Learning Curve of Robotic Lobectomy for the Treatment of Lung Cancer: How Does It Impact on the Autonomic Nervous System of the Surgeon?
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Work-Up
2.2. The Surgeon’s Previous Experience
2.3. Surgical Technique (RATS Lobectomy via Four-Arm Robotic Approach with Utility Incision)
2.4. Patient Outcomes
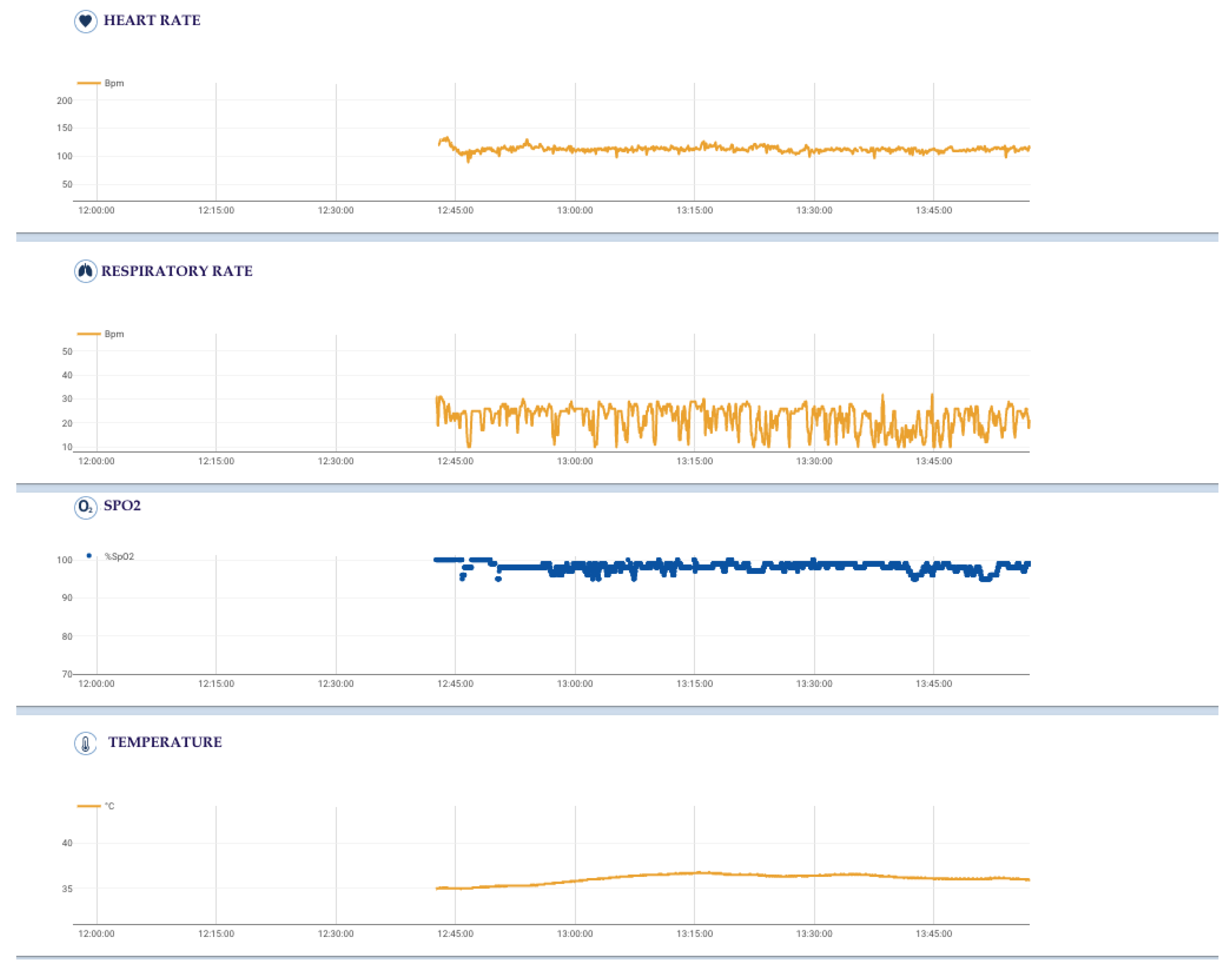
2.5. Cardiovascular and Respiratory Activity of the Surgeon
- -
- Cardiovascular activity (mean, maximum and minimum heart rate), thanks to 6-lead ECG signal at 500 Hz by using 4 ink-based dry electrodes;
- -
- Respiratory activity (mean, maximum and minimum respiratory rate), desaturation and time of desaturation, thanks to 3-channel respiratory signal at 50 Hz from strain circumferential sensors placed at the thoracic, xiphoid and abdominal levels;
- -
- Body activity and temperature (mean, maximum and minimum body temperature) thanks to a contact sensor under the right armpit;
- -
- Blood oxygen saturation (SpO2) (mean, maximum and minimum SpO2, desaturation and time in desaturation) from an optical module under the left armpit;
- -
- Activity level and body position from an inertial measurement unit (IMU) on the back.
