Abstract
This review investigates the convergence of artificial intelligence (AI) and personalized health monitoring through wearable devices, classifying them into three distinct categories: bio-electrical, bio-impedance and electro-chemical, and electro-mechanical. Wearable devices have emerged as promising tools for personalized health monitoring, utilizing machine learning to distill meaningful insights from the expansive datasets they capture. Within the bio-electrical category, these devices employ biosignal data, such as electrocardiograms (ECGs), electromyograms (EMGs), electroencephalograms (EEGs), etc., to monitor and assess health. The bio-impedance and electro-chemical category focuses on devices measuring physiological signals, including glucose levels and electrolytes, offering a holistic understanding of the wearer’s physiological state. Lastly, the electro-mechanical category encompasses devices designed to capture motion and physical activity data, providing valuable insights into an individual’s physical activity and behavior. This review critically evaluates the integration of machine learning algorithms within these wearable devices, illuminating their potential to revolutionize healthcare. Emphasizing early detection, timely intervention, and the provision of personalized lifestyle recommendations, the paper outlines how the amalgamation of advanced machine learning techniques with wearable devices can pave the way for more effective and individualized healthcare solutions. The exploration of this intersection promises a paradigm shift, heralding a new era in healthcare innovation and personalized well-being.
1. Introduction
Recent advances in the development of wearable devices have showcased the integration of machine learning algorithms to enable personalized health monitoring and intervention systems. These systems leverage advanced algorithms to process data from various sensors embedded in wearable devices, such as strain gauges, plastic optical fibers, actuators, and electrochemical sensors, to provide personalized health insights and interventions [1,2,3,4]. The use of machine learning allows these devices to classify and predict various health-related parameters, including blood glucose levels, blood pressure, stress levels, and physical activity, tailored to individual users’ needs and health conditions [5,6,7,8].
Moreover, the application of machine learning in wearable devices has extended to personalized healthcare monitoring for specific medical conditions, such as diabetes, sleep disorders, and neurological rehabilitation [9,10,11]. These systems utilize AI to analyze physiological signals, predict disease states, and recommend personalized interventions, contributing to improved disease management and patient outcomes. The integration of machine learning in these devices enables the real-time monitoring and interpretation of health-related data, leading to actionable insights and personalized recommendations for users’ health management.
Furthermore, the development of personalized wearable devices has been driven by the need to provide tailored solutions for individuals with specific health conditions, such as urinary incontinence, panic attacks, and obsessive compulsive disorder [12,13,14]. Machine learning algorithms have been employed to detect and predict these conditions using sensor data from wearable devices, enabling early intervention and personalized support for individuals with these health challenges. These advancements highlight the potential of personalized wearable devices in addressing specific health needs and improving the quality of life for individuals with diverse health conditions. In recent years, there has been a notable surge in publications addressing the integration of machine learning methodologies in wearable devices. This upward trend, as evidenced by Figure 1, underscores the growing scholarly interest in exploring innovative applications and methodologies at the intersection of wearable technologies and machine learning algorithms. Simultaneously, a conspicuous thematic focus has emerged on personalized wearable devices, reflecting researchers’ increasing attention to the customization of wearable solutions. This indicates the massive potential of personalized wearable devices in patient care and underscores the ongoing evolution of contemporary academic research in wearable technology.
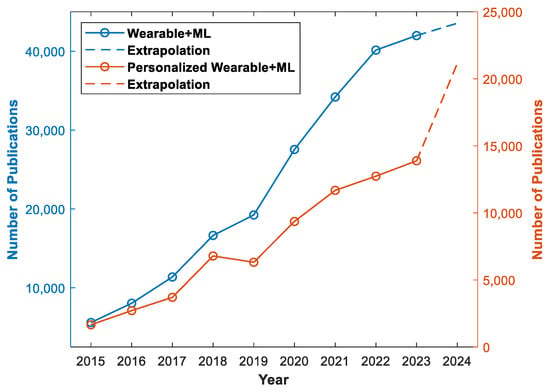
Figure 1.
The blue plot illustrates number of publications on wearable devices utilizing machine learning and the orange plot is number of publications on personalized wearable devices using machine learning [15]. The keywords used were “wearable machine learning” and “personalized wearable machine learning”. The extrapolated data for 2024 were based on the number of publications up to 25 January 2024.
The literature review was carried out by refining the papers through the SCOPUS, Nature, and IEEE-Xplore databases using the search terms “Personalized + Wearable + Machine Learning”. Eventually, the investigation methodically categorizes academic papers into three distinct thematic groups, as illustrated in Figure 2. The first category, designated as “Bio-electrical Wearable Devices”, delves into topics concerning electrical phenomena within biological systems, encompassing ECG, EEG, and EMG, among others. Papers in this category extensively explore the intricacies of bio-electrical processes and their implications. The second category, denoted as “Electro-Chemical and Bio-Impedance”, centers on the nuanced interplay between electrical and chemical processes in biological systems, with a specific emphasis on bio-impedance dynamics. Lastly, the third category, termed “Electro-Mechanical,” encompasses papers that investigate the intersection of electrical and mechanical phenomena within biological contexts. These papers explore electro-mechanical interactions, exemplified by technologies like gait sensors, stretchable sensors, and strain gauges. This tripartite categorization system provides a structured framework, enhancing the comprehension and navigation of the diverse themes presented in the journal papers.
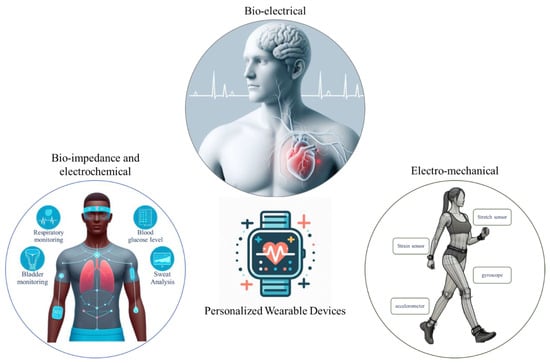
Figure 2.
Illustration of three main categories of personalized wearable devices: bio-electrical, bio-impedance, and electro-chemical and electro-mechanical wearable devices. The figure was generated using the Bing AI chat bot.
2. Bio-Electrical Wearable Devices
Personalized wearable devices that utilize bio-electrical signals, such as ECG, EEG, and EMG, play a crucial role in revolutionizing healthcare and well-being. These devices offer the continuous and non-invasive monitoring of physiological signals, providing valuable insights into an individual’s health status and enabling personalized health management. The integration of machine learning algorithms with these wearable devices enhances their capabilities by enabling the analysis of complex bio-electrical data to detect anomalies, predict health conditions, and provide personalized recommendations. For instance, machine learning models can be trained to classify ECG signals for blood pressure estimation [6], EEG signals for emotion recognition [16], and EMG signals for gesture recognition [17]. This combination of personalized wearable devices and machine learning holds great promise in advancing preventive healthcare, early disease detection, and personalized treatment strategies, ultimately leading to improved patient outcomes and quality of life.
