Abstract
Purpose: Monocyte/HDL cholesterol ratio (MHR) is a novel inflammatory marker that is used as a prognostic factor for cardiovascular diseases and has been studied in many diseases. The aim of this study was to investigate the role of inflammatory factors in schizophrenia patients by examining MHR levels and to compare schizophrenia patients and healthy controls in terms of cardiovascular disease risk. Method: A total of 135 participants between the ages of 18–65, 85 diagnosed with schizophrenia, and 50 healthy individuals in the control group were included in this cross-sectional study. Venous blood samples were taken from the participants and CBC parameters and lipid profiles were analyzed. The sociodemographic and clinical data form and positive and negative symptoms scale (PANSS) were administered to all participants. Results: Although monocyte levels were significantly higher in the patient group, HDL-C levels were lower at significant levels. MHR was found to be higher in the patient group compared to the control group at significant levels. When compared to the control group, total cholesterol, triglyceride, WBC, neutrophil, basophil, and platelet levels were higher in the patient group at significant levels, and RBC, hemoglobin, and hematocrit levels were significantly lower. Conclusion: The elevated MHR in patients with schizophrenia may contribute to our understanding that inflammation plays important roles in the pathophysiology of schizophrenia. Additionally, knowing the levels of MHR and considering the recommendations, such as diet and exercise, in the treatment approaches made us think that it might be beneficial in protecting schizophrenia patients against cardiovascular diseases and early death.
1. Introduction
Schizophrenia is a chronic psychotic disease affecting approximately 1% of the world’s population, beginning in late adolescence or early adulthood generally with positive, negative, and cognitive symptoms [1]. Schizophrenia, a complex disease in which genetic predisposition plays important roles, progresses with neurodegenerative processes and disorders in neurotransmitter systems along with neurodevelopmental deviations. It is already known that exposure to environmental stressors in the early stages of life also plays important roles in the development of schizophrenia. It is considered that exposure to environmental factors from the fetal period may cause neurodevelopmental disorders by increasing the abnormal immune response and increasing the risk of developing schizophrenia [2]. When the broad reflections of the immune response in the organism and its relations with other pathological factors playing roles in schizophrenia are considered, elucidating the role of inflammatory mechanisms in schizophrenia emerges as an important issue. Recent studies suggest that cytokine-mediated inflammatory responses may play an important role in the development of schizophrenia. It is known that the increase in proinflammatory cytokines and the uncontrolled activities of microglia and disorders in neurotransmitter functions affect brain development and cause schizophrenia. [3].
Monocytes and macrophages play important roles in the synthesis and release of pro-inflammatory and prooxidant cytokines [4]. Monocytes, which make up approximately 3–8% of leukocytes in peripheral blood, have important effects in the control of inflammatory processes. It was reported that microglia, which are the monocytes in the brain, can increase inflammatory activation abnormally, affect neuronal growth factors, or be harmful to neurogenesis and synaptogenesis by producing neurotoxic factors and cytokines [5]. Studies conducted on microglia, monocytes, and their products in patient groups and various animal models reported that these cells of the mononuclear phagocyte system play important roles in the pathogenesis of major psychiatric disorders (e.g., schizophrenia, bipolar disorder, and major depressive disorder). It was also reported that activation of microglia and circulating monocytes are elevated in schizophrenia patients and in animal models with schizophrenia-like behaviors, which has important effects on the growth, development, and function of neuronal circuits in the brain [6]. Additionally, it was reported that cytokines, whose blood levels increase after viral or bacterial infections during pregnancy, cross the placenta affecting the brain tissue of the fetus, disrupting the normal neurodevelopmental process, which plays roles in the development of schizophrenia [7].
It was reported in previous studies that high-density lipoprotein cholesterol (HDL-C) protects endothelial cells from the negative effects of low-density lipoprotein cholesterol (LDL-C), inhibiting the oxidation of LDL-C molecules, with antithrombotic, anti-inflammatory, and antioxidant effects [4]. It was also shown that HDL-C reduces the prooxidant and pro-inflammatory effects of monocytes. HDL-C also inhibits the proliferation and differentiation of progenitor cells of monocytes, resulting in decreased monocyte activity. Macrophages differentiating from monocytes take up oxidized LDL-C, and the first foam cells are formed. However, HDL-C molecules remove cholesterol residues from macrophages; in other words, HDL-C molecules exert anti-inflammatory and antioxidant effects through monocytes. For this reason, it is suggested that increased monocytes and decreased HDL-C are associated with inflammation [8]. The relations between macrophage ABCA-1 receptor lipid levels and atherosclerosis were investigated in an experimental study on mice, and it was reported that ABCA-1 deficit increased foam cell accumulation and inflammation; in other words, it had a dual effect on both lipid profile and inflammation suggesting that ABCA1 deficiency is closely related to HDL-C metabolism and inflammation processes [9]. It was reported in a previous study that was conducted in 2020 that there was an increase in the concentrations of atherogenic apoB-containing lipoproteins and a decrease in the concentrations of large HDL-C particles in schizophrenia patients [10]. Additionally, it is already known that schizophrenia patients are prone to dyslipidemia, and antipsychotic drugs may increase the risk of metabolic syndrome, and cardiovascular diseases are increased [11]. It is considered today that the MHR may be a new marker of inflammation and oxidative stress because of the anti-inflammatory and antioxidant effects of HDL-C as well as the pro-inflammatory effect of monocytes [12]. The HDL-C molecule has an anti-inflammatory effect. An increase in monocyte levels indicates increased inflammation. Therefore, an increase in MHR levels indicates increased inflammation. MHR is known both as a marker of inflammation and as a prognostic factor for cardiovascular diseases. MHR has been studied in many medical diseases [13,14,15,16,17,18,19]. However, there are limited studies in the literature on schizophrenia patients [20,21]. For this reason, we preferred to examine the MHR level in this study. In addition, psychiatric diseases and neurological diseases have been detected with artificial intelligence in the literature [1]. Schizophrenia was detected using deep learning techniques.
