Abstract
Acne vulgaris is a common dermatological condition, particularly affecting adolescents during critical developmental stages, which may have lasting psychosocial impacts. Traditional assessments, including global severity grading and lesion counting, are limited by subjectivity and time constraints. Background/Objectives: This review aims to systematically assess the recent advancements in artificial intelligence (AI) applications for acne diagnosis, lesion segmentation/counting, and severity grading, highlighting the potential of AI-driven methods to improve objectivity, reproducibility, and clinical efficiency. Methods: A comprehensive literature search was conducted across PubMed, Scopus, arXiv, Embase, and Web of Science for studies published between 1 January 2017 and 1 March 2025. The search strategy incorporated terms related to “acne” and various AI methodologies (e.g., “neural network”, “deep learning”, “convolutional neural network”). Two independent reviewers screened 345 articles, with 29 studies ultimately meeting inclusion criteria. Data were extracted on study design, dataset characteristics (including internal and publicly available resources such as ACNE04 and AcneSCU), AI architectures (predominantly CNN-based models), and performance metrics. Results: While AI-driven models demonstrated promising accuracy, as high as 97.6% in controlled settings, the limited availability of large public datasets, the predominance of data from specific ethnic groups, and the lack of extensive external validation underscore critical barriers to clinical implementation. Conclusions: The findings indicate that although AI has the potential to standardize acne assessments, reduce observer variability, and enable self-monitoring via mobile platforms, significant challenges remain in achieving robust, real-world applicability. Future research should prioritize the development of large, diverse, and publicly accessible datasets and undertake prospective clinical validations to ensure equitable and effective dermatological care.
1. Introduction
Acne vulgaris is one of the most common dermatological diseases, affecting a significant proportion of the young population, particularly adolescents [1]. Its onset during adolescence, a critical period of individual’s social, emotional, and physical maturation, may negatively impact body image satisfaction, social activities and self-esteem, and often extends into adulthood resulting in impaired quality of life [2].
Accurate measurement and grading of acne are critical for effective management and reproducibility of research [2], but it can be challenging due to spontaneous acne fluctuations and the uneven distribution of lesions.
Traditional acne-severity assessment tools can be categorized into two main approaches: global acne severity grading and lesion counting, both of which have the limitations of subjective assessments and are time-consuming [3]. Global severity grading provides a subjective assessment that compares the clinical presentation with a standard photograph or text description. Currently, the most used acne grading scales include the Investigator Global Assessment (IGA), which features five ordinal grades ranging from 0 to 4 (0, clear; 1, almost clear; 2, mild; 3, moderate; 4, severe) [3] and the Global Acne Severity Scale (GEA), which offers a similar grading scale from 0 to 5 (0, no lesions; 1, nearly no lesions; 2, mild; 3, moderate; 4, severe; 5, very severe) [3].
Another possible approach is lesion counting, exemplified by the Hayashi scale, that evaluates acne severity by counting inflammatory lesions (papules and pustules) on one side of the face and classifies it into four categories: mild (0–5 lesions), moderate (6–20), severe (21–50), and very severe (>50) [3]. Global severity grading tends to be simpler and quicker than lesion counting, but is more operator dependent and less sensitive to disease fluctuation over time. Conversely, lesion counting provides a more objective and reproducible method, but it is often impractical, time-consuming, and does not consider parameters as the pattern distribution, lesions’ size, or the presence of erythema [3]. Both lesion counting and acne severity grading criteria can be further classified into those that consider only the number of lesions and those that incorporate both lesion count and type (e.g., open and closed comedones, papules, pustules, and nodules) [3].
In recent years, the rapid advance of artificial intelligence (AI) and computer vision technologies has opened new avenues for automated and objective acne diagnosis and assessment, through both lesion segmentation/lesion counting and general grading. AI-based systems, trained on vast datasets and sophisticated algorithms to identify and classify acne lesions with high precision, offer the potential for standardized and reproducible ways to assess acne and its response to treatment [4]. The application of AI-driven automated acne-grading algorithms may allow a faster, more precise, reproducible and standardized approach than traditional acne scoring methods, reducing inter- and intra-observer variability [5]. AI algorithms might also lead to self-monitor through mobile applications, assess response to treatments, process enormous amounts of epidemiological data [6], and simplify comparisons across research studies in meta-analyses [7].
This systematic review aims to report recent advancements and implementation of AI applications in acne diagnosis, lesion counting, and severity grading.
2. Materials and Methods
We evaluated methodologies such as convolutional neural networks (CNNs), deep residual networks, and hybrid models, focusing on their architectural designs, dataset composition, and performance metrics (e.g., accuracy, sensitivity, specificity). This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines. A comprehensive search of PubMed, Scopus, arXiv, Embase, and Web of Science was conducted using the following terms: (“acne”) AND (“artificial intelligence” OR “neural network” OR “deep learning” OR “convolutional neural network” OR “transfer learning” OR “machine learning” OR “computer-aided diagnostic” OR “CAD” OR “image classification” OR “image processing” OR “Internet of Things” OR “data mining”) NOT (“Meta-Analysis” OR “Systematic Review”). Studies published between 1 January 2017 and 1 March 2025 were included. Two independent reviewers (D.O.T. and G.P.) screened titles and abstracts to determine the eligibility of studies. We included studies of any design if they enrolled human patients of any age diagnosed with acne vulgaris and evaluated artificial-intelligence or computer-vision algorithms for lesion detection or severity grading. Eligible studies had to benchmark their models against a clinical reference either dermatologist assessments or expert-annotated “ground truth” and report at least one quantitative performance metric (for example, accuracy, area under the receiver-operating-characteristic curve [AUC], Dice coefficient, sensitivity, or specificity). Only retrospective observational investigations published in peer-reviewed journals in English were considered. We excluded conference abstracts, non-English articles, narrative reviews and meta-analyses, and any studies that reused datasets already captured elsewhere to avoid duplication. Because this is a rapidly evolving field, we also extended our search to the preprint server arXiv; however, none of the non-peer-reviewed manuscripts retrieved met our inclusion criteria. The full protocol has been registered on Open Science Framework [8]. Risk of bias and applicability concerns were evaluated for each included study using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool. Detailed ratings are provided in Supplementary Table S1. Full texts of eligible studies were assessed for relevance, with discrepancies resolved through consensus.