The integration of bio-electrical wearables into healthcare has ushered in a new era, with machine learning algorithms enhancing their capabilities across a myriad of applications. In the realm of personalized Parkinson’s disease management, LeMoyne et al. developed a groundbreaking system using the BioStamp nPoint. By adjusting the amplitude of the current applied to deep brain stimulation (4.0 mA, 2.5 mA, 1.0 mA, off), this multilayer neural network was capable of classifying tremor responses with 95% accuracy, demonstrating the potential for tailored interventions [10]. Moving to the domain of rehabilitation, LeMoyne et al. undertook a longitudinal investigation spanning 10 months. During this study, a smartphone affixed to the foot with an armband was employed to capture gyro data and transfer them to the cloud. The gathered data encompassed key metrics such as the maximum, minimum, mean, standard deviation, and coefficient of variation of the gyroscope signal. Subsequently, a support vector machine, facilitated by the Waikato Environment for Knowledge Analysis (WEKA), was employed to classify the gyroscope-acquired data in order to distinguish between the initial and final phases of the therapy regimen. The evaluation of the data demonstrates the effectiveness of the rehabilitation process [18].
Transitioning to cardiovascular health, Banerjee et al. proposed a methodology for blood pressure estimation using ECG data. Utilizing XGBoost for classification and an artificial neural network (ANN) for regression, their system achieved a mean error of 0.89 mm Hg. This application highlights the potential of lightweight ML algorithms in remote health monitoring, particularly in cardiovascular conditions [6]. An example of a wearable cardiovascular healthcare device is the system developed by Chiang et al. for predicting blood pressure (BP) and providing personalized lifestyle recommendations based on ECG data. Utilizing ECG, sleep, and physical activity data collected from smartwatches, the system uses ML models such as random forest and autoregressive integrated moving average (ARIMA) to predict BP and make lifestyle recommendations. The subjects experienced decreased BPs by 3.8 and 2.3 for systolic and diastolic BP. Furthermore, the system used Shapley values to identify lifestyle factors that contribute to high blood pressure [19]. Pramukantoro et al.’s real-time heartbeat monitoring system utilized the Polar H10 wearable device. For classification, they used SVM to categorize the data into five sections: normal, supraventricular, ventricular ectopic, fusion, and unknown. This exemplifies the potential for the accurate classification of heartbeats into five categories. The system’s use of RR interval data and Bluetooth low energy (BLE) enables real-time monitoring, showcasing bio-electrical wearables’ potential in cardiovascular health [20]. To show the unlimited features of an ECG signal, Maged et al. utilized ECG sensors in smartwatches to predict blood glucose levels in diabetic patients. Leveraging machine learning methods for regression, such as LGBM, GBR, AdaBoost, and linear and ridge regressors. and heart rate variability parameters, their system presented a novel approach to health monitoring. This application underscores the versatility of bio-electrical wearables in managing chronic conditions [21].
In the field of predictive healthcare and occupational safety, Shimazaki et al. employed supervised machine learning to prevent heat stroke in hot environments. Based on a personalized heat strain temperature (pHST) meter, their web survey-based automatic annotation system classified workers into thermal and non-thermal groups based on vital data, achieving an 85.2% accuracy in predicting heat stroke. This application highlights the potential of bio-electrical wearables in ensuring safety in challenging occupational environments [22].
Shifting focus to mental health, Campanella et al.’s stress detection system, utilizing physiological signals collected by the Empatica E4 bracelet and machine learning algorithms, introduces an application in stress management. The system collects physiological data through four sensors: a temperature sensor, accelerometer, photoplethysmogram (PPG) sensors, and electrodermal activity (EDA) sensors. Achieving an accuracy range of 70% to 79.17%, this study emphasizes the need for more extensive and diverse datasets to improve model accuracy, showcasing the potential of bio-electrical wearables in mental health [23]. Examining the real-time stress detection domain, Zhu et al. delved into EDA, ECG, and PPG signals from wearable devices. Utilizing six machine learning methods, including support vector machine (SVM) and k-nearest neighbors (KNNs), their stacking ensemble learning method achieved the best accuracy of 86.4% for EDA signals. This application demonstrates the potential of bio-electrical wearables in managing stress, offering real-time insights for users [7]. Tsai et al. devised a 7-day panic attack prediction model by leveraging data from a mobile app and a Garmin Vivosmart 4 smartwatch. The study encompassed 59 participants diagnosed with panic disorder (PD), and data on activity levels, heart rate, sleep patterns, anxiety, and depression scores were collected over a one-year period. Integrating questionnaires and additional physiological and environmental data, including the Air Quality Index (AQI), the researchers employed a random forest model, achieving prediction accuracies ranging from 67.4% to 81.3%. Crucial features such as Beck Anxiety Inventory (BAI), Beck Depression Inventory (BDI), State-Trait Anxiety Inventory (STAI), a Mini International Neuropsychiatric Interview (MINI), heart rate (HR), and deep sleep duration played pivotal roles in ensuring accurate predictions. This underscores the potential for early and personalized mental health interventions for patients diagnosed with panic disorder [13].
On the topic of gesture recognition, Ghaffar Nia et al. developed an artificial neural network (ANN) model with a few control parameters, achieving 98.9% and 93% accuracy in training and testing processes, respectively. The study focused on classifying EMG signals to control assistive devices for individuals with sensory-motor disorders. The ANN model’s application demonstrates the potential of machine learning algorithms in improving the accuracy and efficiency of EMG signal classification [17]. In another work, Avramoni et al. developed a sophisticated algorithm to detect pill intake using a smart wearable device with inertial measurement unit (IMU) sensors by evaluating the associated gestures. Employing supervised machine learning, the algorithm achieved over 99% accuracy in training and validation datasets and 100% accuracy in testing datasets. This application showcases the potential of bio-electrical wearables in gesture recognition and human–computer interaction [24].
Moving to neurological applications, Meisel et al. harnessed wristband sensor data and machine learning to develop a seizure forecasting system. The acquired signals includes EDA, blood volume pulse (BVP), temperature, and accelerometer data. The use of long short-term memory (LSTM) and 1D convolutional neural networks, optimized through grid searches, yielded promising results. The system not only showcased accurate predictions but also hinted at the potential for further improvement through individualized parameter tuning [25]. Transitioning to seizure detection, Jeppesen et al. developed a personalized seizure detection algorithm using patient-adaptive logistic regression machine learning (LRML). Utilizing a wearable ECG device and collecting heart rate variability (HRV) during a long-term video-EEG recording, the system achieved a 78.2% sensitivity and a 31% reduction in false alarm rates. This system contributes to the evolution of personalized healthcare interventions, showcasing the potential of bio-electrical wearables in neurological health [26].
Multiple studies have been conducted on sleep apnea detection and intervention. As an example, Ji et al. developed an airline point-of-care system for hybrid physiological signal monitoring, achieving high accuracy of 84–85% using a long short-term memory recurrent neural network (LSTM-RNN). The system detects electrocardiogram (ECG), breathing, and motion signals, with the diagnosis of sleep apnea-hypopnea syndrome (SAHS) as a key application. The hardware design includes ECG electrodes, flexible piezoelectric belts, and a control box, providing a low-cost, long-term monitoring solution for passengers during flights [27]. In another work, Mohan et al.’s exploration of deep learning and machine learning techniques for sleep apnea detection from single-lead ECG data emphasizes the potential of AI-based bio-signal processing. Their hybrid deep models achieved a sensitivity of 84.26%, a specificity of 92.27%, and an accuracy of 88.13%. This application showcases the potential for bio-electrical wearables in sleep monitoring and respiratory health [9].
The real-time emotion recognition system developed by Mai et al. using an ear-EEG-based on-chip device introduces a compact, battery-powered solution for emotion classification. Leveraging machine learning models such as SVM, MLP, and one-dimensional convolutional neural networks (1D-CNNs), this system utilizes Bluetooth low-energy wireless technology for data transmission, showcasing the potential for bio-electrical wearables in mental health applications [16].