Aims
New studies are important to fully elucidate the role of inflammatory mechanisms, which are increasingly reported in schizophrenia, in understanding the pathophysiology of the disease, to determine the risks of comorbidities, especially cardiovascular disease risks, and to establish effective treatment approaches. Inflammation plays an important role in the etiology of both cardiovascular diseases and schizophrenia. In addition, cardiovascular diseases take the first place among the causes of death in patients with schizophrenia. For this reason, this situation, which causes schizophrenia patients to die in the early period, should be better known, and precautions should be taken in this regard. For these reasons, the aim of this study was to investigate the role of inflammatory factors in schizophrenia patients by examining MHR levels and to compare schizophrenia patients and healthy controls in terms of cardiovascular disease risk.
2. Method
2.1. Participants and Study Design
A total of 85 people, who were diagnosed with schizophrenia according to the diagnostic criteria of The Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5), were hospitalized in the Psychiatry Ward of Elazig Fethi Sekin City Hospital, and who met the study criteria, were included in the study randomly. The healthy control group consisted of 50 patients who met the study criteria and were matched with the patient group in terms of age and gender, without any psychiatric, neurological, or metabolic diseases in their past and present history.
After the purpose and function of the study were explained in detail, written informed consent was obtained from the participants along with a detailed anamnesis. The heights and weights of the participants were measured, and their body mass indices were calculated. Venous blood samples were taken from the participants on an empty stomach, and at the same time of the day, CBC parameters and lipid profile were analyzed. The sociodemographic and clinical data form and positive and negative symptom scale (PANSS) were applied to all patients and the healthy control group. The questionnaire filling process was completed in an average of 30 min. The application and evaluation of the scales were performed by the same psychiatrist. After the scales were filled in, the results of the scales and blood tests were transferred to the electronic medium on a computer on the same day by the relevant psychiatrist and were kept regularly to minimize possible errors and missing data.
2.2. Inclusion and Exclusion Criteria
Inclusion criteria of the patients were being between the ages of 18–65, not having a neurological disease (mental disorders because of brain damage, epilepsy, cerebrovascular disease, dementia, Parkinson’s, etc.) or additional diseases (diabetes, hyperlipidemia, coronary artery disease, malignancy, chronic infection, peripheral artery disease, etc.), and signing the written informed consent form.
For this study, a total of 108 patients were interviewed during the study period, but 4 people were not included in the study due to epilepsy, 8 due to diabetes, 9 due to coronary artery disease, and 3 people because they were illiterate. Although the condition of not having a psychiatric disease was sought in the control group as a criterion, it was considered that the values of individuals with subthreshold psychosis symptoms or individuals with psychotic disease in their close relatives might affect the results. For this reason, the PANSS scale was applied to all participants as it was planned to exclude the patients in the control group with high PANSS values.
The study was conducted in accordance with the principles of the Declaration of Helsinki. Our study was approved by Elazığ Fırat University Clinical Research Ethics Committee (No: 2022/03-30).
2.3. Assessment Scales and Inventories
The sociodemographic and clinical data form: This form was prepared by us in line with the clinical experience and information obtained from the sources and considering the aims of the study that was used. This form is a semi-structured form that includes sociodemographic data, such as age, gender, marital status, educational status, occupation, previous prison history, type of crime alleged to have committed, and clinical evaluation questions, such as the presence of psychiatric illness in the family, additional medical illness, and previous psychiatric treatment history.
Positive and negative symptom scale (PANSS): The scale was developed by Kay et al. (1987) as a semi-structured interview scale consisting of 30 items and a 7-point severity assessment. Among these 30 items, 18 were adapted from the brief psychiatry rating scale (BPRS) and 12 from the psychopathology rating scale. Seven items belong to the positive syndrome subscale, 7 to the negative syndrome subscale, and the remaining 16 to the general psychopathology subscale [22].
2.4. Laboratory Analysis
In the present study, 10 cc venous blood samples were taken from the patients in the patient and control group, after fasting for 8–12 h, into yellow biochemistry tubes and purple blood count tubes. Care was taken to draw blood from all patients at the same time of the day. Then, a complete blood count was analyzed on DXH-800 on the same day, and biochemical parameters were analyzed on Beckman AU-5800 in the biochemistry laboratory of our hospital. Basic hematological parameters, such as monocyte count, were analyzed on an auto analyzer DXH-800 on the same day in the biochemistry laboratory of our hospital, and biochemical parameters (e.g., serum total cholesterol, triglyceride, and HDL-C concentrations) were analyzed on an automatic chemistry analyzer (Beckman AU-5800). Serum low-density lipoprotein cholesterol (LDL-C) values were estimated with the Friedewald Formula or directly measured if triglyceride > 400 mg/dL. The MHR ratio was calculated manually by dividing the monocyte count by HDL-C.