3. Results
3.1. Study Selection and Transparency
The systematic search identified 345 articles, with 98 studies undergoing full-text screening. Ultimately, 29 studies met inclusion criteria (Figure 1). The analysis focused on AI methodologies employed, datasets utilized, and the results achieved in acne diagnosis and severity grading. All the studies included in the review were retrospective. Among them, 13 (44.8%) relied solely on internal datasets, while 16 (55.2%) used publicly available datasets (ACNE04 [9] and AcneSCU [10]). Specifically, seven studies (24.1%) utilized only the ACNE04 dataset, one study (3.4%) used the AcneSCU dataset, five studies (20.7%) integrated ACNE04 with internal data, and one study (3.4%) combined ACNE04, AcneSCU, and internal data.
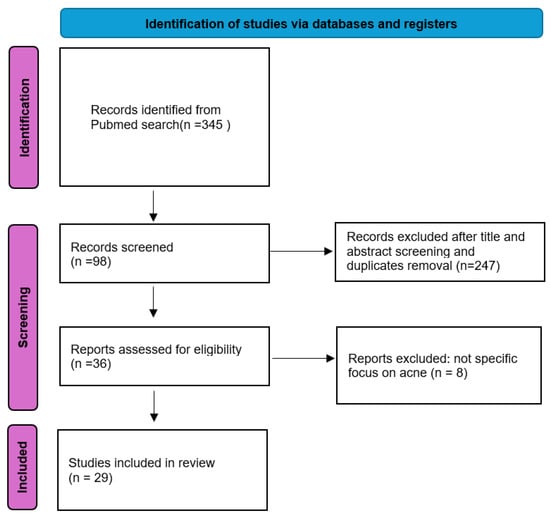
Figure 1.
Our systematic review process following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) framework.
Additionally, one study utilized an AI-generated synthetic dataset, created by training a Generative Adversarial Network (GAN) on ACNE04 [11]. One study [11] (3.4%) used a public dataset of faces (CelebAMask-HQ and 2037 images from the Flickr-Faces-HQ), a minority of whom were acne-affected. It is worth noting that, while most of the studies have used images taken from outpatient clinics photos, seven studies (27.6%) used patient self-taken smartphone photos. Publicly accessible code was available in only four studies (13.8%).
3.2. Participant Demographics and Dataset Diversity
Participant age was reported in only five studies (17.2%). Information on participant ethnicity was available for 16 studies (55.2%), including those relying exclusively on the publicly available ACNE04 dataset. Among these, 15 studies (51.8%) primarily included individuals of East Asian or Chinese ethnicity. The only exception was the study by Seitè et al. [5] that analyzed 5972 images from 1072 acne patients representing different ethnic groups, including Caucasian, African, Asian, Latin American, and Indian populations, across countries such as France, South Africa, China, and India. Images were captured using both iOS and Android smartphones, ensuring broad representation of skin colors and types.
3.3. Algorithms
Among the 29 studies analyzed, 11 (37.9%) utilized multiple deep learning (DL) algorithms, resulting in a total of 47 distinct models. CNNs and deep convolutional neural networks (DCNNs) were the predominant architectures, accounting for 79% (23/29) of all models examined (Table 1). The primary research focus was acne lesion segmentation and counting (12 studies, 41.4%) and severity grading (16 studies, 55.2%), while only one study (3.4%) investigated binary classification (presence vs. absence of acne).
The training datasets exhibited substantial variability, ranging from 1213 to over 150,000 images. Key methodological approaches included CNN-based architectures, semi-supervised learning techniques, and generative adversarial networks (GANs), such as StyleGAN2-ADA, which were employed to generate synthetic datasets, thereby addressing data scarcity and enhancing model generalization. Training data were typically partitioned into training and validation subsets, with 80–90% allocated for training and the remainder reserved for validation.
Model validation was conducted using either k-fold cross-validation or holdout methods to ensure rigorous performance assessment. Metrics such as Dice coefficients, accuracy, and the area under the receiver operating characteristic (AUC-ROC) curve were commonly used to evaluate predictive performance, particularly in lesion detection and acne severity grading (Table 1). Despite the overall robust methodology, 17 studies (58.6%) did not provide detailed information on image resolution or validation set specifications, raising concerns about reproducibility. Reported accuracy varied widely, ranging from 20.5% (ACNet10) to 86.06% (DED framework via Knowledge Distillation [12]). Results are summarized in Table 2.

Table 1.
Characteristics of the included studies.
Table 1.
Characteristics of the included studies.
| Authors | Application Category | Methods | Dataset | Dataset Source | Publicly Available Code | Number of Photos (Total) |
|---|---|---|---|---|---|---|
| Shen et al. [13] (2017) | Automatic Acne Diagnosis | Binary classifier for skin detection and seven-class classifier for acne lesion classification, using CNNs with data augmentation techniques. | Binary classifier: 3000 cutaneous and 3000 non-cutaneous images. Seven-class classifier: 6000 images per class (blackheads, whiteheads, papules, pustules, cysts, nodules, normal skin). | Clinical images, augmented with rotation, shift, shear, scaling, and horizontal flip. | No | Not specified |
| Zhao et al. [14] (2019) | Acne Severity Assessment using Selfie Images | Deep learning model using transfer learning with ResNet 152 and a novel rolling image augmentation approach to improve CNN model generalization. | 4700 selfie images, with 4470 used for training and 230 used for testing. | Selfie images collected by Nestlé SHIELD and labeled by 11 dermatologists. | Code available on GitHub: https://github.com/Microsoft/nestle-acne-assessment (accessed on 5 March 2025) | 4700 |
| Wu et al. [9] (2019) | Joint Acne Image Grading and Counting via Label Distribution Learning | Label Distribution Learning framework that integrates global grading and local lesion counting. | 1457 facial images from the ACNE04 dataset with 18,983 annotated lesions. | Clinical images collected under the Hayashi grading criterion, taken at a 70-degree angle. | Code and dataset available on GitHub: https://github.com/xpwu95/ldl (accessed on 5 March 2025) | 1457 |
| Seitè et al. [5] (2019) | Acne Grading from Smartphone Photographs | AI Algorithm trained on a large and diverse dataset, using deep learning techniques for acne grading and lesion identification. | 5972 images from 1072 patients with acne, including 2939 inflammatory lesions, 7603 non-inflammatory lesions, and 5702 PIHP instances. | Smartphone images (iOS and Android) across various racial groups. | No | 5972 |
| Lim et al. [15] (2019) | Automated Acne Grading using Deep Learning and CNNs | Deep learning models (DenseNet, Inception v4, ResNet18) trained on high-resolution images to automatically calculate IGA scores. | 472 frontal facial images from 416 acne patients, with training set of 314 images and testing set of 98 images, augmented to 6248 images. | Clinical images captured at ~2000 × 3000 pixel resolution. | No | 472 |
