level A, A/B or B levels.
4.2. Determining the Pediatric Exposures to CYP-Associated Drugs
To determine the number of unique pediatric patients exposed to the actionable CYP-associated drugs identified from the CPIC table, we used the Synthetic Derivative (SD), the de-identified electronic health records database at Vanderbilt University Medical Center. The study was reviewed by the Vanderbilt Institutional Review board and determined to be non-human subjects research. Exposure to the medication of interest was defined as one or more mention of the drug name, in conjunction with a dose, route, strength or frequency, using MedEx [118]. For this study, we restricted mentions to those occurring in the 10-year period from 2006 to 2016. These date ranges are approximate given that dates in the SD are de-identified, and every entry in each individual’s record is date shifted with a random (but consistent) number of days up to one year backwards. This date shifting should not meaningfully impact our convenience sample. Individuals were included as pediatric exposures if their age was <18 years on the date of the first exposure to the medication of interest.
To further characterize the pediatric exposures, we collected additional demographic data for each cohort of patients exposed to a drug of interest. The age of each individual on the date of first drug mention was collected and is reported as the median and interquartile range, as well as the distribution of sex, race and ethnicity for those exposed. Race and ethnicity are coded using administratively defined variables in the electronic health record.
4.3. Literature Review for Drug–CYP Interactions in Children
For the CYP-associated drugs with more than 5000 pediatric patients exposed over a 10-year period in our electronic health records cohort, we performed a focused literature review of the evidence for the drug–CYP association in pediatric patients. Reports were included if they were written in English (or an English language translation was available), if some or the entire study cohort was <21 years of age, and if one or more CYP enzyme was evaluated through genotyping or phenotyping. Pharmacokinetic studies that measured drug and metabolite concentrations, pharmacodynamic studies that measured drug response, and clinical implementation studies that used pharmacogenomic data to guide prescribing were included. Findings are described using the standard nomenclature suggested by CPIC [119].
Acknowledgments
This work was supported by the Burroughs Wellcome Fund Innovation in Regulatory Science Award to S.L.V.D.
Author Contributions
S.L.V.D. conceived and designed the manuscript; R.C. extracted data from electronic health records; I.A., C.J.B., A.M.-H., K.A.O. and S.L.V.D. performed literature review; R.C. and S.L.V.D. analyzed the data; S.L.V.D. wrote the paper. I.A., C.J.B., R.C., A.M.-H., K.A.O. and S.L.V.D. critically reviewed and revised the manuscript.
Conflicts of Interest
S.L.V.D. has been an invited speaker to Merck, and received an honorarium. Merck had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Williams, J.A.; Hyland, R.; Jones, B.C.; Smith, D.A.; Hurst, S.; Goosen, T.C.; Peterkin, V.; Koup, J.R.; Ball, S.E. Drug-drug interactions for UDP-glucuronosyltransferase substrates: A pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab. Dispos. 2004, 32, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Shuldiner, A.R.; Relling, M.V.; Peterson, J.F.; Hicks, J.K.; Freimuth, R.R.; Sadee, W.; Pereira, N.L.; Roden, D.M.; Johnson, J.A.; Klein, T.E.; et al. The Pharmacogenomics Research Network Translational Pharmacogenetics Program: Overcoming challenges of real-world implementation. Clin. Pharmacol. Ther. 2013, 94, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Pulley, J.M.; Denny, J.C.; Peterson, J.F.; Bernard, G.R.; Vnencak-Jones, C.L.; Ramirez, A.H.; Delaney, J.T.; Bowton, E.; Brothers, K.; Johnson, K.; et al. Operational Implementation of Prospective Genotyping for Personalized Medicine: The Design of the Vanderbilt PREDICT Project. Clin. Pharmacol. Ther. 2012, 92, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Pestian, J.; Spencer, M.; Matykiewicz, P.; Zhang, K.; Vinks, A.A.; Glauser, T. Personalizing Drug Selection Using Advanced Clinical Decision Support. Biomed. Inform. Insights 2009, 2, 19–29. [Google Scholar] [PubMed]
