Abstract
Coenzyme Q10 (CoQ10) has an important role as an antioxidant. Being that oxidative stress is one of the mechanisms involved in the pathogenesis of Parkinson’s disease (PD) and other neurodegenerative diseases, several studies addressed the concentrations of CoQ10 in the different tissues of patients with PD and other parkinsonian syndromes (PS), trying to elucidate their value as a marker of these diseases. Other studies addressed the potential therapeutic role of CoQ10 in PD and PS. We underwent a systematic review and a meta-analysis of studies measuring tissue CoQ10 concentrations which shows that, compared with controls, PD patients have decreased CoQ10 levels in the cerebellar cortex, platelets, and lymphocytes, increased total and oxidized CoQ10 levels in the cerebrospinal fluid and a non-significant trend toward decreased serum/plasma CoQ10 levels. Patients with multiple system atrophy (MSA) showed decreased CoQ10 levels in the cerebellar cortex, serum/plasma, cerebrospinal fluid, and skin fibroblasts. Patients with Lewy body dementia (LBD) showed decreased cerebellar cortex CoQ10, and those with progressive supranuclear palsy (PSP) had decreased CoQ10 levels in the cerebrospinal fluid. A previous meta-analysis of studies addressing the therapeutic effects of CoQ10 in PD showed a lack of improvement in patients with early PD. Results of the treatment with CoQ10 in PSP should be considered preliminary. The potential role of CoQ10 therapy in the MSA and selected groups of PD patients deserves future studies.
1. Introduction
Coenzyme Q10 (CoQ10, Figure 1), which is also known as ubiquinone, is a 1,4-benzoquinone that is present in the majority of tissues in the human body. It is an important component of the electron transport chain in the mitochondria, participating in the generation of cellular energy through oxidative phosphorylation. In tissues, CoQ10 can be present in three redox states: fully oxidized (ubiquinone), partially oxidized (semiquinone or ubisemiquinone), and fully reduced (ubiquinol). Together with mitochondria, CoQ10 is present in the endoplasmic reticulum, Golgi apparatus, lysosomes, and peroxisomes. CoQ10 has important antioxidant actions (both by scavenging free radicals and by the regeneration of other antioxidants, such as alpha-tocopherol or ascorbate acid), giving protection to cells against oxidative stress processes [1,2].
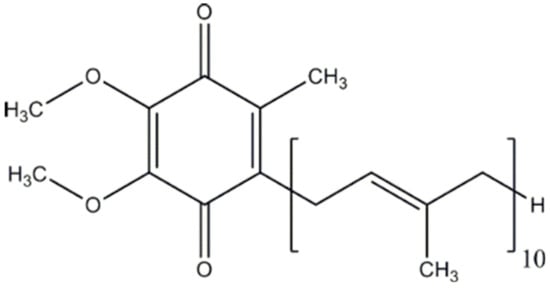
Figure 1.
Chemical structure of coenzyme Q10.
Because oxidative stress is one of the most important pathogenetic mechanisms of Parkinson’s disease (PD) and other neurodegenerative disorders [3,4], and because of the role of CoQ10 as an antioxidant, both the study of CoQ10 concentrations in different tissues of patients diagnosed with PD and/or other parkinsonian syndromes and the potential therapeutic role of CoQ10 in these diseases, have been the matter of several publications over the last two decades. The aim of this systematic review and meta-analysis is to analyze the results of studies addressing the tissular concentrations of CoQ10 in patients diagnosed with parkinsonian syndromes compared to healthy controls and the results of therapeutic trials of CoQ10 in PD and other causes of parkinsonism.
2. Methods
2.1. Search Strategy and Criteria for Eligibility of Studies
A literature search using several well-known databases (PubMed, EMBASE, Web of Science (WOS) Main Collection) from 1966 until 4 May 2022, was performed. The term “coenzyme Q10” was crossed with “Parkinson’s disease” (356, 924, and 303 items were found in PubMed, EMBASE, and WOS, respectively), “parkinsonism” (403, 183, and 39 items were found in PubMed, EMBASE, and WOS, respectively), parkinsonian syndromes (223, 7, and 6 items were found in PubMed, EMBASE, and WOS, respectively), “multiple system atrophy” (36, 125, and 56 items were found in PubMed, EMBASE, and WOS, respectively), “Lewy body dementia” (8, 39, and 10 items were found in PubMed, EMBASE, and WOS, respectively), “Lewy body disease” (8, 62, and 24 items were found in PubMed, EMBASE, and WOS, respectively), “progressive supranuclear palsy” (31, 66, and 9 items were found in PubMed, EMBASE, and WOS, respectively), and “corticobasal degeneration” (4, 28, and 5 items were found in PubMed, EMBASE, and WOS, respectively). A total of 1054 references were retrieved by the whole search and examined one by one, and then those that were strictly related to the proposed topics, without language restrictions, were selected, excluding the duplicated articles and abstracts. The flowcharts for the selection of eligible studies—following the PRISMA guidelines [5]—analyzing tissue CoQ10 concentrations in patients with several types of parkinsonian syndrome and controls, and therapeutic trials with CoQ10 in parkinsonian syndromes, are plotted in Figure 2.
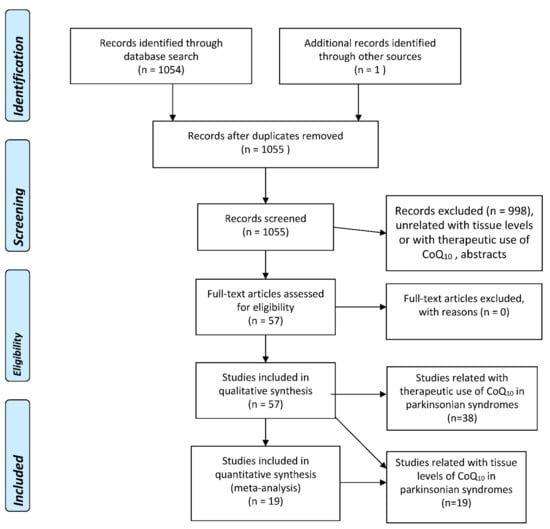
Figure 2.
PRISMA Flowchart for the studies assessing tissue concentrations of coenzyme Q10 in parkinsonian syndromes, and for therapeutic trials with CoQ10 in parkinsonian syndromes.
