Abstract
Chronic kidney disease (CKD) is a major public health concern with an increasing proportion of sufferers progressing to renal replacement therapy (RRT). Early identification of those at risk of disease progression could be key in improving outcomes. We hypothesise that urinary liver-type fatty acid binding protein (uL-FABP) may be a suitable biomarker for CKD progression and can add value to currently established biomarkers such as the urinary protein-to-creatinine ratio (uPCR). A total of 583 participants with CKD 1–5 (not receiving renal replacement therapy) entered a 2 yr prospective longitudinal study. UPCR and uL-FABP were measured at baseline and CKD progression was defined as either (i) a decline in eGFR of >5 mL/min/1.73 m2 or an increase in serum creatinine by 10% at 1 yr; (ii) a decline in eGFR of >6 mL/min/1.73 m2 or an increase in serum creatinine by 20% at 2 yrs; or (iii) the initiation of RRT. A combined outcome of initiating RRT or death was also included. Approximately 40% of participants showed CKD progression. uL-FABP predicted CKD progression at both years 1 and 2 (OR 1.01, p < 0.01). Sensitivity and specificity were comparable to those of uPCR (AUC 0.623 v 0.706) and heat map analysis suggested that uL-FABP in the absence of significant proteinuria can predict an increase in serum creatinine of 10% at 1 yr and 20% at 2 yrs. The risk of the combined outcome of initiating RRT or death was 23% higher in those with high uL-FABP (p < 0.01) independent of uPCR. uL-FABP appears to be a highly sensitive and specific biomarker of CKD progression. The use of this biomarker could enhance the risk stratification of CKD and its progression and should be assessed further.
1. Introduction
Affecting approximately 10–13% of the population worldwide [1], the epidemic of chronic kidney disease (CKD) has soared through the ranking of causes of global mortality, rising from 27th place in 1999 to 18th in 2010 [2,3] to 12th in 2017 [4]. Established as a major public health concern [5], CKD is diagnosed, classified and monitored based on estimates of renal function and creatinine clearance. Using the Kidney Outcomes Quality Initiative (KDOQI) classification [6] CKD can be defined in stages (1–5) according to disease severity, based on estimates of the glomerular filtration rate (eGFR) derived using either the Modification of Diet in Renal Disease Study Group (MDRD) [7] methodology or more recently the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [8] equations and the presence or absence of proteinuria. The risk of disease progression increases at advanced CKD stages with 8.9% of CKD stage 4 patients and 39.1% of CKD stage 5 patients likely to show a decline in eGFR of 5 mL/min/1.73 m2/year or more [9]. However, only 0.5% of the general population progress to these advanced stages of CKD; additionally, the majority of patients with CKD have stage 3 disease (7–9% of the general population; 80–90% of all CKD) and will never progress to end-stage disease [10]. Nevertheless, 6% of CKD stage 3 patients show a decline in eGFR of 5 mL/min/1.73 m2/year [11]. Globally, 50–400 per million CKD patients will become dependent on renal replacement therapy per year [12], alongside an increased risk of cardiovascular disease [13,14]. In this context, early identification of patients at risk of CKD progression is imperative.
Although eGFR is a useful measure for stratifying disease severity, it provides little information about the potential risk of disease progression [15]. Proteinuria has been an important prognostic marker in glomerular pathologies [16], especially diabetic nephropathy [17], but is limited as a biomarker for CKD progression and response to treatment [18]. A number of alternative biomarkers or risk prediction equations based on creatinine and uPCR (Kidney Failure Risk Equation) have been proposed as predictors of disease progression [15]. Urinary liver-type fatty acid binding protein (uL-FABP) is one such biomarker with the potential for predicting CKD progression. Free fatty acids are found in abundance in renal tubules in proteinuric renal disease and they have been implicated in the pathogenesis of proximal tubular injury through their binding to albumin and activation of the inflammatory response [18]. This phenomenon is also present ischemia and under oxidative stress [19,20], in the absence of proteinuria. Free fatty acids are believed to bind to the 14 kDa fatty acid binding protein 1 (also known as L-FABP), found in the cytoplasm of renal tubular cells, before being transported to mitochondria or peroxisomes to be metabolized by oxidation [19,20,21]. uL-FABP is already established as a reliable biomarker of acute kidney injury [21,22] and, in a number of small studies, has been linked with CKD progression in diabetic [23,24,25] and non-diabetic [26] patients.
Our aim was to assess whether uL-FABP levels can predict a decline in renal function, and thus disease progression, in patients with CKD attending outpatient clinics in two large tertiary centres in the United Kingdom.