- Nutescu, E.A.; Drozda, K.; Bress, A.P.; Galanter, W.L.; Stevenson, J.; Stamos, T.D.; Desai, A.A.; Duarte, J.D.; Gordeuk, V.; Peace, D.; et al. Feasibility of Implementing a Comprehensive Warfarin Pharmacogenetics Service. Pharmacotherapy 2013. [Google Scholar] [CrossRef] [PubMed]
- Mega, J.L.; Hochholzer, W.; Frelinger, A.L., 3rd; Kluk, M.J.; Angiolillo, D.J.; Kereiakes, D.J.; Isserman, S.; Rogers, W.J.; Ruff, C.T.; Contant, C.; et al. Dosing clopidogrel based on CYP2C19 genotype and the effect on platelet reactivity in patients with stable cardiovascular disease. JAMA 2011, 306, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Hall-Flavin, D.K.; Winner, J.G.; Allen, J.D.; Jordan, J.J.; Nesheim, R.S.; Snyder, K.A.; Drews, M.S.; Eisterhold, L.L.; Biernacka, J.M.; Mrazek, D.A. Using a pharmacogenomic algorithm to guide the treatment of depression. Transl. Psychiatry 2012, 2, e172. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, O.; Scott, S.A.; Ellis, S.B.; Overby, C.L.; Ludtke, A.; Hulot, J.-S.; Hall, J.; Chatani, K.; Myers, K.; Kannry, J.L.; et al. The CLIPMERGE PGx Program: Clinical implementation of personalized medicine through electronic health records and genomics-pharmacogenomics. Clin. Pharmacol. Ther. 2013, 94, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Dolan, M.E.; Maitland, M.L.; O’Donnell, P.H.; Nakamura, Y.; Cox, N.J.; Ratain, M.J. Institutional Profile: University of Chicago Center for Personalized Therapeutics: Research, education and implementation science. Pharmacogenomics 2013, 14, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Bielinski, S.J.; Olson, J.E.; Pathak, J.; Weinshilboum, R.M.; Wang, L.; Lyke, K.J.; Ryu, E.; Targonski, P.V.; Van Norstrand, M.D.; Hathcock, M.A.; et al. Preemptive genotyping for personalized medicine: Design of the right drug, right dose, right time-using genomic data to individualize treatment protocol. Mayo Clin. Proc. 2014, 89, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.C.; Crews, K.R.; Wilkinson, M.R.; Haidar, C.E.; Hicks, J.K.; Baker, D.K.; Kornegay, N.M.; Yang, W.; Cross, S.J.; Howard, S.C.; et al. Development and use of active clinical decision support for preemptive pharmacogenomics. J. Am. Med. Inform. Assoc. 2013. [Google Scholar] [CrossRef] [PubMed]
- Relling, M.V.; Klein, T.E. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin. Pharmacol. Ther. 2011, 89, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Whirl-Carrillo, M.; McDonagh, E.M.; Hebert, J.M.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Altman, R.B.; Klein, T.E. Pharmacogenomics knowledge for personalized medicine. Clin. Pharmacol. Ther. 2012, 92, 414–417. [Google Scholar] [CrossRef] [PubMed]
- PharmGKB. Available online: https://www.pharmgkb.org/ (accessed on 14 August 2017).
- CPIC. Available online: https://cpicpgx.org/ (accessed on 14 August 2017).
- Van Driest, S.L.; McGregor, T.L. Pharmacogenetics in clinical pediatrics: Challenges and strategies. Pers. Med. 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Wester, C.; Rebeiro, P.F.; Shavor, T.J.; Shepherd, B.E.; McGoy, S.L.; Daley, B.; Morrison, M.; Vermund, S.H.; Pettit, A.C. The 2013 HIV Continuum of Care in Tennessee: Progress Made, but Disparities Persist. Public Health Rep. 2016, 131, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Balyan, R.; Mecoli, M.; Venkatasubramanian, R.; Chidambaran, V.; Kamos, N.; Clay, S.; Moore, D.L.; Mavi, J.; Glover, C.D.; Szmuk, P.; et al. CYP2D6 pharmacogenetic and oxycodone pharmacokinetic association study in pediatric surgical patients. Pharmacogenomics 2017, 18, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.Y.; Svensson, J.O.; Alm, C.; Sjöqvist, F.; Säwe, J. Codeine O-demethylation co-segregates with polymorphic debrisoquine hydroxylation. Br. J. Clin. Pharmacol. 1989, 28, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.G.; Patel, A.; Howard, R.F. Pharmacogenetics of codeine metabolism in an urban population of children and its implications for analgesic reliability. Br. J. Anaesth. 2002, 89, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Koren, G.; Cairns, J.; Chitayat, D.; Gaedigk, A.; Leeder, S.J. Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet 2006, 368, 704. [Google Scholar] [CrossRef]