2.2. Selection of Studies and Methodology for the Meta-Analyses
Meta-analyses of those observational eligible studies that assessed the concentrations of CoQ10 in tissues were performed. The first author, year of publication, country, study design, and quantitative measures were extracted, and the risk of bias was analyzed by using the Newcastle–Ottawa Scale [6]. Data from selected studies analyzing the tissular concentrations of CoQ10 in patients diagnosed with PD compared to controls, patients diagnosed with multiple system atrophy (MSA) compared to controls, and patients with Lewy body dementia (LBD), progressive supranuclear palsy (PSP), and cortical basal degeneration (CBD) compared to healthy controls are summarized, respectively, in Table 1, Table 2 and Table 3. The plasma/serum and CSF levels of coenzyme Q10 were converted to nmol/mL, and brain tissue levels to pmol/mL, when necessary. The meta-analyses followed the PRISMA [5] (Table S1) and MOOSE guidelines [7] (Table S2) and were carried out by using the R software package meta [8]. We applied the random-effects model because of the high heterogeneity across studies, and we used the inverse variance method for the meta-analytical procedure, the DerSimonian-Laird as an estimator for Tau2, the Jackson method for the confidence interval of Tau2 and Tau, and the Hedges’ g (bias-corrected standardized mean difference). We calculated the statistical power to detect differences in mean values (alpha = 0.05) for the pooled samples when stated in the text.

Table 1.
Coenzyme Q10 Concentrations in Several Tissues from Parkinson’s Disease (PD) Patients and Healthy Controls (HC).

Table 2.
Coenzyme Q10 Concentrations in Several Tissues from Patients with Multisystem Atrophy (MSA) and Healthy Controls (HC).

Table 3.
Coenzyme Q10 Concentrations in Several Tissues from Patients with Lewy Body Dementia (LBD), Progressive Supranuclear Palsy, and Cortical Basal Degeneration Compared with Healthy Controls (HC).
3. Results
3.1. Studies Assessing Tissular CoQ10 Concentrations
3.1.1. Parkinson’s Disease
Serum/Plasma
Matsubara et al. [28] reported decreased serum CoQ10 levels in PD patients. However, the comparison group was composed of patients with cerebral infarction instead of healthy controls. The pooled results of the seven studies assessing the serum or plasma total CoQ10 levels in PD patients compared with controls [9,10,11,12,13,14,15], did not show significant differences in this value between the two groups (Table 1, Figure 3a), as was the case with the two studies assessing the serum or plasma CoQ10 corrected to cholesterol levels (Table 1, Figure 3b) [9,14]. However, the serum/plasma oxidized CoQ10/total CoQ10 ratio was found to be significantly higher in PD patients compared with controls in two of these studies (Table 1, Figure 3c) [11,13]. Two studies showed a lack of differences in the serum/plasma oxidized CoQ10 and in the reduced CoQ10 concentrations between PD patients and controls [11,14], although there were substantial differences in these values between these studies.
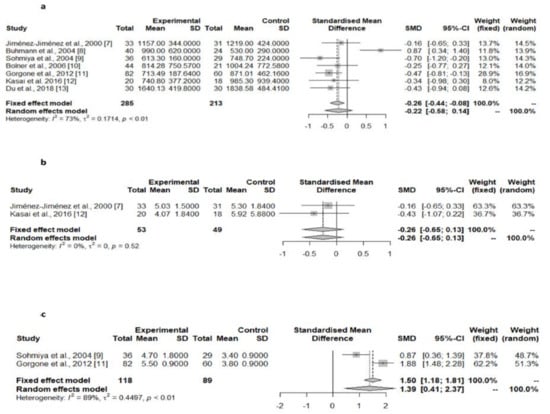
Figure 3.
Meta-analyses of studies assessing serum/plasma total CoQ10 levels [7,8,9,10,11,12,13], serum/plasma CoQ10 corrected to cholesterol levels (b) [7,12], and serum/plasma oxidized CoQ10/total CoQ10 ratio (c) in PD patients compared with controls [9,11].
Blood Cells
Two studies showed decreased CoQ10 concentrations in platelets [16] and lymphocytes [17], respectively, from patients with PD compared with healthy controls (Table 1).
Cerebrospinal Fluid (CSF)
According to two studies, PD patients showed increased total [18,19] and oxidized [18] CSF CoQ10 concentrations (Table 1).
Brain
The pooled data from three studies [20,21,22] showed decreased CoQ10 levels in the cerebellar cortex of PD patients in comparison with controls (Table 1, Figure 4), while concentrations in the cerebral cortex [20,21], striatum [20], and substantia nigra [20] did not differ significantly between the two groups (Table 1).
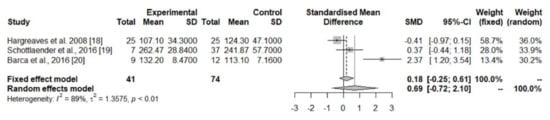
Figure 4.
Meta-analyses of studies assessing CoQ10 concentrations in the cerebellar cortex of PD patients and controls [18,19,20].
Skin Fibroblasts
Del Hoyo et al. [23] reported similar CoQ10 concentrations in skin fibroblasts from PD patients and controls (Table 1).
The pooled data from the three studies, addressing serum/plasma total CoQ10 concentrations [14,15,24], showed a decrease in this value in patients with MSA compared with controls (Table 2, Figure 5). There have been reported decreased CoQ10 concentrations in the CSF [19], cerebellum cortex [21,22], and skin fibroblasts [25] from MSA patients (Table 2), although the pooled data from studies assessing cerebellum cortex CoQ10 levels did not reach statistical significance. In contrast, the cerebral cortex [21,22] and striatum [21] CoQ10 levels were similar in MSA and controls (Table 2).

Figure 5.
Meta-analyses of studies assessing serum/plasma total CoQ10 levels, serum/plasma total CoQ10 concentrations in MSA patients and controls [12,13,22].
3.1.2. Other Parkinsonian Syndromes
The results of studies addressing CoQ10 concentrations in patients with other parkinsonian syndromes, compared with healthy controls, are summarized in Table 3. In summary, patients with Lewy body dementia (DLB) showed similar CoQ10 concentrations to those of the control in serum/plasma [26,27], and in the cerebral cortex [21], but lower CoQ10 concentrations in the cerebellum cortex [21]. Patients diagnosed with progressive supranuclear palsy (PSP) showed decreased CSF CoQ10 levels [19], and patients with cortical basal degeneration showed normal cerebral cortex CoQ10 levels [21].