2.6. Statistical Methods
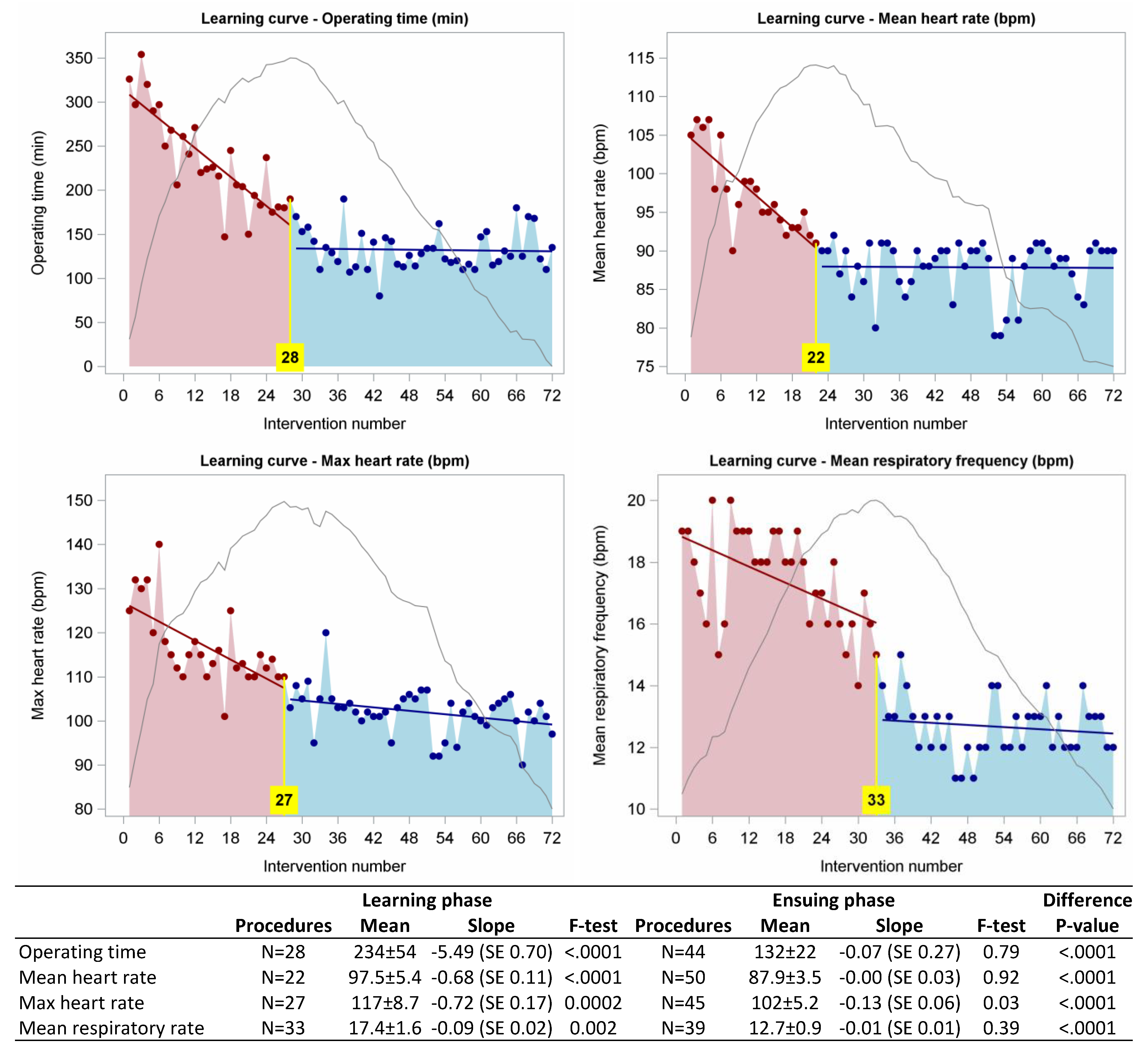
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melfi, F.M.; Menconi, G.F.; Mariani, A.M.; Angeletti, C.A. Early experience with robotic technology for thoracoscopic surgery. Eur. J. Cardio-Thoracic Surg. 2002, 21, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.J.; Sun, Z.; Speicher, P.J.; Saud, S.M.; Gulack, B.C.; Hartwig, M.G.; Harpole Jr, D.H.; Onaitis, M.W.; Tong, B.C.; D’Amico, T.A.; et al. Use and outcomes of minimally invasive lobectomy for stage I non-small cell lung cancer in the national cancer data base. Ann. Thorac. Surg. 2016, 101, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Louie, B.E.; Wilson, J.L.; Kim, S.; Cerfolio, R.J.; Park, B.J.; Farivar, A.S.; Vallières, E.; Aye, R.W.; Burfeind, W.R.; Block, M.I. Comparison of Video-Assisted Thoracoscopic Surgery and Robotic Approaches for Clinical Stage I and Stage II Non-Small Cell Lung Cancer Using the Society of Thoracic Surgeons Database. Ann. Thorac. Surg. 2016, 102, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Xie, L.; Chen, G.; Tang, J.-M.; Ben, X.-S. Robotic thoracic surgery versus video-assisted thoracic surgery for lung cancer: A meta-analysis. Interdiscip. CardioVascular Thorac. Surg. 2015, 21, 409–414. [Google Scholar] [CrossRef]
- Paul, S.; Jalbert, J.; Isaacs, A.J.; Altorki, N.K.; Isom, O.W.; Sedrakyan, A. Comparative effectiveness of robotic-assisted vs thoracoscopic lobectomy. Chest 2014, 146, 1505–1512. [Google Scholar] [CrossRef]
- Augustin, F.; Bodner, J.; Maier, H.; Schwinghammer, C.; Pichler, B.; Lucciarini, P.; Pratschke, J.; Schmid, T. Robotic-assisted minimally invasive vs. thoracoscopic lung lobectomy: Comparison of perioperative results in a learning curve setting. Langenbeck’s Arch. Surg. 2013, 398, 895–901. [Google Scholar] [CrossRef]
- Pan, H.; Gu, Z.; Tian, Y.; Jiang, L.; Zhu, H.; Ning, J.; Huang, J.; Luo, Q. Propensity score-matched comparison of robotic- and video-assisted thoracoscopic surgery, and open lobectomy for non-small cell lung cancer patients aged 75 years or older. Front. Oncol. 2022, 12, 1009298. [Google Scholar] [CrossRef]