- Voronov, P.; Przybylo, H.J.; Jagannathan, N. Apnea in a child after oral codeine: A genetic variant—An ultra-rapid metabolizer. Paediatr. Anaesth. 2007, 17, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Madadi, P.; Ross, C.J.D.; Hayden, M.R.; Carleton, B.C.; Gaedigk, A.; Leeder, J.S.; Koren, G. Pharmacogenetics of neonatal opioid toxicity following maternal use of codeine during breastfeeding: A case-control study. Clin. Pharmacol. Ther. 2009, 85, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Ciszkowski, C.; Madadi, P.; Phillips, M.S.; Lauwers, A.E.; Koren, G. Codeine, ultrarapid-metabolism genotype, and postoperative death. N. Engl. J. Med. 2009, 361, 827–828. [Google Scholar] [CrossRef] [PubMed]
- Ferreirós, N.; Dresen, S.; Hermanns-Clausen, M.; Auwaerter, V.; Thierauf, A.; Müller, C.; Hentschel, R.; Trittler, R.; Skopp, G.; Weinmann, W. Fatal and severe codeine intoxication in 3-year-old twins--interpretation of drug and metabolite concentrations. Int. J. Legal Med. 2009, 123, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.E.; Rieder, M.; van den Anker, J.; Malkin, B.; Ross, C.; Neely, M.N.; Carleton, B.; Hayden, M.R.; Madadi, P.; Koren, G. More codeine fatalities after tonsillectomy in North American children. Pediatrics 2012, 129, e1343–e1347. [Google Scholar] [CrossRef] [PubMed]
- Khetani, J.D.; Madadi, P.; Sommer, D.D.; Reddy, D.; Sistonen, J.; Ross, C.J.D.; Carleton, B.C.; Hayden, M.R.; Koren, G. Apnea and oxygen desaturations in children treated with opioids after adenotonsillectomy for obstructive sleep apnea syndrome: A prospective pilot study. Paediatr. Drugs 2012, 14, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Sistonen, J.; Madadi, P.; Ross, C.J.; Yazdanpanah, M.; Lee, J.W.; Landsmeer, M.L.A.; Nauta, M.; Carleton, B.C.; Koren, G.; Hayden, M.R. Prediction of codeine toxicity in infants and their mothers using a novel combination of maternal genetic markers. Clin. Pharmacol. Ther. 2012, 91, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Friedrichsdorf, S.J.; Nugent, A.P.; Strobl, A.Q. Codeine-associated pediatric deaths despite using recommended dosing guidelines: Three case reports. J. Opioid Manag. 2013, 9, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Prows, C.A.; Zhang, X.; Huth, M.M.; Zhang, K.; Saldaña, S.N.; Daraiseh, N.M.; Esslinger, H.R.; Freeman, E.; Greinwald, J.H.; Martin, L.J.; et al. Codeine-related adverse drug reactions in children following tonsillectomy: A prospective study. Laryngoscope 2014, 124, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Gammal, R.S.; Crews, K.R.; Haidar, C.E.; Hoffman, J.M.; Baker, D.K.; Barker, P.J.; Estepp, J.H.; Pei, D.; Broeckel, U.; Wang, W.; et al. Pharmacogenetics for Safe Codeine Use in Sickle Cell Disease. Pediatrics 2016, 138, e20153479. [Google Scholar] [CrossRef] [PubMed]
- Kearns, G.L.; Leeder, J.S.; Gaedigk, A. Impact of the CYP2C19*17 allele on the pharmacokinetics of omeprazole and pantoprazole in children: Evidence for a differential effect. Drug Metab. Dispos. Biol. Fate Chem. 2010, 38, 894–897. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.J.; Lang, J.E.; Mougey, E.B.; Blake, K.B.; Gong, Y.; Holbrook, J.T.; Wise, R.A.; Teague, W.G. Association of CYP2C19 polymorphisms and lansoprazole-associated respiratory adverse effects in children. J. Pediatr. 2013, 163, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Settin, A.; Abdalla, A.F.; Al-Hussaini, A.S.; El-Baz, R.; Galal, A. Cure rate of Helicobacter pylori infection in Egyptian children related to CYP2C19 gene polymorphism. Indian J. Gastroenterol. 2014, 33, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.E.; Holbrook, J.T.; Mougey, E.B.; Wei, C.Y.; Wise, R.A.; Teague, W.G.; Lima, J.J. American Lung Association-Airways Clinical Research Centers Lansoprazole Is Associated with Worsening Asthma Control in Children with the CYP2C19 Poor Metabolizer Phenotype. Ann. Am. Thorac. Soc. 2015, 12, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, J.P.; Mougey, E.B.; Williams, A.; Gomez-Suarez, R.A.; Thomas, C.; Creech, C.L.; George, K.; Corao, D.; Lima, J.J. Association Between CYP2C19*17 Alleles and pH Probe Testing Outcomes in Children With Symptomatic Gastroesophageal Reflux. J. Clin. Pharmacol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Clement, A.; Raney, J.J.; Wasserman, G.S.; Lowry, J.A. Chronic amitriptyline overdose in a child. Clin. Toxicol. (Phila. Pa.) 2012, 50, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, B.; Olsson, G.; Reis, M.; Walinder, J.; Nordin, C.; Lundmark, J.; Scordo, M.G.; Dahl, M.L.; Bengtsson, F.; Ahlner, J. Enantioselective analysis of citalopram and metabolites in adolescents. Ther. Drug Monit. 2001, 23, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Rudberg, I.; Hendset, M.; Uthus, L.H.; Molden, E.; Refsum, H. Heterozygous mutation in CYP2C19 significantly increases the concentration/dose ratio of racemic citalopram and escitalopram (S-citalopram). Ther. Drug Monit. 2006, 28, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.R.; Najjar, F.; Rubin, L.H.; Guter, S.J.; Owley, T.; Mosconi, M.W.; Jacob, S.; Cook, E.H. Escitalopram pharmacogenetics: CYP2C19 relationships with dosing and clinical outcomes in autism spectrum disorder. Pharmacogenet. Genom. 2015. [Google Scholar] [CrossRef] [PubMed]