2.5. Statistical Analysis
The Statistical Software SPSS for Windows 22 (Statistical Package for Social Sciences for Windows 22) was used to evaluate the data obtained from the participants. Descriptive analyses to give information on the general characteristics of the participants were given as frequency, percentage distribution, and mean ± standard deviation. The data of continuous variables were in the form of mean ± standard deviation. The data on categorical variables were given as n (%). Qualitative variables of the study were given as demographic data, such as gender, age, educational level, socioeconomic status, alcohol, substance use, and whether there was any additional medical disease. Cross-tables and chi-square test were used to evaluate whether there was a relationship between qualitative variables. Quantitative variables were given as the scores obtained from the scales applied to the participants and as blood parameters and MHR values. When evaluating whether there was a relationship between quantitative variables, the independent samples t-test and Pearson correlation analysis were used. When the p value was calculated at less than 0.05, it was considered statistically significant.
3. Results
The present study included 135 participants, 85 of whom were patients diagnosed with schizophrenia according to DSM-5 criteria, and 50 people constituted the healthy control group. The mean age of the patients was 32.77 ± 6.42 (years) and that of the control group was 31.08 ± 7.03 (years), and no significant differences were detected in this respect between the groups (p = 0.155). The majority of the participants were male, and it was found that there were no significant gender differences between the patient group and the control group (p = 0.613). Although the majority of the patients were literate (n = 32, 37.64%), the majority of the control group were university graduates (n = 23, 46%) (p < 0.001). Most of the patients were not working in an income-generating job (n = 45, 52.94%), but all of the control group were working (n = 50, 100%) (p < 0.001). Additionally, 23.3% of the patients (n = 20) had a history of alcohol and substance use, but there was no history of alcohol substance use in the control group (p < 0.001). A total of 28.2% (n = 24) of the patients had self-mutilation, 17.6% (n = 15) of the patients had tattoos, but there were no signs of self-mutilation and tattoos in the control group (p < 0.001, p = 0.002, respectively). The sociodemographic and clinical data of the participants are given in Table 1.

Table 1.
Sociodemographic and clinical characteristics of the patient and control groups.
When the laboratory parameters of the participants were evaluated, monocyte levels were higher in the patient group at a significant level, but HDL-C levels were lower in the patient group at a significant level (p = 0.002, p = 0.004, respectively). MHR was significantly higher in the patient group when compared to the control group (p < 0.001). Total cholesterol, triglyceride, WBC, neutrophil, basophil, and platelet levels were significantly higher in the patient group compared to the control group, (p = 0.046, p < 0.001, p < 0.001, p = 0.014, p < 0.001, p = 0.004, respectively), and RBC, hemoglobin, and hematocrit levels were significantly lower (p = 0.02, p = 0.014, p = 0.034, respectively) (Table 2).

Table 2.
Scale scores and laboratory parameters of the patient and control groups.
When people who had alcohol and substance abuse were excluded from the patient group, monocyte levels were found to be higher in the patient group at significant levels, HDL-C levels were significantly lower in the patient group, and the monocyte/HDL-C ratio was found to be higher in the patient group at a significant level (p = 0.009, p = 0.007, p = 0.001, respectively).
The positive, negative, and general psychopathology subscale scores and total scores of the PANSS scale that were used to measure disease severity were higher in the patient group at a significant level (p < 0.001, p < 0.001, p < 0.001, p < 0.001, respectively) (Table 2).
No significant correlations were detected between PANSS scale scores, monocytes, HDL-C, and monocytes/HDL-C ratios. There was a positive correlation between PANSS positive and general psychopathology subscale scores and blood urea levels, a negative correlation between PANSS negative subscale scores and basophil levels, and also a negative correlation between PANSS total scores and RBC levels. (Table 3).

Table 3.
Pearson Correlation Analysis of Patients’ Scales and Laboratory Parameters.
No significant relations were detected between disease duration and MHR (p = 0.359).
In logistic regression analysis, monocyte/HDL has an effect on schizophrenia (p < 0.05), and monocyte/HDL increases the disease 1.438 times (Table 4).

Table 4.
Logistic Regression Analysis of the Effect of Monocyte/HDL-C on Schizophrenia.