- Casiraghi, M.; Mariolo, A.V.; Mohamed, S.; Sedda, G.; Maisonneuve, P.; Mazzella, A.; Iacono, G.L.; Petrella, F.; Spaggiari, L. Long-Term Outcomes of Robotic-Assisted, Video-Assisted and Open Surgery in Non-Small Cell Lung Cancer: A Matched Analysis. J. Clin. Med. 2022, 11, 3363. [Google Scholar] [CrossRef]
- Kent, M.S.; Hartwig, M.G.; Vallières, E.; Abbas, A.E.; Cerfolio, R.J.; Dylewski, M.R.; Fabian, T.; Herrera, L.J.; Jett, K.G.; Lazzaro, R.S.; et al. Pulmonary Open, Robotic and Thoracoscopic Lobectomy (PORTaL) Study: An Analysis of 5721 Cases. Ann. Surg. 2021. Available online: https://pubmed.ncbi.nlm.nih.gov/34534988/ (accessed on 1 January 2023). [CrossRef]
- Shagabayeva, L.; Fu, B.; Panda, N.; Potter, A.L.; Auchincloss, H.G.; Mansur, A.; Yang, C.-F.J.; Schumacher, L. Open, Video- and Robot-Assisted Thoracoscopic Lobectomy for Stage II-IIIA Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2022, 115, 184–190. [Google Scholar] [CrossRef]
- Mazzella, A.; Olland, A.; Falcoz, P.E.; Renaud, S.; Santelmo, N.; Massard, G. Video-assisted thoracoscopic lobectomy: Which is the learning curve of an experienced consultant? J. Thorac. Dis. 2016, 8, 2444–2453. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, G.; Galetta, D.; Maisonneuve, P.; Melfi, F.; Schmid, R.A.; Borri, A.; Vannucci, F.; Spaggiari, L. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J. Thorac. Cardiovasc. Surg. 2010, 140, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.E.-M.; Ilonen, I.K.; Pälli, O.H.; Salo, J.A.; Räsänen, J.V. Learning curve in robotic-assisted lobectomy for non-small cell lung cancer is not steep after experience in video-assisted lobectomy; single-surgeon experience using cumulative sum analysis. Cancer Treat. Res. Commun. 2021, 27, 100362. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Sun, X.; Miao, S.; Li, S.; Zhao, Y.; Xuan, Y.; Qiu, T.; Niu, Z.; Song, J.; Jiao, W. Learning curve for robot-assisted lobectomy of lung cancer. J. Thorac. Dis. 2019, 11, 2431–2437. [Google Scholar] [CrossRef]