- Troost, P.W.; Lahuis, B.E.; Hermans, M.H.; Buitelaar, J.K.; van Engeland, H.; Scahill, L.; Minderaa, R.B.; Hoekstra, P.J. Prolactin release in children treated with risperidone: Impact and role of CYP2D6 metabolism. J. Clin. Psychopharmacol. 2007, 27, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Aman, M.G.; Vinks, A.A.; Remmerie, B.; Mannaert, E.; Ramadan, Y.; Masty, J.; Lindsay, R.L.; Malone, K. Plasma pharmacokinetic characteristics of risperidone and their relationship to saliva concentrations in children with psychiatric or neurodevelopmental disorders. Clin. Ther. 2007, 29, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.T.; Almeida, J.P.; Santos, P.E.; Sequeira, A.F.; Marques, C.E.; Miguel, T.S.; Abreu, R.L.; Oliveira, G.G.; Vicente, A.M. Pharmacogenetics of risperidone therapy in autism: Association analysis of eight candidate genes with drug efficacy and adverse drug reactions. Pharmacogenom. J. 2010, 10, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, C.M.T.; Saldaña, S.N.; Bies, R.R.; Aman, M.G.; Vinks, A.A. Population pharmacokinetic modeling of risperidone and 9-hydroxyrisperidone to estimate CYP2D6 subpopulations in children and adolescents. Ther. Drug Monit. 2012, 34, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Roke, Y.; van Harten, P.N.; Franke, B.; Galesloot, T.E.; Boot, A.M.; Buitelaar, J.K. The effect of the Taq1A variant in the dopamine D₂ receptor gene and common CYP2D6 alleles on prolactin levels in risperidone-treated boys. Pharmacogenet. Genom. 2013, 23, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Hendset, M.; Molden, E.; Knape, M.; Hermann, M. Serum concentrations of risperidone and aripiprazole in subgroups encoding CYP2D6 intermediate metabolizer phenotype. Ther. Drug Monit. 2014, 36, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Youngster, I.; Zachor, D.A.; Gabis, L.V.; Bar-Chaim, A.; Benveniste-Levkovitz, P.; Britzi, M.; Soback, S.; Ziv-Baran, T.; Berkovitch, M. CYP2D6 genotyping in paediatric patients with autism treated with risperidone: A preliminary cohort study. Dev. Med. Child Neurol. 2014, 56, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, L.A.; Dumitraşcu, V.; Tudor, A.; Grădinaru, R.; Andreescu, N.; Puiu, M. Molecular study of weight gain related to atypical antipsychotics: Clinical implications of the CYP2D6 genotype. Rom. J. Morphol. Embryol. 2014, 55, 877–884. [Google Scholar] [PubMed]
- Dos Santos Júnior, A.; Henriques, T.B.; de Mello, M.P.; Ferreira Neto, A.P.; Paes, L.A.; Della Torre, O.H.; Sewaybricker, L.E.; Fontana, T.S.; Celeri, E.H.R.V.; Guerra Júnior, G.; et al. Hyperprolactinemia in Children and Adolescents with Use of Risperidone: Clinical and Molecular Genetics Aspects. J. Child Adolesc. Psychopharmacol. 2015, 25, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Sukasem, C.; Hongkaew, Y.; Ngamsamut, N.; Puangpetch, A.; Vanwong, N.; Chamnanphon, M.; Chamkrachchangpada, B.; Sinrachatanant, A.; Limsila, P. Impact of Pharmacogenetic Markers of CYP2D6 and DRD2 on Prolactin Response in Risperidone-Treated Thai Children and Adolescents With Autism Spectrum Disorders. J. Clin. Psychopharmacol. 2016, 36, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Vanwong, N.; Ngamsamut, N.; Hongkaew, Y.; Nuntamool, N.; Puangpetch, A.; Chamnanphon, M.; Sinrachatanant, A.; Limsila, P.; Sukasem, C. Detection of CYP2D6 polymorphism using Luminex xTAG technology in autism spectrum disorder: CYP2D6 activity score and its association with risperidone levels. Drug Metab. Pharmacokinet. 2016, 31, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos-Júnior, A.; Henriques, T.B.; de Mello, M.P.; Della Torre, O.H.; Paes, L.A.; Ferreira-Neto, A.P.; Sewaybricker, L.E.; Fontana, T.S.; Celeri, E.H.R.V.; Guerra-Júnior, G.; et al. Pharmacogenetics of Risperidone and Cardiovascular Risk in Children and Adolescents. Int. J. Endocrinol. 2016, 2016, 5872423. [Google Scholar] [CrossRef] [PubMed]
