Comparing CLIF-C ACLF, CLIF-C ACLFlactate, and CLIF-C ACLF-D Prognostic Scores in Acute-on-Chronic Liver Failure Patients by a Single-Center ICU Experience
Abstract
1. Introduction
2. Patients and Methods
2.1. ACLF Definition and Diagnosis
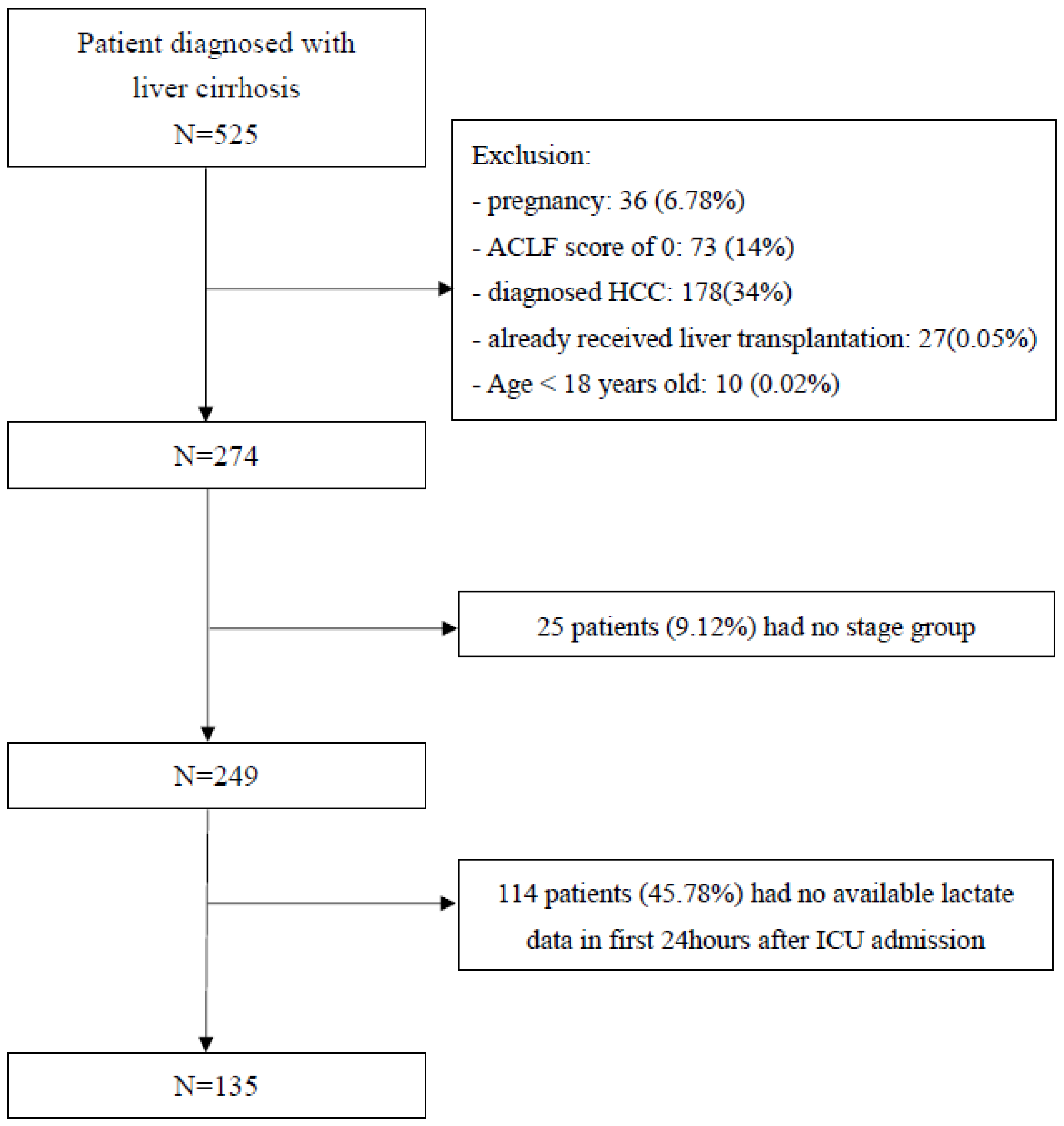
2.2. Patient Selection
2.3. Definition of Liver Cirrhosis and Decompensated Cirrhosis
2.4. Data Source and Collection
2.4.1. Prognostic Score Computation
2.4.2. Primary Outcome and Scheduled Follow-Up Periods
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics of Critically Ill Patients with Cirrhosis and ACLF Admitted to ICU
3.2. Values at 28, 90, 180, and 365 day Follow-Up among Survivors versus Non-Survivors
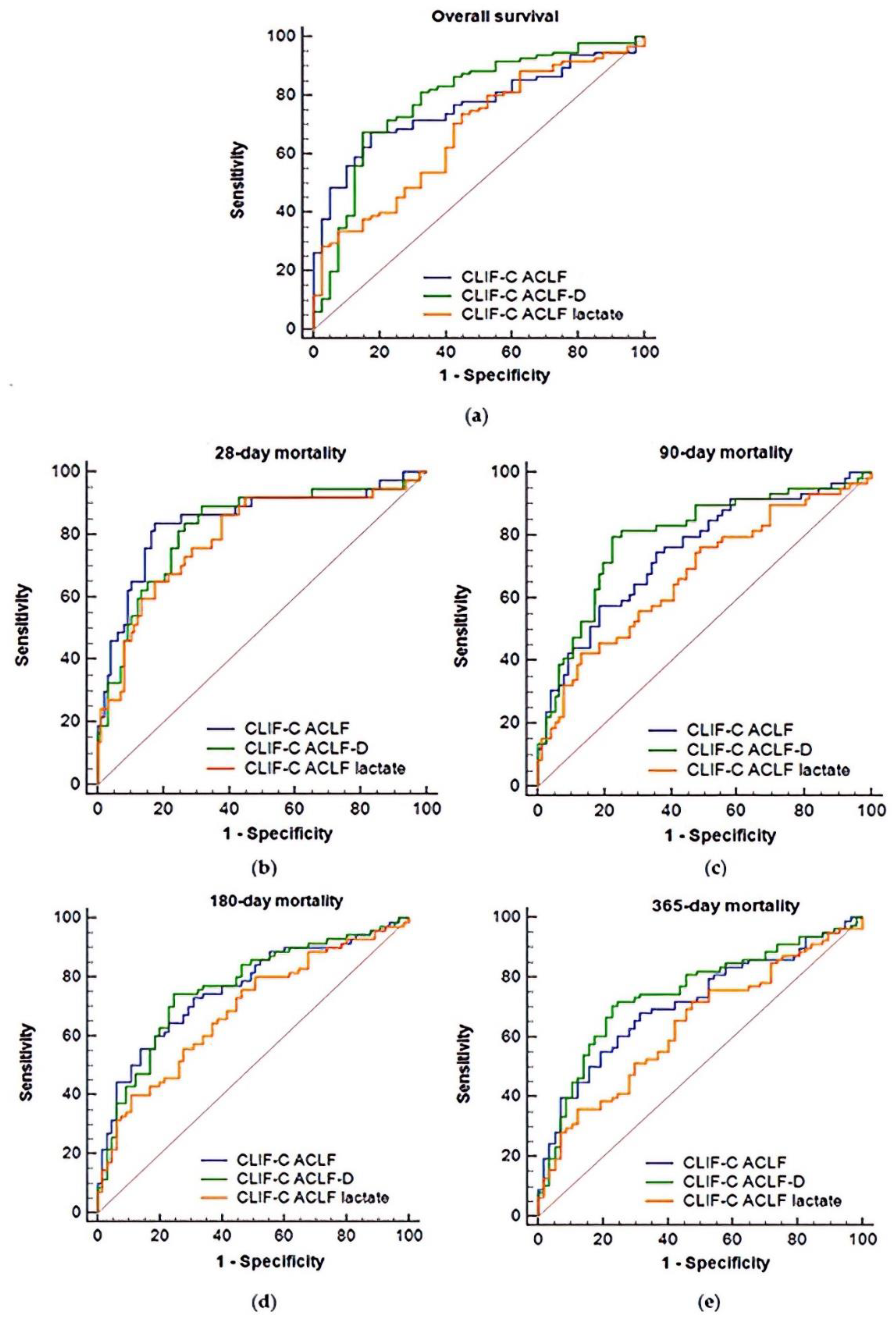
3.3. AUROC Comparisons of Overall Mortality Prediction at Days 28, 90, 180, and 365
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sarin, S.K.; Kumar, A.; Almeida, J.A.; Chawla, Y.K.; Fan, S.T.; Garg, H.; De Silva, H.J.; Hamid, S.S.; Jalan, R.; Komolmit, P.; et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol. Int. 2008, 3, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, V.; Moreau, R.; Jalan, R.; Gines, P. Acute-on-chronic liver failure: A new syndrome that will re-classify cirrhosis. J. Hepatol. 2015, 62, S131–S143. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, V.; Moreau, R.; Kamath, P.S.; Jalan, R.; Ginès, P.; Nevens, F.; Fernández, J.; To, U.; García-Tsao, G.; Schnabl, B. Acute-on-chronic liver failure in cirrhosis. Nat. Rev. Dis. Primers 2016, 2, 16041. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, V.; D’Amico, G.; Fede, G.; Manousou, P.; Tsochatzis, E.; Pleguezuelo, M.; Burroughs, A.K. Infections in patients with cirrhosis increase mortality four-fold and should be used in deter-mining prognosis. Gastroenterology 2010, 139, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Mücke, M.M.; Rumyantseva, T.; Mücke, V.T.; Schwarzkopf, K.; Joshi, S.; Kempf, V.A.J.; Welsch, C.; Zeuzem, S.; Lange, C.M. Bacterial infection-triggered acute-on-chronic liver failure is associated with increased mortality. Liver Int. 2018, 38, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Nusrat, S.; Khan, M.S.; Fazili, J.; Madhoun, M.F. Cirrhosis and its complications: Evidence based treatment. World J. Gastroenterol. 2014, 20, 5442–5460. [Google Scholar] [CrossRef]