- Gómez-Hernández, M.T.; Fuentes, M.G.; Novoa, N.M.; Rodríguez, I.; Varela, G.; Jiménez, M.F. The robotic surgery learning curve of a surgeon experienced in video-assisted thoracoscopic surgery compared with his own video-assisted thoracoscopic surgery learning curve for anatomical lung resections. Eur. J. Cardio-Thoracic Surg. 2021, 61, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, S.P.; Abolhoda, A.; Kirkpatrick, V.E.; Saffarzadeh, A.G.; Thein, M.S.; Wilson, S.E. Learning Curve of Robotic Lobectomy for Early-Stage Non-Small Cell Lung Cancer by a Thoracic Surgeon Adept in Open Lobectomy. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2018, 13, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Power, A.D.; D’Souza, D.M.; Moffatt-Bruce, S.D.; Merritt, R.E.; Kneuertz, P.J. Defining the learning curve of robotic thoracic surgery: What does it take? Surg. Endosc. 2019, 33, 3880–3888. [Google Scholar] [CrossRef]
- Cheufou, D.H.; Mardanzai, K.; Ploenes, T.; Theegarten, D.; Stamatis, G.; Kampe, S.; Aigner, C. Effectiveness of Robotic Lobectomy-Outcome and Learning Curve in a High Volume Center. Thorac. Cardiovasc. Surg. 2019, 67, 573–577. [Google Scholar] [CrossRef]
- Toker, A.; Özyurtkan, M.O.; Kaba, E.; Ayalp, K.; Demirhan, Ö.; Uyumaz, E. Robotic anatomic lung resections: The initial experience and description of learning in 102 cases. Surg. Endosc. 2015, 30, 676–683. [Google Scholar] [CrossRef]
- Ruitenburg, M.M.; Frings-Dresen, M.H.W.; Sluiter, J.K. Physical job demands and related health complaints among surgeons. Int. Arch. Occup. Environ. Health 2012, 86, 271–279. [Google Scholar] [CrossRef]
- Amirian, I.; Andersen, L.; Rosenberg, J.; Gögenur, I. Decreased heart rate variability in surgeons during night shifts. Can. J. Surg. 2014, 57, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Dekker, J.M.; Crow, R.S.; Folsom, A.R.; Hannan, P.J.; Liao, D.; Swenne, C.A.; Schouten, E.G. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: The ARIC study. Circulation 2000, 102, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.I.; Amawi, F.; Bhalla, A.; Peacock, O.; Williams, J.P.; Lund, J.N. Assessing surgeon stress when operating using heart rate variability and the state trait anxiety inventory: Will surgery be the death of us? Color. Dis. 2015, 17, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Ciraulo, L.A.; Robaczewski, M.L.; Ciraulo, N.A.; Ciraulo, R.S.; Robaczewski, G.D.; Falank, C.R.; Ontengco, J.B.; Ciraulo, D.L. Biometric Analysis of Surgeons’ Physiologic Responses During Surgery. Am. Surg. 2020, 86, 1548–1552. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, M.; Galetta, D.; Borri, A.; Tessitore, A.; Romano, R.; Diotti, C.; Brambilla, D.; Maisonneuve, P.; Spaggiari, L. Ten Years’ Experience in Robotic-Assisted Thoracic Surgery for Early Stage Lung Cancer. Thorac. Cardiovasc. Surg. 2019, 67, 564–572. [Google Scholar]
- Galetta, D.; Casiraghi, M.; Pardolesi, A.; Borri, A.; Spaggiari, L. New stapling devices in robotic surgery. J. Vis. Surg. 2017, 3, 45. [Google Scholar] [CrossRef]
- Alicuben, E.T.; Wightman, S.C.; Shemanski, K.A.; David, E.A.; Atay, S.M.; Kim, A.W. Training residents in robotic thoracic surgery. J. Thorac. Dis. 2021, 13, 6169–6178. [Google Scholar] [CrossRef]
- Sier, V.Q.; Schmitz, R.F.; Schepers, A.; van der Vorst, J.R. Exploring the surgical personality. Surgeon 2022. ahead of print. [Google Scholar] [CrossRef]

| Characteristics | All Procedures | First 30 Procedures | Next 42 Procedures | p-Value |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| 72 (100.) | 30 (100.) | 42 (100.) | ||
| Sex | ||||
| Male F | 30 (41.7) | 13 (43.3) | 17 (40.5) | |
| Female M | 42 (58.3) | 17 (56.7) | 25 (59.5) | 0.81 |
| Age | ||||
| <60 | 18 (25.0) | 11 (36.7) | 7 (16.7) | |
| 60–69 | 33 (45.8) | 11 (36.7) | 22 (52.4) | |
| ≥70 | 21 (29.2) | 8 (26.7) | 13 (31.0) | 0.28 |
| Comorbidities * | ||||
| No | 15 (20.8) | 2 (6.7) | 13 (31.0) | |
| Yes | 57 (79.2) | 28 (93.3) | 29 (69.0) | 0.02 |
| Lung | 23 (31.9) | 9 (30.0) | 14 (33.3) | 0.80 |
