Abstract
Background/Objectives: Hydroxychloroquine (HCQ) is widely used in the treatment of autoimmune rheumatologic diseases due to its immunomodulatory and anti-inflammatory properties. However, long-term HCQ therapy carries a risk of irreversible retinal toxicity caused by drug accumulation in the retinal pigment epithelium. The early identification of preclinical retinal changes is essential to prevent permanent visual impairment. Optical coherence tomography (OCT) and OCT-angiography (OCT-A) have emerged as key imaging modalities for the detection of structural and microvascular biomarkers of HCQ retinopathy. A narrative review of the literature was conducted using the PubMed database, focusing on studies published between January 2017 and February 2025. Search terms included “hydroxychloroquine” and “optical coherence tomography.” Eligible studies evaluated HCQ-related retinal toxicity using OCT and/or OCT-A in human subjects. Data were extracted regarding study population characteristics, treatment duration, cumulative HCQ dose, daily dose normalized to real body weight, and reported imaging findings. Results: We identified 223 scientific papers of which 88 studies met the inclusion criteria. Structural OCT parameters—particularly alterations in the ellipsoid zone, outer nuclear layer, and retinal pigment epithelium—were consistently associated with early HCQ toxicity, often preceding functional impairment. OCT-A studies demonstrated microvascular alterations, including reduced vessel density and foveal avascular zone enlargement, though interpretation may be confounded by underlying autoimmune-disease-related vasculopathy. Conclusions: HCQ retinopathy is a potentially vision-threatening condition associated with the cumulative dose, treatment duration, and patient-specific risk factors. OCT and OCT-A provide complementary structural and vascular biomarkers that aid in the detection of subclinical retinal toxicity. The integration of quantitative and automated OCT-derived metrics may improve screening strategies, facilitate early diagnosis, and support personalized care in patients receiving long-term HCQ therapy.
1. Introduction
Hydroxychloroquine (HCQ) is a synthetic antimalarial drug used in the management of autoimmune rheumatologic diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and Sjögren’s syndrome (SJS) due to its well-established immunomodulatory properties and anti-inflammatory effects [1]. By accumulating in lysosomes and autophagosomes, it increases the pH, resulting in the inhibition of the major histocompatibility complex class-II antigen presentation and subsequent activation of T lymphocytes, affecting both the innate and adaptive immune responses. The accumulation of HCQ blocks viral recognition by endosomal toll-like receptors, leading to a reduction in the innate antiviral response (decreased interferon-1 production). HCQ directly affects the adaptive immune system by inhibiting the differentiation and activation of T- and B-cell lymphocytes [2].
In addition to its immunomodulatory functions, HCQ also exerts additional pleiotropic effects including anti-inflammatory, antithrombotic, hypoglycemic, hypolipidemic, anticholinesterase, and photoprotective effects. Although initially proposed as a suppressive treatment for malaria, HCQ today plays an important role in modulating the activity of chronic inflammatory diseases, both as a monotherapy, by reducing disease activity and improving joint status, and in combination with other therapeutic classes, enhancing the overall favorable prognosis. The efficacy of HCQ in RA has been demonstrated both as a monotherapy in mild disease and, more importantly, in combination with other synthetic or biologic disease-modifying antirheumatic drugs in severe forms, in line with EULAR recommendations. In SLE, HCQ is recommended across all disease phenotypes, where it plays a crucial role in long-term disease modulation. Its hypolipidemic effect confers additional cardiovascular protection in RA/SLE patients with concomitant cardiovascular involvement. Recent studies further suggest that HCQ may be indicated in RA with chronic kidney disease, showing no significant impact on creatinine clearance, and may serve as an adjuvant in remission-induction therapy for lupus nephritis [3,4].
Nevertheless, the therapeutic benefits of HCQ must be balanced against its potential adverse effects. Prolonged HCQ exposure carries a well-recognized risk of retinal toxicity affecting the retina, ciliary body, and cornea, manifesting as bull’s-eye maculopathy, color vision disturbances, and paracentral scotomas, which may progress even after treatment discontinuation and can result in irreversible visual impairment. Accordingly, the discontinuation of therapy is warranted upon the onset of pathological ocular changes.
HCQ-induced retinal toxicity is primarily attributed to drug accumulation within melanin rich ocular tissues, particularly the retinal pigment epithelium (RPE). The high lipophilicity of HCQ, its large volume of distribution, and prolonged half-life facilitate intracellular accumulation within lysosomes, where the disruption of autophagic and phagolysosomal pathways in RPE cells impairs photoreceptor (PR) outer segment phagocytosis. This process is further compounded by the inhibition of all-trans retinol recycling via organic-anion-transporting polypeptide 1A2, resulting in early PR dysfunction. These molecular alterations manifest as the parafoveal attenuation or focal disruption of the ellipsoid zone (EZ), reflecting the early compromise of the PR inner segment integrity, followed by the progressive thinning of the outer nuclear layer (ONL), which represents PR cell body loss and is among the earliest quantitative biomarkers of HCQ toxicity. Autophagy-lysosomal defects and an altered sphingolipid metabolism may also contribute to the early involvement of inner retinal (IR) neurons, including retinal ganglion cells (RGC), providing a mechanistic substrate for the subtle IR thinning detected on Optical Coherence Tomography (OCT) in some cohorts. Additional pathways, such as TRPM2-mediated Ca2+ influx and oxidative retinal injury, likely accelerate PR and RPE degeneration, reinforcing the progressive structural changes observed on longitudinal OCT imaging. An additional consideration in minimizing the risk of retinal toxicity is to avoid the concomitant use of HCQ with other agents known to cause ocular damage [5,6]. Given these metabolic alterations, secondary vascular involvement could affect the perfusion of the retinal vascular layers, offering a potential explanation to the preferential toxicity involvement. However, these vascular findings need to be interpreted with caution as systemic autoimmune diseases may lead to choroidal thickness alterations and inflammatory microvascular remodeling in the absence of HCQ involvement.
A critical challenge in HCQ retinopathy is that early structural damage often pre-cedes visual symptoms, rendering the traditional ophthalmic examination insufficient for a timely diagnosis. Consequently, modern screening strategies increasingly rely on advanced retinal imaging modalities capable of detecting subclinical changes before functional loss occurs. The early detection of retinal toxicity is possible through Optical Coherence Tomography (OCT), multifocal electroretinography (mfERG), and fundus autofluorescence (FAF) [7].
Optical Coherence Tomography
OCT is a non-invasive imaging modality based on low-coherence interferometry, widely applied in ophthalmology to generate high-resolution cross-sectional images of retinal layers. It enables a quantitative and qualitative assessment of key structures such as the retinal nerve fiber layer (RNFL), ganglion cell layer (GCL), macular thickness (MT) and architecture, and optic nerve head [8].
In the context of HCQ toxicity, OCT plays a pivotal role in the early identification of subclinical retinal alterations, before the onset of visual symptoms. The characteristic OCT findings associated with HCQ toxicity include the parafoveal disruption of the EZ, RPE degeneration, the thinning of the ONL, and changes in the inner plexiform layer (IPL), all of which represent early structural hallmarks of drug-induced retinal damage [7].
Although retinal thickness (RT) typically remains stable for many years in patients undergoing long-term HCQ therapy, evidence suggests that, beyond a critical cumulative exposure, the retina may begin to thin rapidly [9]. A quantitative analysis of MT across the inner and outer ETDRS rings can reveal these subtle structural alterations several years before the conventional clinical signs of toxicity become apparent. Sequential OCT monitoring therefore provides an objective and sensitive method for detecting early retinal involvement, enabling timely intervention and the adjustment of HCQ therapy. Because these measurements are derived from standard, widely available OCT protocols, their automation and integration into routine ophthalmic practice could substantially enhance the early detection of HCQ-induced retinal damage and improve long-term visual outcomes [10].
The existing literature on HCQ retinopathy assessed by OCT and OCT-A is characterized by considerable heterogeneity, encompassing cross-sectional and retrospective studies, variable imaging protocols, inconsistent segmentation methodologies, and an expanding body of exploratory research on early structural and vascular biomarkers. This methodological fragmentation limits the feasibility of systematic synthesis or meta-analytic comparison. In this context, a narrative review represents an appropriate framework to integrate diverse forms of evidence, contextualize emerging imaging biomarkers, and synthesize current knowledge across structural and vascular parameters. Accordingly, the aim of this review is to synthesize available evidence on OCT- and OCT-A-derived retinal alterations in patients receiving HCQ. Specifically, this review seeks to identify early OCT biomarkers associated with HCQ-induced retinal toxicity, characterize sequential patterns of structural retinal involvement, and evaluate the emerging role of OCT-A in detecting microvascular changes and its complementary value to structural OCT. By integrating data on outer retinal (OR), inner retinal (IR), choroidal, and microvascular changes, this review seeks to clarify the role of OCT-based biomarkers in the early detection, risk stratification, and longitudinal monitoring of HCQ retinopathy, thereby supporting optimized screening strategies and personalized patient care.
2. Materials and Methods
A search was conducted in the PubMed database to identify relevant studies published between January 2017 and February 2025. The search strategy combined free-text keywords and Medical Subject Headings (MeSH), including “hydroxychloroquine,” “chloroquine,” “retinal toxicity,” “maculopathy,” “optical coherence tomography,” “OCT,” “OCT angiography,” and “optical coherence tomography angiography,” using Boolean operators (AND/OR) to maximize sensitivity. Reference lists of all included articles and relevant reviews were manually screened to identify additional pertinent studies not captured by the initial search.