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef]
- Moreau, R.; Jalan, R.; Gines, P.; Pavesi, M.; Angeli, P.; Cordoba, J.; Durand, F.; Gustot, T.; Saliba, F.; Domenicali, M.; et al. Acute-on-Chronic Liver Failure Is a Distinct Syndrome That Develops in Patients with Acute De-compensation of Cirrhosis. Gastroenterology 2013, 144, 1426–1437.e9. [Google Scholar] [CrossRef]
- Jalan, R.; Saliba, F.; Pavesi, M.; Amoros, A.; Moreau, R.; Gines, P.; Levesque, E.; Durand, F.; Angeli, P.; Caraceni, P.; et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J. Hepatol. 2014, 61, 1038–1047. [Google Scholar] [CrossRef]
- Drolz, A.; Horvatits, T.; Rutter, K.; Landahl, F.; Roedl, K.; Meersseman, P.; Wilmer, A.; Kluwe, J.; Lohse, A.W.; Kluge, S.; et al. Lactate Improves Prediction of Short-Term Mortality in Critically Ill Patients with Cirrhosis: A Multinational Study. Hepatology 2019, 69, 258–269. [Google Scholar] [CrossRef]
- Trebicka, J.; Fernandez, J.; Papp, M.; Caraceni, P.; Laleman, W.; Gambino, C.; Giovo, I.; Uschner, F.E.; Jimenez, C.; Mookerjee, R.; et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J. Hepatol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Kumar, M.; Eslam, M.; George, J.; Al Mahtab, M.; Akbar, S.M.F.; Jia, J.; Tian, Q.; Aggarwal, R.; Muljono, D.H.; et al. Liver diseases in the Asia-Pacific region: A Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol. Hepatol. 2020, 5, 167–228. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-H.; Tseng, H.-J.; Chen, W.-T.; Chen, P.-C.; Ho, Y.-P.; Huang, C.-H.; Lin, C.-Y. Comparing Eight Prognostic Scores in Predicting Mortality of Patients with Acute-On-Chronic Liver Failure Who Were Admitted to an ICU: A Single-Center Experience. J. Clin. Med. 2020, 9, 1540. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Concejero, A.M. Liver transplantation for hepatocellular carcinoma in the world: The Taiwan experience. J. Hepato-Biliary-Pancreat. Sci. 2009, 17, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-M.; Kuo, S.-C.; Chiu, Y.-C.; Lin, H.-F.; Kuo, M.-L.; Elsarawy, A.M.; Chen, C.-L.; Lin, C.-C. Cost Analysis and Determinants of Living Donor Liver Transplantation in Taiwan. Transplant. Proc. 2018, 50, 2601–2605. [Google Scholar] [CrossRef]
- Wen, P.; Lu, C.; Strong, C.; Lin, Y.; Chen, Y.; Li, C.; Tsai, C.-C. Demographic and Urbanization Disparities of Liver Transplantation in Taiwan. Int. J. Environ. Res. Public Health 2018, 15, 177. [Google Scholar] [CrossRef]
- Chan, K.; Cheng, C.; Wu, T.; Lee, C.; Wu, T.; Chou, H.; Lee, W. Impact of donor with evidence of bacterial infections on deceased donor liver transplanta-tion: A retrospective observational cohort study in Taiwan. BMJ Open 2019, 9, e023908. [Google Scholar] [CrossRef]
- Wang, T.-H.; Chang, Y.-P.; Chiang, W.-L. Improving Donation Rates in Taiwan. Transplantation 2016, 100, 2235–2237. [Google Scholar] [CrossRef]
- Joel, J.; Michael, B. Cirrhosis and Chronic Liver Failure: Part I. Diagnosis and Evaluation. Am. Fam. Physician 2006, 74, 756–762. [Google Scholar]
- Yeom, S.K.; Lee, C.H.; Cha, S.H.; Park, C.M. Prediction of liver cirrhosis, using diagnostic imaging tools. World J. Hepatol. 2015, 7, 2069–2079. [Google Scholar] [CrossRef]
- Barosa, R.; Ramos, L.R.; Patita, M.; Nunes, G.; Fonseca, J. CLIF-C ACLF score is a better mortality predictor than MELD, MELD-Na and CTP in pa-tients with Acute on chronic liver failure admitted to the ward. Rev. Esp. Enferm. Dig. 2017, 109, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.-B.; Lee, S.-G.; Kang, W.-H.; Song, G.-W.; Jung, D.-H.; Park, G.-C.; Cho, H.-D.; Jwa, E.-K.; Kim, W.-J.; Ha, T.-Y.; et al. Adult Living Donor Liver Transplantation for Acute-on-Chronic Liver Failure in High-Model for End-Stage Liver Disease Score Patients. Arab. Archaeol. Epigr. 2017, 17, 1833–1842. [Google Scholar] [CrossRef]