| Cardiac | 38 (52.8) | 14 (46.7) | 24 (57.1) | 0.47 |
| Metabolic | 35 (48.6) | 13 (43.3) | 22 (52.4) | 0.48 |
| Other | 26 (36.1) | 10 (33.3) | 16 (38.1) | 0.80 |
| Side | ||||
| Right | 48 (66.7) | 23 (76.7) | 25 (59.5) | |
| Left | 24 (33.3) | 7 (23.3) | 17 (40.5) | 0.20 |
| Lobe | ||||
| Inferior | 30 (41.7) | 12 (40.0) | 18 (42.9) | |
| Medial | 4 (5.6) | 4 (13.3) | 0 ( 0.0) | |
| Superior | 38 (52.8) | 14 (46.7) | 24 (57.1) | 0.06 |
| Stage | ||||
| IA | 25 (34.7) | 11 (36.7) | 14 (33.3) | |
| IB | 35 (48.6) | 9 (30.0) | 26 (61.9) | |
| II-III | 10 (13.9) | 8 (26.7) | 2 (4.8) | |
| Benign | 2 (2.8) | 2 (6.7) | 0 (0.0) | 0.004 |
| Grade | ||||
| G1 | 13 (20.0) | 3 (10.0) | 10 (23.8) | |
| G2 | 33 (50.8) | 14 (46.7) | 19 (45.2) | |
| G3 | 19 (29.3) | 8 (26.7) | 11 (26.2) | 0.52 |
| Histology | ||||
| ADK | 50 (69.4) | 19 (63.3) | 31 (73.8) | |
| SCC | 8 (11.1) | 3 (10.0) | 5 (11.9) | |
| Adenosquamous | 1 (1.4) | 1 (3.3) | 0 (0.0) | |
| NSCLC | 1 (1.4) | 1 (3.3) | 0 (0.0) | |
| Large cell | 2 (2.8) | 0 (0.0) | 2 (4.8) | |
| SCLC | 1 (1.4) | 0 (0.0) | 1 (2.4) | |
| Carcinoid | 7 (9.7) | 4 (13.3) | 3 (7.1) | |
| Benign | 2 (2.8) | 2 (6.6) | 0 (0.0) | 0.36 |
| Dimension (mm) | ||||
| Mean ± SD | 22.7 ± 9.0 | 24.9 ± 9.8 | 21.1 ± 8.1 | 0.07 |
| Median (range) | 21 (7–49) | 23.5 (7–49) | 21 (9–38) | 0.12 |
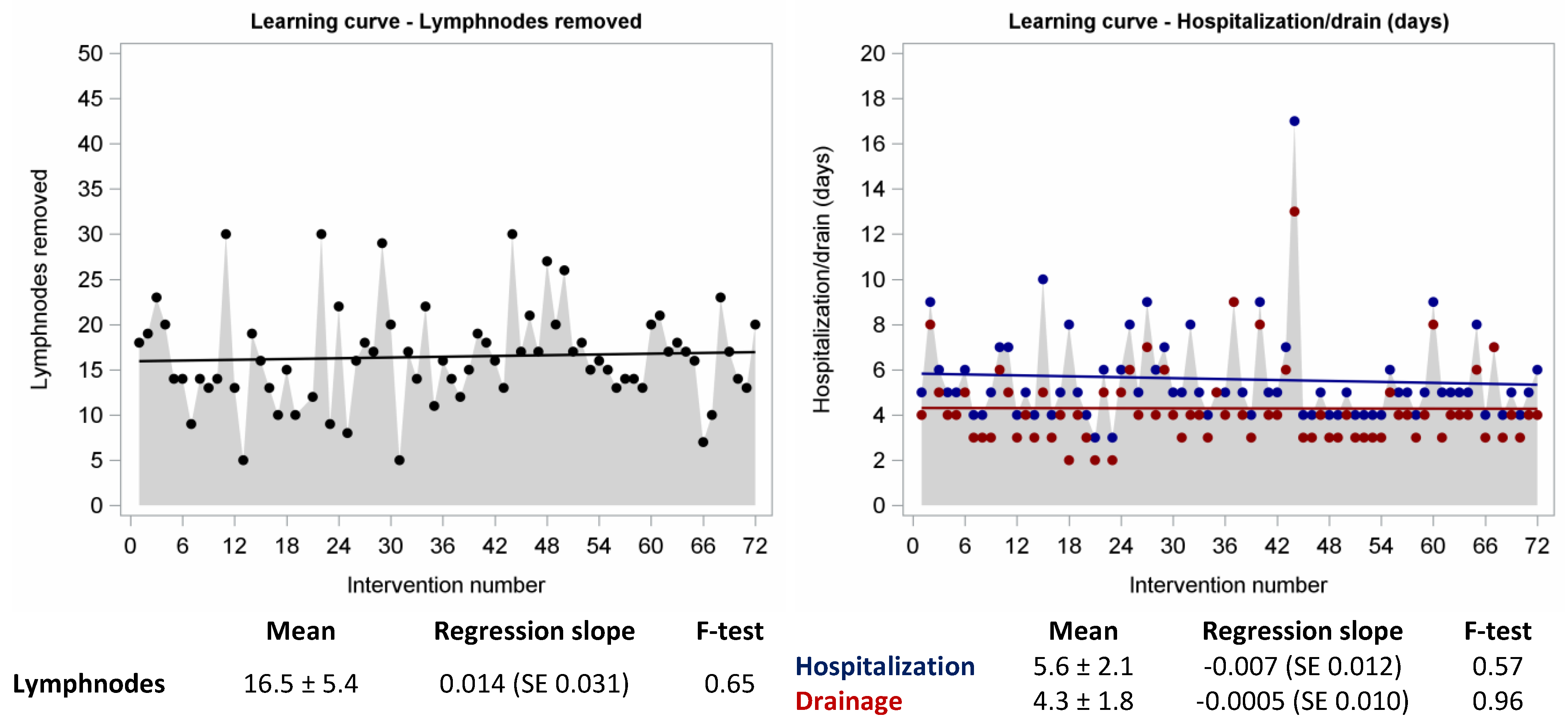
| Harvested lymphnodes | ||||
| Mean ± SD | 16.5 ± 5.4 | 16.2 ± 6.3 | 16.6 ± 4.8 | 0.76 |
| Median (range) | 16 (5–30) | 15 (5–30) | 16.5 (5–30) | 0.42 |
| Conversion | ||||
| No | 70 (97.2) | 29 (96.7) | 41 (97.6) | |
| Yes | 2 (2.8) | 1 (3.3) | 1 (2.4) | 1.00 |
| Complication * | ||||
| No | 55 (77.5) | 21 (70.0) | 34 (81.0) | |
| Yes | 16 (22.5) | 9 (30.0) | 7 (16.7) | 0.25 |
| Air leak | 6 (8.3) | 2 (6.7) | 4 (9.5) | 1.00 |
| AF/arrhythmia | 8 (11.1) | 5 (16.7) | 3 (7.1) | 0.26 |
| Other | 3 (4.2) | 3 (10.0) | 0 (0.0) | 0.07 |
| Hospital stay (days) | ||||
| Mean ± SD | 5.6 ± 2.1 | 5.7 ± 1.8 | 5.5 ± 2.3 | 0.77 |
| Median (range) | 5 (3–17) | 5 (3–10) | 5 (4–17) | 0.16 |