3.2. Studies Assessing Therapeutic Response to CoQ10 Administration
3.2.1. Parkinson’s Disease
The results of the 10 eligible studies addressing the therapeutic response of CoQ10 administration in patients with PD [29,30,31,32,33,34,35,36,37,38] are summarized in Table 4. One of these studies used an open-label design [29], while the others were randomized clinical placebo-controlled trials [30]. CoQ10 was generally well-tolerated, according to the four studies assessing adverse effects [32,33,34,35,36]. Despite five of these studies showing a mild improvement in motor scales in PD patients [30,31,35,36,38], three meta-analyses [39,40,41], one of them including eight randomized clinical trials [41], concluded that CoQ10 was not superior to the placebo in improving motor symptoms.

Table 4.
Studies describing the effects of levodopa and dopamine agonists in patients with RBD.
The study by Yoritaka et al. [38] showed a significant improvement in motor symptoms of PD patients suffering from the “wearing-off” phenomenon, and Li et al. [37] described a positive effect of concomitant CoQ10 and creatine therapy on cognitive impairment, assessed by the Montreal Cognitive Assessment (MoCA). However, these results are based on a small size series.
Mitsui et al. [42] reported the effects of the treatment with CoQ10 1200 mg/day in a patient diagnosed with familial MSA, in an advanced stage, related to the compound heterozygous nonsense (R387X) and missense (V393A) mutations in the COQ2 gene. The administration of CoQ10 resulted in increased serum and CSF total CoQ10 concentrations, the increased cerebral metabolic ratio of the oxygen measured by 15O2 positron emission tomography (PET), and led to stability in several clinical scores (Barthel Index, Scale for the Assessment and Rating of Ataxia—SARA, International Cooperative Ataxia Rating Scale—ICARS, and the Unified Multiple System Atrophy Rating Scale—UMSARS) during 3 years of follow-up.
3.2.2. Progressive Supranuclear Palsy
Two randomized clinical trials studied the effects of CoQ10 in patients diagnosed with PSP. Stamelou et al. [43], in a 6-week, monocenter, double-blind, randomized, placebo-controlled, phase II trial, including 21 clinically probable PSP patients assigned to a liquid nanodispersion of CoQ10 (doses of 5 mg/kg/day) or placebo, showed a mild improvement in a Frontal Assessment Battery and in the total scores of the PSP rating scale (PSPRS) in those assigned to CoQ10, while there were no significant changes in the UPDRS and the Mini-Mental State Examination (MMSE). They did not describe the relevant adverse effects. As should be expected, plasma levels of CoQ10 increased in the treated, but not untreated patients. In patients receiving CoQ10 compared to those receiving the placebo, the ratio of high-energy phosphates to low-energy phosphates (adenosine-triphosphate to adenosine-diphosphate, and phosphocreatine to unphosphorylated creatine) increased significantly in the occipital lobe and showed a consistent trend towards an increase in the basal ganglia. For this reason, the authors suggested a possible disease-modifying neuroprotective of CoQ10.
In contrast, Apetauerova et al. [44], in a one-year, investigator-initiated, multicenter, randomized, placebo-controlled, double-blind clinical trial involving 61 patients diagnosed with PSP assigned to CoQ10 (2400 mg/day) or a placebo, found no significant differences between the two study groups in PSPRS (although there was a non-significant trend toward a slower decline in the CoQ10 group), UPDRS, activities of daily living (ADL), MMSE, the 39-item Parkinson’s Disease Questionnaire (PDQ-39), and the 36-item Short-Form Health Survey (SF-36). Despite CoQ10 being well-tolerated, 41% of participants withdrew from the study for different reasons.
4. Discussion and Conclusions
The possible role of CoQ10 in the pathogenesis, or its value as a diagnostic marker of PD and other parkinsonian syndromes, has not been definitively established. In the case of PD, the pooled analyses of studies measuring CoQ10 concentrations in the brain tissues [20,21,22], showed a significant decrease in the cerebellum cortex of PD patients (Table 1, Figure 4), which was likely related to the concentrations found in the larger control group of one of these studies [21], while CoQ10 concentrations in the striatum, substantia nigra, and cerebral cortex were similar in PD patients and controls. Studies in platelets [16] and lymphocytes [17] showed a consistent decrease in CoQ10 concentrations, while in the CSF, both the total [18,19] and oxidized CoQ10 levels [18] were found to be increased in PD patients. The pooled data of the seven studies assessing serum/plasma CoQ10 levels [9,10,11,12,13,14,15] showed a non-significant trend toward lower concentrations in PD patients compared with controls (Table 1, Figure 3a), while the percentage of oxidized vs. total CoQ10 was increased in PD (Table 1). One study showed a surprisingly very high percentage of oxidized CoQ10, which was related to the easy oxidation of the reduced to oxidized CoQ10 from the moment of sample extraction because precautions were not taken to prevent this oxidation [14]. Finally, CoQ10 levels in the skin fibroblasts were similar in PD patients and controls [23].
In MSA, CoQ10 concentrations were found to be decreased in the cerebellar cortex in two studies (19, 20)—although the results of the pooled data did not reach statistical significance (Table 2)—in the serum/plasma [14,15,24], CSF [19], and skin fibroblasts [25]. Patients with LBD showed decreased cerebellar CoQ10 [21] and patients with PSP showed decreased CoQ10 concentrations [19] in single studies.
Due to their antioxidant actions, it was proposed that CoQ10 administration could be a potential protective therapy in PD and other neurodegenerative diseases [45,46]. Moreover, the administration of CoQ10 has shown neuroprotective effects in several models of experimental parkinsonism:
- (a)
- CoQ10 or idebenone (an analog of CoQ10) attenuates the loss of striatal dopamine and dopaminergic axons, induced by 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine (MPTP) administration in rodents [47,48,49,50] and in monkeys [51].
- (b)
- In rats, both the coadministration of CoQ10 and creatine [52] or CoQ10 and nicotinamide [53] have shown additive neuroprotective effects against striatal dopamine depletion after MPTP administration.