- Artru, F.; Louvet, A.; Ruiz, I.; Levesque, E.; Labreuche, J.; Ursic-Bedoya, J.; Lassailly, G.; Dharancy, S.; Boleslawski, E.; Lebuffe, G.; et al. Liver transplantation in the most severely ill cirrhotic patients: A multicenter study in acute-on-chronic liver failure grade 3. J. Hepatol. 2017, 67, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Shi, Y.; Zhao, H.; Wu, W.; Sheng, J. Acute-on-chronic liver failure in chronic hepatitis B: An update. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Keegan, M.T.; Gajic, O.; Afessa, B. Severity of illness scoring systems in the intensive care unit. Crit. Care Med. 2011, 39, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R. Clinical review: Scoring systems in the critically ill. Crit. Care 2010, 14, 207. [Google Scholar] [CrossRef]
- Li, N.; Huang, C.; Yu, K.-K.; Lu, Q.; Shi, G.-F.; Zheng, J.-M. Validation of prognostic scores to predict short-term mortality in patients with HBV-related acute-on-chronic liver failure. Medicine 2017, 96, e6802. [Google Scholar] [CrossRef]
- Cardoso, F.S.; Abraldes, J.G.; Sy, E.; Ronco, J.J.; Bagulho, L.; McPhail, M.J.; Karvellas, C.J. Lactate and number of organ failures predict intensive care unit mortality in patients with acute-on-chronic liver failure. Liver Int. 2019, 39, 1271–1280. [Google Scholar] [CrossRef]
- Jeppesen, J.B.; Mortensen, C.; Bendtsen, F.; Møller, S. Lactate metabolism in chronic liver disease. Scand. J. Clin. Lab. Investig. 2013, 73, 293–299. [Google Scholar] [CrossRef]
- Haas, S.A.; Lange, T.; Saugel, B.; Petzoldt, M.; Fuhrmann, V.; Metschke, M.; Kluge, S. Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Intensiv. Care Med. 2016, 42, 202–210. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Silva, A.Q.E.; Couto, L., Jr.; Taccone, F.S. The value of blood lactate kinetics in critically ill patients: A systematic review. Crit Care 2016, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-J.; Kao, J.-H.; Chen, D.-S. Therapeutic implications of hepatitis B virus genotypes. Liver Int. 2005, 25, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.H. Impact of hepatitis B vaccination on hepatitis B disease and nucleic acid testing in high-prevalence populations. J. Clin. Virol. 2006, 36, S45–S502. [Google Scholar] [CrossRef]