2. Materials and Methods
This was a 2 yr prospective follow-up study of participants with CKD stages 1–5 (not receiving renal replacement therapy) attending outpatient nephrology clinics in 2 large tertiary university renal centres in the northwest of the UK. The study received approval from the Yorkshire and Humber-Leeds West NHS Research Ethics Committee (15/YH/0516) and was adopted on the NIHR clinical research network portfolio (CRN ID: 20484). Adult patients, with capacity to consent, receiving their care in the participating centres were eligible to participate in the study and were approached during their attendance to clinic. Participation in the study was voluntary. Informed written consent was obtained prior to enrolment.
2.1. Definitions
A number of definitions of CKD progression were considered; however, based on the definitions used by NICE [2,3] and KDIGO [6], for purposes of this study at the time, CKD progression was defined as either:
(A) at 1 yr follow up, (i) a decline in eGFR by the equivalent of 5 mL/min/1.73 m2, (ii) increase in serum creatinine by 10% or (iii) initiation of renal replacement therapy for CKD 5;
(B) at 2 yr follow up, (i) a decline in eGFR by the equivalent of 6 mL/min/1.73 m2, (ii) increase in serum creatinine by 20% or (iii) initiation of renal replacement therapy.
The combined outcome of initiating renal replacement therapy or death was also evaluated.
2.2. Study Population Size
Katoh et al. [27] suggest that a cut-off of 19 μg/g.cr for urinary L-FABP carries 100% sensitivity and 81.8% specificity for the detection of contrast-induced AKI. Their mean study population uL-FABP level was 59.8 ± 45.6 μg/g.cr. Shingai et al. [28] used a detection cut-off 8.4 μg/g.cr based on the study of 420 healthy volunteers by Kamijo-Ikemori et al. [24]. Although published data from Kamijo et al. [26] suggest that a uL-FABP level of 17.4 μg/g.cr can be used as a cut-off for monitoring progression in CKD, the use of uL-FABP as a predictor of progression of CKD is unclear and remains under investigation. Although the literature failed to inform any power calculation, we believe that based on previous study population sizes, a population of 500 CKD participants would provide us with an adequate sample size to evaluate the potential of uL-FABP as a CKD biomarker.
2.3. Data Collection
The past medical history and medication lists were collected directly from participants and through their electronic medical records. These were used to calculate the Davies’ Comorbidity Score for all participants [29] as an indicator of coexisting disease burden. Prospective follow-up data on CKD were collected from patient electronic records at 1 yr and 2 yrs from enrolment.
2.4. Blood Sampling, eGFR Calculation and CKD Classification
Serum samples were collected as part of participants’ routine attendance to clinic and were analysed for urea and creatinine at the individual participating centres’ NHS biochemistry laboratories. Both laboratories comply with the NHS laboratory standardization process for clinical laboratories. The eGFR was calculated using the MDRD [7] formula. Patients were categorised in CKD stages according to KDOQI [30]. The difference in creatinine and eGFR over the 1st and 2nd years of the study was taken to be the difference in the values of these parameters at the data collection time points and at baseline.
2.5. Urine Sampling and Analysis
A 20 mL urine sample was provided as part of the participants’ routine clinical care. Then, 10 mL was analysed by the participating centres’ local NHS biochemistry laboratories to measure urinary protein, creatinine and the protein: creatinine ratio (uPCR). The remaining 10 mL sample was stored at −80° pending measurement of uL-FABP levels using an Enzyme-Linked Immunosorbent Assay (ELISA).
2.6. ELISA Analysis for the Measurement of uL-FABP Levels
Urine samples were processed in batches. Samples were thawed overnight and centrifuged at 2400 rpm at room temperature for 10 min to separate and remove impurities. uL-FABP levels were quantified using a commercially available ELISA kit (CMIC Co Ltd., Tokyo, Japan) as per the manufacturers’ instructions. All samples were assessed in duplicate within the same ELISA plate. The intra-assay coefficient of variation was 5.5%. Ten samples with detectable levels of uL-FABP were analysed separately to assess inter-plate assay variability. The inter-assay coefficient of variance was 7.8%. Calculations of uL-FABP levels were adjusted for urinary creatinine concentration obtained from the uPCR data that were measured within the same sample.