- (c)
- In rats injected with 6-hydroxydopamine (6-OHDA), the coadministration of CoQ10 and a mir-149sp mimic [54], or of CoQ10 and bone marrow stromal cells (BMSC) [55], improves motor symptoms and prevents dopaminergic damage.
- (d)
- CoQ10 administration was also able to prevent iron-induced apoptosis in cultured human dopaminergic (SK-N-SH) neurons, in metallothionein gene-manipulated mice, and in alpha-synuclein knockout (alpha-synko) mice [56].
- (e)
- CoQ10 administration can prevent neurodegeneration and behavioral deterioration in rodents exposed to several toxins causing experimental parkinsonism, such as the pesticides paraquat [57,58], dichlorvos [59], and rotenone [60,61], and showed neuroprotective effects against rotenone in primary rat mesencephalic cultures [62] and human neuroblastoma cells [63]. Interestingly, the exposure of human neuroblastoma SH-SY5Y cells to commonly used organophosphate compounds, such as dichlorvos, methyl-parathion (parathion), and chlorpyrifos (CPF), induces an important decrease in CoQ10 levels and complex II + III activity—both related to a decrease in neuronal cell viability. In this model, CoQ10 supplementation can modestly although significantly increase complex II + III activity [64].
- (f)
- CoQ10 supplementation (with or without the concomitant treatment of levodopa) has shown a protective effect against chlorpromazine-induced parkinsonism in mice, including a reduction in mortality and catalepsy, an increase in dopamine levels, and a decrease in oxidative stress [65]. Similarly, CoQ10 improved the forced swimming test, locomotor activity test, catalepsy, muscle coordination, and akinesia test, and reduced the dopamine depletion in haloperidol-induced parkinsonism in rats [66].
However, CoQ10 had not shown neuroprotective effects in a Drosophila DJ-1 model of PD [67]. Moreover, idebenone can induce apoptotic death cells in human neuroblastoma cells [68]. On the other hand, MPTP and its metabolite 1-methyl-4-phenyl-2,3-dihydropyridinium (MPDP+) are also able to induce a reduction in CoQ10, and a reduction in CoQ10 promotes the conversion of MPDP+ to the active neurotoxin 1-methyl-4-phenylpiridinium (MPP+) and increases its neurotoxicity [69].
Several studies analyzed the effects of CoQ10 administration on serum/plasma and CSF CoQ10 levels. Lönnrot et al. [70] described a significant increase in the plasma CoQ10 concentrations, but a lack of changes in the CSF CoQ10 concentrations in five healthy individuals after oral supplementation with ascorbic acid and CoQ10. Shults et al. [71], described an increase in plasma CoQ10 concentrations in 17 subjects after the administration of an escalating dosage of coenzyme Q10 (1200, 1800, 2400, and 3000 mg/day) with a stable dosage of vitamin E (alpha-tocopherol) 1200 IU/day, reaching the maximum plasma concentration with 2400 mg/day. Nukui et al. [72] reported, both in a double-blind, placebo-controlled study involving 46 healthy volunteers humans and in an acute, single-dose administration study in rats, that the administration of a water-soluble type of CoQ10 reached considerably higher serum CoQ10 concentrations than conventional CoQ10.
Despite the possible beneficial effects of CoQ10 administration, its good absorption, the lack of important adverse effects, and the improvement in PD symptoms suggested by several studies [30,31,35,36,38], data from meta-analyses of randomized clinical trials did not suggest the general usefulness of this therapy in patients with PD [39,40,41]. Several biochemical studies suggest the presence of CoQ10 deficiency in MSA, but the possible role of this compound in the treatment of MSA has not been explored yet. Although short-term use of CoQ10 treatment in PSP showed promising effects [43], the results of a randomized clinical trial involving a small series of patients showed no beneficial effects [44].
Despite all these data, the improvement in motor symptoms reported in a small series of patients with PD and the “wearing-off” phenomenon under CoQ10 therapy [38], and the improvement in cognitive impairment in patients treated with the combination of CoQ10 and creatine [37] suggest that CoQ10 could be useful in selected patients, and the role of personalized medicine could be important. In this regard, Seet et al. [73], in a preliminary study involving 16 PD patients treated with different doses of CoQ10, described that patients who experienced a significant short-term reduction in the UPDRS score had lower baseline plasma ubiquinol and decreased F2-isoprostanes (CoQ10 and F2-isoprostanes increased significantly at a 2400 mg/day dosage of CoQ10), suggesting that the therapeutic response should depend on the baseline levels of these two compounds.
Moreover, a recent double-blind randomized, phase II, placebo-controlled study using an omics-based strategy with CoQ10 has been recently proposed [74]. In this study, the assignation to a treatment group should be done after the stratification by the so-called “mitochondrial risk burden” in homozygous or compound heterozygous Parkin/PINK1 mutation carriers (P++), heterozygous Parkin/PINK1 mutation carriers (P+), and “omics” positive (omics+) and “omics” negative PD patients (omics-), those being omics+ with the highest and those who are omics- with the lowest cumulative burden of common genetic variants in genes that are related to mitochondrial function. Changes in the motor subscore of UPDRS should be the primary endpoint, and the appearance of motor fluctuations and non-motor symptoms in the 31P-magnetic resonance spectroscopy (31P-MRS) imaging results, and changes in structural and functional brain anatomy (MRI), should be the secondary endpoints.med-con
In summary, according to the current data, the possible value of the treatment with CoQ10 in parkinsonian syndromes could deserve further studies, at least in selected subgroups of patients with PD and in patients diagnosed with MSA and PSP.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm12060975/s1. Table S1. PRISMA Checklist. Table S2. MOOSE Checklist.
Author Contributions
F.J.J.-J.: Conceptualization, Methodology, Investigation, Validation, Formal analysis, Writing—original draft, Writing—review and editing, Project administration. H.A.-N.: Conceptualization, Methodology, Investigation, Validation, Formal analysis, Writing—original draft, Writing—review and editing, Project administration. E.G.-M.: Conceptualization, Methodology, Investigation, Validation, Formal analysis, Writing—original draft, Writing—review and editing, Project administration, Obtaining funding. J.A.G.A.: Conceptualization, Methodology, Investigation, Validation, Formal analysis, Writing—original draft, Writing—review and editing, Project administration, Obtaining funding. All authors have read and agreed to the published version of the manuscript.