Studies were considered eligible if they met the following criteria: they included human subjects receiving HCQ therapy; evaluated retinal toxicity or structural and/or vascular retinal changes using (OCT) and/or OCT-A; and reported qualitative and/or quantitative imaging findings relevant to HCQ exposure. Observational study designs were included, such as cross-sectional, cohort, case–control, and retrospective analyses. Studies were excluded if they were limited to isolated case reports or small case series without systematic imaging-based analysis; focused exclusively on non-OCT imaging modalities without OCT or OCT-A correlation; involved experimental animal models or in vitro data; or were not available in the English language.
Titles and abstracts were initially screened for relevance, followed by full-text review of potentially eligible studies. Duplicate records were removed prior to full-text assessment. Study selection and data extraction were performed by the authors through consensus-based review, with discrepancies resolved by discussion.
Data extraction was performed using a predefined framework to ensure consistency across heterogeneous study designs. Imaging findings were collected as reported in the original studies. Structural OCT outcomes were grouped into OR parameters (including EZ integrity and ONL thickness), IR metrics (such as GCL and IPL measurements), and choroidal parameters, while OCT-A outcomes were categorized by vascular plexus involvement and reported quantitative or qualitative perfusion metrics.
Treatment-related variables, including duration of HCQ exposure, cumulative dose, and daily dose normalized to body weight, were extracted when available and interpreted in relative rather than absolute terms, acknowledging variability in reporting thresholds across studies. When quantitative values were not directly comparable due to differences in imaging platforms, segmentation algorithms, or acquisition protocols, findings were synthesized qualitatively based on directional consistency (thinning, disruption, and reduced vessel density) and anatomical localization.
Given the substantial heterogeneity in study design, patient populations, imaging platforms, and outcome reporting, a structured narrative synthesis approach was employed. Rather than pooling results quantitatively, studies were compared and integrated based on shared biological and anatomical targets affected by hydroxychloroquine toxicity. Grouping findings by anatomical domain allowed identification of convergent patterns across diverse methodologies while minimizing bias introduced by platform-specific measurements. Differences related to underlying autoimmune disease, imaging technology, and study design were considered during interpretation and explicitly discussed as potential sources of variability. Findings were grouped according to the primary anatomical region or imaging domain assessed, including OR, IR, EZ, choroid, and retinal microvasculature.
3. Results
The search identified a total of 223 records through the PubMed database. After the removal of 5 duplicate records, 218 unique articles remained and were screened based on the title and abstract. Of these, 130 records were excluded because they were not directly related to HCQ retinal toxicity, did not include OCT or OCT-angiography-based imaging, or involved non-human studies. The remaining 88 articles underwent a full-text assessment for eligibility and met the predefined inclusion criteria. These studies were subsequently included in the qualitative narrative synthesis and formed the basis of the present review. The study selection process is summarized in Figure 1.
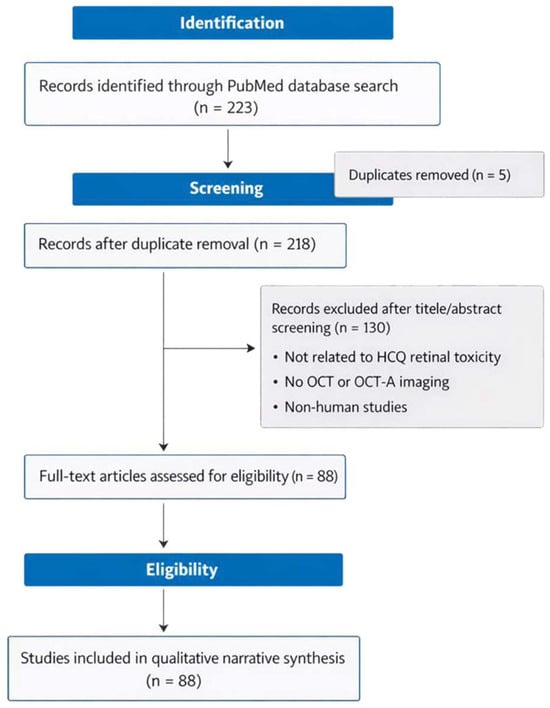
Figure 1.
Flow diagram of study selection.
3.1. Outer Retinal Alterations
Given the HCQ mechanism of action, the OR has been the main target in HCQ retinopathy screening, with OCT being able to provide data regarding thickness measurements in different macular areas, while more subjective analyses were required to identify lesions such as drusen-like deposits (DLDs), EZ hyporeflectivity, or PR loss.
Multiple studies have identified a thinner OR thickness that is correlated with the HCQ dose and treatment duration, with HCQ retinopathy progressing under a certain pattern, the inferior macular quadrant being frequently the most affected (Table 1).

Table 1.
Published studies that analyze HCQ toxicity using OCT focused on OR alterations.
Across the analyzed studies, there is a strong agreement that HCQ exposure is associated with early structural alterations in the OR, even in the absence of clinically apparent retinopathy. The most consistently reported finding is the parafoveal and perifoveal thinning of the ONL and ORT, observed across diverse cohorts and imaging platforms [9,12,15,16,20,23,30].
A preferential topographic pattern was repeatedly observed, with the inferior, nasal, and inferotemporal parafoveal regions most commonly affected, while the superior quadrants often showed no significant changes in the early stages [12,23,25,27]. This sectoral distribution was further supported by the OCT-derived indices demonstrating a higher diagnostic performance in the inferior ETDRS sectors [31].
Several studies reported an association between OR thinning and HCQ exposure metrics, particularly, cumulative dose or long-term treatment. Significant or dose-dependent relationships were described in multiple cases [9,10,11,17,18]. Longitudinal data further suggest that retinal thickness may remain stable for extended periods before accelerated thinning occurs beyond a critical exposure threshold [10].
Advanced imaging studies provided additional structural detail. Garg et al. reported the attenuation of PR outer segments using visible-light OCT, with the cone outer segments affected earlier than rods [13]. Cakir el al. observed differences in the PR segment length in the nasal and temporal regions related to the treatment duration, supporting early PR structural involvement [26].
Despite these convergent observations, not all studies demonstrated a clear correlation between OR changes and the treatment duration or cumulative dose. Trenkic Bozinovic et al. found no significant correlation with the HCQ duration, although greater inner and outer foveal thinning was observed in eyes with established retinopathy compared with those without visible macular changes [25]. Similarly, Allahdina et al. reported no significant differences in treatment duration, cumulative dose, or daily dose between patients with and without retinopathy, despite identifying an increased ONL reflectivity and elevated OCT-based metrics in the affected eyes [31].
Variability was also noted regarding which OR parameter was the most sensitive, with some studies emphasizing ONL thinning [12,20,23], others reporting reduced ORT [9,15], and others focusing on PR segment alterations [13,21,26]. These differences likely reflect the heterogeneity in the imaging protocols, segmentation approaches, and disease stage rather than contradictory biological effects.
Therefore, parafoveal ONL thinning and sectoral OR attenuation are supported as the most consistent structural OCT findings associated with HCQ exposure. The discrepancies across studies mainly concern the strength of the dose correlations and the specific OR metrics used, rather than the presence of early OR involvement itself. These findings support the role of OR OCT parameters as sensitive biomarkers for the early detection and longitudinal monitoring of HCQ-related retinal toxicity.
3.2. Elipsoid Zone
Accumulating evidence suggests a sequential pattern of OR involvement in HCQ retinopathy, in which the disruption of the EZ represents the earliest and most sensitive structural correlate of functional impairment, followed by progressive alterations in the external limiting membrane (ELM) and, ultimately, the RPE, the latter serving as a marker of advanced and often irreversible disease. Once the EZ integrity is compromised, the eyes demonstrate continued progression despite drug cessation, with a subsequent ELM disruption indicating a more severe OR compromise and poorer prognosis. Table 2 summarizes the published studies that analyze the EZ integrity.

Table 2.
Published studies that analyze HCQ toxicity using OCT focused on ellipsoid zone defects.
Multiple studies reported that early HCQ retinopathy exhibits a preferential parafoveal or pericentral EZ involvement, with a progression toward RPE damage in more advanced stages. He et al. observed a pericentral pattern in all early cases, with initial EZ damage followed by RPE involvement, and associated visual acuity reduction [36]. Similarly, Kim et al. reported that pericentral involvement was the most frequent pattern, followed by a parafoveal and mixed distributions, with the inferior temporal sectors most commonly affected [40].
Several investigations identified EZ disruption as a marker of disease severity and progression. Jayakar et al. emphasized that EZ loss reflects significant photoreceptor loss and severe toxicity, while Ahn et al. demonstrated that an increasing EZ defect length and reduced fovea PR defect distance were reliable indicators of progression [42,44]. Quantitative thresholds were also proposed, with one study reporting that a partial EZ attenuation of ≥1.9% achieved a high sensitivity and specificity for toxicity detection [41].
EZ changes were repeatedly shown to correlate with functional impairment, including reduced visual acuity and visual field sensitivity [36,42,44]. Structural–functional correspondence was further supported by studies reporting concordant OCT and visual field abnormalities in parafoveal configurations [42,43]. Kim et al. used a clock-hour analysis of parafoveal OCT scans to map the distribution of HCQ-related OR changes. They found that EZ and RPE alterations were not uniform but demonstrated preferential involvement in certain clock-hour sectors, particularly in the inferotemporal and superotemporal parafovea, regions known to correspond with early functional deficits on VF testing. This topographic approach provides additional sensitivity in detecting localized early toxicity and underscores the value of a spatially resolved analysis when monitoring patients at risk [40].