| Peris Fajarnés et al. [16] (2020) | Segmentation Methods for Acne Vulgaris Images using Fluorescence Imaging | K-means clustering algorithm implemented in MATLAB R2018b for automated segmentation and counting of acne lesions in fluorescence images. | 54 processed fluorescence images of mild acne patients, captured using iPhone X with Wood’s lamp and LED lamp. | Fluorescence images captured using iPhone X smartphone. | No | 54 |
| Rashataprucksa et al. [17] (2021) | Acne Detection | Faster R-CNN and R-FCN models used for acne detection, focusing on various types of acne lesions. | 871 annotated images with 15,917 lesions categorized into four types: Type I, Type III, PIE, and PIH. | Clinical images provided by Pan Rajdhevee Group Public Co., Ltd.; Bangkok, Thailand. | No | 871 |
| Yang et al. [18] (2021) | Acne Vulgaris Assessment | Inception-v3 architecture used for model construction, trained on clinical images categorized into four severity grades. | 5871 clinical images, with 1565 images in the training set, 392 in the validation set, and 40 in the test set. | Clinical images captured with single-lens reflex cameras. | No | 5871 |
| Quattrini et al. [12] (2022) | Facial Acne Classification | Used a VGG-19 based CNN for classification and BiSeNet for semantic segmentation, trained on re-annotated images from the FFHQ dataset. | 2307 re-annotated images from the Flickr-Faces-HQ (FFHQ) dataset, used for acne severity classification. | High-resolution images from the FFHQ dataset, re-annotated for acne severity. | No | 2307 |
| Min et al. [19] (2022) | Acne Detection using Mask-Aware Attention with Dynamic Context Enhancement | ACNet integrates Composite Feature Refinement, Dynamic Context Enhancement, and Mask-Aware Multi-Attention for enhanced feature representation and detection accuracy. | ACNE04 dataset with 1457 facial images and 18,983 annotated lesions. | Clinical photographs with varying resolutions, annotated by dermatologists. | No | 1457 |
| Zhang et al. [20] (2022) | Acne Detection using High-Quality Proposals and Region Proposal Network | Spatial Aware Double Head for classification and localization, Normalized Wasserstein Distance (NWD) for accurate localization confidence prediction. | AcneSCU dataset with 276 high-resolution facial images and 31,777 annotations across 10 lesion categories. | Clinical photographs with high-resolution images, captured using the VISIA complexion analysis system. | Code and dataset available on GitHub: https://github.com/pingguokiller/acnedetection (accessed on 5 March 2025) | 276 |
| Zhang et al. [10] (2022) | Acne Detection | Ensemble neural network composed of ResNet50-based classification and YOLOv5-based localization modules for simultaneous severity, count, and bounding-box prediction | ACNE04: 1457 facial images with expert-annotated lesion counts, severity grades, and bounding boxes | ACNE04 dataset | No 1457 | |
| Wang et al. [21] (2022) | Cell Phone App for Facial Acne Severity Assessment | Acne-RegNet, a lightweight model for lesion detection and classification, combined with metadata for personalized severity assessment. | 1455 images from ACNE04 and 1515 images from a private dataset at Xiangya Hospital, processed into 80 × 80 lesion patches and resized for the model. | Clinical photographs, including metadata, collected from a private dataset and the ACNE04 dataset. | No | 2970 |
| Wang et al. [22] (2022) | Acne Detection and Severity Quantification | Two-stage deep learning scheme | 1803 facial images (1374 for training/test, 429 for clinical validation) | Smartphone images captured with iPhone 5s at Xiangya Hospital, annotated by dermatologists | No | 1803 |
| Huynh et al. [23] (2022) | Automatic Acne Object Detection and Severity Grading Using Smartphone Images and AI | Faster R-CNN deep learning model for acne object detection and LightGBM machine learning model for grading acne severity based on the IGA scale. | 1572 facial images with 41,859 labeled lesions, captured by iOS and Android smartphones. | Smartphone images collected via the ’Skin Detective’ mobile application. | No | 1572 |
| Lin et al. [24] (2022) | Unified Acne Grading Framework on Face Images | KIEGLFN framework with Key Information Enhancement and Global Local Fusion Network, including a transfer fine-tuning strategy for new grading criteria. | 1457 images from the ACNE04 dataset, 200 images from the PLSBRACNE01 dataset, annotated for lesion location, severity, and type. | Clinical images from the ACNE04 and PLSBRACNE01 datasets. | Code available on GitHub: https://github.com/linyi0604/KIEGLFN (accessed on 5 March 2025) | 1657 |
| Liu et al. [25] (2022) | Ensemble Pruning of Deep Learning Base Models for Acne Grading | Ensemble pruning of deep learning models, fivefold cross-validation, and transfer learning applied to both acne and skin cancer datasets. | 1457 images from the ACNE04 dataset, annotated for lesion location and severity grading; 3297 dermoscopic images in the skin cancer dataset, categorized as benign or malignant. | Clinical images from the ACNE04 and skin cancer datasets. | Datasets available at: https://github.com/xpwu95/ldl and https://www.kaggle.com/datasets/fanconic/skin-cancer-malignant-vs-benign (accessed on 5 March 2025) | 4754 |
| Wen et al. [26] (2022) | Acne Detection and Severity Evaluation | Interpretable CNN-based object detection models, with a focus on interpretability | 1457 images from the ACNE04 dataset, with 1222 high-quality images used after data cleaning. | Clinical images with annotations for lesion location, severity, and bounding boxes. | Code available on GitHub: https://github.com/wenh06/acne_detection/ and https://github.com/wenh06/yolov4_acne_torch (accessed on 5 March 2025) | 1457 |
| Kim et al. [27] (2023) | Acne Lesion Detection and Counting for Severity Evaluation | CNN-based algorithm, with a focus on binary and five-class classification of acne lesions. | 1213 images with 20,699 labeled lesions (closed comedones, open comedones, papules, nodules/cysts, pustules). | Clinical images acquired in standardized settings using Canon EOS 550D and NIKON D7100 cameras. | Detailed code and hyperparameters provided in the Electronic Supplementary Materials. | 1213 |
| Wei et al. [28] (2023) | Accurate Acne Detection via Decoupled Sequential Detection Head | Decoupled Sequential Detection Head mechanism applied to mainstream two-stage detectors. | ACNE-DET: 276 high-resolution images with 31,777 labeled lesions; ACNE04: 1457 images with 18,983 bounding box annotations. | Clinical images acquired with VISIA system and annotated by dermatologists. | Code and dataset are publicly available, repository not specified. | 276 |