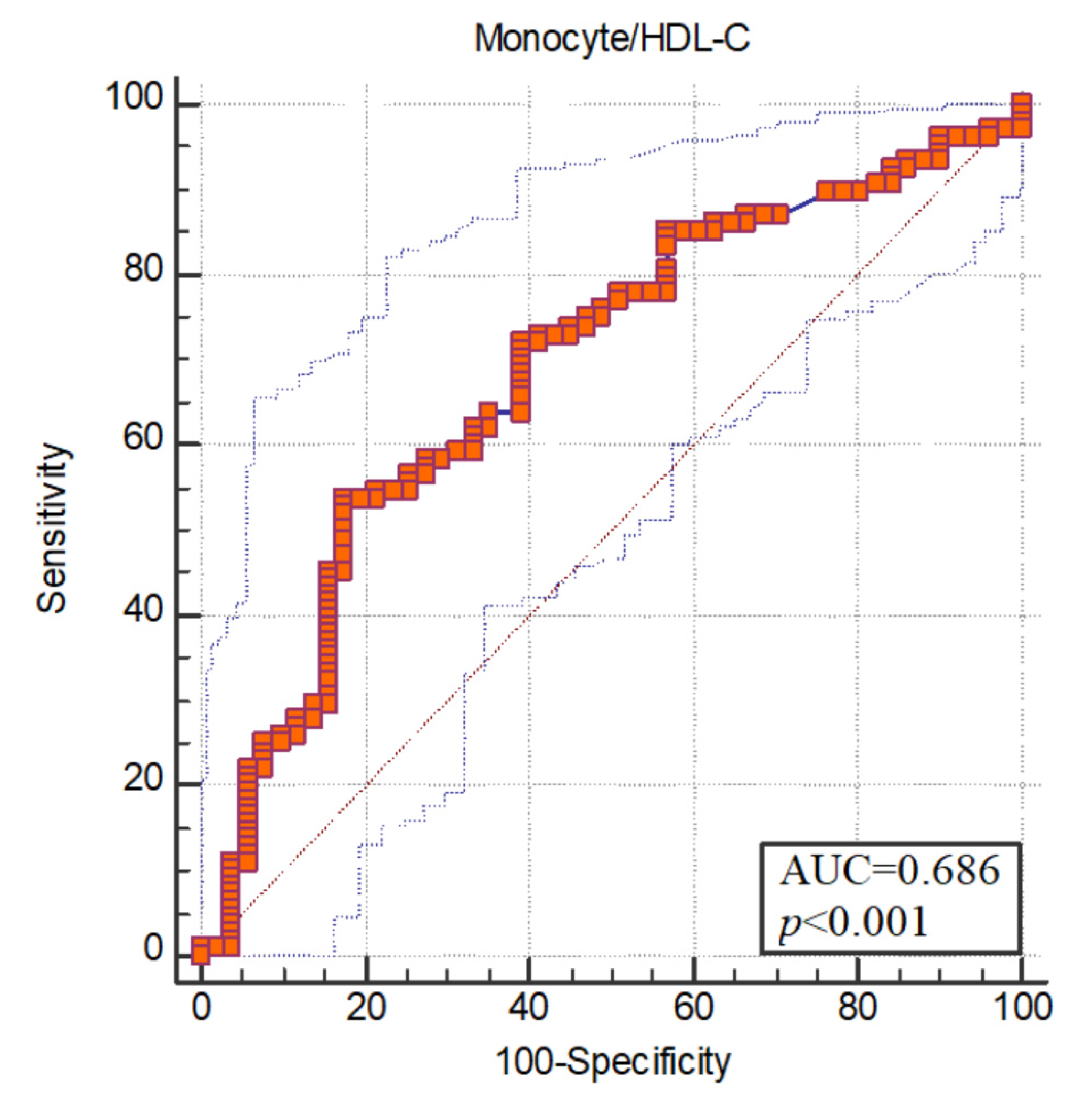
The areas under the receiver operating characteristic (ROC) curve were found to be statistically significant (p < 0.05). ROC curve analysis was used to measure the diagnostic value of the monocyte/HDL-C ratio. The area under the ROC curve of monocyte/HDL for schizophrenia was 0.686. Optimum cut-off value for monocyte/HDL was determined as >0.016. Sensitivity at the breakpoint was 53.70; specificity was set at 82.35 (Figure 1).
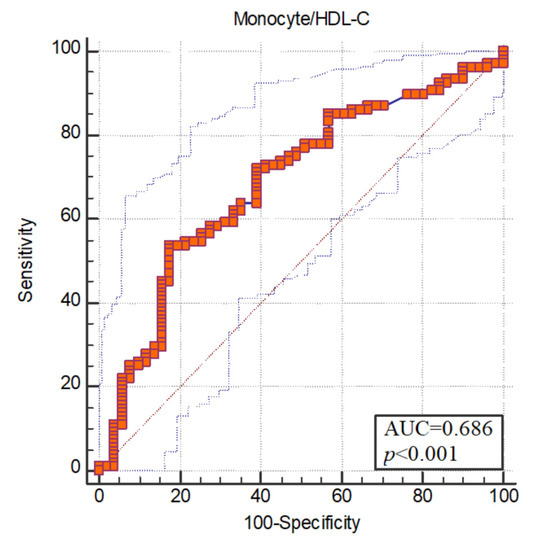
Figure 1.
ROC curve.
4. Discussion
In this study, in which cardiovascular disease risk and inflammatory factors were examined according to MHR levels, MHR levels were found to be significantly higher in the schizophrenia patient group. Additionally, monocyte levels were found to be higher in the patient group at significant levels, HDL-C levels were found to be significantly lower in the patient group. When people who had a history of alcohol and substance use were excluded from the patient group, monocyte levels were significantly higher in the patient group, HDL-C levels were significantly lower in the patient group, and MHR levels were significantly higher in the patient group. It was observed that MHR may be associated with an increased risk of schizophrenia.
Studies that investigate the roles of inflammation in schizophrenia reported that the number of monocytes increases, but monocyte functions are impaired and an increased proinflammatory cytokine signal significantly impairs emotional, cognitive, and social functions, increasing sensitivity to environmental risk factors [23]. It was also reported that children living in neighborhoods where violence was intense were at serious risks for psychiatric disorders, which might be mediated by monocyte activation [24]. In a two-year follow-up study examining inflammatory factors, which were considered to be effective on mental and physical development in children living in neighborhoods with high crime rates, it was found that systemic inflammatory response increased in these children, and monocytes mediated this increased response by activating cellular and molecular pathways [25]. In the present study, monocyte levels were significantly higher in the patient group when compared to the control group, which is consistent with the literature data.