| Parameter | All Patients (135 Patients) | Survivors (98 Patient) | Non-Survivors (37 Patients) | p-Value |
|---|---|---|---|---|
| Male sex | 100 (66.66%) | 79 (80.61%) | 21 (56.75%) | 0.02 |
| Age | 54.76 ± 14.06 | 54.21 ± 13.95 | 56.22 ± 14.46 | 0.50 |
| Etiology | ||||
| HBV | 54 (40.00%) | 39 (39.80%) | 15 (40.54%) | 0.39 |
| HCV | 22 (16.29%) | 12 (12.24%) | 10 (27.02%) | 0.04 |
| ALC | 35 (25.93%) | 32 (32.65%) | 3 (8.11%) | <0.01 |
| HBV + HCV | 6 (4.44%) | 4 (4.09%) | 2 (5.41%) | 0.74 |
| ALC + HCV | 8 (5.93%) | 5 (5.10%) | 3 (8.11%) | 0.51 |
| Others | 10 (7.41%) | 6 (6.12%) | 4 (10.81%) | 0.33 |
| Laboratory values | ||||
| Bilirubin(mg/dl) * | 3.5 (1.7–9.1) | 2.8 (1.68–4.88) | 14.1 (4–26.5) | <0.01 |
| INR * | 1.6 (1.4–2.0) | 1.5 (1.4–1.8) | 2.1 (1.7–3.6) | <0.01 |
| Albumin(g/dl) * | 2.69 (2.40–3.01) | 2.72 (2.41–3.04) | 2.65 (2.21–2.85) | 0.11 |
| Sodium (mEq/L) * | 138 (135–141) | 138 (135–141) | 140 (134–145) | 0.16 |
| Serum Cr (mg/dl) * | 1.08 (0.79–1.73) | 1.00 (0.77–1.28) | 1.63 (1.16–2.26) | <0.01 |
| WBC count (109/L) * | 8.7 (6–11.3) | 8.1 (6–10.8) | 10.4 (6.8–17.5) | 0.01 |
| Arterial PH * | 7.42 (7.34–7.47) | 7.43 (7.35–7.47) | 7.41 (7.34–7.49) | 0.94 |
| HE grade I-II | 56 (41.48%) | 40 (40.81%) | 16 (43.24%) | 0.79 |
| HE grade III-IV | 17 (12.59%) | 6 (6.12%) | 11 (29.73%) | <0.01 |
| ACLF grades of EASL-CLIF consortium | ||||
| ACLF1 | 68 (50.37%) | 58 (59.18%) | 10 (27.03%) | <0.01 |
| ACLF2 | 22 (16.30%) | 15 (15.31%) | 5 (13.51%) | 0.59 |
| ACLF3 | 45 (33.33%) | 25 (25.51%) | 22 (59.46%) | <0.01 |
| Score | AUROC (95%CI) | Pairwise Comparison of ROC Curves | |||
|---|---|---|---|---|---|
| A | B | C | |||
| Overall survival | |||||
| CLIF-C ACLF | A | 0.762 (0.681–0.831) | p < 0.01 | p = 0.53 | |
| CLIF-C ACLFlactate | B | 0.673 (0.587–0.751) | p < 0.01 | p = 0.01 | |
| CLIF-C ACLF-D | C | 0.787 (0.708–0.853) | p = 0.53 | p = 0.01 | |
| 28-day mortality | |||||
| CLIF-C ACLF | A | 0.845 (0.773–0.902) | p = 0.03 | p = 0.57 | |
| CLIF-C ACLFlactate | B | 0.792 (0.713–0.857) | p = 0.03 | p = 0.55 | |
| CLIF-C ACLF-D | C | 0.821 (0.746–0.882) | p = 0.57 | p = 0.55 | |