2.7. Statistical Analysis
The demographic and biochemical characteristics of participants were analysed using descriptive epidemiology. Categorical variables were presented as frequencies and percentages. Continuous variables with normal distribution were presented as means and standard deviation (SD) while variables with skewed distribution were presented as medians with minimum and maximum values. Normality of distribution was assessed using the Shapiro–Wilk method. The relationship between baseline urine uL-FABP levels, serum creatinine, eGFR and urine PCR was explored using stepwise linear regression modelling adjusted for age and sex. Skewed outcome variables underwent logarithmic transformation. Longitudinal analysis was performed from the change in eGFR, increase in serum creatinine, CKD progression and combined renal mortality. Participants that received kidney transplantation between baseline and 1 yr were removed from year one analysis unless they had initiated dialysis prior to transplantation. The same was true for participants receiving renal transplantation between years 1 and 2 for year 2 analysis. The association between uL-FABP and uPCR and the change in serum creatinine and eGFR was assessed using stepwise linear regression modelling adjusted for age and sex. The demographic characteristics and biochemical profiles of participants that exhibited CKD progression were compared to those without disease progression using group comparison. Categorical variables were compared using Pearson’s Chi2 with observation of the standardised residuals as post hoc analysis. Continuous variables were skewed in distribution, so differences between the assessed groups were analysed using the Kruskal–Wallis test. The predictive potential of uL-FABP was evaluated using logistic regression modelling adjusted for age, sex and proteinuria. The predictive sensitivity and specificity of uL-FABP were evaluated through ROC curve analysis and were compared to that of uPCR and uPCR in combination with uL-FABP. The interaction between uL-FABP and uPCR and changes in creatinine was also explored using heat map assessment. Subgroup analysis stratified by individuals’ CKD stage at baseline and the absence of proteinuria was performed using the same logistic regression model utilised for the entire study population. Combined outcome analysis was performed using Cox regression modelling using 8 mcg/gCr of uL-FABP as a cut-off for a significant level. Models were adjusted for age, sex and uPCR. Statistical analyses were performed by IBM SPSS Statistics, version 23 (IBM Corp., Armonk, NY, USA).
3. Results
A total of 641 patients were recruited into the study. Of those recruited, the data of 583 participants were analysed at baseline, while 484 were available in year one and 335 in year two. Participants’ exclusions and dropout reasons are shown in Figure 1 and Figure 2. The demographic characteristics and biochemical profiles of these participants are shown in Table 1 and Figure 3. No participants were noted to have liver disease.
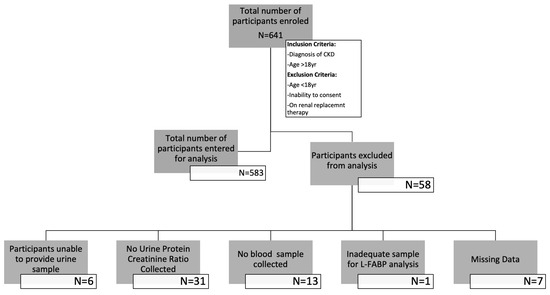
Figure 1.
Flow diagram illustrating the number of participants enrolled in the study and those whose data were entered for analysis.
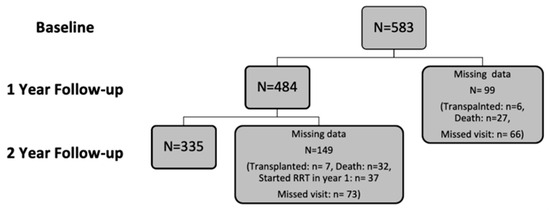
Figure 2.
Flow diagram illustrating the number of participants analysed per year of study and study dropout.

Table 1.
Demographic and biochemical characteristics at baseline of participants entered for baseline and longitudinal analysis.
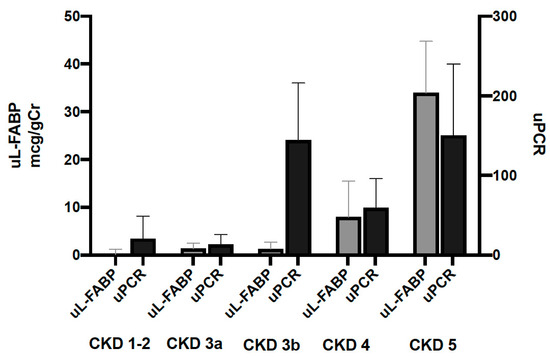
Figure 3.
Urinary levels of L-FABP and PCR at baseline stratified by CKD stage. Bars represent median biomarker levels and whiskers indicate the 95% confidence interval.
At baseline, uL-FABP levels correlated to those of uPCR (beta = 0.383, CI 0.305; 0.457, p ≤ 0.001, R2 = 0.142). uL-FABP also correlated to serum creatinine (beta = 0.379, CI 0.303; 0.455, p ≤ 0.001, R2 = 0.145) and eGFR (beta = −0.380, CI −0.454; −0.302, p ≤ 0.001, R2 = 0.143). This association was independent of proteinuria, age and sex (linear regression models shown in Table 2).