Figure 3 represents instances of the examined studies highlighting the utilization of bio-electrical wearable devices employing machine learning techniques.
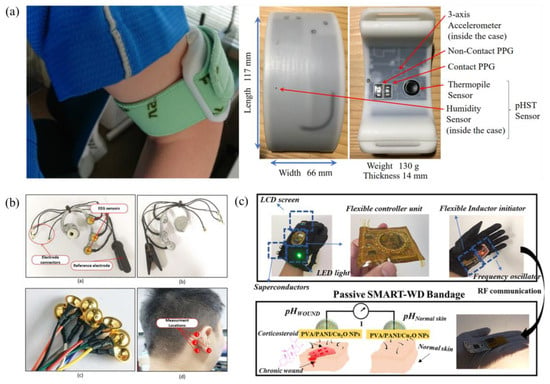
Figure 3.
Schematics depicting the sensor setups for: (a) a system to prevent heat stroke in hot environments [22], (b) a real-time emotion recognition system [16], and (c) an intelligent wearable system for wound monitoring [28]. (c) is adapted by permission from [28]. Copyright 2022 American Chemical Society.
Table 1 reviews recent research on personalized wearable bio-electrical devices from 2020 to 2023. The vast majority (86.6%) of the papers discuss devices customized to individual users. These personalized wearables leverage sensors like IMUs, ECGs, and EDAs to monitor physiological signals and detect conditions accurately, often with over 90% accuracy. For example, personalized devices using IMUs and ECGs can detect panic attacks, dehydration, wound healing, and Parkinson’s with over 90% accuracy [10,28,29,30]. Other applications include monitoring heart rate variability, sleep apnea, and stress levels [7,9,26]. The research shows a trend towards more personalized and accurate wearable sensors over time. While earlier papers from 2022 focus on non-personalized devices [6,23], the most recent 2023 studies emphasize personalized wearables [16,29]. In summary, Table 1 demonstrates personalized health monitoring wearables using sensors like IMUs and ECGs can provide highly accurate and customized detection for a variety of conditions.

Table 1.
Summary of the reviewed papers with bio-electrical sensors.
3. Bio-Impedance and Electro-Chemical Wearables
Wearable devices utilizing electro-chemical and bio-impedance sensors have gained significant importance in healthcare and personalized health monitoring. These devices enable the non-invasive and continuous monitoring of various physiological parameters, such as glucose levels, electrolyte biomarkers, and tissue regeneration, providing valuable insights into an individual’s health status. The integration of machine learning algorithms with these wearable devices allows for the accurate interpretation of the collected data, leading to real-time health assessments and predictive analytics. The combination of wearable devices with electro-chemical and bio-impedance sensors, along with machine learning, holds great promise for revolutionizing personalized healthcare and improving overall well-being.
On impedance-based flow cytometry, Annabestani et al. introduced a sheath-free microfluidic system that employs machine learning to estimate the size and quantity of particles passing through the channel. This innovative approach utilizes a set of variables termed “Basis Impedances” and a memory-less version of a polynomial-based nonlinear auto-regressive with exogenous inputs (NARX) model to predict the total output impedance of multiparticle systems [31]. Shifting focus to respiratory monitoring, Rozo et al. developed machine learning models to assess thoracic bio-impedance (BioZ) measurements. Using SVM and CNN classifiers, transfer learning, and feature-based classification, they evaluated the impact of different breathing patterns on model performance [32].
Continuing the exploration of non-invasive health monitoring, Chahine et al. developed a wearable system utilizing electromagnetic sensors and artificial intelligence to non-invasively monitor glucose levels. This comprehensive system integrates environmental and physiological sensors to account for temperature, humidity, sweat, and motion effects, achieving high fidelity in tracking glucose variations with low error and good prediction accuracy [33]. Following the topic, Islam et al. designed a non-invasive glucose monitoring system using PPG and galvanic skin response (GSR) sensors, implementing a deep learning algorithm for improved prediction accuracy. The system collected data from 10 volunteers over 2 days and 15 patients over 1 day, using a total of 210 sample data points for training and testing the deep learning model. The deep learning model consisted of three stages: feature extraction, global average pooling, and regression. The results showed that the predicted blood glucose levels were accurate, with 80% of the training data and 40% of the testing data falling within acceptable error margins [5]. Another compelling area of research revolves around initiatives dedicated to wound monitoring and tissue regeneration. Kalasin et al. developed a contactless wearable system. This system, incorporating AI-enabled sensors and advanced wound dressing bandages, utilized an artificial neural network algorithm and a pH-responsive mechanism for wound monitoring. The integration of these elements showcases the potential for bio-electrical wearables in advanced healthcare applications [28].
Advancing the field of non-invasive sweat sensors, Sankhala et al. introduced a platform using electrochemical impedance spectroscopy and machine learning to report glucose concentrations. Employing ensemble and decision tree regression algorithms with k-fold cross-validation, the system optimizes models and prevents overfitting. The machine learning algorithm accurately interprets the progression trend of glucose levels, making it a valuable tool for lifestyle management [3]. As another example on sweat analysis, Wang et al. developed a patch with printed electrochemical sensors to monitor sweat biomarkers and predict core body temperature using machine learning algorithms. The system employs printed sensor patches with integrated microfluidics, utilizing machine learning to predict core body temperature based on real-time sweat biomarker measurements [34]. Nyein et al. developed a microfluidic patch for continuous sweat analysis during rest. The patch collects sweat from different body sites, enabling noninvasive monitoring of sweat rate, pH, and chloride levels. The study emphasizes machine learning for real-time data analysis. The patch’s potential for continuous metabolite monitoring makes it a promising tool for health and fitness applications [35]. Khosravi et al. developed a flexible electrochemical glucose sensor screen-printed onto a textile substrate, demonstrating a linear response in the range of 20–1000 µM of glucose concentration with high sensitivity (18.41 µA mM−1 cm−2, R2 = 0.996). The sensor showed high selectivity toward glucose and excellent stability over 30 days of storage. The study evaluated the successful immobilization of glucose oxidase and the sensor’s response to repeated glucose measurements [36].
The exploration of skin hydration levels by Liaqat et al. introduced a hybrid algorithm combining machine learning and deep learning methods. The collection of data from various postures and fasting durations facilitated the estimation of skin hydration levels with an impressive accuracy of around 97%. This application showcases the potential of bio-electrical wearables in non-invasive monitoring for skincare [30]. As another approach to applications of electro-dermal sensors, Almadhor et al.’s proposed federated learning framework for stress prediction showcases the potential for collaborative AI models based on wrist-worn sensor data. Achieving improved stress detection accuracy compared to traditional approaches, this system ensures privacy by training local models before sending parameters to a global model [37].
In the domain of real-time bladder monitoring, Zhang et al. proposed a wearable utilizing bio-impedance data and a random forest machine learning algorithm. Achieving over 90% accuracy in predicting bladder fullness, this system holds promise for aiding in urinary incontinence [12]. To mention another example on the topic, Dheman et al. developed a non-invasive bladder volume estimation system using tetrapolar bio-impedance measurements and a deep learning algorithm. The system uses a wearable sensor node and AI-based artefact suppression to provide quantitative bladder volume measures. The algorithm demonstrated feasibility and comparability to commercial portable ultrasound devices [38].