| 90-day mortality | |||||
| CLIF-C ACLF | A | 0.747 (0.665–0.818) | p < 0.01 | p = 0.23 | |
| CLIF-C ACLFlactate | B | 0.673 (0.587–0.751) | p < 0.01 | p < 0.01 | |
| CLIF-C ACLF-D | C | 0.795 (0.717–0.860) | p = 0.23 | p < 0.01 | |
| 180-day mortality | |||||
| CLIF-C ACLF | A | 0.759 (0.678–0.828) | p < 0.01 | p = 0.91 | |
| CLIF-C ACLFlactate | B | 0.685 (0.600–0.763) | p < 0.01 | p = 0.07 | |
| CLIF-C ACLF-D | C | 0.763 (0.683–0.832) | p = 0.91 | p = 0.07 | |
| 365-day mortality | |||||
| CLIF-C ACLF | A | 0.711 (0.626–0.786) | p = 0.01 | p = 0.07 | |
| CLIF-C ACLFlactate | B | 0.636 (0.548–0.717) | p = 0.01 | p = 0.01 | |
| CLIF-C ACLF-D | C | 0.744 (0.662–0.815) | p = 0.07 | p = 0.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, C.-C.; Huang, C.-H.; Chang, C.; Chen, P.-C.; Chen, B.-H.; Chen, W.-T.; Ho, Y.-P. Comparing CLIF-C ACLF, CLIF-C ACLFlactate, and CLIF-C ACLF-D Prognostic Scores in Acute-on-Chronic Liver Failure Patients by a Single-Center ICU Experience. J. Pers. Med. 2021, 11, 79. https://doi.org/10.3390/jpm11020079
Kuo C-C, Huang C-H, Chang C, Chen P-C, Chen B-H, Chen W-T, Ho Y-P. Comparing CLIF-C ACLF, CLIF-C ACLFlactate, and CLIF-C ACLF-D Prognostic Scores in Acute-on-Chronic Liver Failure Patients by a Single-Center ICU Experience. Journal of Personalized Medicine. 2021; 11(2):79. https://doi.org/10.3390/jpm11020079
Chicago/Turabian StyleKuo, Chao-Cheng, Chien-Hao Huang, Ching Chang, Pin-Cheng Chen, Bo-Huan Chen, Wei-Ting Chen, and Yu-Pin Ho. 2021. "Comparing CLIF-C ACLF, CLIF-C ACLFlactate, and CLIF-C ACLF-D Prognostic Scores in Acute-on-Chronic Liver Failure Patients by a Single-Center ICU Experience" Journal of Personalized Medicine 11, no. 2: 79. https://doi.org/10.3390/jpm11020079
APA StyleKuo, C.-C., Huang, C.-H., Chang, C., Chen, P.-C., Chen, B.-H., Chen, W.-T., & Ho, Y.-P. (2021). Comparing CLIF-C ACLF, CLIF-C ACLFlactate, and CLIF-C ACLF-D Prognostic Scores in Acute-on-Chronic Liver Failure Patients by a Single-Center ICU Experience. Journal of Personalized Medicine, 11(2), 79. https://doi.org/10.3390/jpm11020079