Table 2.
Unadjusted and adjusted linear regression models of uL-FABP and uPCR with serum creatinine and eGFR.
3.1. Year 1 and 2 Analysis
The median percentage increase in serum creatinine between baseline and year 1 was 3.5% (−6.9; 18.8) with a median decrease in MDRD eGFR of 1 mL/min (−2.0; 5.0).
Both uL-FABP (beta = 0.140, CI 0.045; 0.203, p = 0.002, R2 = 0.020) and uPCR (beta = 0.202, CI 0.102; 0.258, p < 0.001, R2 = 0.041) correlated with increases in serum creatinine. UPCR (beta = 0.101, CI 0.010; 0.159, p = 0.026, R2 = 0.010) predicted a decrease in eGFR over 1 year, but this relationship was not observed with uL-FABP (beta = −0.045, CI −0.112; 0.037, p = 0.324, R2 = 0.002).
By the end of year 1, 208 participants (43%) showed progression of CKD defined as a reduction in eGFR by 5 mL/min, an increase in serum creatinine by 10% or initiation of renal replacement therapy. Their characteristics are described in Table 3. Both uL-FABP and uPCR predicted CKD progression (logistic regression model adjusted for sex and age) (Table 4).

Table 3.
Participants’ demographic characteristics and biochemical profiles stratified by CKD progression by the end of year 1 of follow-up.

Table 4.
Logistic regression models for the prediction of CKD progression by uL-FABP and uPCR during the first year of follow-up.
By the end of year 2, 153 participants (42%) showed CKD progression defined as a reduction in eGFR by 6 mL/min, an increase in creatinine by 20% and start of renal replacement therapy. uL-FABP did not correlate with the increase in creatinine over 2 years (beta = 0.041, p = 0.464) or the decrease in eGFR (beta = −0.030, p = 0.602) over the same period. uPCR did correlate with both of these outcomes in a linear model adjusted for age, sex and uL-FABP (ΔCreatinine: beta = 0.310, p < 0.001, ΔeGFR; beta = 0.253, p < 0.001). Both L-FABP and uPCR predicted CKD progression (logistic regression model adjusted for sex and age). The results are shown in Supplementary Table S1.
3.2. Sensitivity and Specificity
The area under the curve for sensitivity and specificity of uL-FABP in predicting CKD progression was calculated using ROC curve analysis as 0.623 (CI 0.572; 0.675, p < 0.01) while that for uPCR was 0.706 (CI 0.658; 0.753, p < 0.01) (Figure 4a,b). Heat map analysis (Figure 5) also showed that uL-FABP in the absence of significant proteinuria can predict modest increases in serum creatinine (10% at 1 year and 20% at 2 years). The combined elevation of uL-FABP and uPCR had the highest predictive power for increases in creatinine at both 1 year (≤20% increase; Figure 5a) and 2 years (≤40% increase; Figure 5b).
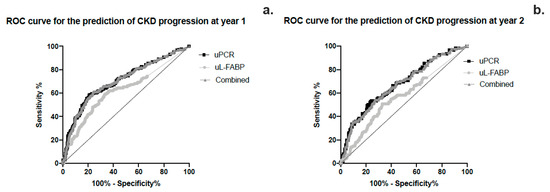
Figure 4.
(a,b) ROC curve analysis of the sensitivity and specificity of urinary L-FABP, PCR and the combined effect of the 2 biomarkers in predicting CKD progression at years 1 and 2.
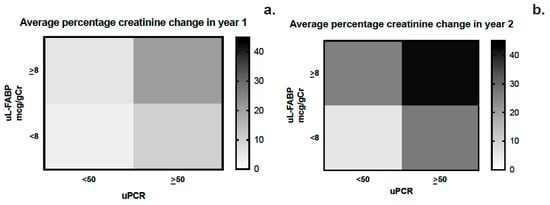
Figure 5.
(a,b) Heat map analysis of the average change in creatinine at years 1 and 2 stratified by urinary L-FABP and PCR levels.
3.3. Mortality and RRT Initiation Risk Analysis
Cox regression for the combined outcomes of death and initiation of RRT over the 2-year study period showed that the cumulative risk for patients with high uL-FABP levels (using uL-FABP of 8 as the differentiator) was significantly higher than those with low levels (p < 0.001) in a model adjusted for age, sex and uPCR (Figure 6).