Finally, in the realm of wireless biomedical monitoring, Yang et al. introduced a non-printed integrated-circuit textile (NIT). This innovative textile, woven with sensors, logic computing, wireless transmission, and power supply, utilizes AI for continuous on-body monitoring and logical codes for emergency assistance. The NIT can monitor body movement, sweat, and light, sending wireless signals for various emergency scenarios. Powered by solar energy harvesting, it serves as a 24/7 private AI nurse [39].
Figure 4 provides visual depictions illustrating the practical application of the described wearable technologies. Following this, the subsequent discourse will conduct a comprehensive review of recent advanced research dedicated to implementing bio-impedance and electro-chemical wearable devices through the incorporation of machine learning methodologies.
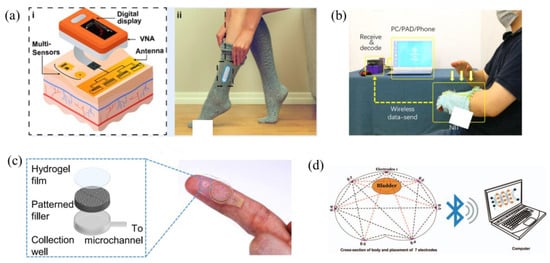
Figure 4.
Illustration of examples on bio-impedance and electro-chemical wearables as presented in the literature: (a) a wearable system utilizing electromagnetic sensors to non-invasive glucose level measuring [33], (b) a non-printed integrated-circuit textile [39], (c) a microfluidic patch for continuous sweat analysis [35], and (d) an impedance-based wearable for real-time bladder monitoring [12].
Among the examined papers on wearable sensors for health monitoring, 75% were identified as employing personalized devices, while the remaining 25% demonstrated potential for personalization (Table 2). The personalized devices utilized a variety of sensors, including photoplethysmography, galvanic skin response, smart textiles, sweat sensors, electromagnetic sensors, printed sensors, and impedance sensors to monitor biomarkers such as blood glucose, respiratory rate, sweat composition, core body temperature, and bladder volume. Reported accuracy ranged from 74.6% to over 99%. The studies spanned publication dates from 2019 to 2023, reflecting the recent progress in the development of personalized wearable health sensors. Overall, this review of the recent literature demonstrates a substantial advancement of personalized wearable devices for continuous health monitoring and their potential to provide individualized care.

Table 2.
Synopsis of wearable devices employing electro-chemical and bio-impedance technologies.
4. Electro-Mechanical Wearable Devices
The utilization of electro-mechanical elements in wearable devices for the analysis of gait and recognition of motion holds substantial significance in diverse fields, including healthcare, rehabilitation, and robotics. These devices, encompassing soft sensors [40,41] and strain gauges [1,42], facilitate the non-invasive monitoring of human movement patterns, providing invaluable insights for gait analysis and motion tracking. Soft sensors integrated into wearable systems, for example, have the capacity to capture nuanced changes in joint movements and muscle activities, enabling the assessment of gait patterns and the detection of abnormalities in movement. Furthermore, these devices contribute to the creation of intelligent wearable systems, serving purposes such as fall detection, silent communication, and human activity recognition. The incorporation of machine learning algorithms with these wearable devices further amplifies their capabilities, enabling the precise and real-time analysis of gait and motion data. These advancements have the potential to transform personalized healthcare, enhance rehabilitation outcomes, and advance the development of intelligent robotic systems.
Advancing the field of hand gesture recognition, Ferrone et al. developed a wearable wristband equipped with strain sensors. The system utilized strain gauge sensors, machine learning algorithms such as linear discriminant analysis (LDA) and support vector machine (SVM) as well as a leap motion system for validation. Featuring stretchable strain gauge sensors and readout electronics, the wristband achieved a reproducibility of over 98% using the LDA classifier [42]. Another approach was based on an e-textile, where Zeng et al. developed a highly conductive carbon-based e-textile for gesture recognition using heat transfer printing and screen printing. The system uses AI to recognize eight different gestures with 96.58% accuracy [43]. One of the applications of hand gesture recognition was introduced by DelPreto et al., who developed a smart glove with resistive sensors and an accelerometer, using machine learning to classify American Sign Language poses and gestures in real time with high accuracy (96.3%). The system utilizes a strain-sensitive resistive knit for postural information and an accelerometer for motion, with a small custom PCB and microcontroller reading sensors, performing feature extraction, and running a pre-trained neural network [44]. Another interesting application of hand motion detection was introduced during the COVID-19 pandemic. Marullo et al. developed No Face-Touch, a system that uses wearable devices and machine learning to detect hand motions ending in face-touches. The system utilizes a recurrent neural network (RNN) with long short-term memory (LSTM) cells and accelerometer data to detect face-touches, achieving a high true detections rate, low false detection rate, and short time to detect the contact. The system is designed to run on smartwatches and low-cost devices, with a focus on battery consumption and generalization to different users [45]. In silent communication, Smith et al. developed a wearable patch with a graphene-based strain gauge sensor and haptic feedback for silent communication. They used machine learning algorithms, including neural networks, to classify throat movements and predict spoken words with 82% accuracy for movements and 51% for words. They handcrafted a dataset with 15 words and four movements, and used a sensor attached to the throat to collect resistance readings for training and testing the algorithms [1]. In a more recent approach, Tashakori et al. achieved the precise real-time tracking of hand and finger movements using stretchable, washable smart gloves embedded with helical sensor yarns and inertial measurement units. The sensor yarns exhibit a high dynamic range and stability during use and washing. Through multi-stage machine learning, the system achieves low joint-angle estimation errors of 1.21° and 1.45° for intra- and inter-participant validation, matching costly motion-capture cameras’ accuracy. A data augmentation technique enhances robustness to noise, enabling accurate tracking during object interactions and diverse applications, including typing on a simulated keyboard, recognizing dynamic and static gestures from American Sign Language, and object identification [46].
Exploring joint analysis, Gholami et al. developed a fabric-based strain sensor system for knee-joint angle estimation. Implementing machine learning algorithms, including random forest and neural networks, the system processed sensor data and achieved an accuracy of around 6 degrees. The study highlighted the potential applications in healthcare, virtual reality, and robotics [47]. Following knee flexion and adduction moments estimation, Stetter et al. developed an artificial neural network (ANN) using wearable sensors to estimate knee flexion and adduction moments (KFM and KAM) during various locomotion tasks. The ANN was trained with IMU signals and biomechanical data, and the model architecture included two hidden layers with 100 and 20 neurons. The study used a leave-one-subject-out cross-validation method to evaluate the ANN’s performance. The ANN approach does not require musculoskeletal modeling and can provide accurate predictions for new data [48].
Shifting to fall detection, Desai et al. developed a wearable belt using machine learning and signal processing algorithms. With the ability to detect falls within 0.25 s, the system achieved high accuracy using a logistic regression classifier and triggered alerts via a GSM module upon fall detection [49]. In medication adherence monitoring, Cheon et al. utilized sensor data from an Apple Watch to detect low medication states in prescription bottles. Employing machine learning, specifically a gradient-boosted tree model, the system predicted low pill counts with high accuracy and F1 scores. The system involved preprocessing sensor data, extracting summary statistics and training the model using Apache Spark’s MLlib platform [50].