- Mannheimer, B.; Haslemo, T.; Lindh, J.D.; Eliasson, E.; Molden, E. Risperidone and Venlafaxine Metabolic Ratios Strongly Predict a CYP2D6 Poor Metabolizing Genotype. Ther. Drug Monit. 2016, 38, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Medhasi, S.; Pinthong, D.; Pasomsub, E.; Vanwong, N.; Ngamsamut, N.; Puangpetch, A.; Chamnanphon, M.; Hongkaew, Y.; Pratoomwun, J.; Limsila, P.; et al. Pharmacogenomic Study Reveals New Variants of Drug Metabolizing Enzyme and Transporter Genes Associated with Steady-State Plasma Concentrations of Risperidone and 9-Hydroxyrisperidone in Thai Autism Spectrum Disorder Patients. Front. Pharmacol. 2016, 7, 475. [Google Scholar] [CrossRef] [PubMed]
- Vanwong, N.; Ngamsamut, N.; Medhasi, S.; Puangpetch, A.; Chamnanphon, M.; Tan-Kam, T.; Hongkaew, Y.; Limsila, P.; Sukasem, C. Impact of CYP2D6 Polymorphism on Steady-State Plasma Levels of Risperidone and 9-Hydroxyrisperidone in Thai Children and Adolescents with Autism Spectrum Disorder. J. Child Adolesc. Psychopharmacol. 2017, 27, 185–191. [Google Scholar] [CrossRef] [PubMed]
- DailyMed. ONDANSETRON—Ondansetron Hydrochloride Injection. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e0050959-c14c-41b6-9a92-fadc5f6feff3 (accessed on 12 September 2017).
- DailyMed. ONDANSETRON—Ondansetron Hydrochloride Solution. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac3dfb81-a4c7-4147-9374-c234153b7c51 (accessed on 12 September 2017).
- Bell, G.C.; Caudle, K.E.; Whirl-Carrillo, M.; Gordon, R.J.; Hikino, K.; Prows, C.A.; Gaedigk, A.; Agundez, J.; Sadhasivam, S.; Klein, T.E.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 genotype and use of ondansetron and tropisetron. Clin. Pharmacol. Ther. 2017, 102, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.C.; Marsh, S.A.; Zaya, M.J.; Regina, K.J.; Divakaran, K.; Le, M.; Hines, R.N. Developmental changes in human liver CYP2D6 expression. Drug Metab. Dispos. 2008, 36, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- DailyMed. CODEINE SULFATE—Codeine Sulfate Tablet. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fa3ed180-298a-4f9d-9d05-15182d7218bf (accessed on 12 September 2017).
- DailyMed. OXYCODONE HYDROCHLORIDE—Oxycodone Hydrochloride Solution. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c193a56a-bb8f-476d-b1ec-789fd2aaef7e (accessed on 12 September 2017).
- DailyMed. Acetaminophen and Codeine Phosphate—Acetaminophen and Codeine Phosphate Liquid. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7bca1d24-a317-44c4-8db2-68030eb961ed (accessed on 12 September 2017).
- Crews, K.R.; Gaedigk, A.; Dunnenberger, H.M.; Leeder, J.S.; Klein, T.E.; Caudle, K.E.; Haidar, C.E.; Shen, D.D.; Callaghan, J.T.; Sadhasivam, S.; et al. Clinical Pharmacogenetics Implementation Consortium Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin. Pharmacol. Ther. 2014, 95, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Crews, K.R.; Gaedigk, A.; Dunnenberger, H.M.; Klein, T.E.; Shen, D.D.; Callaghan, J.T.; Kharasch, E.D.; Skaar, T.C. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for codeine therapy in the context of cytochrome P450 2D6 (CYP2D6) genotype. Clin. Pharmacol. Ther. 2012, 91, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Barron, J.J.; Tan, H.; Spalding, J.; Bakst, A.W.; Singer, J. Proton pump inhibitor utilization patterns in infants. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Rudolph, C.D.; Di Lorenzo, C.; Hassall, E.; Liptak, G.; Mazur, L.; Sondheimer, J.; Staiano, A.; Thomson, M.; Veereman-Wauters, G.; et al. Pediatric gastroesophageal reflux clinical practice guidelines: Joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J. Pediatr. Gastroenterol. Nutr. 2009, 49, 498–547. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Nakayama, K.; Yasuda, H.; Yoshida, M.; Asamura, T.; Ohrui, T.; Arai, H.; Araya, J.; Kuwano, K.; Yamaya, M. A randomized, single-blind study of lansoprazole for the prevention of exacerbations of chronic obstructive pulmonary disease in older patients. J. Am. Geriatr. Soc. 2009, 57, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- DailyMed. PRILOSEC—Omeprazole Magnesium Granule, Delayed Release. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b6761f84-53ac-4745-a8c8-1e5427d7e179 (accessed on 12 September 2017).