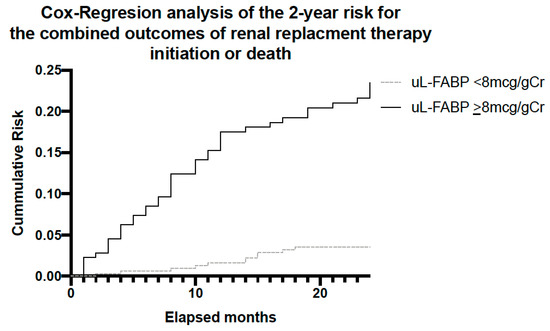
Figure 6.
Analysis for the combined outcome of RRT initiation or death over the 2-year follow-up period stratified by participants uL-FABP levels at baseline.
4. Discussion
We describe the findings of a prospective 2-year follow-up study exploring the potential of uL-FABP as a biomarker for disease progression in CKD. This is the largest study of a CKD population using uL-FABP. Our baseline analysis demonstrated an association between uL-FABP levels and disease severity, as demonstrated both by raised creatinine and reduced eGFR but also proteinuria. The presence of elevated levels of uL-FABP at baseline appears to predict CKD progression both at 1 and 2 years of follow-up. Although proteinuria appears to have a higher sensitivity and specificity than uL-FABP alone in predicting CKD progression, our ROC curve analysis would suggest that the best prediction for sensitivity and specificity is offered by the combination of the two biomarkers. As apparent from the heat map analysis, patients with elevated uL-FABP levels were more likely to experience a rise in serum creatinine both at 1 and 2 years of follow-up. In addition to disease progression, elevated uL-FABP carried a 24% higher risk of RRT and overall mortality independent of the presence of proteinuria.
Serum creatinine levels, and by extension creatinine-based estimations of GFR, might portray a reliable insight into CKD severity but their overall value in predicting disease progression is limited [15]. uPCR and its risk prediction equations (KFRE [31]) remain the gold standard in identifying individuals more likely to progress [18,32,33,34] but our findings suggest that the absence of proteinuria does not exclude progressive pathology. In these individuals, the presence of elevated uL-FABP might be a better predictor of disease progression.
Urinary PCR and L-FABP biomarkers are likely to represent different pathological processes involving the kidney. While uPCR tends to be predominantly glomerular in its origin in the early stages of CKD [16], uL-FABP is produced by the renal tubules in response to ischemia and oxidative stress. A number of studies have linked urinary levels of L-FABP with tubular injury both in the acute and chronic phases of kidney disease. In health, L-FABP is produced in the proximal renal tubule and binds fatty acids reabsorbed by the renal tubular cells enabling their transposition into the tubular mitochondria [20]. Under ischaemic conditions, this process is compromised by lipid peroxidation products binding to L-FABP instead, leading to increased secretion of L-FABP into the urine [20]. Whether this process is pathological or protective is unclear. All studies to date have shown that high levels of uL-FABP are generally linked with adverse outcomes. This has been predominantly in the setting of acute kidney injury [35,36,37,38,39], perioperative ischaemic [40,41], contrast-induced acute tubular injury [42] and peri-haematopoietic stem cell transplantation-associated acute kidney injury [28]. In the setting of CKD, studies have been few, with limited study populations [23,26,38,43]. Some studies have investigated specific CKD groups such as those with diabetes or cardiovascular disease [23,26,38]. They do all, however, conclude that uL-FABP is a useful biomarker in CKD [20] and its potential should be explored further. Our results are suggestive that in addition to an additive predictive value to that of uPCR, uL-FABP might play a role in risk stratification of patients, especially with negligible or absent proteinuria. Our attempts to characterise this group were limited by a smaller subgroup size (Supplementary Table S2).
Although the association between uL-FABP and ESKD/RRT has been noted before in a study of diabetic Pima Indians [38], this is the first time that such an association has been shown in a large population of unselected patients with CKD.
Another potential benefit when considering uL-FABP as a potential biomarker of CKD progression in the clinical setting is its potential availability as a semi-quantitative point-of-care test. The technology can be adapted for screening the CKD population in clinics.
The main limitation of our study was the lack of repeated measurements of uL-FABP during the follow-up period. The limited number of studies in the field made the design of this study and any population size estimation difficult. Efforts were made to minimise selection biases during the recruitment of participants. Participants were randomly approached for recruitment during their attendance at routine CKD clinical appointments. Laboratory analysis was also blinded to the personal information of participants.