Another interesting application of gait analysis was introduced by Kirsten et al., who developed a sensor-based OCD detection system using AI, personalized federated learning, and motion sensors. The system achieved high AUPRCs and demonstrated privacy-preserving model training [14]. Meanwhile, Chee et al. explored gait analysis and machine learning for diabetes detection. They emphasized the potential of deep learning models like CNN and LSTM in analyzing gait data. The paper highlights the use of gait sensors and features, as well as the need to implement DL models for improved accuracy [11]. In another approach, Igene et al.’s SVM model, utilizing accelerometer data, showcased an accuracy of 94.4% in predicting Parkinson’s disease. Employing ANOVA, PCA, and grid search for feature selection and hyperparameter tuning, this application emphasizes the potential of electro-mechanical wearables in early disease detection and monitoring [29]. Li et al. developed a multimodal sensor glove to assess Parkinson’s disease symptoms in patients’ hands. They used various algorithms to process signals, achieving a 95.83% accuracy in identifying tremor signals. The glove assessed flexibility, muscle strength, and stability, showing high consistency with clinical observations. The system’s reliability was confirmed through repeated experiments, with intraclass correlation coefficients exceeding 0.9 [51].
Moving to stretchable sensors, Nguyen et al. developed a stretchable gold nanowire sensor for motion tracking. They used a machine learning algorithm to characterize the sensor’s response, achieving a high gauge factor of 12 and an error of less than 2 degrees in measuring bending motion [52]. As another example on stretchable sensors, Feng et al. developed a sensing-actuation unit for force estimation in soft stretch sensors. They used deep learning methods, including LSTM and Informer, to calibrate and predict force, achieving a mean square error (MSE) of less than 0.28 N2 and normalized root mean square error (NRMSE) of less than 2.0%. The unit has adjustable stiffness and is promising for applications like lightweight flexible exoskeletons [53]. Another soft sensor for gait generation was introduced by Kim et al., who introduced a semi-supervised deep learning model using microfluidic soft sensors. Leveraging a deep autoencoder, the model embedded gait motion into a latent motion manifold, reducing the need for a large calibration dataset. The system utilized AI to generate natural human gait motion from sensor outputs [40]. Transitioning to upper-limb posture detection, Giorgino et al. introduced a system utilizing conductive elastomer sensors for neurological rehabilitation. Employing machine learning for posture classification, the system addressed challenges related to sensor noise and generalization, achieving high recognition performance for real-time classification [54].
Exploring material surface recognition, Liu et al. developed smart gloves with ZNS-01 sensors to recognize five material surfaces. They used machine learning algorithms like XGBoost to achieve 98% classification accuracy. The system extracts time and frequency domain characteristics to train the models [55]. Introducing an advanced system for the ongoing wireless monitoring of arterial blood pressure, this technology, created by Li et al., features a thin, soft, and miniaturized design. The system incorporates a sensing module, active pressure adaptation module, and data processing module to identify the blood pulse wave, apply back pressure, and extract the pulse transit time interval. Employing a sophisticated multiple-feature fusion framework and ensemble learning, particularly extreme gradient boosting, the system constructs an estimation model. This model integration includes AI techniques, ensuring meticulous control over blood pressure [56].
Shifting to body movement detection, Wang et al. developed a wearable plastic optical fiber sensing system for human motion recognition using machine learning. The system uses AI, such as support vector machines and convolutional neural networks, to analyze motion signals and achieve high recognition accuracy. The system’s key parameters include feature vectors, cumulative contribution rate, and time consumed for recognition [2]. On another approach, Mani et al. developed a conductive fabric-based suspender system for human activity recognition (HAR) using machine learning and deep learning techniques. The system achieved an accuracy of 98.11% using eight different classifiers, including KNN, SVM, RF, and LSTM [57]. Utilizing MXene technology, Yang et al. developed wearable Ti3C2Tx MXene sensor modules with in-sensor machine learning (ML) models for full-body motion classifications and avatar reconstruction. The sensors exhibited ultrahigh sensitivities within user-designated working windows, and the ML chip enabled in-sensor reconstruction of high-precision avatar animations with an average error of 3.5 cm. The ML models achieved 100% accuracy for full-body motion classification without using image/video data. The edge sensor module with ML chip allowed the real-time and high-accuracy determination of 15 avatar joint locations, leading to personalized avatar animations. The integration of wearable sensors with ML chip for in-sensor machine learning and avatar reconstruction is a significant advancement in the field of wearable sensors and human–machine interaction [58]. Jiang et al. summarized the benefits of using advanced algorithms in wearable tactile sensors, including time series models and classification algorithms based on machine learning and signal processing. They discussed the integration of AI in the system, including the use of machine learning for motion recognition and voice recognition [59].
Vasdekis et al. developed WeMoD, an AI-based approach for predicting daily step count and setting personalized physical activity goals using a combination of physiological, psychological, and contextual features. They utilized ML algorithms such as ridge regression, decision tree, random forest, and gradient boosting regressor to achieve a mean absolute error of 1908 steps [8]. Papaleonidas et al.’s focus on high-accuracy human activity recognition models introduces the potential for health monitoring and smart home management. Utilizing machine learning and raw signals from wearables, the model achieved 99.9% accuracy. The integration of ML algorithms and variable segmentation methodology showcases the versatility of electro-mechanical wearables in recognizing activities [60].
Visual representations exemplifying the application of these wearable technologies are presented in Figure 5. The subsequent discussion will delve into a review of recent advanced research focused on the implementation of electro-mechanical wearable devices utilizing machine learning techniques.
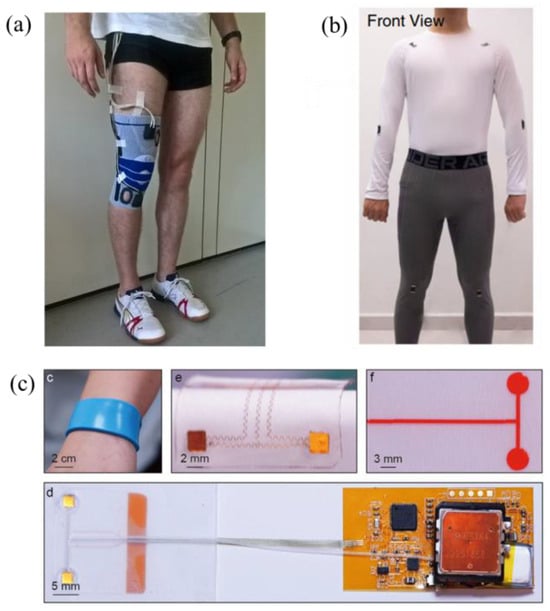
Figure 5.
Setup for various electro-mechanical wearable devices: (a) a set of wearable sensors to estimate knee flexion [48], (b) a thin, soft, and miniaturized design for arterial blood pressure [56], and (c) Ti3C2Tx MXene sensor modules for full-body motion classifications [58].
Table 3 reviews recent progress in wearable sensors for personalized health monitoring. Among the 23 papers reviewed, 78% (18 papers) demonstrated wearable devices personalized for individual users, while 22% (5 papers) showed potential for personalization but did not implement it. The personalized devices targeted a wide range of applications including posture, gesture, and motion tracking; fall detection; medication monitoring; knee and joint movement; touch sensing; muscle activity; step counting; blood pressure; and diabetes detection. Sensing modalities included strain gauges, stretchable sensors, microfluidics, IMUs, EMG, tactile sensors, and optical fibers. Reported accuracy ranged from 75–100%, with 78% of papers achieving over 90% accuracy. The high accuracy and focus on personalization in the majority of surveyed devices highlights the growing ability of wearable sensors to provide customized real-time health insights for individual users. This progress suggests personalized wearable health monitoring will continue expanding in the coming years.

Table 3.
Overview of wearable devices employing electro-mechanical technology.