Funding
The work at the authors’ laboratory is supported in part by Grants RETICS RD16/0006/0004 (ARADyAL), PI18/00540 and PI21/01683 from Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, Madrid, Spain and IB20134 and GR21073 from Junta de Extremadura, Mérida, Spain. This study was partially funded with FEDER funds.
Institutional Review Board Statement
Not applicable because this is a review article.
Informed Consent Statement
Not applicable because this is a review article.
Data Availability Statement
All data related to the current study, intended for reasonable use, is available from J.A.G. Agúndez (University Institute of Molecular Pathology Biomarkers, University of Extremadura -UNEx ARADyAL Instituto de Salud Carlos III, Av/de la Universidad S/N, E10071 Cáceres. Spain) and F.J. Jiménez-Jiménez (Section of Neurology, Hospital del Sureste, Arganda del Rey, Madrid, Spain).
Acknowledgments
We recognize the efforts of the personnel at the Library of Hospital Universitario del Sureste, Arganda del Rey, who retrieved an important number of papers for us.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Crane, F.L. Biochemical functions of coenzyme Q10. J. Am. Coll. Nutr. 2001, 20, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Heaton, R.A.; Hargreaves, I.P. Coenzyme Q10, Ageing and the Nervous System, An Overview. Antioxidants 2021, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Navarro, H.; Jiménez-Jiménez, F.J.; Pilo de la Fuente, B.; Plaza-Nieto, J.F. Mecanismos patogénicos de la enfermedad de Parkinson. In Tratado de los Trastornos del Movimiento, 2nd ed.; Jiménez-Jiménez, F.J., Luquin, M.R., Molina, J.A., Linazasoro, G., Eds.; Viguera Editores: Barcelona, Spain, 2008; Volume 1, pp. 425–485. [Google Scholar]
- Jurcau, A. Insights into the Pathogenesis of Neurodegenerative Diseases, Focus on Mitochondrial Dysfunction and Oxidative Stress. Int. J. Mol. Sci. 2021, 22, 11847. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses, the PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 10 May 2022).
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology, a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2009, 283, 2008–2012. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid Based Ment Health. 2019, 22, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Jiménez, F.J.; Molina, J.A.; de Bustos, F.; García-Redondo, A.; Gómez-Escalonilla, C.; Martínez-Salio, A.; Berbel, A.; Camacho, A.; Zurdo, M.; Barcenilla, B.; et al. Serum levels of coenzyme Q10 in patients with Parkinson’s disease. J. Neural. Transm. 2000, 107, 177–181. [Google Scholar] [CrossRef]
- Buhmann, C.; Arlt, S.; Kontush, A.; Möller-Bertram, T.; Sperber, S.; Oechsner, M.; Stuerenburg, H.J.; Beisiegel, U. Plasma and CSF markers of oxidative stress are increased in Parkinson’s disease and influenced by antiparkinsonian medication. Neurobiol. Dis. 2004, 15, 160–170. [Google Scholar] [CrossRef]
- Sohmiya, M.; Tanaka, M.; Tak, N.W.; Yanagisawa, M.; Tanino, Y.; Suzuki, Y.; Okamoto, K.; Yamamoto, Y. Redox status of plasma coenzyme Q10 indicates elevated systemic oxidative stress in Parkinson’s disease. J. Neurol. Sci. 2004, 223, 161–166. [Google Scholar] [CrossRef]
- Bolner, A.; Micciolo, R.; Bosello, O.; Nordera, G.P. A Panel of Oxidative Stress Markers in Parkinson’s Disease. Clin. Lab. 2016, 62, 105–112. [Google Scholar] [CrossRef]
- Gorgone, G.; Currò, M.; Ferlazzo, N.; Parisi, G.; Parnetti, L.; Belcastro, V.; Tambasco, N.; Rossi, A.; Pisani, F.; Calabresi, P.; et al. Coenzyme Q10, hyperhomocysteinemia and MTHFR C677T polymorphism in levodopa-treated Parkinson’s disease patients. Neuromolecular Med. 2012, 14, 84–90. [Google Scholar] [CrossRef]
- Kasai, T.; Tokuda, T.; Ohmichi, T.; Ishii, R.; Tatebe, H.; Nakagawa, M.; Mizuno, T. Serum Levels of Coenzyme Q10 in Patients with Multiple System Atrophy. PLoS One 2016, 11, e0147574. [Google Scholar] [CrossRef]
- Du, J.; Wang, T.; Huang, P.; Cui, S.; Gao, C.; Lin, Y.; Fu, R.; Shen, J.; He, Y.; Tan, Y.; et al. Clinical correlates of decreased plasma coenzyme Q10 levels in patients with multiple system atrophy. Parkinsonism Relat. Disord. 2018, 57, 58–62. [Google Scholar] [CrossRef]
- Götz, M.E.; Gerstner, A.; Harth, R.; Dirr, A.; Janetzky, B.; Kuhn, W.; Riederer, P.; Gerlach, M. Altered redox state of platelet coenzyme Q10 in Parkinson’s disease. J. Neural. Transm. 2000, 107, 41–48. [Google Scholar]
- Mischley, L.K.; Allen, J.; Bradley, R. Coenzyme Q10 deficiency in patients with Parkinson’s disease. J. Neurol. Sci. 2012, 318, 72–75. [Google Scholar] [CrossRef] [Green Version]
- Isobe, C.; Murata, T.; Sato, C.; Terayama, Y. Increase of oxidized/total coenzyme Q-10 ratio in cerebrospinal fluid in patients with Parkinson’s disease. J. Clin. Neurosci. 2007, 14, 340–343. [Google Scholar] [CrossRef]