| Drain (days) | ||||
| Mean ± SD | 4.3 ± 1.8 | 4.2 ± 1.4 | 4.4 ± 2.0 | 0.70 |
| Median (range) | 4 (2–13) | 4 (2–8) | 4 (3–13) | 0.37 |
| Characteristics | All Procedures | First 30 Procedures | Next 42 Procedures | p-Value |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| 72 (100.) | 30 (100.) | 42 (100.) | ||
| Mean heart rate | ||||
| Mean ± SD | 90.8 ± 6.1 | 95.0 ± 6.3 | 87.8 ± 3.6 | <0.0001 |
| Median (range) | 90 (79–107) | 94.5 (84–107) | 89 (79–91) | <0.0001 |
| Min heart rate | ||||
| Mean ± SD | 82.3 ± 7.7 | 87.7 ± 6.9 | 78.5 ± 5.6 | <0.0001 |
| Median (range) | 82 (68–98) | 89 (75–98) | 80.5 (68–85) | 0.005 |
| Max heart rate | ||||
| Mean ± SD | 107.6 ± 9.8 | 115.6 ± 9.0 | 101.8 ± 5.3 | <0.0001 |
| Median (range) | 105 (90–140) | 113.5 (101–140) | 102 (90–120) | <0.0001 |
| Mean respiratory rate | ||||
| Mean ± SD | 14.8 ± 2.7 | 17.6 ± 1.6 | 12.9 ± 1.3 | <0.0001 |
| Median (range) | 14 (11–20) | 18 (14–20) | 13 (11–17) | <0.0001 |
| Min respiratory rate * | ||||
| Mean ± SD | 10.2 ± 1.1 | 10.3 ± 1.5 | 10.1 ± 0.7 | 0.55 |
| Median (range) | 10 (8–13) | 10 (8–13) | 10 (8–12) | 0.92 |
| Max respiratory rate | ||||
| Mean ± SD | 23.9 ± 2.0 | 24.2 ± 1.6 | 23.7 ± 2.3 | 0.27 |
| Median (range) | 24 (21–33) | 24 (21–28) | 23.5 (21–33) | 0.02 |
| Mean body temperature | ||||
| Mean ± SD | 36.4 ± 0.2 | 36.4 ± 0.2 | 36.4 ± 0.3 | 0.49 |
| Median [range] | 36.3 [35.6–36.9] | 36.5 [36.1–36.7] | 36.3 [35.6–36.9] | 0.15 |
| Min body temperature | ||||
| Mean ± SD | 36.0 ± 0.5 | 36.1 ± 0.3 | 35.9 ± 0.5 | 0.11 |
| Median (range) | 36.0 (34.5–37.1) | 36.1 (35.2–37.1) | 35.9 (34.5–36.8) | 0.11 |
| Max body temperature | ||||
| Mean ± SD | 36.8 ± 0.3 | 36.8 ± 0.3 | 36.8 ± 0.3 | 0.82 |
| Median (range) | 36.8 (35.7–37.4) | 36.8 (35.7–37.2) | 36.8 (36.3–37.4) | 0.24 |
| Mean saturation | ||||
| Mean ± SD | 98.1 ± 0.2 | 98.1 ± 0.3 | 98.0 ± 0.2 | 0.22 |
| Median (range) | 98 (98–99) | 98 (98–99) | 98 (98–99) | 0.17 |
| Min saturation | ||||
| Mean ± SD | 91.4 ± 2.6 | 91.1 ± 2.3 | 91.7 ± 2.8 | 0.30 |