5. Conclusions
In conclusion, recent strides in wearable device development underscore the integration of machine learning algorithms, ushering in a new era of personalized health monitoring and intervention systems. The comprehensive review of literature in this field, with a particular emphasis on personalized wearables, revealed a noteworthy finding: 78.5% of the scrutinized articles showcased the incorporation of personalized features, while the remaining articles demonstrated the potential for personalization. These wearable systems leverage sophisticated algorithms to process diverse sensor data, ranging from strain gauges to electrochemical sensors, enabling the provision of tailored health insights and interventions. The application of machine learning extends beyond general health monitoring, delving into personalized healthcare solutions for specific medical conditions such as diabetes, sleep disorders, and neurological rehabilitation. The analytical power of AI facilitates the interpretation of physiological signals, the prediction of disease states, and the delivery of personalized interventions, thereby enhancing disease management and patient outcomes. Furthermore, the development of personalized wearables addresses specific health challenges, exemplified by conditions like urinary incontinence, panic attacks, and obsessive compulsive disorder. Machine learning algorithms play a pivotal role in detecting and predicting these conditions, enabling early intervention and personalized support. These collective advancements underscore the immense potential of personalized wearable devices in catering to individual health needs and ultimately elevating the quality of life for individuals with diverse health conditions.
Author Contributions
Conceptualization, methodology, formal analysis, investigation, and writing have been performed by A.O. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ravenscroft, D.; Prattis, I.; Kandukuri, T.; Samad, Y.A.; Occhipinti, L.G. A Wearable Graphene Strain Gauge Sensor with Haptic Feedback for Silent Communications. In Proceedings of the 2021 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Manchester, UK, 20–23 June 2021; pp. 1–4. [Google Scholar]
- Wang, S.; Liu, B.; Wang, Y.-L.; Hu, Y.; Liu, J.; He, X.-D.; Yuan, J.; Wu, Q. Machine-Learning-Based Human Motion Recognition via Wearable Plastic-Fiber Sensing System. IEEE Internet Things J. 2023, 10, 17893–17904. [Google Scholar] [CrossRef]
- Sankhala, D.; Sardesai, A.U.; Pali, M.; Lin, K.-C.; Jagannath, B.; Muthukumar, S.; Prasad, S. A Machine Learning-Based on-Demand Sweat Glucose Reporting Platform. Sci. Rep. 2022, 12, 2442. [Google Scholar] [CrossRef]
- Annabestani, M.; Esmaeili-Dokht, P.; Nejad, S.K.; Fardmanesh, M. NAFAS: Non-Rigid Air Flow Active Sensor, a Cost-Effective, Wearable, and Ubiquitous Respiratory Bio-Sensor. IEEE Sens. J. 2021, 21, 9530–9537. [Google Scholar] [CrossRef]
- Islam, M.M.; Manjur, S.M. Design and Implementation of a Wearable System for Non-Invasive Glucose Level Monitoring. In Proceedings of the 2019 IEEE International Conference on Biomedical Engineering, Computer and Information Technology for Health (BECITHCON), Dhaka, Bangladesh, 28–30 November 2019; pp. 29–32. [Google Scholar]
- Banerjee, S.; Kumar, B.; James, A.P.; Tripathi, J.N. Blood Pressure Estimation from ECG Data Using XGBoost and ANN for Wearable Devices. In Proceedings of the 2022 29th IEEE International Conference on Electronics, Circuits and Systems (ICECS), Glasgow, UK, 24–26 October 2022; pp. 1–4. [Google Scholar]
- Zhu, L.; Spachos, P.; Gregori, S. Multimodal Physiological Signals and Machine Learning for Stress Detection by Wearable Devices. In Proceedings of the 2022 IEEE International Symposium on Medical Measurements and Applications, MeMeA 2022—Conference Proceedings, Messina, Italy, 22–24 June 2022. [Google Scholar]
- Vasdekis, D.; Yfantidou, S.; Efstathiou, S.; Vakali, A. WeMoD: A Machine Learning Approach for Wearable and Mobile Physical Activity Prediction. In Proceedings of the 2022 IEEE International Conference on Pervasive Computing and Communications Workshops and other Affiliated Events (PerCom Workshops), Pisa, Italy, 21–25 March 2022; pp. 385–390. [Google Scholar]
- Mohan S, A.; Akash, P.; Ranjani, M. Sleep Apnea Detection from Single-Lead ECG A Comprehensive Analysis of Machine Learning and Deep Learning Algorithms. In Proceedings of the 2023 International Conference on Recent Advances in Electrical, Electronics, Ubiquitous Communication, and Computational Intelligence (RAEEUCCI), Chennai, India, 19–21 April 2023; pp. 1–7. [Google Scholar]
- LeMoyne, R.; Mastroianni, T.; Whiting, D.; Tomycz, N. Parametric Evaluation of Deep Brain Stimulation Parameter Configurations for Parkinson’s Disease Using a Conformal Wearable and Wireless Inertial Sensor System and Machine Learning. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 3606–3611. [Google Scholar]
- Chee, L.Z.; Hwong, H.H.; Sivakumar, S. Diabetes Detection Using Gait Analysis and Machine Learning. In Proceedings of the 2023 International Conference on Digital Applications, Transformation & Economy (ICDATE), Miri, Malaysia, 14–16 July 2023; pp. 1–7. [Google Scholar]
- Zhang, R.; Fang, R.; Zhang, Z.; Hosseini, E.; Orooji, M.; Homayoun, H.; Goncu-Berk, G. Short: Real-Time Bladder Monitoring by Bio-Impedance Analysis to Aid Urinary Incontinence. In Proceedings of the 2023 IEEE/ACM Conference on Connected Health: Applications, Systems and Engineering Technologies (CHASE), Orlando, FL, USA, 21–23 June 2023; pp. 138–142. [Google Scholar]
- Tsai, C.-H.; Chen, P.-C.; Liu, D.-S.; Kuo, Y.-Y.; Hsieh, T.-T.; Chiang, D.-L.; Lai, F.; Wu, C.-T. Panic Attack Prediction Using Wearable Devices and Machine Learning: Development and Cohort Study. JMIR Med. Inform. 2022, 10, e33063. [Google Scholar] [CrossRef]
- Kirsten, K.; Pfitzner, B.; Löper, L.; Arnrich, B. Sensor-Based Obsessive-Compulsive Disorder Detection With Personalised Federated Learning. In Proceedings of the 2021 20th IEEE International Conference on Machine Learning and Applications (ICMLA), Pasadena, CA, USA, 13–16 December 2021; pp. 333–339. [Google Scholar]
- Dimensions Dimensions. Available online: https://www.dimensions.ai/ (accessed on 25 January 2024).