- DailyMed. LANSOPRAZOLE—Lansoprazole Capsule, Delayed Release. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fb26cbe4-d17a-41f7-8654-edebd8665f76 (accessed on 12 September 2017).
- DailyMed. DEXILANT—Dexlansoprazole Capsule, Delayed Release. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9819f033-3bbe-442e-8e92-45fec77b237d (accessed on 12 September 2017).
- DailyMed. ESOMEPRAZOLE MAGNESIUM—Esomeprazole Magnesium Capsule, Delayed Release Pellets. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=eb8f8b81-d482-4b94-be87-c766f49e753f (accessed on 12 September 2017).
- DailyMed. PANTOPRAZOLE SODIUM—Pantoprazole Tablet, Delayed Release. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f3ded82a-cf0d-4844-944a-75f9f9215ff0 (accessed on 12 September 2017).
- DailyMed. RABEPRAZOLE SODIUM—Rabeprazole Tablet, Delayed Release. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=951caefe-6e18-48ff-9567-beb545b09c25 (accessed on 12 September 2017).
- Proton Pump Inhibitors: Use in Pediatric Patients-ppi-pediatric-factsheet11-14.pdf. Available online: https://www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/Medicaid-Integrity-Education/Pharmacy-Education-Materials/Downloads/ppi-pediatric-factsheet.pdf (accessed on 12 September 2017).
- Wedlund, P.J. The CYP2C19 enzyme polymorphism. Pharmacology 2000, 61, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, R.M.; Ohlsson, S.; Pedersen, R.S.; Mwinyi, J.; Ingelman-Sundberg, M.; Eliasson, E.; Bertilsson, L. Increased omeprazole metabolism in carriers of the CYP2C19*17 allele; a pharmacokinetic study in healthy volunteers. Br. J. Clin. Pharmacol. 2008, 65, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, S.J.; Begg, E.J. Pharmacogenetics, drug-metabolizing enzymes, and clinical practice. Pharmacol. Rev. 2006, 58, 521–590. [Google Scholar] [CrossRef] [PubMed]
- Shirai, N.; Furuta, T.; Moriyama, Y.; Okochi, H.; Kobayashi, K.; Takashima, M.; Xiao, F.; Kosuge, K.; Nakagawa, K.; Hanai, H.; et al. Effects of CYP2C19 genotypic differences in the metabolism of omeprazole and rabeprazole on intragastric pH. Aliment. Pharmacol. Ther. 2001, 15, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Kearns, G.L.; Blumer, J.; Schexnayder, S.; James, L.P.; Adcock, K.G.; Reed, M.D.; Daniel, J.F.; Gaedigk, A.; Paul, J. Single-dose pharmacokinetics of oral and intravenous pantoprazole in children and adolescents. J. Clin. Pharmacol. 2008, 48, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Knebel, W.; Tammara, B.; Udata, C.; Comer, G.; Gastonguay, M.R.; Meng, X. Population pharmacokinetic modeling of pantoprazole in pediatric patients from birth to 16 years. J. Clin. Pharmacol. 2011, 51, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.M.; Tammara, B.; Sullivan, S.E.; Stewart, D.L.; Rath, N.; Meng, X.; Maguire, M.K.; Comer, G.M. Single-dose, multiple-dose, and population pharmacokinetics of pantoprazole in neonates and preterm infants with a clinical diagnosis of gastroesophageal reflux disease (GERD). Eur. J. Clin. Pharmacol. 2010, 66, 555–561. [Google Scholar] [CrossRef] [PubMed]
- DailyMed. SERTRALINE HYDROCHLORIDE—Sertraline Hydrochloride Solution. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7f144b68-ae90-483c-b030-f6824662a734 (accessed on 12 September 2017).