In summary, uL-FABP appears to be a highly sensitive and specific biomarker of renal dysfunction and an effective predictor of CKD progression. A rapid assay of elevated uL-FABP in routine CKD care could improve rapid diagnosis and feasibility in clinical practice, especially in CKD patients with minimal or no proteinuria.
Further studies involving large registries are needed to evaluate the role of uL-FABP in the risk stratification of CKD patients and disease progression. Future research should also investigate interventions that can modify the expression of the biomarker and help improve the prognosis of chronic kidney disease.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm13101481/s1, Table S1: Logistic regression models for the prediction of CKD progression by uLFABP and uPCR over the 2 years of follow-up; Table S2: Subgroup analysis of demographic and biochemical characteristics of participants’ with no proteinuria (uPCR < 50) and high uL-FABP levels (>8 mcg/gCr) that showed progression at year 1 of follow-up.
Author Contributions
Conceptualization, N.M. and S.M.; methodology, N.M. and S.M.; validation, A.S., S.H. and N.M.; formal analysis, N.M.; investigation, N.M., A.S. and S.H.; resources, S.M. and P.A.K.; data curation, A.S. and N.M.; writing—original draft preparation, N.M. and V.M.; writing—review and editing, N.M., V.M., P.A.K. and S.M.; supervision, S.M.; project administration, N.M. and A.S.; funding acquisition, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by CMIC Holding. The funder had no involvement in the design of the study, data analysis or authorship of this manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and received approval from the Yorkshire and Humber-Leeds West NHS Research Ethics Committee (15/YH/0516).
Informed Consent Statement
Written informed consent was obtained from all participant in the study, prior to their participation.
Data Availability Statement
Data cannot be made publicly available until the completion of all work relating to this study.
Acknowledgments
We would like to thank the clinical and technical staff at the participating units, the Manchester Renal Research and Transplant Laboratory and Caroline Monroe (CMIC Holding project adviser) for their help and support. Sandip Mitra is supported by the National Institute for Health Research (NIHR) Devices for Dignity Healthcare Technology Co-operative. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflicts of Interest
The authors declare no conflict of interest.
References
- James, M.T.; Hemmelgarn, B.R.; Tonelli, M. Early recognition and prevention of chronic kidney disease. Lancet 2010, 375, 1296–1309. [Google Scholar] [CrossRef]
- NICE. Chronic Kidney Disease in Adults: Assessment and Management|Guidance and Guidelines|NICE. Chronic Kidney Dis. Adults Assess. Manag. 2014, 1–63. Available online: https://www.nice.org.uk/guidance/cg182/chapter/1-Recommendations#referral-criteria (accessed on 24 July 2017).
- Recommendations|Chronic Kidney Disease: Assessment and Management|Guidance|NICE. Available online: https://www.nice.org.uk/guidance/ng203/chapter/Recommendations#managing-anaemia (accessed on 6 October 2023).
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- De Nicola, L.; Zoccali, C. Chronic kidney disease prevalence in the general population: Heterogeneity and concerns. Nephrol. Dial. Transplant. 2016, 31, 331–335. [Google Scholar] [CrossRef]
- Inker, L.A.; Astor, B.C.; Fox, C.H.; Isakova, T.; Lash, J.P.; Peralta, C.A.; Kurella Tamura, M.; Feldman, H.I. KDOQI US Commentary on the 2012 KDIGO Clinical Practice Guideline for the Evaluation and Management of CKD. Am. J. Kidney Dis. 2014, 63, 713–735. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A More Accurate Method to Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Zhang, A.-H.; Tam, P.; LeBlanc, D.; Zhong, H.; Chan, C.T.; Bargman, J.M.; Oreopoulos, D.G. Natural history of CKD stage 4 and 5 patients following referral to renal management clinic. Int. Urol. Nephrol. 2009, 41, 977–982. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Eriksen, B.; Ingebretsen, O. The progression of chronic kidney disease: A 10-year population-based study of the effects of gender and age. Kidney Int. 2006, 69, 375–382. [Google Scholar] [CrossRef]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Garg, A.X.; Clark, W.F.; Haynes, R.B.; House, A.A. Moderate renal insufficiency and the risk of cardiovascular mortality: Results from the NHANES I. Kidney Int. 2002, 61, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.E.; Tighiouart, H.; Amin, M.G.; Stark, P.C.; MacLeod, B.; Griffith, J.L.; Salem, D.N.; Levey, A.S.; Sarnak, M.J. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J. Am. Soc. Nephrol. 2004, 15, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Venuthurupalli, S.K.; Gobe, G.C.; Coombes, J.S.; Cooper, M.A.; Hoy, W.E. Biomarkers in chronic kidney disease: A review. Kidney Int. 2011, 80, 806–821. [Google Scholar] [CrossRef]
- Cameron, S. Proteinuria and Progression in Human Glomerular Diseases. Am. J. Nephrol. 1990, 10, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Keane, W.F.; Brenner, B.M.; de Zeeuw, D.; Grunfeld, J.-P.; McGill, J.; Mitch, W.E.; Ribeiro, A.B.; Shahinfar, S.; Simpson, R.L.; Snapinn, S.M.; et al. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: The RENAAL Study. Kidney Int. 2003, 63, 1499–1507. [Google Scholar] [CrossRef]
- Levey, A.S.; Cattran, D.; Friedman, A.; Miller, W.G.; Sedor, J.; Tuttle, K.; Kasiske, B.; Hostetter, T. Proteinuria as a Surrogate Outcome in CKD: Report of a Scientific Workshop Sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am. J. Kidney Dis. 2009, 54, 205–226. [Google Scholar] [CrossRef]
- Ichikawa, D.; Kamijo-Ikemori, A.; Sugaya, T.; Shibagaki, Y.; Yasuda, T.; Hoshino, S.; Katayama, K.; Igarashi-Migitaka, J.; Hirata, K.; Kimura, K. Human liver-type fatty acid–binding protein protects against tubulointerstitial injury in aldosterone-induced renal injury. Am. J. Physiol. Physiol. 2015, 308, F114–F121. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xie, Y.; Shao, X.; Ni, Z.; Mou, S. L-FABP: A novel biomarker of kidney disease. Clin. Chim. Acta 2015, 445, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Susantitaphong, P.; Siribamrungwong, M.; Doi, K.; Noiri, E.; Terrin, N.; Jaber, B.L. Performance of Urinary Liver-Type Fatty Acid–Binding Protein in Acute Kidney Injury: A Meta-analysis. Am. J. Kidney Dis. 2013, 61, 430–439. [Google Scholar] [CrossRef]
- Suzuki, G.; Ichibayashi, R.; Yamamoto, S.; Nakamichi, Y.; Watanabe, M.; Honda, M. Clinical significance of urinary L-FABP in the emergency department. Int. J. Emerg. Med. 2019, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Kamijo-Ikemori, A.; Sugaya, T.; Ichikawa, D.; Hoshino, S.; Matsui, K.; Yokoyama, T.; Yasuda, T.; Hirata, K.; Kimura, K. Urinary liver type fatty acid binding protein in diabetic nephropathy. Clin. Chim. Acta 2013, 424, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Kamijo-Ikemori, A.; Sugaya, T.; Yasuda, T.; Kawata, T.; Ota, A.; Tatsunami, S.; Kaise, R.; Ishimitsu, T.; Tanaka, Y.; Kimura, K. Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients. Diabetes Care 2011, 34, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Ngan, D.T.T.; Binh, N.G.; Lan, L.T.H.; Nguyen, C.T.T.; Huong, P.T. Evaluation of urinary L-FABP as an early marker for diabetic nephropathy in type 2 diabetic patients. J. Med. Biochem. 2019, 39, 224–230. [Google Scholar] [CrossRef]
- Kamijo, A.; Sugaya, T.; Hikawa, A.; Yamanouchi, M.; Hirata, Y.; Ishimitsu, T.; Numabe, A.; Takagi, M.; Hayakawa, H.; Tabei, F.; et al. Urinary liver-type fatty acid binding protein as a useful biomarker in chronic kidney disease. Mol. Cell. Biochem. 2006, 284, 175–182. [Google Scholar] [CrossRef]
- Katoh, H.; Nozue, T.; Kimura, Y.; Nakata, S.; Iwaki, T.; Kawano, M.; Kawashiri, M.-A.; Michishita, I.; Yamagishi, M. Elevation of urinary liver-type fatty acid-binding protein as predicting factor for occurrence of contrast-induced acute kidney injury and its reduction by hemodiafiltration with blood suction from right atrium. Heart Vessel. 2013, 29, 191–197. [Google Scholar] [CrossRef]