Many studies investigate HDL-C ratios in patients with schizophrenia. It was reported in a meta-analysis conducted in schizophrenia patients with first-episode psychosis who did not use medication that HDL-C and total cholesterol ratios were low, and triglyceride ratios were significantly higher [26]. In a one-year follow-up study that was conducted in our country, it was reported that there were increased inflammatory activities in patients with first-episode psychosis, which continued despite the regression in the symptoms of the patients at the end of a one-year follow-up period [27]. It was reported that the increased serum HDL-C levels with the use of antipsychotics that did not cause weight gain for one year after the first episode of psychosis reduced negative symptoms [28]. In a meta-analysis study investigating the effects of pharmacological interventions for the treatment of dyslipidemia because of antipsychotics in patients with schizophrenia, it was reported that the drugs used could decrease LDL-C, triglyceride, and total cholesterol levels while increasing HDL-C levels [29]. In the present study, it was found that HDL-C ratios were significantly lower in patients with schizophrenia, and triglyceride and cholesterol levels were found to be higher, in line with the literature data.
According to recent studies, MHR, which is easy to use, non-invasive, and can be obtained with a simple calculation method, was reported to be a prognostic factor in cardiovascular diseases in addition to being a new inflammatory marker [30]. As the disease progresses in schizophrenia patients, the risk of cardiovascular disease increases because of an unhealthy lifestyle and poor eating habits, as well as the side effects of the antipsychotics used [31]. It is also known that the atypical antipsychotics cause weight gain, hyperlipidemia, diabetes, hypertension, cardiovascular diseases, and premature death. In other words, this risk increases with the course of the disease and the treatments used [32]. It was reported that life expectancy is shorter in schizophrenia patients when compared to the general population, and cardiovascular diseases are among the leading causes of mortality [33]. For this reason, investigating the presence of atherosclerosis in these patients must be determined before the development of coronary heart disease symptoms. In a study investigating atherosclerosis risk factors in schizophrenia patients, it was reported that biochemical parameters, such as P Selectin, IL-6, MCP-1, and CD 40L, which are markers of atherosclerosis, were elevated [34]. In another study investigating arterial stiffness in schizophrenia patients, it was reported that these patients were more prone to develop arterial stiffness because of atherosclerosis, either because of the nature of the disease or the effect of antipsychotic treatment [35]. It was reported in a previous study that examined the relationship between inflammatory factors and cardiovascular diseases that WBC, CRP, and monocyte levels might be risk determinants for cardiovascular diseases [36]. It was shown in another study with cytokines that low-level inflammation increases the cardiometabolic risk [37]. In other words, even if there is no diagnosed coronary heart disease, elevated MHR levels might be an indication that these patients are at serious risk for coronary heart diseases.
MHR was investigated in many diseases in which inflammation is effective. It was reported that it might be a prognostic factor predicting mortality in patients with acute pulmonary embolism [13]. It was also reported that it can be used to predict end-organ damage in hypertensive patients [14]. In another study, it was reported that MHR could be a useful tool for diagnosing obstructive sleep apnoea syndrome (OSAS) and even used for OSAS classification [15]. It was emphasized in another study that MHR rates were lower in women with high physical activity, and MHR could be reduced with physical activity [16]. In another study that evaluated the effects of subclinical hyperthyroidism on lipids and inflammation markers in patients with newly diagnosed polycystic ovary syndrome, MHR was found to be high [17]. In a study that examined MHR in familial Mediterranean fever (FMF) patients, it was reported that a positive correlation was detected between inflammation parameters (e.g., CRP, serum amyloid A, fibrinogen, erythrocyte sedimentation rate, and MHR) [18]. It was also reported that MHR is elevated in patients with acute ischemic stroke and might be an indicator of chronic inflammation [19].
It was found that there are only two studies in the literature examining MHR in schizophrenia patients. In one study, schizophrenic patients with stable coronary disease (Group 1) and without the coronary disease (Group 2) were compared with healthy controls (Group 3), and MHR was found as Group 1 > Group 2 > Group 3. It was reported that MHR is an important independent marker showing inflammation and oxidative stress in patients with schizophrenia and schizophrenia without the stable coronary disease [20]. In another study, 75 schizophrenia patients and 74 healthy controls were compared, and monocyte and MHR values were found to be significantly higher in schizophrenia patients, and a positive correlation was detected between MHR values and age, body mass index, and PANSS scores. However, no significant differences were detected between HDL-C values [21]. In the present study, schizophrenia patients without coronary disease were compared with healthy controls, and MHR values were found to be significantly higher in patients with schizophrenia.
In the present study, when confounding factors, such as alcohol and substance use, were excluded, monocyte and MHR values were significantly higher in patients with schizophrenia, and HDL-C values were significantly lower. There is no study in the literature examining the relations between alcohol, substance use, and MHR. However, it was reported that MHR is elevated in smoking individuals [38].