- Compta, Y.; Giraldo, D.M.; Muñoz, E.; Antonelli, F.; Fernández, M.; Bravo, P.; Soto, M.; Cámara, A.; Torres, F.; Martí, M.J.; et al. Cerebrospinal fluid levels of coenzyme Q10 are reduced in multiple system atrophy. Parkinsonism Relat. Disord. 2018, 46, 16–23. [Google Scholar] [CrossRef]
- Hargreaves, I.P.; Lane, A.; Sleiman, P.M. The coenzyme Q10 status of the brain regions of Parkinson’s disease patients. Neurosci Lett. 2008, 447, 17–19. [Google Scholar] [CrossRef]
- Schottlaender, L.V.; Bettencourt, C.; Kiely, A.P.; Chalasani, A.; Neergheen, V.; Holton, J.L.; Hargreaves, I.; Houlden, H. Coenzyme Q10 Levels Are Decreased in the Cerebellum of Multiple-System Atrophy Patients. PLoS One 2016, 11, e0149557. [Google Scholar] [CrossRef] [Green Version]
- Barca, E.; Kleiner, G.; Tang, G.; Ziosi, M.; Tadesse, S.; Masliah, E.; Louis, E.D.; Faust, P.; Kang, U.J.; Torres, J.; et al. Decreased Coenzyme Q10 Levels in Multiple System Atrophy Cerebellum. J. Neuropathol. Exp. Neurol. 2016, 75, 663–672. [Google Scholar] [CrossRef] [Green Version]
- Del Hoyo, P.; García-Redondo, A.; de Bustos, F.; Molina, J.A.; Sabed, Y.; Alonso-Navarro, H.; Caballero, L.; Arenas, J.; Agúndez, J.A.; Jiménez-Jiménez, F.J. Oxidative stress in skin fibroblasts cultures from patients with Parkinson’s disease. BMC Neurol. 2010, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Mitsui, J.; Matsukawa, T.; Yasuda, T.; Ishiura, H.; Tsuji, S. Plasma Coenzyme Q10 Levels in Patients With Multiple System Atrophy. JAMA Neurol. 2016, 73, 977–980. [Google Scholar] [CrossRef] [Green Version]
- Compagnoni, M.C.; Kleiner, G.; Bordoni, A.; Fortunato, F.; Ronchi, D.; Salani, S.; Guida, M.; Corti, C.; Pichler, I.; Bergamini, C.; et al. Mitochondrial dysfunction in fibroblasts of Multiple System Atrophy. Biochim. Biophys. Acta Mol. Basis. Dis. 2018, 1864, 3588–3597. [Google Scholar] [CrossRef]
- Molina, J.A.; de Bustos, F.; Ortiz, S.; Del Ser, T.; Seijo, M.; Benito-Léon, J.; Oliva, J.M.; Pérez, S.; Manzanares, J. Serum levels of coenzyme Q in patients with Lewy body disease. J. Neural. Transm. 2002, 109, 1195–1201. [Google Scholar] [CrossRef]
- Gironi, M.; Bianchi, A.; Russo, A.; Alberoni, M.; Ceresa, L.; Angelini, A.; Cursano, C.; Mariano, E.; Nemni, R.; Kullmann, C.; et al. Oxidative imbalance in different neurodegenerative diseases with memory impairment. Neurodegener. Dis. 2011, 8, 129–137. [Google Scholar] [CrossRef]
- Matsubara, T.; Azuma, T.; Yoshida, S.; Yamagami, T.; Okamoto, T.; Kishi, T. Serum coenzyme Q-10 level in Parkinson syndrome. In Biomedical and Clinical Aspects of Coenzyme Q; Folkers, K., Littarru, G.P., Yamagami, T., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1991; pp. 159–166. [Google Scholar]
- Strijks, E.; Kremer, H.P.; Horstink, M.W. Q10 therapy in patients with idiopathic Parkinson’s disease. Mol. Aspects Med. 1997, 18, S237–S240. [Google Scholar] [CrossRef]
- Shults, C.; Oakes, D.; Kieburtz, K.; Beal, M.F.; Haas, R.; Plumb, S.; Juncos, J.L.; Nutt, J.; Shoulson, I.; Carter, J.; et al. Effects of coenzyme Q10 in early Parkinson disease, evidence of slowing of the functional decline. Arch. Neurol. 2002, 59, 1541–1550. [Google Scholar] [CrossRef]
- Müller, T.; Büttner, T.; Gholipour, A.F.; Kuhn, W. Coenzyme Q10 supplementation provides mild symptomatic benefit in patients with Parkinson’s disease. Neurosci. Lett. 2003, 341, 201–204. [Google Scholar] [CrossRef]
- NINDS NET-PD Investigators. A randomized clinical trial of coenzyme Q10 and GPI-1485 in early Parkinson disease. Neurology 2007, 68, 20–28. [Google Scholar]
- Storch, A.; Jost, W.H.; Vieregge, P.; Spiegel, J.; Greulich, W.; Durner, J.; Müller, T.; Kupsch, A.; Henningsen, H.; Oertel, W.H.; et al. Randomized, double-blind, placebo-controlled trial on symptomatic effects of coenzyme Q(10) in Parkinson disease. Arch. Neurol. 2007, 64, 938–944. [Google Scholar] [CrossRef] [Green Version]
- Parkinson Study Group QE3 Investigators; Beal, M.F.; Oakes, D.; Shoulson, I.; Henchcliffe, C.; Galpern, W.R.; Haas, R.; Juncos, J.L.; Nutt, J.G.; Voss, T.S.; et al. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease, no evidence of benefit. JAMA Neurol. 2014, 71, 543–552. [Google Scholar]
- Jie, Z. Clinical effects and safety of coenzyme Q10 in Parkinson disease. China Foreign Med. Treat. 2014, 23, 79–80. [Google Scholar]
- Wang, X.Y.; Yang, Z.M.; Zhang, X.J.; Xi, S.; Liu, C.; Li, J.; Wang, Q.; Wang, L. Clinical observation of coenzyme Q10 in Parkinson disease. HeBei J. TCM 2014, 36, 151–153. [Google Scholar]
- Li, Z.; Wang, P.; Yu, Z.; Cong, Y.; Sun, H.; Zhang, J.; Zhang, J.; Sun, C.; Zhang, Y.; Ju, X. The effect of creatine and coenzyme q10 combination therapy on mild cognitive impairment in Parkinson’s disease. Eur. Neurol. 2015, 73, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Yoritaka, A.; Kawajiri, S.; Yamamoto, Y.; Nakahara, T.; Ando, M.; Hashimoto, K.; Nagase, M.; Saito, Y.; Hattori, N. Randomized, double-blind, placebo-controlled pilot trial of reduced coenzyme Q10 for Parkinson’s disease. Parkinsonism Relat. Disord. 2015, 21, 911–916. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Zhan, S.Y.; Xia, Y. Coenzyme Q10 for Parkinson’s disease. Cochrane Database Syst. Rev. 2011, 12, CD008150, Update in Cochrane Database Syst. Rev. 2012, 5, CD008150. [Google Scholar]
- Negida, A.; Menshawy, A.; El Ashal, G.; Elfouly, Y.; Hani, Y.; Hegazy, Y.; El Ghonimy, S.; Fouda, S.; Rashad, Y. Coenzyme Q10 for Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis. CNS Neurol. Disord. Drug Targets 2016, 15, 45–53. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Sun, M.X.; Zhang, W.L.; Wang, W.W.; Jin, Y.M.; Xie, C.L. The efficacy and safety of coenzyme Q10 in Parkinson’s disease: A meta-analysis of randomized controlled trials. Neurol. Sci. 2017, 38, 215–224. [Google Scholar] [CrossRef]
- Mitsui, J.; Koguchi, K.; Momose, T.; Takahashi, M.; Matsukawa, T.; Yasuda, T.; Tokushige, S.I.; Ishiura, H.; Goto, J.; Nakazaki, S.; et al. Three-Year Follow-Up of High-Dose Ubiquinol Supplementation in a Case of Familial Multiple System Atrophy with Compound Heterozygous COQ2 Mutations. Cerebellum. 2017, 16, 664–672. [Google Scholar] [CrossRef] [Green Version]
- Stamelou, M.; Reuss, A.; Pilatus, U.; Magerkurth, J.; Niklowitz, P.; Eggert, K.M.; Krisp, A.; Menke, T.; Schade-Brittinger, C.; Oertel, W.H.; et al. Short-term effects of coenzyme Q10 in progressive supranuclear palsy: A randomized, placebo-controlled trial. Mov. Disord. 2008, 23, 942–949. [Google Scholar] [CrossRef]
- Apetauerova, D.; Scala, S.A.; Hamill, R.W.; Simon, D.K.; Pathak, S.; Ruthazer, R.; Standaert, D.G.; Yacoubian, T.A. CoQ10 in progressive supranuclear palsy: A randomized, placebo-controlled, double-blind trial. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e266. [Google Scholar] [CrossRef] [Green Version]
- Beal, M.F. Coenzyme Q10 administration and its potential for treatment of neurodegenerative diseases. Biofactors 1999, 9, 261–266. [Google Scholar] [CrossRef]
- Shults, C.W.; Haas, R.H.; Beal, M.F. A possible role of coenzyme Q10 in the etiology and treatment of Parkinson’s disease. Biofactors 1999, 9, 267–272. [Google Scholar] [CrossRef]
- Beal, M.F.; Matthews, R.T.; Tieleman, A.; Shults, C.W. Coenzyme Q10 attenuates the 1-methyl-4-phenyl-1.;2.;3.; Tetrahydropyridine (MPTP) induced loss of striatal dopamine and dopaminergic axons in aged mice. Brain Res. 1998, 783, 109–114. [Google Scholar] [CrossRef]
- Cleren, C.; Yang, L.; Lorenzo, B.; Calingasan, N.Y.; Schomer, A.; Sireci, A.; Wille, E.J.; Beal, M.F. Therapeutic effects of coenzyme Q10 (CoQ10) and reduced CoQ10 in the MPTP model of Parkinsonism. J. Neurochem. 2008, 104, 1613–1621. [Google Scholar] [CrossRef]
- Kobayashi, S.; Muroyama, A.; Matsushima, H.; Yoshimura, I.; Mitsumoto, Y. Oral administration of coenzyme Q10 reduces MPTP-induced loss of dopaminergic nerve terminals in the striatum in mice. Neurol. Sci. 2012, 33, 195–199. [Google Scholar] [CrossRef]
- Yan, A.; Liu, Z.; Song, L.; Wang, X.; Zhang, Y.; Wu, N.; Lin, J.; Liu, Y.; Liu, Z. Idebenone Alleviates Neuroinflammation and Modulates Microglial Polarization in LPS-Stimulated BV2 Cells and MPTP-Induced Parkinson’s Disease Mice. Front. Cell. Neurosci. 2019, 12, 529. [Google Scholar] [CrossRef]
- Horvath, T.L.; Diano, S.; Leranth, C.; Garcia-Segura, L.M.; Cowley, M.A.; Shanabrough, M.; Elsworth, J.D.; Sotonyi, P.; Roth, R.H.; Dietrich, E.H.; et al. Coenzyme Q induces nigral mitochondrial uncoupling and prevents dopamine cell loss in a primate model of Parkinson’s disease. Endocrinology 2003, 144, 2757–2760. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Calingasan, N.Y.; Wille, E.J.; Cormier, K.; Smith, K.; Ferrante, R.J.; Beal, M.F. Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson’s and Huntington’s diseases. J. Neurochem. 2009, 109, 1427–1439. [Google Scholar] [CrossRef] [Green Version]
- Schulz, J.B.; Henshaw, D.R.; Matthews, R.T.; Beal, M.F. Coenzyme Q10 and nicotinamide and a free radical spin trap protect against MPTP neurotoxicity. Exp. Neurol. 1995, 132, 279–283. [Google Scholar] [CrossRef]
- Ghasemloo, E.; Mostafavi, H.; Hosseini, M.; Forouzandeh, M.; Eskandari, M.; Mousavi, S.S. Neuroprotective effects of coenzyme Q10 in Parkinson’s model via a novel Q10/miR-149-5p/MMPs pathway. Metab. Brain Dis. 2021, 36, 2089–2100. [Google Scholar] [CrossRef]
- Nezhadi, A.; Ghazi, F.; Rassoli, H.; Bakhtiari, M.; Ataiy, Z.; Soleimani, S.; Mehdizadeh, M. BMSC and CoQ10 improve behavioural recovery and histological outcome in rat model of Parkinson’s disease. Pathophysiology 2011, 18, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Kooncumchoo, P.; Sharma, S.; Porter, J.; Govitrapong, P.; Ebadi, M. Coenzyme Q(10) provides neuroprotection in iron-induced apoptosis in dopaminergic neurons. J. Mol. Neurosci. 2006, 28, 125–141. [Google Scholar] [CrossRef]