| Median (range) | 92 (84–96) | 91 (84–96) | 92 (84–95) | 0.03 |
| Max saturation | ||||
| Mean ± SD | 98.7 ± 0.5 | 98.8 ± 0.4 | 98.6 ± 0.5 | 0.05 |
| Median (range) | 99 (98–99) | 99 (98–99) | 99 (98–99) | 0.05 |
| Desaturation * | ||||
| Mean ± SD | 69.9 ± 41.2 | 57.1 ± 23.4 | 79.1 ± 48.4 | 0.001 |
| Median (range) | 60 (17–243) | 54 (20–146) | 71 (17–243) | 0.01 |
| Mean desaturation | ||||
| Mean ± SD | 3.0 ± 0.7 | 2.7 ± 0.6 | 3.3 ± 0.7 | 0.0002 |
| Median (range) | 3 (2–5) | 3 (2–4) | 3 (2–5) | 0.0003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzella, A.; Mohamed, S.; Maisonneuve, P.; Sedda, G.; Cara, A.; Casiraghi, M.; Petrella, F.; Donghi, S.M.; Lo Iacono, G.; Spaggiari, L. Learning Curve of Robotic Lobectomy for the Treatment of Lung Cancer: How Does It Impact on the Autonomic Nervous System of the Surgeon? J. Pers. Med. 2023, 13, 193. https://doi.org/10.3390/jpm13020193
Mazzella A, Mohamed S, Maisonneuve P, Sedda G, Cara A, Casiraghi M, Petrella F, Donghi SM, Lo Iacono G, Spaggiari L. Learning Curve of Robotic Lobectomy for the Treatment of Lung Cancer: How Does It Impact on the Autonomic Nervous System of the Surgeon? Journal of Personalized Medicine. 2023; 13(2):193. https://doi.org/10.3390/jpm13020193
Chicago/Turabian StyleMazzella, Antonio, Shehab Mohamed, Patrick Maisonneuve, Giulia Sedda, Andrea Cara, Monica Casiraghi, Francesco Petrella, Stefano Maria Donghi, Giorgio Lo Iacono, and Lorenzo Spaggiari. 2023. "Learning Curve of Robotic Lobectomy for the Treatment of Lung Cancer: How Does It Impact on the Autonomic Nervous System of the Surgeon?" Journal of Personalized Medicine 13, no. 2: 193. https://doi.org/10.3390/jpm13020193
APA StyleMazzella, A., Mohamed, S., Maisonneuve, P., Sedda, G., Cara, A., Casiraghi, M., Petrella, F., Donghi, S. M., Lo Iacono, G., & Spaggiari, L. (2023). Learning Curve of Robotic Lobectomy for the Treatment of Lung Cancer: How Does It Impact on the Autonomic Nervous System of the Surgeon? Journal of Personalized Medicine, 13(2), 193. https://doi.org/10.3390/jpm13020193