5. Limitations and Strengths
The first was the small sampling size. The second was that it was not evaluated whether the antipsychotics used had effects on MHR. Thirdly, confounding factors, such as smoking, physical activity level, and diet, which might affect the MHR levels, were not evaluated. Additionally, the fact that there were male and female genders and the majority of the participants were males might have had a limited effect on the comparisons in terms of gender. Another limitation was that parameters, such as C-reactive protein and inflammatory cytokines were not studied. These limits the interpretation and generalization of the results. For these findings to gain importance, further studies with larger sample groups are needed.
6. Conclusions
The present study is the first one in the literature to investigate the MHR levels in schizophrenic patients without known cardiovascular disease. The elevated MHR, which is a new marker of inflammation in patients with schizophrenia, might contribute to our understanding of the important role of inflammation in the pathophysiology of schizophrenia and cardiovascular disease risk. Additionally, because of the low cost and widespread use of blood count tests, knowing the MHR levels and considering them in the treatment approaches, such as diet and exercise, made us think that it might be beneficial in protecting schizophrenia patients against comorbid diseases, especially cardiovascular disease and early death. In addition, the detection of diseases can be detected by artificial intelligence techniques. In addition, the detection of diseases with OCT, EEG, ECG, MRI and scattergram images can be detected with artificial intelligence techniques [39,40,41,42,43,44,45,46,47,48,49]. In the future, schizophrenia can be detected with inflammatory markers or scattergram images with artificial intelligence techniques.
Author Contributions
Methodology, G.T. and S.K.; Conceptualization, N.K., G.T., S.Y., P.Ö. and S. K; Writing—original draft, G.T. and S.K.; Writing—review and editing, N.K., S.Y. and P.Ö.; Supervision, G.T. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by Fırat University Clinical Research Ethics Committee on 24.02.2022 with the number 2022/03-30 and was conducted in accordance with the Declaration of Helsinki in a prospective design, in the form of case-control.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are not publicly available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tandon, R.; Keshavan, M.S.; Nasrallah, H.A. Schizophrenia, “just the facts”: What we know in 2008: Part 1: Overview. Schizophr. Res. 2008, 100, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Paquin, V.; Lapierre, M.; Veru, F.; King, S. Early environmental upheaval and the risk for schizophrenia. Annu. Rev. Clin. Psychol. 2021, 17, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Erkmen, T.; Şahin, C.; ARICIOĞLU, F. Şizofreni’de inflamatuvar mekanizmaların yeri. Clin. Exp. Health Sci. 2015, 5, 134–139. [Google Scholar]
- Han, C.Y.; Tang, C.; Guevara, M.E.; Wei, H.; Wietecha, T.; Shao, B.; Subramanian, S.; Omer, M.; Wang, S.; O’Brien, K.D. Serum amyloid A impairs the antiinflammatory properties of HDL. J. Clin. Investig. 2016, 126, 266–281. [Google Scholar] [CrossRef]
- Ekdahl, C.; Kokaia, Z.; Lindvall, O. Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience 2009, 158, 1021–1029. [Google Scholar] [CrossRef]
- Beumer, W.; Gibney, S.M.; Drexhage, R.C.; Pont-Lezica, L.; Doorduin, J.; Klein, H.C.; Steiner, J.; Connor, T.J.; Harkin, A.; Versnel, M.A. The immune theory of psychiatric diseases: A key role for activated microglia and circulating monocytes. J. Leukoc. Biol. 2012, 92, 959–975. [Google Scholar] [CrossRef]
- Massarali, A.; Adhya, D.; Srivastava, D.P.; Baron-Cohen, S.; Kotter, M.R. Virus-Induced Maternal Immune Activation as an Environmental Factor in the Etiology of Autism and Schizophrenia. Front. Neurosci. 2022, 16, 834058. [Google Scholar] [CrossRef] [PubMed]
- Şerifler, S. Investigation of the Effect of Monocyte/HDL and Monocyte/lymphocyte Parameters on the Stage of the Disease and Prognosis in Peripheral Facial Paralysis; Ankara Yıldırım Beyazıt Üniversitesi Tıp Fakültesi: Ankara, Turkiye, 2020. [Google Scholar]
- Aiello, R.J.; Brees, D.; Francone, O.L. ABCA1-deficient mice: Insights into the role of monocyte lipid efflux in HDL formation and inflammation. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 972–980. [Google Scholar] [CrossRef]
- Luquain-Costaz, C.; Kockx, M.; Anastasius, M.; Chow, V.; Kontush, A.; Jessup, W.; Kritharides, L. Increased ABCA1 (ATP-Binding Cassette Transporter A1)-specific cholesterol efflux capacity in schizophrenia. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2728–2737. [Google Scholar] [CrossRef]
- Mhalla, A.; Mensi, R.; Amamou, B.; Messaoud, A.; Gassab, L.; Douki, W.; Najjar, M.; Gaha, L. Lipid profile in schizophrenia: Case control study. La Tunis. Med. 2018, 96, 22–29. [Google Scholar]
- Negi, G.; Kumar, A.; Joshi, R.P.; Sharma, S.S. Oxidative stress and Nrf2 in the pathophysiology of diabetic neuropathy: Old perspective with a new angle. Biochem. Biophys. Res. Commun. 2011, 408, 1–5. [Google Scholar] [CrossRef]
- Efe, T.H.; Arslan, E.D.; Ertem, A.G.; Yayla, Ç.; Şahan, H.F.; Felekoğlu, M.A.; Algül, E.; Genç, S.; Yeter, E. PP-103 The Prognostic Value of the Monocyte/HDL ratio in Predicting Short-term Mortality in Patients with Acute Pulmonary Embolism. Am. J. Cardiol. 2016, 117, S76. [Google Scholar] [CrossRef]
- Kaplan, I.; Kaplan, M.; Abacioglu, O.; Yavuz, F.; Saler, T. Monocyte/HDL ratio predicts hypertensive complications. Bratisl. Lek. Listy 2020, 121, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Acat, M.; Yazici, O. The Monocyte/HDL Cholesterol Ratio in Obstructive Sleep Apnea Syndrome/Obstruktif Uyku Apne Sendromunda Monosit/HDL Kolesterol Orani. Meandros Med. Dent. J. 2019, 20, 204–209. [Google Scholar] [CrossRef]
- Doğan, H.; Çaltekin, M.D. Comparison of Sleep Quality and Monocyte/High Density Lipoprotein Ratio by Physical Activity Level in Healthy Women. Kırıkkale Üniversitesi Tıp Fakültesi Derg. 2021, 23, 522–529. [Google Scholar] [CrossRef]
- Cakir, I.; Simsek, Y. Total cholesterol/HDL cholesterol ratio and monocyte/HDL cholesterol ratio are related with subclinical hypothyroidism in polycystic ovary syndrome. Turk. J. Biochem. 2022, 47, 65–69. [Google Scholar]
- Gunes, H.; Duksal, F.; Parlak, M. Can monocyte to HDL ratio be used as an inflammatory marker in children with familial mediterranean fever? Ann. Med. Res. 2019, 26, 1453–1457. [Google Scholar] [CrossRef]
- Kosovali, B.D. Monosit Sayısının Yüksek Yoğunluklu Lipoproteine Oranı Akut İskemik İnmede İnflamasyon Belirteci Midir? Abant Tıp Dergisi 2020, 9, 31–34. [Google Scholar]
- CANDEMİR, M.; CANSIZ, A. Predictive Value of Monocyte to High-Density Lipoprotein Cholesterol Ratio (MHR) in Schizophrenia Patients with Stable Coronary Artery Disease. Genel Tıp Derg. 2022, 32, 85–91. [Google Scholar] [CrossRef]
- Sahpolat, M.; Ayar, D.; Ari, M.; Karaman, M.A. Elevated Monocyte to High-density Lipoprotein Ratios as an Inflammation Markers for Schizophrenia Patients. Clin. Psychopharmacol. Neurosci. 2021, 19, 112. [Google Scholar] [CrossRef]
- First, M.; Spitzer, R.; Gibbon, M.; Williams, J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. Schizophr Bull. 1987, 13, 261–276. [Google Scholar]
- Müller, N.; Wagner, J.K.; Krause, D.; Weidinger, E.; Wildenauer, A.; Obermeier, M.; Dehning, S.; Gruber, R.; Schwarz, M.J. Impaired monocyte activation in schizophrenia. Psychiatry Res. 2012, 198, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, P. Uneasy Peace: The Great Crime Decline, the Renewal of City Life, and the Next War on Violence; WW Norton & Company: New York, NY, USA, 2018. [Google Scholar]
- Miller, G.E.; Chen, E.; Finegood, E.; Shimbo, D.; Cole, S.W. Prospective associations between neighborhood violence and monocyte pro-inflammatory transcriptional activity in children. Brain Behav. Immun. 2022, 100, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wysokiński, A.; Strzelecki, D.; Kłoszewska, I. Levels of triglycerides, cholesterol, LDL, HDL and glucose in patients with schizophrenia, unipolar depression and bipolar disorder. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 168–176. [Google Scholar] [CrossRef]
- Özkalayci, Ö. Investigation of Immunological Parameters in the First Episode Psychosis Patients after 6 Months from the Beginning of the Treatment. 2020. Available online: https://www.morressier.com/o/event/5c3da4fd9ae8fb00131ce43e/article/5c642be19ae8fb00131cec5a (accessed on 20 December 2022).
- Gjerde, P.B.; Dieset, I.; Simonsen, C.; Hoseth, E.Z.; Iversen, T.; Lagerberg, T.V.; Lyngstad, S.H.; Mørch, R.H.; Skrede, S.; Andreassen, O.A. Increase in serum HDL level is associated with less negative symptoms after one year of antipsychotic treatment in first-episode psychosis. Schizophr. Res. 2018, 197, 253–260. [Google Scholar] [CrossRef]
- Kanagasundaram, P.; Lee, J.; Prasad, F.; Costa-Dookhan, K.A.; Hamel, L.; Gordon, M.; Remington, G.; Hahn, M.K.; Agarwal, S.M. Pharmacological interventions to treat antipsychotic-induced dyslipidemia in schizophrenia patients: A systematic review and meta analysis. Front. Psychiatry 2021, 12, 642403. [Google Scholar] [CrossRef]
- Ganjali, S.; Gotto Jr, A.M.; Ruscica, M.; Atkin, S.L.; Butler, A.E.; Banach, M.; Sahebkar, A. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J. Cell. Physiol. 2018, 233, 9237–9246. [Google Scholar] [CrossRef]
- İpekçioğlu, D.; Kendirlioğlu, B.K. Physical comorbidity and causes of death among schizophrenia patients: A retrospective descriptive study. Bakirkoy Tip Derg. 2019, 15, 103. [Google Scholar] [CrossRef]
- Newcomer, J.W. Second-generation (atypical) antipsychotics and metabolic effects. CNS Drugs 2005, 19, 1–93. [Google Scholar] [CrossRef]
- Delibaş, D.H.; Aydin, M.; Sati-Kirkan, T.; Oğuz, E.G.; Karasu, U.; Şimşek, Y.; Kiliç, C.; Kirci-Ercan, S.; Bayrakçi, A.; Talas-Özçimen, A. Clinical Characteristics, Comorbid Medical Diagnoses, and Causes of Death of Individuals with Severe Mental Illness Who Died During Follow-up in Community Mental Health Centers: A Multicenter, Retrospective Study. Turk. Psikiyatr. Derg. 2021, 32, 246. [Google Scholar] [CrossRef]
- Açıkgöz, H.E. Cardiovascular risk factors in obese and non-obese patients with spectrum disorder schizophrenia. West Indian Med. J. 2013, 21. [Google Scholar]
- Baykara, S.; Bozdağ, P.G.; Baykara, M.; Namlı, M.N. Evaluation of arterial stiffness in patients with schizophrenia. J. Clin. Neurosci. 2020, 79, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.J.; Kandhal, P.; Rapaport, M.H.; Mellor, A.; Buckley, P. Total and differential white blood cell counts, high-sensitivity C-reactive protein, and cardiovascular risk in non-affective psychoses. Brain Behav. Immun. 2015, 45, 28–35. [Google Scholar] [CrossRef]
- Balõtšev, R.; Koido, K.; Vasar, V.; Janno, S.; Kriisa, K.; Mahlapuu, R.; Ljubajev, U.; Parksepp, M.; Veiksaar, P.; Volke, V. Inflammatory, cardio-metabolic and diabetic profiling of chronic schizophrenia. Eur. Psychiatry 2017, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Köylü, E.; Kurtoğlu, Y.K. Relationship between elevated monocyteHDL ratio, an inflammatory marker, with smoking. J. Turk. Fam. Physician 2021, 12, 22–31. [Google Scholar]
- Demir, F.; Taşcı, B. An effective and robust approach based on r-cnn+ lstm model and ncar feature selection for ophthalmological disease detection from fundus images. J. Pers. Med. 2021, 11, 1276. [Google Scholar] [CrossRef]
- Taşcı, B.; Acharya, M.R.; Barua, P.D.; Yildiz, A.M.; Gun, M.V.; Keles, T.; Dogan, S.; Tuncer, T. A new lateral geniculate nucleus pattern-based environmental sound classification using a new large sound dataset. Appl. Acoust. 2022, 196, 108897. [Google Scholar] [CrossRef]
- Macin, G.; Tasci, B.; Tasci, I.; Faust, O.; Barua, P.D.; Dogan, S.; Tuncer, T.; Tan, R.-S.; Acharya, U.R. An accurate multiple sclerosis detection model based on exemplar multiple parameters local phase quantization: ExMPLPQ. Appl. Sci. 2022, 12, 4920. [Google Scholar] [CrossRef]
- Tasci, B.; Tasci, I. Deep feature extraction based brain image classification model using preprocessed images: PDRNet. Biomed. Signal Process. Control. 2022, 78, 103948. [Google Scholar] [CrossRef]
- Dogan, S.; Baygin, M.; Tasci, B.; Loh, H.W.; Barua, P.D.; Tuncer, T.; Tan, R.-S.; Acharya, U.R. Primate brain pattern-based automated Alzheimer’s disease detection model using EEG signals. Cogn. Neurodynamics 2022, 1–13. [Google Scholar] [CrossRef]
- Tasci, G.; Loh, H.W.; Barua, P.D.; Baygin, M.; Tasci, B.; Dogan, S.; Tuncer, T.; Palmer, E.E.; Tan, R.-S.; Acharya, U.R. Automated accurate detection of depression using twin Pascal’s triangles lattice pattern with EEG Signals. Knowl.-Based Syst. 2023, 260, 110190. [Google Scholar] [CrossRef]
- Tasci, B. Automated ischemic acute infarction detection using pre-trained CNN models’ deep features. Biomed. Signal Process. Control. 2023, 82, 104603. [Google Scholar] [CrossRef]
- Demir, F.; Akbulut, Y.; Taşcı, B.; Demir, K. Improving brain tumor classification performance with an effective approach based on new deep learning model named 3ACL from 3D MRI data. Biomed. Signal Process. Control. 2023, 81, 104424. [Google Scholar] [CrossRef]
- Tasci, B.; Tasci, G.; Dogan, S.; Tuncer, T. A novel ternary pattern-based automatic psychiatric disorders classification using ECG signals. Cogn. Neurodynamics 2022, 1–14. [Google Scholar] [CrossRef]
- Tasci, B. Classification of Skin Lesion Images Using Feature Selection Algorithm in Pre-Trained Convolutional Neural Network Models. Fırat Üniversitesi Mühendislik Bilim. Derg. 2020, 34, 541–552. [Google Scholar]
- Tasci, İ.; Tasci, B.; Doğan, S.; Tuncer, T. A new dataset for EEG abnormality detection MTOUH. Turk. J. Sci. Technol. 2022, 17, 135–141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).