| Li et al. [29] (2023) | AI-Powered Acne Grading System with Lesion Identification | ResNet50 architecture, with 945 images used for training and 240 for testing, incorporating lesion identification and severity grading. | 1501 standardized photos from 1501 acne patients, with detailed labeling of 15,922 lesions across 10 categories. | Clinical images obtained using the VISIA complexion analysis system. | No | 1501 |
| Lin et al. [30] (2023) | Acne Severity Grading via Diagnostic Evidence Distillation | Teacher-student structure using CNNs for both global estimation and lesion detection, integrating lesion counting and grading. | ACNE04: 1457 facial images with 13,633 lesions; PLSBRACNE01: 200 images categorized by severity. | Clinical images labeled according to Hayashi and Pillsbury criteria. | Code available on GitHub: https://github.com/linyi0604/DED (accessed on 5 March 2025) | 1457 |
| Li et al. [6] (2023) | AI Analysis of Dark Circles and Facial Skin Aging in Chinese Population | CNNs based system for grading facial features from selfies, validated against dermatologist assessments. | Over 1.9 million selfies from 1,939,586 participants, graded for facial aging indicators. | Selfies taken with smartphones, collected via a mobile app. | No | 1,939,586 |
| Li et al. [31] (2024) | Acne severity assessment | CNN-based automatic grading | 1,156,703 women aged 18–85 | Selfies taken with smartphones, collected via a mobile app. Annotated by dermatologists. | No | 1,156,703 |
| Kim et al. [32] (2024) | Facial Acne Segmentation | Semi-supervised learning using bidirectional copy–paste and deep learning with U-Net. | Acne images cropped from facial images | Clinical images (likely taken with specialized skin diagnostic equipment) | No | 2000 |
| Zhang et al. [33] (2024) | Acne Detection using CenterNet | CenterNet-based deep learning framework, utilizing deep hierarchical aggregation strategies for feature extraction, combined with anchor-free detection for improved accuracy in locating and identifying acne lesions. | 153,183 images collected from 15 hospitals in different provinces of China using smartphones. | A mix of clinical and smartphone images | No | 153,183 |
| Prokhorov et al. [34] (2024) | Acne Image Grading using Label Distribution Smoothing | Gaussian label distribution generation and label smoothing techniques combined with a scale-adaptive approach, validated on the ACNE04 dataset using ResNet-50 architecture. | ACNE04 dataset with 1457 images and 18,983 bounding boxes of lesions. | Clinical images from the ACNE04 dataset, specifically annotated for acne grading. | Source code is publicly available at http://github.com/openface-io/acne-lds | 1457 |
| Zein et al. [11] (2024) | Acne Dataset Generation Using Generative Adversarial Networks (GANs) | GAN-based method with StyleGAN2 to generate synthetic acne images at different severity levels, used for training a CNN-based classification system. | 1473 real images initially collected, with additional synthetic images generated across Mild, Moderate, and Severe severity levels. | Synthetic images generated using GANs based on clinical images. | Code and dataset available on GitHub: https://github.com/hz01/AcneGAN/tree/main | 1473 |
| Gao et al. [35] (2025) | Acne lesion detection and severity grading in online and offline scenarios | Deep learning model (AcneDGNet) that combines a vision transformer–based feature extractor with a CNN-based lesion detection module and a severity grading module | 2157 facial acne images compiled from two public datasets (ACNE04, AcneSCU) and three self-built datasets (AcnePA1, AcnePA2, AcnePKUIH) | Mixed capture sources: digital cameras, VISIA system, and smartphone images | No | 2157 |

Table 2.
Results of the included studies.
Table 2.
Results of the included studies.
| Study | Task | Models | Dataset | Validation | Metrics |
|---|---|---|---|---|---|
| Shen et al. (2018) [13] | Binary acne detection and 7-class acne classification | Custom CNN; pre-trained VGG16 | Binary: 3000 skin + 3000 non-skin patches; Seven-class: 6000 patches per class (7 classes); Not specified | Train/validation/test split (80/10/10%) for both classifiers | Binary acne detection: accuracy 92%, 7-class acne classification: per-class accuracy > 81% |
| Lim et al. (2019) [15] | Investigator’s Global Assessment (IGA) grading | CNN architectures (DenseNet, Inception v4, ResNet18) | 472 frontal images (374 train, 60 val, 98 test), augmented to 6248 train, 1200 val; Not specified | Hold-out validation | Best classification accuracy 67% |
| Seité et al. (2019) [5] | Global Acne Severity (GEA) grading and lesion segmentation | Not specified | 5972 smartphone photos | Not specified | GEA grading accuracy 68%; |
| Zhao et al. (2019) [14] | Acne severity assessment from selfies | ResNet-152 pre-trained + fully connected regression CNN | 4470 training images; 230 test images; | Hold-out golden set labeled by 11 dermatologists | Root-mean-square error 0.482 (baseline 0.78; without augmentation 0.72); |
| Wu et al. (2019) [9] | Joint acne grading and lesion counting | Label Distribution Learning; CNN backbone (ResNet-50) | 1457 images (1165 train; 292 test); ACNE04 dataset | 5-fold cross-validation | Accuracy 84.11%; |
| Rashataprucksa et al. (2020) [17] | Acne lesion detection | Faster R-CNN; R-FCN | 871 annotated facial images (783 train; 88 test); | Hold-out test set evaluation | Mean average precision: Faster R-CNN 0.233; R-FCN 0.283 |
| Peris Fajarnés et al. (2020) [16] | Acne lesion segmentation and counting | k-means clustering segmentation | Fluorescence images of mild acne patients; validation on 54 images; Fluorescence imaging (Wood’s lamp and LED with filters) | Comparison to manual expert counts | Segmentation effectiveness > 90%; discrepancy ≤ 11.2% |
| Yang et al. (2021) [18] | Acne severity grading | Inception-v3 CNN (transfer learning) | 5871 clinical images (1565 train; 392 val; 40 test); Clinical photos, Chinese Academy of Medical Sciences | Hold-out test set against attending dermatologists | Average F1 = 0.80; weighted kappa = 0.791 |
| Zhang et al. (2022) [20] | High-quality proposal generation for acne detection | Spatial Aware Double Head and NWD prediction on RPN | 276 images; 31,777 annotations; AcneSCU dataset | 90/10 train:test split; separate patients | Improved proposal AR and detection mean average precision (specifics N/A) |
| Zhang et al. (2022) [10] | Acne severity classification and lesion localization | Ensemble NN with ResNet50 classifier and YOLOv5 localization | 1457 facial images; ACNE04 public dataset | Hold-out test set (80/20 split) | Best accuracy 90.67% |
| Lin et al. (2022) [24] | Unified acne grading across criteria | KIEGLFN (Key Information Enhancement + GLFNet) | 1457 ACNE04 images + 200 other images (PLSBRACNE01 dataset) | 5-fold CV on ACNE04; 80/20 split on PLSBRACNE01 | Accuracy 84.52% on ACNE04; 59.35% on PLSBRACNE01 |
| Wang et al. (2022) [22] | Smartphone-based acne severity assessment | Acne-RegNet (lightweight CNN with attention) | 1455 ACNE04 images + 1515 internal Xiangya Hospital images | Hold-out evaluation | Severity accuracy 94.56% on internal dataset |
| Liu et al. (2022) [25] | Acne grading via ensemble pruning | Ensemble pruning of deep learning base models (CNNs) | 1457 ACNE04 images; 3297 skin cancer dermoscopic images; ACNE04 dataset; public skin cancer dataset (dermoscopic) | 5-fold cross-validation on ACNE04; hold-out test on cancer dataset | Accuracy 85.82% on ACNE04 |
| Wen et al. (2022) [26] | Acne lesion detection and severity evaluation | Faster R-CNN (ResNet101) and other interpretable CNNs | 1222 images (Filtered ACNE04 dataset) | Not reported | mAP = 0.536; MAE = 3.49 |
| Huynh et al. (2022) [23] | Acne lesion detection and severity grading | Faster R-CNN (ResNet50 backbone) for detection; LightGBM for grading | 1572 smartphone facial images | Hold-out evaluation | mAP = 0.54 (detection); accuracy = 0.85 (grading) |
| Min et al. (2022) [19] | Acne lesion detection | ACNet (Composite Feature Refinement + DCE + MAMA) | ACNE04 public dataset | 80/20 hold-out split | Detection mAP = 20.5% |
| Quattrini et al. (2022) [12] | Acne classification and semantic segmentation | Semantic Segmentation + DenseNet121 | 2307 FFHQ images re-annotated; 30,000 CelebAMask-HQ masks | Hold-out test set for segmentation and classification | Segmentation F1 avg 86.1%; Classification F1 avg 60.84% |
| Wang et al. (2023) [21] | Acne detection and severity quantification | Localization-DL (SE-ResNet-50 teacher + MobileNetV2 student) and ClassSeg pipelines | 1374 self-collected clinical facial images | Hold-out test set; ablation and comparison vs. dermatologists | Severity quantification accuracy 90.91% vs. dermatologists (93.01%, 87.41%, 74.83%) |
| Kim et al. (2023) [27] | Automated lesion detection and counting for severity evaluation | PP-YOLO (improved YOLOv3) for five-class and binary lesion detection | 1213 images; 20,699 manually labeled lesions; Clinical facial acne photography sets | Hold-out test set evaluation and reader test | Binary detection mAP 28.48% |
| Li et al. (2023) [29] | AI-powered acne severity grading incorporating lesion identification | ResNet-50 with fixed and learnable lesion-count integration | 1501 VISIA images (276 for lesion ID, 1185 for grading); Standardized VISIA clinical photos from 1501 acne patients | Hold-out test sets | Lesion identification: precision 0.507, Recall 0.775; severity kappa: baseline 0.652, fixed weights 0.737, learnable weights 0.696 |
| Wei et al. (2023) [28] | Fine-grained acne lesion detection | Decoupled Sequential Detection Head (DSDH) on two-stage detectors | Internal dataset of 276 images (241 train/35 test) and 31,777 lesion instances; + ACNE04 | Hold-out test set on ACNE-DET | ACNE-DET—AP 45.6%, APS 44.3%, APM 46.9%, APL 34.1% (best config) |
| Lin et al. (2023) [30] | Acne severity grading across varied criteria | Diagnostic Evidence Distillation (DED) teacher–student CNN framework | ACNE04: 1457 images + 200 images | Hold-out test sets on both datasets | ACNE04—Precision 85.31%, Accuracy 86.06%; |
| PLSBRACNE01—Precision 69.16%, Accuracy 67.56% | |||||
| Li et al. (2023) [6] | Automatic grading of facial signs including acne vulgaris | ResNet50V2 transfer learning | 1,939,586 selfie images (100,589 men; 1,838,997 women) from a smartphone app | Internal validation against dermatologist scores | Dark circles classification accuracy 91.5%; acne vulgaris grading metrics not separately reported |
| Zhang et al. (2024) [33] | Multi-task acne detection (quality control, classification, localization, segmentation) | CenterNet with DLA34 backbone and custom segmentation submodule | 153,183 acne lesion images (150,219 train; 2322 val); 642 independent clinical test images; smartphone and clinical images from 15 hospitals in China (Jan 2020–Oct 2022) | Independent clinical test set; comparison against ResNet18 and dermatologists | Lesion categorization accuracy 83%; lesion stratification precision 76% |
| Li et al. (2024) [34] | Epidemiological analysis of adult female acne severity | ResNet-50 + FPN + RPN + ROI head CNN | 1,156,703 high-res smartphone selfies from a smartphone app | 8:1:1 train:val:test split; physician consensus on 100 samples | Inter-rater ICC 0.887; intra-rater ICC 0.900 |
| Kim et al. (2024) [32] | Facial acne lesion segmentation | Semi-supervised U-Net with bidirectional copy–paste framework | Proprietary facial acne image dataset | Comparison with previous SSL methods across varying labeled proportions | Dice score 0.5205 with 3% labeled images (improvement 0.0151–0.0473) |
| Zein et al. (2024) [11] | Synthetic acne face dataset generation and CNN classification | StyleGAN-based GANs; InceptionResNetV2 classifier | ACNE04 public dataset | Not specified | Accuracy 97.6% |
| Prokhorov et al. (2024) [34] | Acne image grading with scale-adaptive label distribution smoothing | Scale-adaptive LDL + weighted label smoothing | ACNE04 public dataset | 5-fold cross-validation | Accuracy 84.11 ± 1.94%; Precision 83.11 ± 2.56%; |
| Gao et al. (2025) [22] | Acne lesion detection and severity grading in online and offline scenarios | AcneDGNet (Swin Transformer + CNN modules) | ACNE04 + AcneSCU + internal datasets (AcnePA1, AcnePA2, AcnePKUIH) | Hold-out evaluations: online (AcnePA1 and PA2) and offline (ACNE04, AcneSCU, AcnePKUIH); | Online accuracy 89.5%; offline accuracy 89.8%; |
4. Discussion
AI-based systems for acne grading are expected to improve dermatological care by providing standardized assessments, reducing inter-observer variability, and enhancing clinical efficiency. However, challenges such as data imbalance, limited dataset diversity, and difficulties integrating these tools into existing clinical workflows continue to limit their wider adoption.
The widespread application of AI-based acne grading might standardize dermatological research, making studies conducted in different regions and clinical settings more comparable and facilitating the development of therapeutic strategies based on robust and reproducible data. Furthermore, AI’s ability to process vast amounts of data from diverse skin types can enhance our understanding of acne’s incidence and geographical distribution. It can also help identify new risk factors, support the development of updated epidemiological registries, and contribute more effectively to health policy. Li et al. [6] used AI to analyze facial acne in adult women across China, involving 1,156,703 participants. The study employed a smartphone application, “You Look Good Today,” to collect high-resolution selfies and user data through questionnaires, including information on age, skin sensitivity, dietary habits, and environmental factors. The AI algorithm, based on a ResNet-50 architecture combined with a Feature Pyramid Network and a Region Proposal Network, classified acne severity into four grades. The Overall Severity Score (OSS) was calculated using the following formula: OSS = 100 × (∑(Si2))1/2.
In this formula, Si represents the severity score assigned to each acne lesion detected by the CNN in a selfie. The scores were defined as follows: 1 for comedones only, 2 for papules, 3 for pustules, and 4 for nodules and cysts. The study revealed that the OSS decreases from age 25, reaches its lowest point between ages 40 and 44, and then gradually increases again. The analysis indicated that oily and sensitive skin, frequent use of cosmetic products, and unhealthy dietary habits significantly contribute to higher acne severity. Environmental factors, such as the level of urban development, season, altitude, and solar radiation, also impacted acne severity, with more severe acne observed in developed cities and during autumn and winter seasons [6]. The study further found a positive correlation between acne severity and other facial issues, such as enlarged pores, blackheads, skin roughness, and dark circles [6].
However, while some models have demonstrated high accuracy in controlled environments [35], translating these results into clinical practice remains challenging: to date, none of the proposed algorithms has received clinical validation and no study has been published regarding real-life settings. Prospective studies with real-world testing are needed to validate the effectiveness of these AI systems in routine dermatological care.
Unlike other dermatological conditions, which have extensive public databases supporting standardized AI algorithm development, acne remains underrepresented in these resources [36]. Privacy concerns surrounding the public sharing of images of acne-affected faces continue to be a major obstacle to building large, publicly accessible datasets. Consequently, most datasets used in acne-related studies are not publicly accessible [12].
Quattrini et al. [12] used a public dataset of faces: Flickr-Faces-HQ (FFHQ), consisting of 70,000 high-quality PNG images of human faces at 1024×1024 resolution and containing considerable variation in terms of age, ethnicity and image background. However, because most images did not show acne, the dataset was highly unbalanced: about 88% of the images fell into the “No Acne” category. This imbalance restricted the study to simply detecting the presence of acne rather than assessing its severity.
The ACNE04 dataset is a notable exception [30]. This public dataset contains 1457 facial images with acne annotations, each labeled with acne severity and lesion count by professional dermatologists. However, the dataset has several limitations, including an imbalance in lesion counts, with most images containing fewer than 10 lesions and few images with high lesion counts (40 to 50), and it consists solely of Han Chinese subjects [30].
Similarly, another public resource is represented by the AcneSCU dataset [10], which includes 276 annotated facial images of acne, but its limited size and focus solely on Han Chinese patients also constrain its broader applicability.
A novel approach to address the challenges posed by the limited availability of public acne datasets has been recently proposed [11] using GANs to create AI-generated synthetic and anonymized datasets of acne-affected faces. In this study, a small initial dataset consisting of 1473 images from the ACNE04 dataset and additional sources was pre-processed and augmented to generate high-resolution synthetic images (1024 × 1024) representing three levels of acne severity: mild, moderate, and severe. By generating realistic images with different grades of acne severity, the study not only avoids the ethical and legal challenges associated with sharing medical images but also contributes to the diversification of available data, as the synthetic datasets are not confined to any specific ethnicity or lesion distribution. The generated datasets were then used to train three different CNNs (InceptionResNetV2, ResNet50V2, and ResNet152V2) solely on synthetic images. Then, real acne images were used to test these models. The best-performing model, InceptionResNetV2, achieved a grading accuracy of 97.6% when validated on real images, demonstrating the viability of this approach for future research on this topic. It should also be noted that the accuracy of the estimation of acne severity may be influenced by lighting conditions, facial expressions, and individual variations in skin type [13].
Ethnic diversity also remains another major limitation: the two largest public image datasets (ACNE04 and AcneSCU) comprise only Han Chinese subjects, and most published studies focus on East Asian individuals. In other dermatology applications, lack of skin-type diversity has been shown to reduce algorithmic accuracy on under-represented group in training sets [3]: acne detection and grading models trained predominantly on East Asian data are likely to underperform in other ethnic populations.
Another aspect revealed by this review, and a further obstacle for the real-life applications of automatized acne grading systems, is the pervasive lack of transparency—58.6% of studies failed to report crucial details such as image resolution or the makeup of their independent validation sets, severely undermining both reproducibility and inter-study comparability. Moreover, only 13.8% have made their source code publicly available, and nearly half (44.8%) rely exclusively on internal, non-public datasets. Practical implementation of external validation could involve a hold-out set drawn from partner dermatology clinics, with subsequent cross-site performance benchmarking and domain-adaptation techniques to identify and mitigate dataset biases. Addressing these shortcomings through open data, shared code, and prospective, multicenter clinical trials will be crucial to developing unbiased, generalizable tools.
Other crucial issues include data privacy and security (transmitting sensitive facial images), data interoperability (integrating AI outputs into electronic health records or telehealth systems) and regulatory approval. In future, privacy-preserving methods like federated learning may help address confidentiality concerns [37]. Ultimately, adoption will depend on evidence of benefit in real-world workflows and compliance with evolving regulations and ethical guidelines.
In conclusion, to date none of the published algorithms have demonstrated the generalizability, reproducibility, or external and prospective clinical validation necessary for integration into routine dermatological practice. Future work in AI-driven acne diagnosis and severity grading should address these gaps, particularly the need for real-world prospective clinical trials to evaluate model performance in real-world settings [35]; the creation of large, publicly accessible, and demographically diverse imaging repositories [11]; the exploration of federated learning to enable privacy-preserving cross-institutional model training; and the integration of explainable AI methods to enhance clinician interpretability and adoption.
Despite current limitations, however, the results are encouraging: AI-driven tools promise to transform acne research and clinical care, reducing inter- and intra-observer variability and enabling truly personalized, timely therapies. By refining model architectures, broadening and diversifying available datasets, and rigorously validating performance in real-world settings, the field can unleash AI’s full potential in this condition.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jpm15060238/s1, Table S1: QUADAS2 assessment of the included studies.
Author Contributions
Conceptualization, D.O.T.; methodology, D.O.T. and G.P.; validation, C.G. and K.P.; formal analysis, D.O.T., G.P. and K.P.; investigation, D.O.T. and G.P.; data curation, D.O.T.; writing—original draft preparation, D.O.T. and G.P.; writing—review and editing, C.G. and K.P.; supervision K.P.; project administration, K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
K. Peris has received consulting fees and honoraria for Advisory Board Meetings from Abbvie, Almirall, Beiersdorf, BMS, Galderma, Leo Pharma, Lilly, Novartis, Sun-Pharma, MSD, Philogen, Pierre Fabre, Regeneron, and Sanofi, outside of the submitted work. D.O. Traini, G. Palmisano, and C. Guerriero declare that they have no conflicts of interest relevant to this manuscript.
Abbreviations
The following abbreviations are used in this manuscript:
| IGA | Investigator Global Assessment |
| AI | Artificial Intelligence |
| CNN | Convolutional Neural Network |
| GAN | Generative Adversarial Networks |
| OSS | Overall Severity Score |
References
- Chen, H.; Zhang, T.C.; Yin, X.L.; Man, J.Y.; Yang, X.R.; Lu, M. Magnitude and temporal trend of acne vulgaris burden in 204 countries and territories from 1990 to 2019: An analysis from the Global Burden of Disease Study 2019*. Br. J. Dermatol. 2021, 186, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.V.; Yeung, H.; Cheng, C.E.; Cook-Bolden, F.; Desai, S.R.; Druby, K.M.; Freeman, E.E.; Keri, J.E.; Gold, L.F.S.; Tan, J.K.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2024, 90, 1006.e1–1006.e30. [Google Scholar] [CrossRef]
- Bae, I.H.; Kwak, J.H.; Na, C.H.; Kim, M.S.; Shin, B.S.; Choi, H. A Comprehensive Review of the Acne Grading Scale in 2023. Ann. Dermatol. 2024, 36, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.A.; Chan, H.W.H.; Baker, M.A.B. Machine learning: Applications of artificial intelligence to imaging and diagnosis. Biophys. Rev. 2018, 11, 111–118. [Google Scholar] [CrossRef]
- Seité, S.; Khammari, A.; Benzaquen, M.; Moyal, D.; Dréno, B. Development and accuracy of an artificial intelligence algorithm for acne grading from smartphone photographs. Exp. Dermatol. 2019, 28, 1252–1257. [Google Scholar] [CrossRef]
- Li, J.; Du, D.; Zhang, J.; Liu, W.; Wang, J.; Wei, X.; Xue, L.; Li, X.; Diao, P.; Zhang, L.; et al. Development and validation of an artificial intelligence-powered acne grading system incorporating lesion identification. Front. Med. 2023, 10, 1255704. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.K.; Park, C.; Henao, R.; Kheterpal, M. Deep Learning in Dermatology: A Systematic Review of Current Approaches, Outcomes, and Limitations. JID Innov. 2022, 3, 100150. [Google Scholar] [CrossRef]
- Van den Akker, O.R.; Peters, G.Y.; Bakker, C.; Carlsson, R.; Coles, N.A.; Corker, K.S.; Feldman, G.; Moreau, D.; Nordström, T.; Pickering, J.S.; et al. Generalized Systematic Review Registration Form. Available online: https://osf.io/e8rhd (accessed on 5 March 2025).
- Wu, X.; Wen, N.; Liang, J.; Lai, Y.-K.; She, D.; Cheng, M.-M.; Yang, J. Joint Acne Image Grading and Counting via Label Distribution Learning. In Proceedings of the 2019 IEEE/CVF International Conference on Computer Vision (ICCV), Seoul, Republic of Korea, 27 October–2 November 2019; pp. 10641–10650. [Google Scholar]
- Zhang, J.; Zhang, L.; Wang, J.; Wei, X.; Li, J.; Jiang, X.; Du, D. SA-RPN: A Spacial Aware Region Proposal Network for Acne Detection. IEEE J. Biomed. Health Inform. 2023, 27, 5439–5448. [Google Scholar] [CrossRef]
- Zein, H.; Chantaf, S.; Fournier, R.; Nait-Ali, A. Generative adversarial networks for anonymous acneic face dataset generation. PLoS ONE 2024, 19, e0297958. [Google Scholar] [CrossRef]
- Quattrini, A.; Boër, C.; Leidi, T.; Paydar, R. A Deep Learning-Based Facial Acne Classification System. Clin. Cosmet. Investig. Dermatol. 2022, ume 15, 851–857. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, J.; Yan, C.; Zhou, H. An Automatic Diagnosis Method of Facial Acne Vulgaris Based on Convolutional Neural Network. Sci. Rep. 2018, 8, 5839. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, H.; Spoelstra, J. A Computer Vision Application for Assessing Facial Acne Severity from Selfie Images. arXiv 2019, arXiv:1907.07901. [Google Scholar]
- Lim, Z.V.; Akram, F.; Ngo, C.P.; Winarto, A.A.; Lee, W.Q.; Liang, K.; Oon, H.H.; Thng, S.T.G.; Lee, H.K. Automated grading of acne vulgaris by deep learning with convolutional neural networks. Skin Res. Technol. 2019, 26, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Fajarnés, G.P.; Santonja, M.M.; García, B.D.; Lengua, I.L. Segmentation methods for acne vulgaris images: Proposal of a new methodology applied to fluorescence images. Skin Res. Technol. 2020, 26, 734–739. [Google Scholar] [CrossRef]
- Rashataprucksa, K.; Chuangchaichatchavarn, C.; Triukose, S.; Nitinawarat, S.; Pongprutthipan, M.; Piromsopa, K. Acne Detection with Deep Neural Networks. In Proceedings of the 2nd International Conference on Image Processing and Machine Vision, 2nd International Conference on Image Processing and Machine Vision, Bangkok, Thailand, 5–7 August 2020. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, L.; Wu, Q.; Zhang, M.; Zeng, R.; Ding, H.; Zheng, H.; Xie, J.; Li, Y.; Ge, Y.; et al. Construction and Evaluation of a Deep Learning Model for Assessing Acne Vulgaris Using Clinical Images. Dermatol. Ther. 2021, 11, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Kong, H.; Yoon, C.; Kim, H.C.; Suh, D.H. Development and evaluation of an automatic acne lesion detection program using digital image processing. Skin Res. Technol. 2012, 19, e423–e432. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, T. Acne Detection by Ensemble Neural Networks. Sensors 2022, 22, 6828. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Wang, Z.; Hounye, A.H.; Li, Z.; Kong, M.; Hou, M.; Zhang, J.; Qi, M. A novel automatic acne detection and severity quantification scheme using deep learning. Biomed. Signal Process. Control. 2023, 84, 104803. [Google Scholar] [CrossRef]
- Wang, J.; Luo, Y.; Wang, Z.; Hounye, A.H.; Cao, C.; Hou, M.; Zhang, J. A cell phone app for facial acne severity assessment. Appl. Intell. 2022, 53, 7614–7633. [Google Scholar] [CrossRef]
- Huynh, Q.T.; Nguyen, P.H.; Le, H.X.; Ngo, L.T.; Trinh, N.-T.; Tran, M.T.-T.; Nguyen, H.T.; Vu, N.T.; Nguyen, A.T.; Suda, K.; et al. Automatic Acne Object Detection and Acne Severity Grading Using Smartphone Images and Artificial Intelligence. Diagnostics 2022, 12, 1879. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, J.; Ma, Z.; Chen, D.; Guan, Y.; You, H.; Cheng, X.; Liu, B.; Luo, G. KIEGLFN: A unified acne grading framework on face images. Comput. Methods Programs Biomed. 2022, 221, 106911. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fan, Y.; Duan, M.; Wang, Y.; Su, G.; Ren, Y.; Huang, L.; Zhou, F. AcneGrader: An ensemble pruning of the deep learning base models to grade acne. Skin Res. Technol. 2022, 28, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Yu, W.; Wu, Y.; Zhao, J.; Liu, X.; Kuang, Z.; Fan, R. Acne detection and severity evaluation with interpretable convolutional neural network models. Technol. Health Care 2022, 30, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Sun, S.; Cho, S.I.; Kong, H.-J.; Lee, J.W.; Lee, J.H.; Suh, D.H. Automated Facial Acne Lesion Detecting and Counting Algorithm for Acne Severity Evaluation and Its Utility in Assisting Dermatologists. Am. J. Clin. Dermatol. 2023, 24, 649–659. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, L.; Zhang, J.; Wang, J.; Liu, W.; Li, J.; Jiang, X. Decoupled Sequential Detection Head for accurate acne detection. Knowledge-Based Syst. 2023, 284, 111305. [Google Scholar] [CrossRef]
- Li, T.; Ma, X.; Li, Z.; Yu, N.; Song, J.; Ma, Z.; Ying, H.; Zhou, B.; Huang, J.; Wu, L.; et al. Artificial intelligence analysis of over a million Chinese men and women reveals level of dark circle in the facial skin aging process. Skin Res. Technol. 2023, 29, e13492. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, J.; Chen, D.; Ma, Z.; Guan, Y.; Liu, X.; You, H.; Yang, J. DED: Diagnostic Evidence Distillation for acne severity grading on face images. Expert Syst. Appl. 2023, 228, 120312. [Google Scholar] [CrossRef]
- Li, T.; Ma, X.; Li, Z.; Yu, N.; Song, J.; Ma, Z.; Ying, H.; Zhou, B.; Huang, J.; Wu, L.; et al. Facial adult female acne in China: An analysis based on artificial intelligence over one million. Skin Res. Technol. 2024, 30, e13693. [Google Scholar] [CrossRef]
- Kim, S.; Yoon, H.; Lee, J. Semi-Supervised Facial Acne Segmentation Using Bidirectional Copy–Paste. Diagnostics 2024, 14, 1040. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Wang, J.; Wei, X.; Li, J.; Jiang, X.; Du, D. Learning High-quality Proposals for Acne Detection. arXiv 2022, arXiv:2207.03674. [Google Scholar]
- Prokhorov, K.; Kalinin, A.A. Improving Acne Image Grading with Label Distribution Smoothing. In Proceedings of the 2024 IEEE International Symposium on Biomedical Imaging (ISBI), Athens, Greece, 27–30 May 2024; pp. 1–5. [Google Scholar]
- Gao, N.; Wang, J.; Zhao, Z.; Chu, X.; Lv, B.; Han, G.; Ni, Y.; Xie, G. Evaluation of an acne lesion detection and severity grading model for Chinese population in online and offline healthcare scenarios. Sci. Rep. 2025, 15, 1119. [Google Scholar] [CrossRef] [PubMed]
- Choy, S.P.; Kim, B.J.; Paolino, A.; Tan, W.R.; Lim, S.M.L.; Seo, J.; Tan, S.P.; Francis, L.; Tsakok, T.; Simpson, M.; et al. Systematic review of deep learning image analyses for the diagnosis and monitoring of skin disease. npj Digit. Med. 2023, 6, 180. [Google Scholar] [CrossRef] [PubMed]
- Haggenmüller, S.; Schmitt, M.; Krieghoff-Henning, E.; Hekler, A.; Maron, R.C.; Wies, C.; Utikal, J.S.; Meier, F.; Hobelsberger, S.; Gellrich, F.F.; et al. Federated Learning for Decentralized Artificial Intelligence in Melanoma Diagnostics. JAMA Dermatol. 2024, 160, 303–311. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).