- Mai, N.-D.; Nguyen, H.-T.; Chung, W.-Y. Real-Time On-Chip Machine-Learning-Based Wearable Behind-The-Ear Electroencephalogram Device for Emotion Recognition. IEEE Access 2023, 11, 47258–47271. [Google Scholar] [CrossRef]
- Nia, N.G.; Kaplanoglu, E.; Nasab, A. EMG-Based Hand Gestures Classification Using Machine Learning Algorithms. In Proceedings of the SoutheastCon 2023, Orlando, FL, USA, 1–16 April 2023; pp. 787–792. [Google Scholar]
- LeMoyne, R.; Mastroianni, T. Longitudinal Evaluation of Diadochokinesia Characteristics for Hemiplegic Ankle Rehabilitation by Wearable Systems with Machine Learning. In Proceedings of the 2022 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 17–18 November 2022; pp. 1–4. [Google Scholar]
- Chiang, P.-H.; Wong, M.; Dey, S. Using Wearables and Machine Learning to Enable Personalized Lifestyle Recommendations to Improve Blood Pressure. IEEE J. Transl. Eng. Health Med. 2021, 9, 2700513. [Google Scholar] [CrossRef] [PubMed]
- Pramukantoro, E.S.; Gofuku, A. A Real-Time Heartbeat Monitoring Using Wearable Device and Machine Learning. In Proceedings of the 2022 IEEE 4th Global Conference on Life Sciences and Technologies (LifeTech), Osaka, Japan, 7–9 March 2022; pp. 270–272. [Google Scholar]
- Maged, Y.; Atia, A. The Prediction Of Blood Glucose Level By Using The ECG Sensor of Smartwatches. In Proceedings of the 2022 2nd International Mobile, Intelligent, and Ubiquitous Computing Conference (MIUCC), Cairo, Egypt, 8–9 May 2022; pp. 406–411. [Google Scholar]
- Shimazaki, T.; Anzai, D.; Watanabe, K.; Nakajima, A.; Fukuda, M.; Ata, S. Heat Stroke Prevention in Hot Specific Occupational Environment Enhanced by Supervised Machine Learning with Personalized Vital Signs. Sensors 2022, 22, 395. [Google Scholar] [CrossRef] [PubMed]
- Campanella, S.; Altaleb, A.; Belli, A.; Pierleoni, P.; Palma, L. A Method for Stress Detection Using Empatica E4 Bracelet and Machine-Learning Techniques. Sensors 2023, 23, 3565. [Google Scholar] [CrossRef] [PubMed]
- Avramoni, D.; Virlan, R.; Prodan, L.; Iovanovici, A. Detection of Pill Intake Associated Gestures Using Smart Wearables and Machine Learning. In Proceedings of the 2022 IEEE 22nd International Symposium on Computational Intelligence and Informatics and 8th IEEE International Conference on Recent Achievements in Mechatronics, Automation, Computer Science and Robotics (CINTI-MACRo), Budapest, Hungary, 21–22 November 2022; pp. 251–256. [Google Scholar]
- Kalasin, S.; Sangnuang, P.; Surareungchai, W. Intelligent Wearable Sensors Interconnected with Advanced Wound Dressing Bandages for Contactless Chronic Skin Monitoring: Artificial Intelligence for Predicting Tissue Regeneration. Anal. Chem. 2022, 94, 6842–6852. [Google Scholar] [CrossRef]
- Meisel, C.; El Atrache, R.; Jackson, M.; Schubach, S.; Ufongene, C.; Loddenkemper, T. Machine Learning from Wristband Sensor Data for Wearable, Noninvasive Seizure Forecasting. Epilepsia 2020, 61, 2653–2666. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.; Christensen, J.; Johansen, P.; Beniczky, S. Personalized Seizure Detection Using Logistic Regression Machine Learning Based on Wearable ECG-Monitoring Device. Seizure—Eur. J. Epilepsy 2023, 107, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Rao, Z.; Zhang, W.; Liu, C.; Wang, Z.; Zhang, S.; Zhang, B.; Hu, M.; Servati, P.; Xiao, X. Airline Point-of-Care System on Seat Belt for Hybrid Physiological Signal Monitoring. Micromachines 2022, 13, 1880. [Google Scholar] [CrossRef] [PubMed]
- Igene, L.; Alim, A.; Imtiaz, M.H.; Schuckers, S. A Machine Learning Model for Early Prediction of Parkinson’s Disease from Wearable Sensors. In Proceedings of the 2023 IEEE 13th Annual Computing and Communication Workshop and Conference (CCWC), Las Vegas, NV, USA, 8–11 March 2023; pp. 734–737. [Google Scholar]
- Liaqat, S.; Dashtipour, K.; Rizwan, A.; Usman, M.; Shah, S.A.; Arshad, K.; Assaleh, K.; Ramzan, N. Personalized Wearable Electrodermal Sensing-Based Human Skin Hydration Level Detection for Sports, Health and Wellbeing. Sci. Rep. 2022, 12, 3715. [Google Scholar] [CrossRef] [PubMed]
- Annabestani, M.; Shaegh, A.M.; Esmaeili-Dokht, P.; Fardmanesh, M. An Intelligent Machine Learning-Based Sheath-Free Microfluidic Impedance Flow Cytometer. In Proceedings of the 2020 10th International Conference on Computer and Knowledge Engineering (ICCKE), Mashhad, Iran, 29–30 October 2020; pp. 284–288. [Google Scholar]
- Rozo, A.; Buil, J.; Moeyersons, J.; Morales, J.; Van Der Westen, R.G.; Lijnen, L.; Smeets, C.; Jantzen, S.; Monpellier, V.; Ruttens, D.; et al. Controlled Breathing Effect on Respiration Quality Assessment Using Machine Learning Approaches. In Proceedings of the 2021 Computing in Cardiology (CinC), Brno, Czech Republic, 13–15 September 2021; Volume 48, pp. 1–4. [Google Scholar]
- Hanna, J.; Tawk, Y.; Azar, S.; Ramadan, A.H.; Dia, B.; Shamieh, E.; Zoghbi, S.; Kanj, R.; Costantine, J.; Eid, A.A. Wearable Flexible Body Matched Electromagnetic Sensors for Personalized Non-Invasive Glucose Monitoring. Sci. Rep. 2022, 12, 14885. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Rovira, M.; Demuru, S.; Lafaye, C.; Kim, J.; Kunnel, B.P.; Besson, C.; Fernandez-Sanchez, C.; Serra-Graells, F.; Margarit-Taulé, J.M.; et al. Multisensing Wearables for Real-Time Monitoring of Sweat Electrolyte Biomarkers During Exercise and Analysis on Their Correlation With Core Body Temperature. IEEE Trans. Biomed. Circuits Syst. 2023, 17, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Nyein, H.Y.Y.; Bariya, M.; Tran, B.; Ahn, C.H.; Brown, B.J.; Ji, W.; Davis, N.; Javey, A. A Wearable Patch for Continuous Analysis of Thermoregulatory Sweat at Rest. Nat. Commun. 2021, 12, 1823. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, S.; Soltanian, S.; Servati, A.; Khademhosseini, A.; Zhu, Y.; Servati, P. Screen-Printed Textile-Based Electrochemical Biosensor for Noninvasive Monitoring of Glucose in Sweat. Biosensors 2023, 13, 684. [Google Scholar] [CrossRef] [PubMed]
- Almadhor, A.; Sampedro, G.A.; Abisado, M.; Abbas, S.; Kim, Y.-J.; Khan, M.A.; Baili, J.; Cha, J.-H. Wrist-Based Electrodermal Activity Monitoring for Stress Detection Using Federated Learning. Sensors 2023, 23, 3984. [Google Scholar] [CrossRef]
- Dheman, K.; Walser, S.; Mayer, P.; Eggimann, M.; Kozomara, M.; Franke, D.; Hermanns, T.; Sax, H.; Schürle, S.; Magno, M. Non-Invasive Urinary Bladder Volume Estimation with Artefact-Suppressed Bio-Impedance Measurements. IEEE Sens. J. 2023, 24, 1633–1643. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, X.; Zhang, N.; Zheng, J.; Chen, X.; Wen, Q.; Luo, X.; Lee, C.-Y.; Liu, X.; Zhang, X.; et al. A Non-Printed Integrated-Circuit Textile for Wireless Theranostics. Nat. Commun. 2021, 12, 4876. [Google Scholar] [CrossRef]
- Kim, D.; Kim, M.; Kwon, J.; Park, Y.-L.; Jo, S. Semi-Supervised Gait Generation With Two Microfluidic Soft Sensors. IEEE Robot. Autom. Lett. 2019, 4, 2501–2507. [Google Scholar] [CrossRef]
- Annabestani, M.; Esmaeili-Dokht, P.; Olyanasab, A.; Orouji, N.; Alipour, Z.; Sayad, M.H.; Rajabi, K.; Mazzolai, B.; Fardmanesh, M. A New 3D, Microfluidic-Oriented, Multi-Functional, and Highly Stretchable Soft Wearable Sensor. Sci. Rep. 2022, 12, 20486. [Google Scholar] [CrossRef]
- Ferrone, A.; Maita, F.; Maiolo, L.; Arquilla, M.; Castiello, A.; Pecora, A.; Jiang, X.; Menon, C.; Ferrone, A.; Colace, L. Wearable Band for Hand Gesture Recognition Based on Strain Sensors. In Proceedings of the 2016 6th IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob), Singapore, 26–29 June 2016; pp. 1319–1322. [Google Scholar]
- Zeng, X.; Hu, M.; He, P.; Zhao, W.; Dong, S.; Xu, X.; Dai, G.; Sun, J.; Yang, J. Highly Conductive Carbon-Based E-Textile for Gesture Recognition. IEEE Electron Device Lett. 2023, 44, 825–828. [Google Scholar] [CrossRef]
- DelPreto, J.; Hughes, J.; D’Aria, M.; de Fazio, M.; Rus, D. A Wearable Smart Glove and Its Application of Pose and Gesture Detection to Sign Language Classification. IEEE Robot. Autom. Lett. 2022, 7, 10589–10596. [Google Scholar] [CrossRef]
- Marullo, S.; Baldi, T.L.; Paolocci, G.; D’Aurizio, N.; Prattichizzo, D. No Face-Touch: Exploiting Wearable Devices and Machine Learning for Gesture Detection. In Proceedings of the 2021 IEEE International Conference on Robotics and Automation (ICRA), Xi’an, China, 30 May–5 June 2021; pp. 4187–4193. [Google Scholar] [CrossRef]
- Tashakori, A.; Jiang, Z.; Servati, A.; Soltanian, S.; Narayana, H.; Le, K.; Nakayama, C.; Yang, C.; Wang, Z.J.; Eng, J.J.; et al. Capturing Complex Hand Movements and Object Interactions Using Machine Learning-Powered Stretchable Smart Textile Gloves. Nat. Mach. Intell. 2024, 6, 106–118. [Google Scholar] [CrossRef]
- Gholami, M.; Ejupi, A.; Rezaei, A.; Ferrone, A.; Menon, C. Estimation of Knee Joint Angle Using a Fabric-Based Strain Sensor and Machine Learning: A Preliminary Investigation. In Proceedings of the 2018 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (Biorob), Enschede, The Netherlands, 26–29 August 2018; pp. 589–594. [Google Scholar]
- Stetter, B.J.; Krafft, F.C.; Ringhof, S.; Stein, T.; Sell, S. A Machine Learning and Wearable Sensor Based Approach to Estimate External Knee Flexion and Adduction Moments During Various Locomotion Tasks. Front. Bioeng. Biotechnol. 2020, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.; Mane, P.; Dsilva, M.; Zare, A.; Shingala, P.; Ambawade, D. A Novel Machine Learning Based Wearable Belt For Fall Detection. In Proceedings of the 2020 IEEE International Conference on Computing, Power and Communication Technologies (GUCON), Greater Noida, India, 2–4 October 2020; pp. 502–505. [Google Scholar]
- Cheon, A.; Jung, S.Y.; Prather, C.; Sarmiento, M.; Wong, K.; Woodbridge, D.M. A Machine Learning Approach to Detecting Low Medication State with Wearable Technologies. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 4252–4255. [Google Scholar]
- Li, Y.; Yin, J.; Liu, S.; Xue, B.; Shokoohi, C.; Ge, G.; Hu, M.; Li, T.; Tao, X.; Rao, Z.; et al. Learning Hand Kinematics for Parkinson’s Disease Assessment Using a Multimodal Sensor Glove. Adv. Sci. 2023, 10, 2206982. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.A.; Gong, S.; Cheng, W.; Chauhan, S. A Stretchable Gold Nanowire Sensor and Its Characterization Using Machine Learning for Motion Tracking. IEEE Sens. J. 2021, 21, 15269–15276. [Google Scholar] [CrossRef]
- Feng, L.; Gui, L.; Yan, Z.; Yu, L.; Yang, C.; Yang, W. Force Calibration and Prediction of Soft Stretch Sensor Based on Deep Learning. In Proceedings of the 2023 International Conference on Advanced Robotics and Mechatronics (ICARM), Sanya, China, 8–10 July 2023; pp. 852–857. [Google Scholar]
- Giorgino, T.; Quaglini, S.; Lorassi, F.; Rossi, D.D. Experiments in the Detection of Upper Limb Posture through Kinestetic Strain Sensors. In Proceedings of the International Workshop on Wearable and Implantable Body Sensor Networks (BSN’06), Cambridge, MA, USA, 3–5 April 2006; pp. 4–12. [Google Scholar]
- Liu, Y.; Zhang, S.; Luo, Q. Recognition of Material Surfaces with Smart Gloves Based on Machine Learning. In Proceedings of the 2021 4th World Conference on Mechanical Engineering and Intelligent Manufacturing (WCMEIM), Shanghai, China, 12–14 November 2021; pp. 224–228. [Google Scholar]
- Li, J.; Jia, H.; Zhou, J.; Huang, X.; Xu, L.; Jia, S.; Gao, Z.; Yao, K.; Li, D.; Zhang, B.; et al. Thin, Soft, Wearable System for Continuous Wireless Monitoring of Artery Blood Pressure. Nat. Commun. 2023, 14, 5009. [Google Scholar] [CrossRef] [PubMed]
- Mani, N.; Haridoss, P.; George, B. Evaluation of a Combined Conductive Fabric-Based Suspender System and Machine Learning Approach for Human Activity Recognition. IEEE Open J. Instrum. Meas. 2023, 2, 2500310. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Xiao, X.; Wang, J.; Li, Y.; Li, K.; Li, Z.; Yang, H.; Wang, Q.; Yang, J.; et al. Topographic Design in Wearable MXene Sensors with In-Sensor Machine Learning for Full-Body Avatar Reconstruction. Nat. Commun. 2022, 13, 5311. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, R.; Zhu, H. Recent Progress in Wearable Tactile Sensors Combined with Algorithms Based on Machine Learning and Signal Processing. APL Mater. 2021, 9, 30906. [Google Scholar] [CrossRef]
- Papaleonidas, A.; Psathas, A.P.; Iliadis, L. High Accuracy Human Activity Recognition Using Machine Learning and Wearable Devices’ Raw Signals. J. Inf. Telecommun. 2022, 6, 237–253. [Google Scholar] [CrossRef]
- Obwaller, N.; Langer, J.; Eibensteiner, F. Smart Clothing for Detecting Pressure-Sensitive Gestures. In Proceedings of the 2019 IEEE 13th International Conference on Application of Information and Communication Technologies (AICT), Baku, Azerbaijan, 23–25 October 2019; pp. 1–6. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).