Despite the usefulness of the EZ in detecting HCQ retinopathy, it requires a subjective evaluation of the damage extent, being, therefore, subjective to errors such as observer-related errors. Talcott et al. introduced the EZ At-Risk parameter as an automated OCT biomarker for HCQ retinopathy, quantifying areas of subclinical EZ alteration likely to undergo progression. In a cohort of HCQ users, the mean EZ At-Risk burden was significantly greater in eyes with established toxicity compared with non-toxic HCQ eyes and healthy controls, and this parameter demonstrated a strong association with the HCQ dosage when normalized to both the actual and ideal body weight. These findings support the utility of automated EZ metrics for early risk stratification and objective monitoring. The authors suggest that additional research is required to clarify the longitudinal behavior of this novel biomarker in patients receiving HCQ, to compare it with other quantitative measures, to investigate potential threshold values relevant for toxicity risk stratification, and to strengthen correlations with disease severity [38].
In a similar manner, Ugwuegbu et al. applied a semi-automated segmentation approach to OCT macular cube scans, deriving volumetric and en-face measurements of ORL. Eyes with HCQ retinopathy demonstrated a significant thinning of the ONL/HFL-EZ complex in parafoveal regions as well as quantifiable EZ-RPE attenuation on en-face mapping, changes that were detectable even in early disease. A longitudinal assessment further confirmed the progressive structural decline in the affected eyes [48].
These studies highlight the potential of both automated and semi-automated OCT-derived biomarkers to identify the early structural alterations, quantify the disease burden, and monitor the progression beyond conventional clinical and functional testing.
Yucel Gencoglu et al. reported a reduced reflectivity of the EZ, ELM, and RPE in the parafoveal and perifoveal regions of HCQ users compared with controls, while the central foveal reflectivity was often preserved or even increased. Reflectivity losses in the ELM and RPE were most pronounced in eyes with an established EZ disruption, and such alterations were closely associated with worse visual outcomes, highlighting their prognostic significance [37].
Evidence across multiple studies supports EZ disruption as a pivotal diagnostic marker in HCQ retinopathy, representing a critical transition from sub-clinical structural alteration to clinically relevant disease. Partial attenuation or focal EZ discontinuity consistently precedes ELM and RPE involvement, and shows strong correlations with VF sensitivity loss and reduced VA. Importantly, EZ integrity appears to predict disease progression even after drug discontinuation. While the traditional EZ assessment relies on qualitative interpretation, emerging automated and quantitative metrics offer new directions for objective risk stratification and longitudinal monitoring.
3.3. Inner Retinal Alterations
Across studies, findings related to the inner retinal layer (IRL) involvement in HCQ exposure are more heterogeneous than those reported for OR alterations. While several studies identified the thinning of the GCL, GCIPL, or RNFL, others failed to detect significant IR changes, particularly in early or asymptomatic patients (Table 3).

Table 3.
Published studies that analyze HCQ toxicity using OCT focused on IR alterations.
Multiple investigations reported GCL or GCIPL thinning in HCQ-treated patients, including those without clinically evident retinopathy, suggesting that IR involvement may occur before overt toxicity in the selected cohorts [51,53,63,65]. Sectoral thinning was frequently observed, affecting the parafoveal or perifoveal regions, rather than the foveal center [53,60]. Sonalcan et al. reported a reduced mean and temporal RNFL thickness as well as decreased GCIPL thickness in HCQ users, although no differences were observed between HCQ subgroups stratified by treatment duration [51].
Several studies found associations between IR thinning and a longer HCQ exposure or higher cumulative dose. Agcayazi et al. reported correlations between GCL thinning and HCQ intake duration, while Kim et al. observed macular GCL and RNFL thinning predominantly in patients with established retinopathy [53,54]. Godinho et al. found a negative correlation between HCQ treatment duration and foveal GCL thickness, further supporting a potential exposure-related effect [58].
Longitudinal and severity-based analyses suggest that IR thinning may be more prominent in advanced disease stages. Membreno et al. showed that IR thinning was largely absent in early disease but became evident in more severe HCR groups, whereas OR thinning was present even in mild cases [56]. Similarly, Mondal et al. reported damage involving both IR neurons and ORL in long-term HCQ users [55].
In contrast, several studies reported no significant IR differences between HCQ-treated patients and controls. Jung et al. found no differences in macular GCL thickness between groups despite detecting early functional abnormalities on mfERG [50]. Mimier Janczak et al., similarly, reported no OCT detectable differences in retinal, RNFL, GCL, or GCIPL thickness between SLE patients receiving HCQ and controls, and no correlation between HCQ treatment duration and retinal parameters [52].
Findings vary when analyzing IR involvement in HCQ retinopathy. While some studies did not report any significant findings regarding GCL thickness between HCQ patients and controls [23,50,52], others found an early thinning in the perifoveal GCL thickness as a sign of early degeneration [53,65], with signs of correlations between HCQ intake and IR thinning [57,58,60]. IR thinning appears more consistently associated with advanced or established toxicity, suggesting that IR OCT parameters may function as markers of disease severity rather than early detection. Peripapillary retinal nerve fiber layer measurements show limited diagnostic reliability and should not be used in isolation for toxicity screening [62,63,64].
3.4. Choroid
Studies showed that, in HCQ retinopathy, the first affected layer in the choroid is the choriocapillaris (CC), objectifying the importance of OCT examination of this area (Table 4). The CC is the source of nutrients for the ORL, including RPE and PRL.

Table 4.
Published studies that analyze HCQ toxicity using OCT focused on choroidal alterations.
Choroidal thickness (CT) findings in HCQ-intake users are heterogeneous, with reports of both choroidal thinning and relative thickening depending on cohort characteristics, disease activity, and retinopathy status. Overall, the data suggest that choroidal impairment is more consistently observed in established or advanced HCQ retinopathy, whereas earlier-stage or short-duration HCQ cohorts show more variable patterns. While most authors declared a lower CT to be associated with HCQ intake, the underlying disease may lead to other choroidal modifications that could either mask or influence these results. Braga et al. found an increase in CT in SLE associated with lupus nephritis that was not linked to disease duration [75].
Several studies reported reduced CT or signs of choroidal impairment in eyes with HCQ retinopathy, particularly in advanced disease. Ahn et al. found significantly thinner CT at most measured locations in eyes with HCQ retinopathy compared with eyes without, with severity significantly associated with reduced CT. CT was also lower in areas exhibiting OR defects compared with areas without [73]. Halouani et al. similarly reported lower subfoveal and mean choroidal parameters in advanced retinopathy compared with controls, supporting the concept of choroidal involvement in more severe disease [68].
Additional studies suggested a link between HCQ exposure and reduced choroidal metrics. Hasan et al. reported a relationship between an increased cumulative HCQ dose and decreased choroidal volume, and found that choroidal vascular parameters were significantly lower in the study group than controls [67]. Polat et al. reported reduced subfoveal CT in HCQ patients compared with controls, and further described decreased temporal CT in patients with >5 years of HCQ use. These results suggest that early parafoveal RPE thickening may represent an adaptive or preclinical structural response to drug exposure, preceding the reflectivity loss and disruption observed in manifest toxicity [71].
Arias-Peso et al. reported variable CT alterations in patients with SLE under HCQ treatment. Their results indicate that patients treated with HCQ for less than five years exhibited a thicker choroid compared to control subjects, particularly in the central, nasal, and superior sectors. Conversely, individuals receiving HCQ therapy for more than five years demonstrated a significant reduction in CT, suggesting a thinning effect associated with prolonged treatment duration. Their results also revealed sectoral differences, with thinner CT observed in the temporal and inferior regions in association with HCQ exposure. Moreover, a positive correlation was identified between disease activity (measured by the SLEDAI score) and HCQ dosage, implying that higher doses of HCQ may be required to achieve adequate disease activity control [66].
The relationship between CT and HCQ exposure metrics remains inconsistent. Ahn et al. reported a positive correlation between subfoveal choriocapillaris thickness and HCQ duration and cumulative dose adjusted for body weight [73], while Ru reported no correlation between CT and HCQ duration or cumulative dose in the pediatric population [69]. One study reported a decreased choroidal volume with a higher cumulative dose [67], while another emphasized the influence of disease activity and short-duration thickening patterns [66].
Choroidal involvement in HCQ appears to reflect drug-related effects with disease-specific inflammatory or microvascular processes. While several studies report reduced CT, CVI, and CC perfusion in patients with long-term HCQ exposure or established retinopathy, others describe early CT potentially related to inflammatory activity. These discrepancies highlight the susceptibility of choroidal metrics to systemic confounding factors. Consequently, OCT parameters may serve as adjunctive biomarkers of disease progression rather than primary diagnostic indicators when analyzing the choroid, with CVI emerging as a potentially more stable metric than absolute thickness measurements.
3.5. OCT-A Findings
The evaluation of retinal vascular alterations using optical coherence tomography angiography (OCT-A) has become increasingly important in the early detection of HCQ-induced retinal toxicity. OCT-A provides a non-invasive, high-resolution assessment of the retinal microvasculature, allowing a detailed visualization of both superficial (SVP) and deep (DVP) capillary plexuses. Since vascular affliction may precede overt structural or functional changes detectable using imaging or VF testing, OCT-A can serve as a sensitive biomarker for subclinical toxicity. A quantitative analysis of the vessel density (VD) and perfusion parameters enables the identification of subtle microvascular disturbances, particularly in the parafoveal and perifoveal regions, which are preferentially affected in HCQ toxicity. Table 5 offers the parameters of the published studies that analyzed OCT-A in HCQ patients.

Table 5.
Published studies that analyze HCQ toxicity focused on OCT-Angiography alterations.
Most studies agree on a decrease in VD across the SVP and DVP in patients with HCQ intake > 5 years. While some authors found a decrease in VD compared to healthy controls regardless of treatment duration [93], other studies support these findings by objectifying a correlation with the cumulative HCQ dosage regardless of treatment duration [90]. However, there are separate studies that suggest the lack of any type of vascular involvement in HCQ toxicity, with their studies finding no statistical difference between the HCQ and control groups [83], or even an increase in VD in the HCQ groups compared to controls.
Leclaire et al. highlighted that SLE patients are predisposed to a reduction in retinal VD due to pathological alterations caused by the disease and not HCQ toxicity [79]. Subasi et al. investigated the retinal microvascular changes in patients with SLE, reporting significant reductions in VD within both SVP and DVP compared to healthy controls. The decrease was particularly pronounced in the perifoveal regions of the deep plexus, while the superior parafoveal and temporal areas were relatively spared. Furthermore, a subgroup analysis revealed no structural differences between SLE patients with or without renal involvement, though perifoveal DVP alterations in the superior quadrant were more evident in those with nephropathy, indicating potential diagnostic value for systemic disease activity [86]. These results suggest that microvascular impairment in SLE primarily affects the deep retinal circulation and may reflect disease-related vascular pathology rather than HCQ toxicity, underscoring the relevance of OCT-A in the early detection of subclinical vascular changes in SLE.
Yu et al. reported a reduction in retinal microvascular density in SJS patients, with and without HCQ treatment, compared to healthy controls, with an even greater decrease observed in the HCQ-treated group. Visual acuity was significantly reduced in both the SJS and HCQ groups relative to controls, and more severely affected in patients receiving HCQ. A quantitative OCT-A analysis further revealed reductions in both the superficial and deep microvascular indices in the HCQ group compared to SJS alone. VD was significantly decreased in multiple parafoveal and perifoveal sectors and in the central regions in SJS versus controls, with more extensive reductions in the HCQ-treated cohort [85]. These findings highlight OCT-A-derived VD as a promising non-invasive biomarker for evaluating disease severity and vascular compromise in SJS and its modulation by systemic therapy.
Similarly, Liu et al. observed comparable microvascular alterations in patients with RA, with further reductions associated with long-term CQ therapy. Their study demonstrated that RA patients exhibited significantly decreased retinal VD compared to healthy controls, particularly within the SVP, while those treated with CQ showed an additional decline in both SVP and DVP parameters. The reduction in VD was most evident in the parafoveal and perifoveal regions, as well as in the superior and temporal retinal sectors. Conjunctival VD was likewise diminished in both RA- and CQ-treated groups, suggesting that the microvascular compromise extends beyond the retina. These findings indicate that CQ exposure may exacerbate the retinal and conjunctival vascular rarefaction in autoimmune disease, and further support the utility of OCT-A as a sensitive imaging tool for detecting early, subclinical vascular changes related to disease progression and antimalarial drug toxicity [84].
Multiple studies observed an increase in the foveal avascular zone (FAZ) size which was positively correlated with the cumulative HCQ dose and duration. Furthermore, a decrease in FAZ VD in both SVP and DVP has been objectified in patients with >5 years of HCQ intake. Despite these findings, alterations in FAZ did not correlate with clinical symptoms as there were no significant differences when evaluating VD parameters between normal and abnormal mfERG in HCQ patients, suggesting that microvascular alterations could appear as a marker of subclinical toxicity or as a consequence of disease activity [89].
The use of inter-eye symmetry may provide additional data regarding VD as macular VD was shown to be reduced in HCQ patients compared with healthy controls in the absence of HCQ retinopathy [76].
Halouani et al. investigated CC alterations in patients receiving long-term HCQ therapy and found significant flow-related deficits (FDs) in eyes with HCQ retinopathy. Compared with both HCQ-treated eyes without signs of toxicity and healthy controls, eyes with confirmed HCQ retinopathy showed a significantly higher percentage of CC FD, a larger mean FD size, and a lower FD number, indicating more extensive and confluent areas of CC nonperfusion. The total area of flow deficits was also increased in the toxicity group relative to controls, whereas no significant differences were observed between the non-toxicity and control groups for most CC parameters. These findings provide strong evidence of CC involvement in the pathophysiology of HCQ-induced retinal toxicity, suggesting that CC flow impairment may occur secondary to OR and RPE damage or as a direct vascular effect of the drug. The authors proposed that a quantitative CC flow-deficit analysis may offer valuable insight into the microvascular alterations underlying HCQ retinopathy and may aid in detecting early choroidal involvement before irreversible structural damage develops [87].
The OCT-A studies highlight microvascular alterations in HCQ patients, particularly the reduced VD and enlarged FAZ after prolonged exposure. However, the interpretation of these findings is complicated by the intrinsic microvascular involvement of underlying autoimmune diseases, such as SLE and RA. While the reduced macular VD and CC hypoperfusion may reflect an early-toxicity-related vascular compromise, these changes lack sufficient specificity when considered in isolation. Therefore, OCT-A parameters should be interpreted as complementary diagnostic biomarkers, integrated with structural OCT findings and clinical context, rather than used as standalone screening tools.
4. Discussion
HCQ retinopathy represents a diagnostic challenge because structural retinal damage may develop insidiously and progress despite treatment discontinuation, often before patients report visual symptoms. The present narrative review synthesizes the current evidence on OCT- and OCT-A-derived biomarkers associated with HCQ exposure, highlighting their respective diagnostic roles, limitations, and clinical applicability across disease stages.
When considered together, the available evidence indicates that structural OCT demonstrates a greater consistency and robustness than OCT angiography (OCT-A) for the detection of HCQ-related retinal toxicity, particularly in the early or subclinical stages. Across multiple studies, OCT-derived structural biomarkers, most notably, sectoral OR, ONL thinning, and EZ alterations, show reproducible patterns that correlate with cumulative exposure, disease stage, and functional impairment. In contrast, the OCT-A findings, including reduced macular VD and FAZ enlargement, exhibit substantial inter-study variability and are strongly influenced by the underlying autoimmune disease, inflammatory activity, and imaging methodology.
While OCT-A can reveal microvascular alterations and may detect changes in selected high-risk or advanced cases, these vascular metrics lack specificity for HCQ toxicity when used in isolation. Consequently, OCT-A appears best suited as a complementary modality, providing supportive microvascular information alongside structural OCT, rather than as a stand-alone screening tool. This hierarchical performance supports current screening strategies that prioritize structural OCT while reserving OCT-A for selected cases requiring additional pathophysiological or prognostic insight.
Membreno et al. proposed a classification of HCQ retinopathy severity based on the extent of EZ disruption measured on OCT. Patients were divided into four groups: group 1, with <100 μm of EZ loss; group 2, with 100–1000 μm of EZ loss; group 3, with >1000 μm of EZ loss but the preservation of a central foveal EZ island > 500 μm; and group 4, with <500 μm of residual foveal EZ. Their analysis showed that groups 1 and 2 did not differ significantly from the control subjects in terms of visual acuity, whereas patients in groups 3 and 4 exhibited measurable declines in visual function [56]. In a similar approach, Allahdina et al. categorized the affected eyes into four OCT-based stages: stage 1 with subtle parafoveal alterations, stage 2 with definite localized parafoveal damage but preserved fovea, stage 3 with extensive parafoveal involvement and intact fovea, and stage 4 with foveal involvement. A longitudinal follow-up after drug cessation revealed that eyes in stages 1 and 2 generally maintained stable VA and VF sensitivity, with stage 1 cases even demonstrating functional improvement on mfERG parameters. By contrast, eyes in stages 3 and 4 showed progressive structural deterioration and significant vision loss. Notably, in stages 2 and 3, the length of the central EZ island progressively shortened in the majority of eyes (approximately two-thirds of stage 2 and nearly nine-tenths of stage 3), indicating that, beyond the earliest stage, the discontinuation of HCQ does not fully prevent retinopathy progression [28]. This aspect aligns with other studies that confirm that HCQ maculopathy advances despite drug cessation [25,29,44].
The 2016 screening recommendations for CQ and HCQ retinopathy proposed by the Marmor et al. support screening at baseline followed by annual screening after 5 years for patients without additional risk factors such as a high daily dosage (>5 mg/kg real body weight for HCQ and >2.3 mg/kg real body weight in CQ), a longer duration of use (>5 years), concomitant renal disease with a subnormal filtration rate, the use of other retinotoxic drugs such as Tamoxifen, or the presence of a concomitant macular disease that could influence susceptibility to HCQ retinopathy. In these high-risk patients, screening should be performed annually [98]. The recently published 2025 revisions continue to follow these recommendations, adding initiation at an advanced age as another high-risk factor. They propose annual screening using OCT, wide-pattern autofluorescence as the primary annual screening techniques with visual field and ERG as secondary, confirmatory techniques, while OCT-A was not recommended for annual screening [99].
Some societies recommend that the initial ophthalmological consult not be mandatory for HCQ toxicity screening, as the clinical benefit is insufficient to justify the required costs [100], while others still recommend baseline testing to rule out confounding diseases [101]. These contradictory statements arise from the socio-economic costs that may burden the national health services, therefore highlighting the need for better toxicity biomarkers. Furthermore, a lack of baseline OCT data useful in future comparisons may lead to a false-positive HCQ retinopathy diagnosis, taking the patient off an effective, well-tolerated treatment regimen and starting more expensive treatments which require additional investigations [102].
Despite the existing protocols and guidelines, multiple atypical presentations have been reported, spanning from rapid onset [103,104,105,106,107], peripheral alterations [108,109], and unilateral or asymmetric ocular toxicity [110,111]. While the recommendations for screening are after 5 years of HCQ therapy, other confounding factors may lead to faster toxic retinal modifications [112,113]. A retrospective analysis performed on 95 Asian patients diagnosed with HCQ retinopathy over 13 years identified 14 cases with atypical presentations, with the authors highlighting the need for vigilance when performing screening for HCQ toxicity [114].
Mohapatra et al. identified nine cases of accelerated HCQ toxicity, the fastest appearing in 2 months of drug intake at a cumulative dose of 18 g, with the other eight cases appearing in under 12 months of treatment [107]. Similar reports [103,104,105,106] have been published with early macular toxicity that may have been caused by an increased bioavailability of HCQ due to decreased cytochrome p450 enzyme activity that could be affected by other medications such as nonsteroidal anti-inflammatory drugs or genetic predisposition. Lee et al. highlighted the existence of genetic polymorphisms in Korean SLE patients taking HCQ that led to different HCQ blood level concentrations despite a similar prescribed dosage [115].
While Petri et al. found a relationship between HCQ blood levels and toxicity [116], other studies analyzed HCQ blood levels to correlate with retinal structural and vascular toxicity but did not observe any significant relationships between these parameters [80,81,92]. Liu et al. highlighted that SLE patients with a high concentration of blood HCQ presented a better controlled disease activity, being negatively correlated with disease severity scores and inflammation markers [81].
Multiple studies that focused on the incidence of HCQ retinopathy among different populations reported that the prevalence of maculopathy in recent years was lower than previously reported [39,46,117]. Different Asian populations reported a pericentral or mixed pattern in the progression of HCQ retinopathy [33,35,36,40,44,73,118], drawing attention to the need for a wider structural and functional analysis in these populations compared to the standard “bull’s eye” maculopathy [34]. Short obese women may be more susceptible to HCQ overdose as an ideal body weight may be better in evaluating the daily dose in these patients. Daily dosing based on the older 6.5 mg/kg ideal weight threshold is safer in women with a BMI of 30 kg/m2 or more [47,119].
In contrast to the initial thickening of OR structures seen in patients with drusen-like deposits (DLDs), subsequent degenerative processes may lead to the thinning of the ONL, photoreceptor layer (PRL), and other ORL. As the inflammatory insult from vasculitis subsides or becomes chronic, ischemia, immunocomplex deposition, and microvascular compromise may trigger the apoptosis or shrinkage of PR cell bodies and supporting Müller cells. Over time, this secondary degeneration leads to measurable reductions in ONL thickness and PRL thickness. In parallel, decreased perfusion and chronic stress may impair the RPE and outer segment integrity, further contributing to the structural atrophy of the OR. These thinning changes may lag behind the earlier thickening, but mark a transition from active inflammation toward permanent tissue loss. Although Kitay et al. observed no significant change in mfERG between groups, implying a preserved cone function despite structural differences, the structural thinning could signal the declining resilience of PR before the functional loss becomes detectable [14].
Kukan et al. found that SLE patients with DLD exhibited lower CVI values compared to healthy controls, while no significant difference was observed between SLE eyes with and without DLD. Eyes with DLD showed a thicker GCL but the thinning of the OR and PR relative to those without DLD, indicating concomitant neurodegenerative changes. The authors proposed that the presence of DLD may reflect a more active inflammatory disease state, characterized by choroidal vascular alterations associated with immune complex deposition and vasculitis-type processes. These findings suggest that DLD could serve as an indicator of heightened inflammatory activity and evolving retinal degeneration in SLE [70].
Zirtiloglu et al. evaluated the RNFL thickness and radial peripapillary capillary (RPC) VD in patients with systemic sclerosis (SS) using OCT-A and found no significant overall differences compared with healthy controls. However, several microstructural and vascular parameters showed correlations with disease activity and treatment profiles. Specifically, the superior RNFL, whole, peripapillary, superior and inferior RPCvalues significantly decreased with increasing antinuclear antibody titers, while the nasal RNFL thickness declined with a longer disease duration, suggesting a progressive microvascular compromise with the disease severity. Interestingly, patients using HCQ demonstrated higher RPC inside values than those not on HCQ, and the RPC whole, RPC peripapillary, and RPC nasal densities were significantly higher in corticosteroid users compared to non-users [120]. These results indicate that, although global peripapillary perfusion and RNFL thickness remain preserved in SS, localized vascular attenuation correlates with serologic disease activity and duration, whereas certain systemic therapies such as HCQ and corticosteroids may exert a protective or modulatory effect on peripapillary microcirculation.
HCQ retinopathy has been shown to have racial variations. While, most often, the defect follows a parafoveal pattern, being a specific clinical finding, Melles and Marmor found that, in the Asian population, it can have a pericentral disposition [121]. This finding highlights the need for an extensive level of attention when evaluating HCQ toxicity in certain populations as smaller OCT B-scans or 10-2 perimetry may overlook existing peripheral lesions and delay drug cessation.
Understanding the mechanisms of HCQ toxicity may provide additional guidelines that could be used in the pediatric population. Bousquet et al. reported a case of toxic retinopathy in a 23-year-old patient undergoing under 200 mg/day HCQ for 13 years, finding specific retinal damage on OCT, highlighting the need for screening guidelines in the pediatric population [122]. Lu et al. draw attention to the need for a close ophthalmological examination in order to diagnose adverse effects in children, as, in spite of the low HCQ dose, renal impairment caused visual loss in a 14-year-old patient [123]. A group led by AlAhmed evaluated the screening rate for HCQ retinopathy in the pediatric rheumatology department and developed an algorithm that led to the increase from 65% to 85% in 12 months, emphasizing the importance of interdisciplinarity in increasing awareness among the medical provider and patients [124].
Ru et al. found that CT is lower in juvenile SLE under HCQ than in heathy age-matched controls with a negative correlation between CT and the systemic cytokine profile [69]. Given that alterations in CT have been shown to appear secondary either to HCQ or to the autoimmune disease itself, in the absence of structural and/or functional retinal damage, CT cannot be used alone as a biomarker. Furthermore, physiological fluctuations have been shown to influence CT [125]; therefore, it has a low reliability in detecting HCQ toxicity, especially in the presence of a systemic inflammatory disorder.
Cystoid macular edema has also been reported in rare cases as a side effect of HCQ treatment. A proposed mechanism is that the accumulation of HCQ in the RPE provokes a disruption in the pump function that will lead to intraretinal fluid accumulation [126,127]. Given this mechanism, carbonic anhydrase inhibitors have been used to increase the RPE pump function and stimulate fluid absorption [128]. Dexamethasone has also been suggested in refractory cases [32,129].
While HCQ is mainly used in autoimmune and inflammatory diseases, it has been described in other pathologies as well. The SARS-CoV-2 pandemic caused an increase in the search of new immunomodulatory medicine, HCQ being proposed as a prophylactic drug used to reduce the risk of severe acute respiratory syndrome [130]. Clinical trials have described using HCQ as an adjunctive therapy for advanced metastatic melanoma in association with other drugs, now known to cause retinal toxicity [131]. Understanding the molecular mechanisms and evolution of HCQ retinopathy may also lead to safety profiles regarding the use of this drug in other diseases.
4.1. Toward a Diagnostic Hierarchy and Personalized Screening
The collective evidence supports a hierarchical diagnostic framework in which structural OCT biomarkers, particularly the ORT metrics and EZ integrity, form the foundation of early toxicity detection. IR and choroidal changes provide additional information regarding disease severity and progression, while the OCT-A findings offer complementary insights into vascular involvement. This layered approach aligns with current screening recommendations and underscores the importance of integrating multiple imaging domains rather than relying on single parameters.
Advances in automated OCT analysis and artificial-intelligence-assisted segmentation hold promise for improving reproducibility, reducing observer variability, and enabling personalized screening strategies.
Future automated machine-learning techniques are required for the proper quantification of EZ loss, RPE damage, and the effects of the systemic autoimmune disease on the retina. While some studies have described using automated machine learning for evaluating HCQ toxicity [38,41,43], researchers have attempted to use automated retinal vessel analysis to measuring venular dilation and assess rheumatic disease inflammatory activity [132]. Quantitative metrics such as automated EZ attenuation, ORT mapping, and standardized sectoral analysis may facilitate earlier detection and more accurate risk stratification in patients receiving long-term HCQ therapy.
4.2. Limitations
This review has several limitations. As a narrative synthesis, it does not provide quantitative pooled estimates or a formal risk-of-bias assessment. Considerable heterogeneity exists among the included studies with respect to the imaging protocols, segmentation algorithms, patient populations, and definitions of toxicity. Additionally, the lack of a separate-disease-grouped analysis may lead to confounding effects of the underlying autoimmune disease activity that limit the specificity of our findings. Nevertheless, the consistency of key structural OCT biomarkers across diverse cohorts strengthens the clinical relevance of the conclusions drawn.
Most included studies were observational in design, predominantly cross-sectional or retrospective, which limits causal inference and the ability to assess the temporal progression or predictive performance of individual biomarkers. Variability in OCT and OCT-A devices and technology, image acquisition parameters, and post-processing methods further complicates the direct comparison of outcomes and may contribute to inconsistent findings, particularly for the choroidal and microvascular parameters. Moreover, OCT-A-derived vascular metrics are especially susceptible to disease-related and systemic confounders, underscoring the need for cautious interpretation when attributing these changes solely to HCQ toxicity.
Future prospective studies using standardized imaging protocols and unified diagnostic criteria are required to validate proposed biomarkers and to clarify their role in risk stratification and screening strategies.
5. Conclusions
HCQ retinopathy is a potentially vision-threatening complication that is correlated with the cumulative dose, treatment duration and specific risk factors. This narrative review highlights the role of OCT-derived structural biomarkers in the early detection and monitoring of HCQ-related retinal toxicity.
Alterations in the ORL, particularly EZ attenuation and ONL thinning, could be used as indicators of early toxicity, often preceding functional impairment. IR and choroidal changes appear to reflect more advanced disease stages and provide complementary information regarding severity and progression. OCT-A VD metrics have been considered useful in revealing microvascular alterations associated with prolonged exposure; however, these findings lack sufficient specificity when considered in isolation and should be interpreted within the context of structural OCT findings and the underlying autoimmune disease activity. An integrated, multimodal imaging approach that prioritizes structural OCT biomarkers and incorporates OCT-A as a complementary tool offers the most reliable diagnostic strategy for HCQ retinopathy.
While current OCT-derived biomarkers show promise in detecting HCQ retinopathy, the future implementation of automated OCT analysis and artificial-intelligence-assisted biomarker quantification may enhance screening precision, reduce observer variability, and enable personalized risk stratification, ultimately improving patient safety and long-term visual outcomes.
Author Contributions
Conceptualization, A.L.D. and C.A.; methodology, A.L.D., V.C.D., and C.A.; validation, C.P., I.M.B., and A.I.A.; formal analysis, C.M.B.; investigation, A.L.D. and V.C.D.; resources, A.L.D.; data curation, C.A.S., M.R., and V.L.; writing—original draft preparation, A.L.D. and V.C.D.; writing—review and editing, C.M.B., A.I.A., and C.P.; supervision, C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BCVA | Best corrected visual acuity |
| CC | Choriocapillaris |
| CCP | Choriocapillaris plexus |
| CCT | Choriocapillaris thickness |
| CMT | Central macula thickness |
| CQ | Chloroquine |
| CRP | C Reactive Protein |
| CT | Choroidal thickness |
| CVI | Choroidal vascular index |
| DAI | Disease activity index |
| DLD | Drusen-like deposit |
| DMIR | Deep macular ischemia ratio |
| DTMI | Deep total microvascular index |
| DVP | Deep vascular plexus |
| ELM | External limiting membrane |
| ESR | Erythrocyte sedimentation rate |
| EZ | Ellipsoid zone |
| FAF | Fundus autofluorescence |
| FAZ | Foveal avascular zone |
| FFA | Fundus fluorescein angiography |
| FD | Flow-related deficits |
| FT | Foveal thickness |
| G | Group |
| GCL | Ganglion cell layer |
| GCIPL | Ganglion cell inner-plexiform layer |
| HCR | Hydroxychloroquine retinopathy |
| HCQ | Hydroxychloroquine |
| HFL | Henle fiber layer |
| ID | Interdigitation |
| IL | Interleukin |
| Ig | Immunoglobulin |
| INL | Inner nuclear layer |
| IPL | Inner plexiform layer |
| IR | Inner retina |
| IRL | Inner retinal layers |
| IRT | Inner retina thickness |
| IS | Inner segment |
| JSLE | Juvenile systemic lupus erythematosus |
| LCA | Luminal choroidal area |
| mfERG | Multifocal electroretinogram |
| MI | Macular integrity |
| MT | Macular thickness |
| NIR | Near-infrared reflectance |
| NIR-AF | Near-infrared autofluorescence |
| ONL | Outer nuclear layer |
| OCT | Optical coherence tomography |
| OCT-A | Optical coherence tomography angiography |
| OR | Outer retina |
| ORL | Outer retinal layers |
| ORT | Outer retina thickness |
| OS | Outer segment |
| PR | Photoreceptor |
| PRL | Photoreceptor layer |
| pRNFL | Peripapillary retinal nerve fiber layer |
| QOL | Quality of life |
| RA | Rheumatoid arthritis |
| RGC | Retinal ganglion cell |
| RPC | Radial peripapillary capillaries |
| RPE | Retinal pigmented epithelium |
| RNFL | Retinal nerve fiber layer |
| RT | Retinal thickness |
| SCA | Stromal choroidal area |
| SFCT | Subfoveal choroidal thickness |
| SJS | Sjögren’s syndrome |
| SLE | Systemic lupus erythematosus |
| SLICC-SDI | Systemic Lupus International Collaborating Clinics–Systemic Damage Index |
| SMAR | Superficial macular area ratio |
| SMIR | Superficial macular ischemia ratio |
| SS | Systemic sclerosis |
| STMI | Superficial total microvascular index |
| SVP | Superficial vascular plexus |
| SW-AF | Short-wave autofluorescence |
| TCA | Total choroidal area |
| V | Visit |
| VA | Visual acuity |
| VD | Vessel density |
| VF | Visual field |
| VIS | Visible-light |
| VRQOL | Vision-related quality of life |
References
- Ferreira, A.; Anjos, R.; José-Vieira, R.; Afonso, M.; Abreu, A.C.; Monteiro, S.; Macedo, M.; Andrade, J.P.; Furtado, M.J.; Lume, M. Application of optical coherence tomography angiography for microvascular changes in patients treated with hydroxychloroquine: A systematic review and meta-analysis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 2221–2233. [Google Scholar] [CrossRef]
- Yusuf, I.H.; Issa, P.C.; Ahn, S.J. Hydroxychloroquine-induced Retinal Toxicity. Front. Pharmacol. 2023, 14, 1196783. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Kostopoulou, M.; Andersen, J.; Aringer, M.; Arnaud, L.; Bae, S.-C.; Boletis, J.; Bruce, I.N.; Cervera, R.; Doria, A.; et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann. Rheum. Dis. 2024, 83, 15–29. [Google Scholar] [CrossRef]
- Jones, P.; Kalra, G.; Al-Sheikh, M.; Chhablani, J. Mimickers of hydroxychloroquine retinal toxicity. Clin. Exp. Ophthalmol. 2024, 52, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Snow, Z.; Seely, K.; Barrett, S.; Pecha, J.; Goldhardt, R. Target in Sight: A Comprehensive Review of Hydroxychloroquine-Induced Bull’s Eye Maculopathy. Curr. Ophthalmol. Rep. 2024, 12, 38–48. [Google Scholar] [CrossRef]
- Cheong, K.; Ong, C.; Chandrasekaran, P.; Zhao, J.; Teo, K.; Mathur, R. Review of Retinal Imaging Modalities for Hydroxychloroquine Retinopathy. Diagnostics 2023, 13, 1752. [Google Scholar] [CrossRef] [PubMed]
- Donica, V.C.; Alexa, A.I.; Pavel, I.A.; Danielescu, C.; Ciapă, M.A.; Donica, A.L.; Bogdănici, C.M. The Evolvement of OCT and OCT-A in Identifying Multiple Sclerosis Biomarkers. Biomedicines 2023, 11, 3031. [Google Scholar] [CrossRef] [PubMed]
- Montesel, A.; Sacconi, R.; La Rubia, P.; Querques, G. Impact of cumulative dose of hydroxychloroquine on retinal structures. Graefe’s Arch. Clin. Exp. Ophthalmol. 2024, 263, 235–237. [Google Scholar] [CrossRef]
- Melles, R.B.; Marmor, M.F. Rapid Macular Thinning Is an Early Indicator of Hydroxychloroquine Retinal Toxicity. Ophthalmology 2022, 129, 1004–1013. [Google Scholar] [CrossRef]
- Farvardin, M.; Peiravian, P.; Ravankhah, M.; Nowroozzadeh, M.H. Evaluation of changes in thickness of macular sublayers in patients using Hydroxychloroquine: A cross sectional case-control study and literature review. Cutan. Ocul. Toxicol. 2024, 44, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Salameh, N.; Doumit, C.A.; Jalkh, E.; Nehme, J. Association between hydroxychloroquine intake and damage to the outer nuclear layer in eyes without manifest retinal toxicity. BMC Ophthalmol. 2024, 24, 414. [Google Scholar] [CrossRef]
- Garg, A.K.; Wang, J.; Alonzo, B.; Yi, J.; Kashani, A.H. Photoreceptor outer segment reflectivity with ultrahigh resolution visible light optical coherence tomography in systemic hydroxychloroquine use. Transl. Vis. Sci. Technol. 2025, 14, 2. [Google Scholar] [CrossRef]
- Kitay, A.M.; Hanson, J.V.M.; Hasan, N.; Driban, M.; Chhablani, J.; Barthelmes, D.; Gerth-Kahlert, C.; Al-Sheikh, M. Functional and Morphological Characteristics of the Retina of Patients with Drusen-like Deposits and Systemic Lupus Erythematosus Treated with Hydroxychloroquine: A Retrospective Study. Biomedicines 2023, 11, 1629. [Google Scholar] [CrossRef]
- Tarassoly, K.; Miraftabi, A.; Sedaghat, A.; Parvaresh, M.M. Analysis of the outer retinal thickness pixel maps for the screening of hydroxychloroquine retinopathy. Int. Ophthalmol. 2022, 43, 1737–1743. [Google Scholar] [CrossRef]
- Borrelli, E.; Battista, M.; Cascavilla, M.L.; Viganò, C.; Borghesan, F.; Nicolini, N.; Clemente, L.; Sacconi, R.; Barresi, C.; Marchese, A.; et al. Impact of Structural Changes on Multifocal Electroretinography in Patients With Use of Hydroxychloroquine. Investig. Opthalmology Vis. Sci. 2021, 62, 28. [Google Scholar] [CrossRef]
- Sallam, M.A.; Beltagi, A.S.; Abdellatif, M.A.; Awadalla, M.A. Visual Impact of Early Hydroxychloroquine-Related Retinal Structural Changes in Patients with Systemic Lupus Erythematosus. Ophthalmologica 2021, 244, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Manoj, M.; Sahoo, R.R.; Singh, A.; Hazarika, K.; Bafna, P.; Kaur, A.; Wakhlu, A. Prevalence of hydroxychloroquine retinopathy with long-term use in a cohort of Indian patients with rheumatic diseases. Rheumatol. Int. 2021, 41, 929–937. [Google Scholar] [CrossRef]
- Kim, K.E.; Ahn, S.J.; Woo, S.J.; Park, K.H.; Lee, B.R.; Lee, Y.-K.; Sung, Y.-K. Use of OCT Retinal Thickness Deviation Map for Hydroxychloroquine Retinopathy Screening. Ophthalmology 2021, 128, 110–119. [Google Scholar] [CrossRef]
- Hasan, H.; Lotery, A.; Price, E.J.; Smith, G.T. An objective method of diagnosing hydroxychloroquine maculopathy. Eye 2020, 35, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Kumar, P.; Moulick, P.S.; Vats, S. Spectral domain optical coherence tomography–based prevalence of hydroxychloroquine maculopathy in Indian patients on hydroxychloroquine therapy: A utopia of underdiagnosis. Med. J. Armed Forces India 2020, 76, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Dias-Santos, A.; Ferreira, J.T.; Pinheiro, S.; Cunha, J.P.; Alves, M.; Papoila, A.L.; Moraes-Fontes, M.F.; Proença, R. Ocular involvement in systemic lupus erythematosus patients: A paradigm shift based on the experience of a tertiary referral center. Lupus 2020, 29, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Casado, A.; López-de-Eguileta, A.; Fonseca, S.; Muñoz, P.; Demetrio, R.; Gordo-Vega, M.A.; Cerveró, A. Outer Nuclear Layer Damage for Detection of Early Retinal Toxicity of Hydroxychloroquine. Biomedicines 2020, 8, 54. [Google Scholar] [CrossRef]
- Cabral, R.T.D.S.; Klumb, E.M.; Couto, M.I.N.N.; Carneiro, S. Evaluation of toxic retinopathy caused by antimalarial medications with spectral domain optical coherence tomography. Arq. Bras. Oftalmol. 2019, 82, 12–17. [Google Scholar] [CrossRef]
- Božinovic, M.S.T.; Babic, G.S.; Petrovic, M.; Karadžic, J.; Vulovic, T.Š.; Trenkic, M. Role of optical coherence tomography in the early detection of macular thinning in rheumatoid arthritis patients with chloroquine retinopathy. J. Res. Med. Sci. 2019, 24, 55. [Google Scholar]
- Cakir, A.; Ozturan, Ş.G.; Yildiz, D.; Erden, B.; Bolukbasi, S.; Tascilar, E.K.; Yanmaz, M.N.; Elcioglu, M.N. Evaluation of photoreceptor outer segment length in hydroxychloroquine users. Eye 2019, 33, 1321–1326. [Google Scholar] [CrossRef]
- Garrity, S.T.; Jung, J.Y.; Zambrowski, O.; Pichi, F.; Su, D.; Arya, M.; Waheed, N.K.; Duker, J.S.; Chetrit, Y.; Miserocchi, E.; et al. Early hydroxychloroquine retinopathy: Optical coherence tomography abnormalities preceding Humphrey visual field defects. Br. J. Ophthalmol. 2019, 103, 1600–1604. [Google Scholar] [CrossRef]
- Allahdina, A.M.; Chen, K.G.; Alvarez, J.A.; Wong, W.T.; Chew, E.Y.; Cukras, C.A. Longitudinal Changes in Eyes with Hydroxychloroquine Retinal Toxicity. Retina 2019, 39, 473–484. [Google Scholar] [CrossRef]
- Pham, B.H.; Marmor, M.F. Sequential Changes in Hydroxychloroquine Retinopathy up to 20 Years After Stopping the Drug: Implications for Mild Versus Severe Toxicity. Retina 2019, 39, 492–501. [Google Scholar] [CrossRef]
- Ruberto, G.; Bruttini, C.; Tinelli, C.; Cavagna, L.; Bianchi, A.; Milano, G. Early morpho-functional changes in patients treated with hydroxychloroquine: A prospective cohort study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 2201–2210. [Google Scholar] [CrossRef]
- Allahdina, A.M.; Stetson, P.F.; Vitale, S.; Wong, W.T.; Chew, E.Y.; Ferris, F.L., III; Sieving, P.A.; Cukras, C. Optical Coherence Tomography Minimum Intensity as an Objective Measure for the Detection of Hydroxychloroquine Toxicity. Investig. Opthalmology Vis. Sci. 2018, 59, 1953. [Google Scholar] [CrossRef]
- Ahn, S.J.; Joung, J.; Lee, S.H.; Lee, B.R. Intravitreal dexamethasone implant therapy for the treatment of cystoid macular Oedema due to hydroxychloroquine retinopathy: A case report and literature review. BMC Ophthalmol. 2018, 18, 310. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Kim, Y.Y.; Lee, H.; Park, S.-H.; Kim, S.-K.; Choe, J.-Y. Risk of Retinal Toxicity in Longterm Users of Hydroxychloroquine. J. Rheumatol. 2017, 44, 1674–1679. [Google Scholar] [CrossRef]
- Ahn, S.J.; Joung, J.; Lim, H.W.; Lee, B.R. Optical Coherence Tomography Protocols for Screening of Hydroxychloroquine Retinopathy in Asian Patients. Am. J. Ophthalmol. 2017, 184, 11–18. [Google Scholar] [CrossRef]
- Eo, D.-R.; Lee, M.G.; Ham, D.-I.; Kang, S.W.; Lee, J.; Cha, H.S.; Koh, E.; Kim, S.J. Frequency and Clinical Characteristics of Hydroxychloroquine Retinopathy in Korean Patients with Rheumatologic Diseases. J. Korean Med. Sci. 2017, 32, 522. [Google Scholar] [CrossRef] [PubMed]
- He, S.-K.; Lai, T.-T.; Hsieh, Y.-T. Optical coherence tomography characteristics in hydroxychloroquine retinopathy and the correlations with visual deterioration in Taiwanese. Taiwan J. Ophthalmol. 2024, 14, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Gencoglu, A.Y.; Ağın, A.; Colak, D.; Un, Y.; Ozturk, Y. Decreased peri-parafoveal RPE, EZ and ELM intensity: A novel predictive biomarker for hydroxychloroquine retinal toxicity. Graefe’s Arch. Clin. Exp. Ophthalmol. 2024, 262, 3543–3552. [Google Scholar] [CrossRef]
- Talcott, K.E.; Kalra, G.; Cetin, H.; Cakir, Y.; Whitney, J.; Budrevich, J.; Reese, J.L.; Srivastava, S.K.; Ehlers, J.P. Automated Evaluation of Ellipsoid Zone At-Risk Burden for Detection of Hydroxychloroquine Retinopathy. J. Pers. Med. 2024, 14, 448. [Google Scholar] [CrossRef]
- Alieldin, R.A.; Boonarpha, N.; Saedon, H. Outcomes of screening for hydroxychloroquine retinopathy at the Manchester Royal Eye Hospital: 2 years’ audit. Eye 2022, 37, 1410–1415. [Google Scholar] [CrossRef]
- Kim, K.E.; Kim, J.H.; Kim, Y.H.; Ahn, S.J. Clock-hour topography and extent of outer retinal damage in hydroxychloroquine retinopathy. Sci. Rep. 2022, 12, 11809. [Google Scholar] [CrossRef]
- Kalra, G.; Talcott, K.E.; Kaiser, S.; Ugwuegbu, O.; Hu, M.; Srivastava, S.K.; Ehlers, J.P. Machine Learning–Based Automated Detection of Hydroxychloroquine Toxicity and Prediction of Future Toxicity Using Higher-Order OCT Biomarkers. Ophthalmol. Retin. 2022, 6, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Jayakar, G.; De Silva, T.; Cukras, C.A. Visual Field Sensitivity Prediction Using Optical Coherence Tomography Analysis in Hydroxychloroquine Toxicity. Investig. Opthalmology Vis. Sci. 2022, 63, 15. [Google Scholar] [CrossRef]
- Dabit, J.Y.; Hocaoglu, M.; Moder, K.G.; Barkmeier, A.J.; Smith, W.M.; O’Byrne, T.J.; Crowson, C.S.; Duarte-García, A. Risk of hydroxychloroquine retinopathy in the community. Rheumatology 2021, 61, 3172–3179. [Google Scholar] [CrossRef]
- Ahn, S.J.; Seo, E.J.; Kim, K.E.; Kim, Y.J.; Lee, B.R.; Kim, J.-G.; Yoon, Y.H.; Lee, J.Y. Long-Term Progression of Pericentral Hydroxychloroquine Retinopathy. Ophthalmology 2021, 128, 889–898. [Google Scholar] [CrossRef]
- Jauregui, R.; Parmann, R.; Nuzbrokh, Y.; Tsang, S.H.; Sparrow, J.R. Spectral-Domain Optical Coherence Tomography Is More Sensitive for Hydroxychloroquine-Related Structural Abnormalities Than Short-Wavelength and Near-Infrared Autofluorescence. Transl. Vis. Sci. Technol. 2020, 9, 8. [Google Scholar] [CrossRef]
- Gobbett, A.; Kotagiri, A.; Bracewell, C.; Smith, J. Two years’ experience of screening for hydroxychloroquine retinopathy. Eye 2020, 35, 1171–1177. [Google Scholar] [CrossRef]
- Browning, D.J.; Easterbrook, M.; Lee, C. The 2016 American Academy of Ophthalmology Hydroxychloroquine Dosing Guidelines For Short, Obese Patients. Ophthalmol. Retin. 2019, 3, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Ugwuegbu, O.; Uchida, A.; Singh, R.P.; Beven, L.; Hu, M.; Kaiser, S.; Srivastava, S.K.; Ehlers, J.P. Quantitative assessment of outer retinal layers and ellipsoid zone mapping in hydroxychloroquine retinopathy. Br. J. Ophthalmol. 2018, 103, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Arndt, C.; Costantini, M.; Chiquet, C.; Afriat, M.; Berthemy, S.; Vasseur, V.; Ducasse, A.; Mauget-Faÿsse, M. Comparison between multifocal ERG and C-Scan SD-OCT (“en face” OCT) in patients with a suspicion of antimalarial retinal toxicity: Preliminary results. Doc. Ophthalmol. 2018, 136, 97–111. [Google Scholar] [CrossRef]
- Jung, S.H.; Park, Y.-H.; Park, Y.G. Subclinical Detection of Hydroxychloroquine-Induced Retinopathy in Patients with Systemic Lupus Erythematous Using Multifocal Electroretinography and Optical Coherence Tomography. J. Clin. Med. 2024, 13, 7663. [Google Scholar] [CrossRef]
- Sonalcan, V.; Çakir, B.; Aksoy, N.Ö.; Gündoğdu, K.Ö.; Şen, E.B.T.; Alagöz, G. The assessment of structural and functional test results for early detection of hydroxychloroquine macular toxicity. Int. Ophthalmol. 2024, 44, 370. [Google Scholar] [CrossRef]
- Mimier-Janczak, M.K.; Kaczmarek, D.; Proc, K.; Misiuk-Hojło, M.; Kaczmarek, R. Subclinical retinopathy in systemic lupus erythematosus patients—Optical Coherence Tomography study. Rheumatology 2023, 61, 161. [Google Scholar] [CrossRef]
- Agcayazi, S.B.E.; Gurlu, V.; Alacamli, G. Decreased perifoveal ganglion cell complex thickness—A first sign for macular damage in patients using hydroxychloroquine. Rom. J. Ophthalmol. 2023, 67, 146–151. [Google Scholar]
- Kim, K.E.; Kim, Y.H.; Kim, J.; Ahn, S.J. Macular Ganglion Cell Complex and Peripapillary Retinal Nerve Fiber Layer Thicknesses in Hydroxychloroquine Retinopathy. Am. J. Ophthalmol. 2023, 245, 70–80. [Google Scholar] [CrossRef]
- Mondal, K.; Porter, H.; Cole, J., II; Pandya, H.K.; Basu, S.K.; Khanam, S.; Chiu, C.-Y.; Shah, V.; Stephenson, D.J.; Chalfant, C.E.; et al. Correction: Hydroxychloroquine Causes Early Inner Retinal Toxicity and Affects Autophagosome–Lysosomal Pathway and Sphingolipid Metabolism in the Retina. Mol. Neurobiol. 2022, 59, 6611. [Google Scholar] [CrossRef] [PubMed]
- Membreno, R.F.; De Silva, T.; Agrón, E.; Keenan, T.D.L.; Cukras, C.A. Quantitative analysis of optical coherence tomography imaging in patients with different severities of hydroxychloroquine toxicity. Br. J. Ophthalmol. 2022, 107, 849–855. [Google Scholar] [CrossRef]
- Kurna, S.A.; Kanar, H.S.; Garlı, M.; Çakır, N. Evaluation of the role of spectral-domain optical coherence tomography in the early detection of macular and ganglion cell complex thickness changes in patients with rheumatologic diseases taking hydroxychloroquine. Photodiagn. Photodyn. Ther. 2022, 38, 102741. [Google Scholar] [CrossRef] [PubMed]
- Godinho, G.; Madeira, C.; Falcão, M.; Penas, S.; Dinah-Bragança, T.; Brandão, E.; Carneiro, Â.; Santos-Silva, R.; Falcão-Reis, F.; Beato, J. Longitudinal Retinal Changes Induced by Hydroxychloroquine in Eyes without Retinal Toxicity. Ophthalmic Res. 2020, 64, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Martín-Iglesias, D.; Artaraz, J.; Fonollosa, A.; Ugarte, A.; Arteagabeitia, A.; Ruiz-Irastorza, G. Evolution of retinal changes measured by optical coherence tomography in the assessment of hydroxychloroquine ocular safety in patients with systemic lupus erythematosus. Lupus 2019, 28, 555–559. [Google Scholar] [CrossRef]
- Gil, P.; Nunes, A.; Farinha, C.; Teixeira, D.; Pires, I.; Silva, R. Structural and Functional Characterization of the Retinal Effects of Hydroxychloroquine Treatment in Healthy Eyes. Ophthalmologica 2019, 241, 226–233. [Google Scholar] [CrossRef]
- Conigliaro, P.; Triggianese, P.; Draghessi, G.; Canofari, C.; Aloe, G.; Chimenti, M.; Valeri, C.; Nucci, C.; Perricone, R.; Cesareo, M. Evidence for the Detection of Subclinical Retinal Involvement in Systemic Lupus Erythematosus and Sjögren Syndrome: A Potential Association with Therapies. Int. Arch. Allergy Immunol. 2018, 177, 45–56. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, S.J.; Han, J.C.; Eo, D.R.; Lee, M.G.; Ham, D.-I.; Kang, S.W.; Kee, C.; Lee, J.; Cha, H.-S.; et al. Peripapillary Retinal Nerve Fiber Layer Thicknesses Did Not Change in Long-term Hydroxychloroquine Users. Korean J. Ophthalmol. 2018, 32, 459. [Google Scholar] [CrossRef]
- Bulut, M.; Kazım, E.R.O.L.M.; Toslak, D.; Akidan, M.; Başar, E.K.A.Y.A.; Fatih, Ç.A.Y.H. A New Objective Parameter in Hydroxychloroquine-Induced Retinal Toxicity Screening Test: Macular Retinal Ganglion Cell-Inner Plexiform Layer Thickness. Arch. Rheumatol. 2018, 33, 052–058. [Google Scholar] [CrossRef]
- Telek, H.H.; Yesilirmak, N.; Sungur, G.; Ozdemir, Y.; Yesil, N.K.; Ornek, F. Retinal toxicity related to hydroxychloroquine in patients with systemic lupus erythematosus and rheumatoid arthritis. Doc. Ophthalmol. 2017, 135, 187–194. [Google Scholar] [CrossRef]
- Kan, E.; Yakar, K.; Demirag, M.D.; Gok, M. Macular ganglion cell–inner plexiform layer thickness for detection of early retinal toxicity of hydroxychloroquine. Int. Ophthalmol. 2017, 38, 1635–1640. [Google Scholar] [CrossRef]
- Arias-Peso, B.; González, M.C.; García-Navarro, D.; del Tiempo, M.P.R.; Barón, N.P.; Sáez-Comet, L.; Ruiz-Moreno, O.; Bartol-Puyal, F.; Méndez-Martínez, S.; Júlvez, L.P. Automated analysis of choroidal thickness in patients with systemic lupus erithematosus treated with hydroxychloroquine. Int. Ophthalmol. 2024, 44, 40. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Driban, M.; Mohammed, A.R.; Schwarz, S.; Yoosuf, S.; Barthelmes, D.; Vupparaboina, K.K.; Al-Sheikh, M.; Chhablani, J. Effects of hydroxychloroquine therapy on choroidal volume and choroidal vascularity index. Eye 2023, 38, 620–624. [Google Scholar] [CrossRef]
- Halouani, S.; Le, H.M.; Querques, G.; Borrelli, E.; Sacconi, R.; Battista, M.; Jung, C.; Souied, E.H.; Miere, A. Choroidal Vascularity Index in Hydroxychloroquine Toxic Retinopathy: A Quantitative Comparative Analysis Using Enhanced Depth Imaging In Spectral Domain Optical Coherence Tomography. Retina 2023, 43, 94–101. [Google Scholar] [CrossRef]
- Ru, L.; Xu, J.; Lin, Z.; Cao, L.; Zhang, L. Analysis of choroidal thickness in juvenile systemic lupus erythematosus and its correlation with laboratory tests. BMC Ophthalmol. 2023, 23, 148. [Google Scholar] [CrossRef] [PubMed]
- Kukan, M.; Driban, M.; Vupparaboina, K.K.; Schwarz, S.; Kitay, A.M.; Rasheed, M.A.; Busch, C.; Barthelmes, D.; Chhablani, J.; Al-Sheikh, M. Structural Features of Patients with Drusen-like Deposits and Systemic Lupus Erythematosus. J. Clin. Med. 2022, 11, 6012. [Google Scholar] [CrossRef] [PubMed]
- Polat, O.A.; Okçu, M.; Yılmaz, M. Hydroxychloroquine treatment alters retinal layers and choroid without apparent toxicity in optical coherence tomography. Photodiagn. Photodyn. Ther. 2022, 38, 102806. [Google Scholar] [CrossRef]
- Ahn, S.J.; Ryu, S.J.; Lim, H.W.; Lee, B.R. Toxic Effects of Hydroxychloroquine on the Choroid: Evidence From Multimodal Imaging. Retina 2019, 39, 1016–1026. [Google Scholar] [CrossRef]
- Ahn, S.J.; Ryu, S.J.; Joung, J.Y.; Lee, B.R. Choroidal Thinning Associated With Hydroxychloroquine Retinopathy. Am. J. Ophthalmol. 2017, 183, 56–64. [Google Scholar] [CrossRef]
- Karti, O.; Ayhan, Z.; Zengin, M.O.; