Development and Validation of Predictive Assessment of Complicated Diverticulitis Score
Abstract
1. Introduction
2. Results
2.1. Demographic Data of the Derivation Cohort
2.2. Parameters Associated with Complicated AD in Univariate Analysis
2.3. Parameters Associated with Complicated AD in Multivariate Analysis
2.4. Outcomes
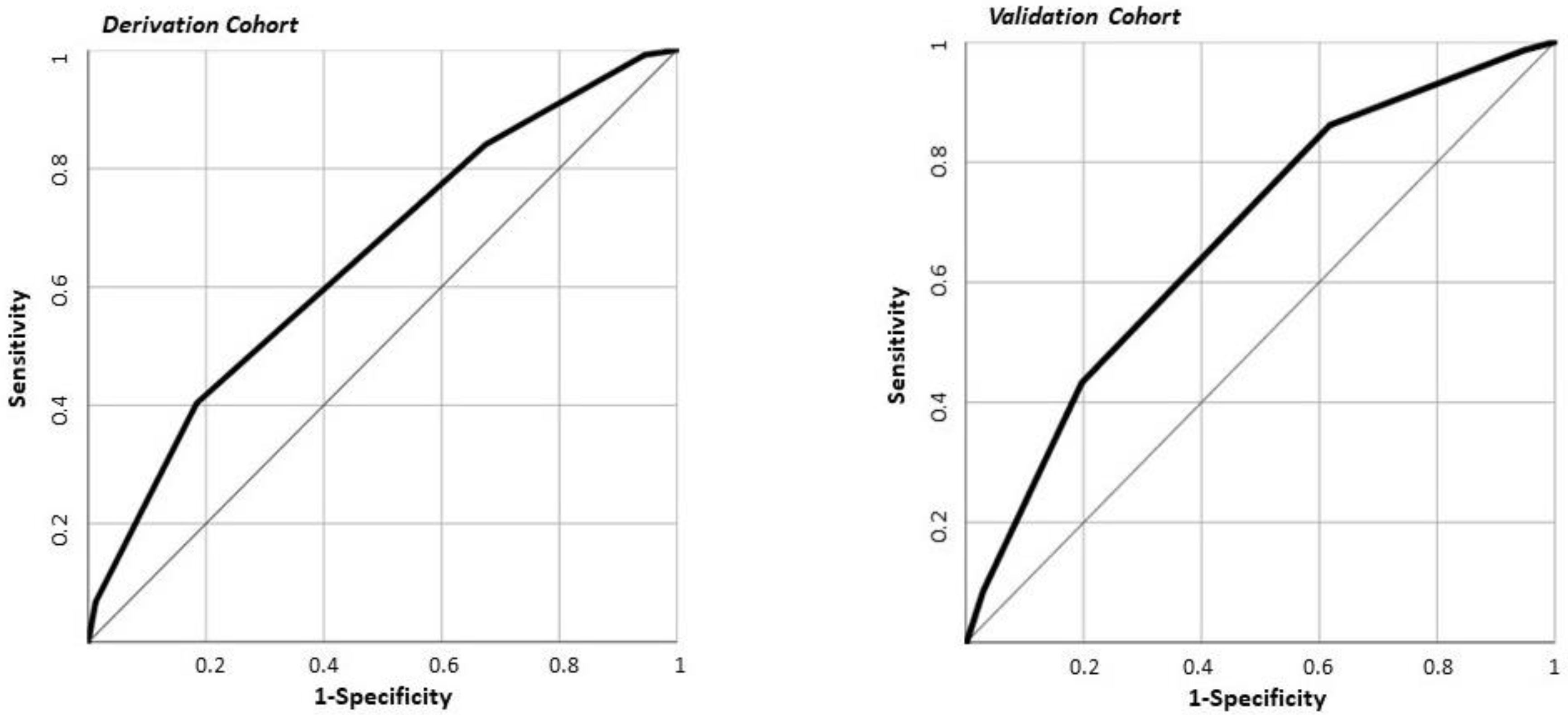
2.5. The PACO-D Score Development and Application in the Derivation Cohort
2.6. Demographic, Clinical Data, and Outcomes of the Patients Included in the Validation Cohort
2.7. The PACO-D Score Application in the Validation Cohort
2.8. The PACO-D Score and Cumulative Major Complications
3. Discussion
4. Materials and Methods
4.1. Study Design, Patient Enrollment, and Selection
4.2. Data Collection
4.3. Outcomes
4.4. Statistical Analysis
4.5. Development of the Predictive Score
4.6. Score Validation
4.7. Sample Size
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tursi, A.; Papa, A.; Danese, S. Review article: The pathophysiology and medical management of diverticulosis and diverticular disease of the colon. Aliment. Pharmacol. Ther. 2015, 42, 664–684. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Scarpignato, C.; Strate, L.L.; Lanas, A.; Kruis, W.; Lahat, A.; Danese, S. Colonic diverticular disease. Nat. Rev. Dis. Primers 2020, 26, 20. [Google Scholar] [CrossRef] [PubMed]
- Humes, D.J.; West, J. Role of acute diverticulitis in the development of complicated colonic diverticular disease and 1-year mortality after diagnosis in the UK: Population-based cohort study. Gut 2012, 61, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Mataloni, F.; Bruzzone, M.; Carabotti, M.; Cirocchi, R.; Nascimbeni, R.; Gambassi, G.; Vettoretto, N.P.; Pinnarelli, L.; Cuomo, R.; et al. Hospital admission for complicated diverticulitis is increasing in Italy, especially in younger patients: A national database study. Tech. Coloproctol. 2020, 24, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Hupfeld, L.; Pommergaard, H.-C.; Burcharth, J.; Rosenberg, J. Emergency admissions for complicated colonic diverticulitis are increasing: A nationwide register-based cohort study. Int. J. Colorectal Dis. 2018, 33, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Jeyarajah, S.; Faiz, O.; Bottle, A.; Aylin, P.; Bjarnason, I.; Tekkis, P.P.; Papagrigoriadis, S. Diverticular disease hospital admissions are increasing, with poor outcomes in the elderly and emergency admissions. Aliment. Pharm. Ther. 2009, 30, 1171–1182. [Google Scholar] [CrossRef]
- Papa, A.; Papa, V. The economic burden of diverticular disease. J. Clin. Gastroenterol. 2016, 50, S2–S3. [Google Scholar] [CrossRef]
- Mennini, F.; Sciattella, P.; Marcellusi, A.; Toraldo, B.; Koch, M. Economic burden of diverticular disease: An observational analysis based on real world data from an Italian region. Dig. Liver Dis. 2017, 49, 1003–1008. [Google Scholar] [CrossRef]
- Biondo, S.; Golda, T.; Kreisler, E.; Espin, E.; Vallribera, F.; Oteiza, F.; Codina-Cazador, A.; Pujadas, M.; Flor, B. Outpatient versus hospitalization management for uncomplicated diverticulitis. A prospective, multicenter randomized clinical trial (DIVER trial). Ann. Surg. 2014, 259, 38–44. [Google Scholar] [CrossRef]
- Tursi, A.; Brandimarte, G.; di Mario, F.; Lanas, A.; Scarpignato, C.; Bafutto, M.; Barbara, G.; Bassotti, G.; Binda, G.A.; Biondi, A.; et al. International consensus on diverticulosis and diverticular disease. Statements from the 3rd International Symposium on Diverticular Disease. J. Gastrointest. Liver Dis. 2019, 28 (Suppl. 4), 57–66. [Google Scholar] [CrossRef]
- Covino, M.; Petruzziello, C.; Onder, G.; Migneco, A.; Simeoni, B.; Franceschi, F.; Ojetti, V. A 12-year retrospective analysis of differences between elderly and oldest old patients referred to the emergency department of a large tertiary hospital. Maturitas 2019, 120, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Swanson, S.M.; Strate, L.L. Acute colonic diverticulitis. Ann. Intern. Med. 2018, 168, ITC65–ITC80, Erratum in Ann. Intern. Med. 2020, 172, 640. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, A.E.; Parthasarathy, G.; Ditah, I.; Fletcher, J.G.; Ewelukwa, O.; Pendlimari, R.; Yawn, B.P.; Melton, L.J.; Schleck, C.; Zinsmeister, A.R. Temporal trends in the incidence and natural history of diverticulitis: A population-based study. Am. J. Gastroenterol. 2015, 110, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Humes, D.J.; Solaymani-Dodaran, M.; Fleming, K.M.; Simpson, J.; Spiller, R.C.; West, J. A population-based study of perforated diverticular disease incidence and associated mortality. Gastroenterology 2009, 136, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.M.; Machicado, G.A.; Jutabha, R.; Kovacs, T.O.G. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N. Engl. J. Med. 2000, 342, 78–82. [Google Scholar] [CrossRef]
- Kinjo, K.; Matsui, T.; Hisabe, T.; Ishihara, H.; Kojima, T.; Chuman, K.; Yasukawa, S.; Beppu, T.; Koga, A.; Ishikawa, S.; et al. Risk factors for severity of colonic diverticular hemorrhage. Intest. Res. 2018, 16, 458–466. [Google Scholar] [CrossRef]
- Lee, K.K.; Shah, S.M.; Moser, M.A. Risk factors predictive of severe diverticular hemorrhage. Int. J. Surg. 2011, 9, 83–85. [Google Scholar] [CrossRef]
- Laméris, W.; van Randen, A.; van Gulik, T.M.; Busch, O.R.C.; Winkelhagen, J.; Bossuyt, P.M.M.; Stoker, J.; Boermeester, M.A. A clinical decision rule to establish the diagnosis of acute diverticulitis in the emergency department. Dis. Colon Rectum. 2010, 53, 896–904. [Google Scholar]
- Andeweg, C.S.; Knobben, L.; Hendriks, J.C.; Bleichrodt, R.P.; van Goor, H. How to diagnose acute left-sided colonic diverticulitis: Proposal for a clinical scoring system. Ann. Surg. 2011, 253, 940–946. [Google Scholar] [CrossRef]
- Sartelli, M.; Weber, D.G.; Kluger, Y.; Ansaloni, L.; Coccolini, F.; Abu-Zidan, F.; Augustin, G.; Ben-Ishay, O.; Biffl, W.L.; Bouliaris, K.; et al. 2020 update of the WSES guidelines for the management of acute colonic diverticulitis in the emergency setting. World J. Emerg. Surg. 2020, 15, 32. [Google Scholar] [CrossRef]
- Schultz, J.K.; Azhar, N.; Binda, G.A.; Barbara, G.; Biondo, S.; Boermeester, M.A.; Chabok, A.; Consten, E.C.J.; Van Dijk, S.T.; Johanssen, A.; et al. European Society of Coloproctology: Guidelines for the management of diverticular disease of the colon. Colorectal Dis. 2020, 22, 5–28. [Google Scholar] [CrossRef]
- Kaiser, A.M.; Jiang, J.-K.; Lake, J.P.; Ault, G.; Artinyan, A.; Gonzalez-Ruiz, C.; Essani, R.; Beart, R.W. The management of complicated diverticulitis and the role of computed tomography. Am. J. Gastroenterol. 2005, 100, 910–917. [Google Scholar] [CrossRef]
- Umezawa, S.; Nagata, N.; Arimoto, J.; Uchiyama, S.; Higurashi, T.; Nakano, K.; Ishii, N.; Sakurai, T.; Moriyasu, S.; Takeda, Y.; et al. Contrast-enhanced CT for colonic diverticular bleeding before colonoscopy: A prospective multicenter study. Radiology 2018, 288, 755–761. [Google Scholar] [CrossRef]
- Tan, J.P.; Barazanchi, A.W.; Singh, P.P.; Hill, A.G.; Maccormick, A.D. Predictors of acute diverticulitis severity: A systematic review. Int. J. Surg. 2016, 26, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Bolkenstein, H.E.; Van De Wall, B.J.M.; Consten, E.C.J.; Broeders, I.A.M.J.; Draaisma, W.A. Risk factors for complicated diverticulitis: Systematic review and meta-analysis. Int. J. Colorectal Dis. 2017, 32, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, G.F.; Iyer, R.L.; Chu, L.-H.X.; Chen, W.; Yen, L.S.; Hodgkins, P.; Kawatkar, A.A. Acute diverticulitis: Demographic, clinical and laboratory features associated with computed tomography findings in 741 patients. Aliment. Pharm. Ther. 2012, 36, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Nizri, E.; Spring, S.; Ben-Yehuda, A.; Khatib, M.; Klausner, J.; Greenberg, R. C-reactive protein as a marker of complicated diverticulitis in patients on anti-inflammatory medications. Tech. Coloproctol. 2013, 18, 145–149. [Google Scholar] [CrossRef]
- Mäkelä, J.T.; Klintrup, K.; Takala, H.; Rautio, T. The role of C-reactive protein in prediction of the severity of acute diverticulitis in an emergency unit. Scand. J. Gastroenterol. 2015, 50, 536–541. [Google Scholar] [CrossRef]
- Van de Wall, B.J.; Draaisma, W.A.; van der Kaaij, R.T.; Consten, E.C.; Wiezer, M.J.; Broeders, I.A. The value of inflammation markers and body temperature in acute diverticulitis. Colorectal Dis. 2013, 15, 621–626. [Google Scholar] [CrossRef]
- Käser, S.A.; Fankhauser, G.; Glauser, P.M.; Toia, D.; Maurer, C.A. Diagnostic value of inflammation markers in predicting perforation in acute sigmoid diverticulitis. World J. Surg. 2010, 34, 2717–2722. [Google Scholar] [CrossRef]
- Ho, B.-L.; Hu, H.-Y.; Chang, S.-S. Association between use of proton pump inhibitors and occurrence of colon diverticulitis. J. Chin. Med. Assoc. 2016, 79, 5–10. [Google Scholar] [CrossRef]
- Sbeit, W.; Khoury, T.; Kadah, A.; Asadi, W.; Shahin, A.; Lubany, A.; Safadi, M.; Haddad, H.; Ahmad, R.A.; el Hija, S.A.; et al. Proton Pump inhibitor use may increase the risk of diverticulitis but not it’s severity among patients with colonic diverticulosis: A multicenter study. J. Clin. Med. 2020, 9, 2966. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Violi, A.; Cambie, G.; Franceschi, M.; Baldassarre, G.; Rodriguez, K.I.; Miraglia, C.; Brandimarte, G.; Elisei, W.; Picchio, M.; et al. Risk factors for endoscopic severity of diverticular disease of the colon and its outcome: A real-life case-control study. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Le Bastard, Q.; Al-Ghalith, G.A.; Grégoire, M.; Chapelet, G.; Javaudin, F.; Dailly, E.; Batard, E.; Knights, D.; Montassier, E. Systematic review: Human gut dysbiosis induced by non-antibiotic prescription medications. Aliment. Pharm. Ther. 2018, 47, 332–345. [Google Scholar] [CrossRef]
- Ojetti, V.; Petruzziello, D.S.C.; Cardone, S.; Saviano, L.; Migneco, A.; Santarelli, L.; Gabrielli, M.; Zaccaria, R.; Lopetuso, L.; Covino, M.; et al. The use of probiotics in different phases of diverticular disease. Rev. Recent Clin. Trials 2018, 13, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
| Variable | All Population n = 1089 | Uncomplicated Diverticulitis n = 793 | Complicated Diverticulitis n = 296 | p-Value |
|---|---|---|---|---|
| Age (Years), median (IQR) | 66 (53–77) | 65 (53–77) | 67 (54–79) | 0.106 |
| Sex (Male), n (%) | 501 (46.0) | 341 (43.0) | 160 (54.1) | 0.001 |
| Presentation, n (%) | ||||
| Fever | 429 (39.4) | 324 (40.9) | 105 (35.5) | 0.106 |