- Rudberg, I.; Hermann, M.; Refsum, H.; Molden, E. Serum concentrations of sertraline and N-desmethyl sertraline in relation to CYP2C19 genotype in psychiatric patients. Eur. J. Clin. Pharmacol. 2008, 64, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Liu, Z.Q.; Wang, W.; Chen, X.P.; Shu, Y.; He, N.; Zhou, H.H. Pharmacokinetics of sertraline in relation to genetic polymorphism of CYP2C19. Clin. Pharmacol. Ther. 2001, 70, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwerburgh, F.C.W.; Denys, D.A.J.P.; Westenberg, H.G.M.; Deforce, D.L.D. Response to serotonin reuptake inhibitors in OCD is not influenced by common CYP2D6 polymorphisms. Int. J. Psychiatry Clin. Pract. 2009, 13, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Brandl, E.J.; Tiwari, A.K.; Zhou, X.; Deluce, J.; Kennedy, J.L.; Müller, D.J.; Richter, M.A. Influence of CYP2D6 and CYP2C19 gene variants on antidepressant response in obsessive-compulsive disorder. Pharmacogenom. J. 2014, 14, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Grasmäder, K.; Verwohlt, P.L.; Rietschel, M.; Dragicevic, A.; Müller, M.; Hiemke, C.; Freymann, N.; Zobel, A.; Maier, W.; Rao, M.L. Impact of polymorphisms of cytochrome-P450 isoenzymes 2C9, 2C19 and 2D6 on plasma concentrations and clinical effects of antidepressants in a naturalistic clinical setting. Eur. J. Clin. Pharmacol. 2004, 60, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.W.F.; Gaedigk, A.; Ereshefsky, L.; Alfaro, C.L.; Simpson, J. CYP2D6 inhibition by selective serotonin reuptake inhibitors: Analysis of achievable steady-state plasma concentrations and the effect of ultrarapid metabolism at CYP2D6. Pharmacotherapy 2002, 22, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.K.; Bishop, J.R.; Sangkuhl, K.; Müller, D.J.; Ji, Y.; Leckband, S.G.; Leeder, J.S.; Graham, R.L.; Chiulli, D.L.; LLerena, A.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clin. Pharmacol. Ther. 2015. [Google Scholar] [CrossRef] [PubMed]
- DailyMed. AMITRIPTYLINE HYDROCHLORIDE—Amitriptyline Hydrochloride Tablet, Film Coated. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1e6d2c80-fbc8-444e-bdd3-6a91fe1b95bd (accessed on 12 September 2017).
- Hicks, J.K.; Swen, J.J.; Thorn, C.F.; Sangkuhl, K.; Kharasch, E.D.; Ellingrod, V.L.; Skaar, T.C.; Müller, D.J.; Gaedigk, A.; Stingl, J.C. Clinical Pharmacogenetics Implementation Consortium Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clin. Pharmacol. Ther. 2013, 93, 402–408. [Google Scholar] [CrossRef] [PubMed]
- DailyMed. CITALOPRAM HYDROBROMIDE—Citalopram Tablet. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a8e8fde2-8392-4d5b-902b-e2b9dd55a02d (accessed on 12 September 2017).
- DailyMed. ESCITALOPRAM—Escitalopram Oxalate Tablet, Film Coated. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c7561244-68c9-6889-65b1-4ab8d1e09d2a (accessed on 12 September 2017).
- Amitai, M.; Kronenberg, S.; Carmel, M.; Michaelovsky, E.; Frisch, A.; Brent, D.; Apter, A.; Chen, A.; Weizman, A.; Fennig, S. Pharmacogenetics of citalopram-related side effects in children with depression and/or anxiety disorders. J. Neural Transm. 2016, 123, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Tybring, G.; Dahl, M.-L.; Lindh, J.D. Impact of cytochrome P450 2C19 polymorphisms on citalopram/escitalopram exposure: A systematic review and meta-analysis. Clin. Pharmacokinet. 2014, 53, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Germann, D.; Kurylo, N.; Han, F. Risperidone. Profiles Drug Subst. Excip. Relat. Methodol. 2012, 37, 313–361. [Google Scholar] [CrossRef] [PubMed]
- Masri, B.; Salahpour, A.; Didriksen, M.; Ghisi, V.; Beaulieu, J.-M.; Gainetdinov, R.R.; Caron, M.G. Antagonism of dopamine D2 receptor/β-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc. Natl. Acad. Sci. USA 2008, 105, 13656–13661. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Takano, H.; Takahashi, H.; Arakawa, R.; Miyoshi, M.; Kodaka, F.; Okumura, M.; Otsuka, T.; Suhara, T. Effects of the antipsychotic risperidone on dopamine synthesis in human brain measured by positron emission tomography with l-[β-11C]DOPA: A stabilizing effect for dopaminergic neurotransmission? J. Neurosci. 2009, 29, 13730–13734. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Bourin, M.; Baker, G.B. Metabolism of risperidone to 9-hydroxyrisperidone by human cytochromes P450 2D6 and 3A4. Naunyn. Schmiedebergs Arch. Pharmacol. 1999, 359, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Dean, L. Risperidone Therapy and CYP2D6 Genotype. In Medical Genetics Summaries; Pratt, V., McLeod, H., Dean, L., Malheiro, A., Rubinstein, W., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012. [Google Scholar]
- DailyMed. RISPERIDONE—Risperidone Tablet. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1c3250f3-d291-4280-97d5-da3bb171e2b6 (accessed on 12 September 2017).