- Attia, H.N.; Maklad, Y.A. Neuroprotective effects of coenzyme Q10 on paraquat-induced Parkinson’s disease in experimental animals. Behav. Pharmacol. 2018, 29, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Somayajulu-Niţu, M.; Sandhu, J.K.; Cohen, J.; Sikorska, M.; Sridhar, T.S.; Matei, A.; Borowy-Borowski, H.; Pandey, S. Paraquat induces oxidative stress.; Neuronal loss in substantia nigra region and parkinsonism in adult rats, neuroprotection and amelioration of symptoms by water-soluble formulation of coenzyme Q10. BMC Neurosci. 2009, 10, 88. [Google Scholar] [CrossRef] [Green Version]
- Binukumar, B.K.; Gupta, N.; Bal, A.; Gill, K.D. Protection of dichlorvos induced oxidative stress and nigrostriatal neuronal death by chronic coenzyme Q10 pretreatment. Toxicol Appl. Pharmacol. 2011, 256, 73–82. [Google Scholar] [CrossRef]
- Abdin, A.A.; Hamouda, H.E. Mechanism of the neuroprotective role of coenzyme Q10 with or without L-dopa in rotenone-induced parkinsonism. Neuropharmacology. 2008, 55, 1340–1346. [Google Scholar] [CrossRef]
- Avcı, B.; Günaydın, C.; Güvenç, T.; Yavuz, C.K.; Kuruca, N.; Bilge, S.S. Idebenone Ameliorates Rotenone-Induced Parkinson’s Disease in Rats Through Decreasing Lipid Peroxidation. Neurochem. Res. 2021, 46, 513–522. [Google Scholar] [CrossRef]
- Moon, Y.; Lee, K.H.; Park, J.H.; Geum, D.; Kim, K. Mitochondrial membrane depolarization and the selective death of dopaminergic neurons by rotenone, protective effect of coenzyme Q10. J. Neurochem. 2005, 93, 1199–1208. [Google Scholar] [CrossRef]
- McCarthy, S.; Somayajulu, M.; Sikorska, M.; Borowy-Borowski, H.; Pandey, S. Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble Coenzyme Q10. Toxicol. Appl. Pharmacol. 2004, 201, 21–31. [Google Scholar] [CrossRef]
- Turton, N.; Heaton, R.A.; Ismail, F.; Roberts, S.; Nelder, S.; Phillips, S.; Hargreaves, I.P. The Effect of Organophosphate Exposure on Neuronal Cell Coenzyme Q10 Status. Neurochem. Res. 2001, 46, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Onaolapo, O.J.; Odeniyi, A.O.; Jonathan, S.O.; Samuel, M.O.; Amadiegwu, D.; Olawale, A.; Tiamiyu, A.O.; Ojo, F.O.; Yahaya, H.A.; Ayeni, O.J.; et al. An Investigation of the Anti-Parkinsonism Potential of Co-enzyme Q10 and Co-enzyme Q10/Levodopa-carbidopa Combination in Mice. Curr. Aging Sci. 2021, 14, 62–75. [Google Scholar] [CrossRef]
- Gupta, B.K.; Kumar, S.; Kaur, H.; Ali, J.; Baboota, S. Attenuation of Oxidative Damage by Coenzyme Q10 Loaded Nanoemulsion Through Oral Route for the Management of Parkinson’s Disease. Rejuvenation Res. 2018, 21, 232–248. [Google Scholar] [CrossRef]
- Faust, K.; Gehrke, S.; Yang, Y.; Yang, L.; Beal, M.F.; Lu, B. Neuroprotective effects of compounds with antioxidant and anti-inflammatory properties in a Drosophila model of Parkinson’s disease. BMC Neurosci. 2009, 10, 109. [Google Scholar] [CrossRef] [Green Version]
- Tai, K.K.; Pham, L.; Truong, D.D. Idebenone induces apoptotic cell death in the human dopaminergic neuroblastoma SHSY-5Y cells. Neurotox. Res. 2011, 20, 321–328. [Google Scholar] [CrossRef]
- Shi, H.; Noguchi, N.; Xu, Y.; Niki, E. 1-Methyl-4-phenyl-2.;3-dihydropyridinium is transformed by ubiquinone to the selective nigrostriatal toxin 1-methyl-4-phenylpyridinium. FEBS Lett. 1999, 461, 196–200. [Google Scholar] [CrossRef] [Green Version]
- Lönnrot, K.; Metsä-Ketelä, T.; Molnár, G.; Ahonen, J.P.; Latvala, M.; Peltola, J.; Pietilä, T.; Alho, H. The effect of ascorbate and ubiquinone supplementation on plasma and CSF total antioxidant capacity. Free Radic. Biol. Med. 1996, 21, 211–217. [Google Scholar] [CrossRef]
- Shults, C.W.; Flint Beal, M.; Song, D.; Fontaine, D. Pilot trial of high dosages of coenzyme Q10 in patients with Parkinson’s disease. Exp. Neurol. 2004, 188, 491–494. [Google Scholar] [CrossRef]
- Nukui, K.; Yamagishi, T.; Miyawaki, H.; Kettawan, A.; Okamoto, T.; Belardinelli, R.; Tiano, L.; Littarru, G.P.; Sato, K. Blood CoQ10 levels and safety profile after single-dose or chronic administration of PureSorb-Q40, animal and human studies. Biofactors. 2008, 32, 209–219. [Google Scholar] [CrossRef]
- Seet, R.C.; Lim, E.C.; Tan, J.J.; Quek, A.M.; Chow, A.W.; Chong, W.L.; Ng, M.P.; Ong, C.N.; Halliwell, B. Does high-dose coenzyme Q10 improve oxidative damage and clinical outcomes in Parkinson’s disease? Antioxid. Redox Signal. 2014, 21, 211–217. [Google Scholar] [CrossRef]
- Prasuhn, J.; Brüggemann, N.; Hessler, N.; Berg, D.; Gasser, T.; Brockmann, K.; Olbrich, D.; Ziegler, A.; König, I.R.; Klein, C.; et al. An omics-based strategy using coenzyme Q10 in patients with Parkinson’s disease, concept evaluation in a double-blind randomized placebo-controlled parallel group trial. Neurol. Res. Pract. 2019, 1, 31. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).