| Abdominal pain | 753 (69.1) | 567 (71.5) | 186 (62.8) | 0.006 |
| Vomit | 205 (18.8) | 153 (19.3) | 52 (17.6) | 0.517 |
| Constipation | 144 (10.5) | 89 (9.3) | 55 (13.3) | 0.029 |
| Diarrhea | 151 (13.9) | 111 (14.0) | 40 (13.5) | 0.837 |
| Weight loss | 27 (2.5) | 20 (2.5) | 7 (2.4) | 0.882 |
| Therapy, n (%) | ||||
| PPIs | 135 (12.4) | 114 (14.4) | 21 (7.1) | 0.001 |
| Aspirin | 151 (13.9) | 117 (14.8) | 34 (11.5) | 0.165 |
| NSAIDs in previous week | 50 (4.6) | 35 (4.4) | 15 (5) | 0.646 |
| Steroids | 49 (4.5) | 35 (4.4) | 14 (4.7) | 0.823 |
| Anticoagulation (VKA) | 46 (4.2) | 34 (4.3) | 12 (4.1) | 0.865 |
| Statin | 62 (5.7) | 51 (6.4) | 11 (3.7) | 0.085 |
| Laboratory Values, Median (IQR) | ||||
| Hemoglobin (g/dL) | 13.3 (11.9–14.5) | 13.5 (12.3–14.6) | 12.8 (10.9–14.1) | <0.001 |
| WBC (×109/L) | 9.0 (6.2–12.0) | 9.0 (6.1–12.0) | 10.1 (6.5–13.6) | 0.009 |
| Fibrinogen (mg/dL) | 459 (341–604) | 446 (335–582) | 497 (362–642) | 0.001 |
| C-reactive protein (mg/L) | 46 (23–80) | 45 (21–74) | 48 (26–106) | <0.001 |
| Comorbidities | ||||
| Charlson comorbidity index, median (IQR) | 2 (1–4) | 2 (1–4) | 2 (1–4) | 0.426 |
| First episode of AD, n (%) | 773 (71.0) | 547 (69.0) | 226 (76.4) | 0.017 |
| Hypertension, n (%) | 149 (13.7) | 93 (11.7) | 56 (18.9) | 0.002 |
| Obesity, n (%) | 10 (0.9) | 4 (0.5) | 6 (2.0) | 0.019 |
| Heavy smoker, n (%) | 73 (6.7) | 43 (5.4) | 30 (10.1) | 0.006 |
| Factor | ß | SE | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Sex (Male) | 0.548 | 0.150 | 1.73 (1.29–2.32) | <0.001 |
| Abdominal Pain | −0.228 | 0.164 | 0.79 (0.58–1.09) | 0.163 |
| Constipation | 0.650 | 0.214 | 1.95 (1.26–2.91) | 0.002 |
| Not on PPIs | 0.766 | 0.262 | 2.15 (1.29–3.59) | 0.003 |
| Hemoglobin < 11.9 (g/dL) | 0.876 | 0.165 | 2.40 (1.74–3.32) | <0.001 |
| WBC > 12.9 (×109/L) | 0.147 | 0.177 | 1.16 (0.82–1.64) | 0.407 |
| Fibrinogen > 623 (mg/dL) | 0.171 | 0.177 | 1.16 (0.84–1.68) | 0.334 |
| C-reactive protein > 80 (mg/L) | 0.612 | 0.169 | 1.84 (1.32–2.57) | <0.001 |
| First episode of AD | 0.221 | 0.173 | 1.25 (0.88–1.75) | 0.203 |
| Hypertension | 0.310 | 0.216 | 1.36 (0.89–2.08) | 0.151 |
| Obesity | 1.350 | 0.688 | 3.86 (1.01–14.85) | 0.049 |
| Heavy smoker | 0.367 | 0.282 | 1.44 (0.83–2.51) | 0.194 |
| Variable | All Population n = 1089 | Uncomplicated Diverticulitis n = 793 | Complicated Diverticulitis n = 296 | p-Value |
|---|---|---|---|---|
| Death, n (%) | 11 (1.0) | 3 (0.4) | 8 (2.7) | 0.001 |
| Sepsis, n (%) | 14 (1.3) | 7 (0.9) | 7 (2.4) | 0.053 |
| Mechanical ventilation, n (%) | 9 (0.8) | 0 | 9 (3.0) | <0.001 |
| Major complications †, n (%) | 25 (2.3) | 10 (1.3) | 15 (5.1) | <0.001 |
| Any surgical procedure, n (%) | 95 (8.7) | 11 (1.4) | 84 (28.4) | <0.001 |
| Major surgery, n (%) | 82 (7.5) | 7 (0.9) | 75 (25.3) | <0.001 |
| Percutaneous drainage, n (%) | 13 (1.2) | 4 (0.5) | 9 (3.0) | <0.001 |
| Colostomy, n (%) | 33 (3.0) | 2 (0.3) | 31 (10.5) | <0.001 |
| Total LOS ‡, median (interquartile range) | 2.7 (0.3–5.8) | 0.8 (0.3–3.8) | 6.9 (4.4–10.3) | <0.001 |
| 4-A Derivation Cohort (1089 Patients) | |||||
| PACO Risk Class | Score Values | Complicated Diverticulitis n (%) | Relative Risk OR (95% CI) | Cumulative Major Complications n (%) | Relative Risk OR (95% CI) |
| Low Risk | 0–1 | 41 (11.9) | Reference | 4 (1.2) | Reference |
| Moderate Risk | 2–3 | 230 (33.0) | 3.6 (2.5–5.2) | 18 (2.6) | 2.2 (0.7–6.7) |
| High Risk | ≥4 | 25 (52.1) | 8.0 (4.2–5.4) | 3 (6.3) | 5.7 (1.2–26.1) |
| 4-B Validation Cohort (282 Patients) | |||||
| PACO Risk Class | Score Values | Complicated Diverticulitis n (%) | Relative Risk OR (95% CI) | Cumulative Major Complications n (%) | Relative Risk OR (95% CI) |
| Low Risk | 0–1 | 17 (22.4) | Reference | 1 (1.3) | Reference |
| Moderate Risk | 2–3 | 53 (42.4) | 2.4 (1.4–4.9) | 4 (3.2) | 2.5 (0.3–22.6) |
| High Risk | ≥4 | 49 (60.5) | 5.3 (2.6–0.7) | 8 (9.9) | 8.2 (1.1–7.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covino, M.; Papa, V.; Tursi, A.; Simeoni, B.; Lopetuso, L.R.; Vetrone, L.M.; Franceschi, F.; Rapaccini, G.; Gasbarrini, A.; Papa, A. Development and Validation of Predictive Assessment of Complicated Diverticulitis Score. J. Pers. Med. 2021, 11, 80. https://doi.org/10.3390/jpm11020080
Covino M, Papa V, Tursi A, Simeoni B, Lopetuso LR, Vetrone LM, Franceschi F, Rapaccini G, Gasbarrini A, Papa A. Development and Validation of Predictive Assessment of Complicated Diverticulitis Score. Journal of Personalized Medicine. 2021; 11(2):80. https://doi.org/10.3390/jpm11020080
Chicago/Turabian StyleCovino, Marcello, Valerio Papa, Antonio Tursi, Benedetta Simeoni, Loris Riccardo Lopetuso, Lorenzo Maria Vetrone, Francesco Franceschi, Gianludovico Rapaccini, Antonio Gasbarrini, and Alfredo Papa. 2021. "Development and Validation of Predictive Assessment of Complicated Diverticulitis Score" Journal of Personalized Medicine 11, no. 2: 80. https://doi.org/10.3390/jpm11020080
APA StyleCovino, M., Papa, V., Tursi, A., Simeoni, B., Lopetuso, L. R., Vetrone, L. M., Franceschi, F., Rapaccini, G., Gasbarrini, A., & Papa, A. (2021). Development and Validation of Predictive Assessment of Complicated Diverticulitis Score. Journal of Personalized Medicine, 11(2), 80. https://doi.org/10.3390/jpm11020080