- Correll, C.U.; Penzner, J.B.; Parikh, U.H.; Mughal, T.; Javed, T.; Carbon, M.; Malhotra, A.K. Recognizing and monitoring adverse events of second-generation antipsychotics in children and adolescents. Child Adolesc. Psychiatr. Clin. N. Am. 2006, 15, 177–206. [Google Scholar] [CrossRef] [PubMed]
- De Leon, J.; Susce, M.T.; Pan, R.-M.; Fairchild, M.; Koch, W.H.; Wedlund, P.J. The CYP2D6 poor metabolizer phenotype may be associated with risperidone adverse drug reactions and discontinuation. J. Clin. Psychiatry 2005, 66, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-F. Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part I. Clin. Pharmacokinet. 2009, 48, 689–723. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-F. Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part II. Clin. Pharmacokinet. 2009, 48, 761–804. [Google Scholar] [CrossRef] [PubMed]
- Lane, H.-Y.; Liu, Y.-C.; Huang, C.-L.; Chang, Y.-C.; Wu, P.-L.; Lu, C.-T.; Chang, W.-H. Risperidone-related weight gain: Genetic and nongenetic predictors. J. Clin. Psychopharmacol. 2006, 26, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Novalbos, J.; López-Rodríguez, R.; Román, M.; Gallego-Sandín, S.; Ochoa, D.; Abad-Santos, F. Effects of CYP2D6 genotype on the pharmacokinetics, pharmacodynamics, and safety of risperidone in healthy volunteers. J. Clin. Psychopharmacol. 2010, 30, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Puangpetch, A.; Vanwong, N.; Nuntamool, N.; Hongkaew, Y.; Chamnanphon, M.; Sukasem, C. CYP2D6 polymorphisms and their influence on risperidone treatment. Pharmacogenom. Pers. Med. 2016, 9, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Cabaleiro, T.; Ochoa, D.; López-Rodríguez, R.; Román, M.; Novalbos, J.; Ayuso, C.; Abad-Santos, F. Effect of polymorphisms on the pharmacokinetics, pharmacodynamics, and safety of risperidone in healthy volunteers. Hum. Psychopharmacol. 2014, 29, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Dodgen, T.M.; Eloff, A.; Mataboge, C.; Roos, L.J.L.; van Staden, W.C.W.; Pepper, M.S. Risperidone-associated adverse drug reactions and CYP2D6 polymorphisms in a South African cohort. Appl. Transl. Genom. 2015, 5, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Calarge, C.A.; Miller, D.D. Predictors of risperidone and 9-hydroxyrisperidone serum concentration in children and adolescents. J. Child Adolesc. Psychopharmacol. 2011, 21, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Mummaneni, P.; Kim, I.W.; Oh, J.M.; Pacanowski, M.; Burckart, G.J. Pharmacogenomic information in FDA-approved drug labels: Application to pediatric patients. Clin. Pharmacol. Ther. 2016, 99, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Hines, R.N. Ontogeny of human hepatic cytochromes P450. J. Biochem. Mol. Toxicol. 2007, 21, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Relling, M.V.; Gardner, E.E.; Sandborn, W.J.; Schmiegelow, K.; Pui, C.-H.; Yee, S.W.; Stein, C.M.; Carrillo, M.; Evans, W.E.; Hicks, J.K.; et al. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin. Pharmacol. Ther. 2013, 93, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Relling, M.V.; Gardner, E.E.; Sandborn, W.J.; Schmiegelow, K.; Pui, C.-H.; Yee, S.W.; Stein, C.M.; Carrillo, M.; Evans, W.E.; Klein, T.E. Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin. Pharmacol. Ther. 2011, 89, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Li, G.-F.; Markowitz, J.S. Atomoxetine: A Review of Its Pharmacokinetics and Pharmacogenomics Relative to Drug Disposition. J. Child Adolesc. Psychopharmacol. 2016, 26, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Caudle, K.E.; Gong, L.; Whirl-Carrillo, M.; Stein, C.M.; Scott, S.A.; Lee, M.T.; Gage, B.F.; Kimmel, S.E.; Perera, M.A.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Pharmacogenetics-Guided Warfarin Dosing: 2017 Update. Clin. Pharmacol. Ther. 2017, 102, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Stenner, S.P.; Doan, S.; Johnson, K.B.; Waitman, L.R.; Denny, J.C. MedEx: A medication information extraction system for clinical narratives. J. Am. Med. Inform. Assoc. 2010, 17, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Caudle, K.E.; Dunnenberger, H.M.; Freimuth, R.R.; Peterson, J.F.; Burlison, J.D.; Whirl-Carrillo, M.; Scott, S.A.; Rehm, H.L.; Williams, M.S.; Klein, T.E.; et al. Standardizing terms for clinical pharmacogenetic test results: Consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet. Med. 2017, 19, 215–223. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).