- Shingai, N.; Morito, T.; Najima, Y.; Igarashi, A.; Kobayashi, T.; Doki, N.; Kakihana, K.; Ohashi, K.; Ando, M. Urinary Liver-Type Fatty Acid-Binding Protein Linked with Increased Risk of Acute Kidney Injury after Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2014, 20, 2010–2014. [Google Scholar] [CrossRef]
- Davies, S.J.; Russell, L.; Bryan, J.; Phillips, L.; Russell, G.I. Comorbidity, urea kinetics, and appetite in continuous ambulatory peritoneal dialysis patients: Their interrelationship and prediction of survival. Am. J. Kidney Dis. 1995, 26, 353–361. [Google Scholar] [CrossRef]
- National Kidney Foundation. K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Clasification and Stratification. Am. J. Kidney Dis. 2002, 39 (Suppl. S1), S1–S266. [Google Scholar]
- Tangri, N.; Stevens, L.A.; Griffith, J.; Tighiouart, H.; Djurdjev, O.; Naimark, D.; Levin, A.; Levey, A.S. A Predictive Model for Progression of Chronic Kidney Disease to Kidney Failure. JAMA 2011, 305, 1553–1559. [Google Scholar] [CrossRef]
- Viswanathan, G.; Upadhyay, A. Assessment of Proteinuria. Adv. Chronic Kidney Dis. 2011, 18, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Cravedi, P.; Remuzzi, G. Pathophysiology of proteinuria and its value as an outcome measure in CKD. Br. J. Clin. Pharmacol. 2013, 76, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Sarnak, M.J.; Astor, B.C. Implications of Proteinuria: CKD Progression and Cardiovascular Outcomes. Adv. Chronic Kidney Dis. 2011, 18, 258–266. [Google Scholar] [CrossRef]
- Pajek, J.; Škoberne, A.; Šosterič, K.; Adlešič, B.; Leskošek, B.; Pajek, M.B.; Osredkar, J.; Lindič, J. Non-inferiority of creatinine excretion rate to urinary L-FABP and NGAL as predictors of early renal allograft function. BMC Nephrol. 2014, 15, 117. [Google Scholar] [CrossRef]
- Zeng, X.-F.; Li, J.-M.; Tan, Y.; Wang, Z.-F.; He, Y.; Chang, J.; Zhang, H.; Zhao, H.; Bai, X.; Xie, F.; et al. Performance of urinary NGAL and L-FABP in predicting acute kidney injury and subsequent renal recovery: A cohort study based on major surgeries. Clin. Chem. Lab. Med. 2014, 52, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Holzscheiter, L.; Beck, C.; Rutz, S.; Manuilova, E.; Domke, I.; Guder, W.G.; Hofmann, W. NGAL, L-FABP, and KIM-1 in comparison to established markers of renal dysfunction. Clin. Chem. Lab. Med. 2014, 52, 537–546. [Google Scholar] [CrossRef]
- Fufaa, G.D.; Weil, E.J.; Nelson, R.G.; Hanson, R.L.; Bonventre, J.V.; Sabbisetti, V.; Waikar, S.S.; Mifflin, T.E.; Zhang, X.; Xie, D.; et al. Association of urinary KIM-1, L-FABP, NAG and NGAL with incident end-stage renal disease and mortality in American Indians with type 2 diabetes mellitus. Diabetologia 2015, 58, 188–198. [Google Scholar] [CrossRef]
- Parr, S.K.; Clark, A.J.; Bian, A.; Shintani, A.K.; Wickersham, N.E.; Ware, L.B.; Ikizler, T.A.; Siew, E.D. Urinary L-FABP predicts poor outcomes in critically ill patients with early acute kidney injury. Kidney Int. 2015, 87, 640–648. [Google Scholar] [CrossRef]
- Parikh, C.R.; Thiessen-Philbrook, H.; Garg, A.X.; Kadiyala, D.; Shlipak, M.G.; Koyner, J.L.; Edelstein, C.L.; Devarajan, P.; Patel, U.D.; Zappitelli, M.; et al. Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin. J. Am. Soc. Nephrol. 2013, 8, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Kokot, M.; Biolik, G.; Ziaja, D.; Fojt, T.; Kedzierski, L.; Antoniak, K.; Janowska, M.; Pawlicki, K.; Ziaja, K.; Dulawa, J. Assessment of subclinical acute kidney injury after abdominal aortic aneurysm surgery using novel markers: L-FABP and H-FABP. Nefrologia 2014, 34, 628–636. [Google Scholar]
- Hwang, Y.J.; Hyun, M.C.; Choi, B.S.; Chun, S.Y.; Cho, M.H. Acute kidney injury after using contrast during cardiac catheterization in children with heart disease. J. Korean Med. Sci. 2014, 29, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, A.; Kimura, K.; Sugaya, T.; Yamanouchi, M.; Hikawa, A.; Hirano, N.; Hirata, Y.; Goto, A.; Omata, M. Urinary fatty acid–binding protein as a new clinical marker of the progression of chronic renal disease. J. Lab. Clin. Med. 2004, 143, 23–30. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).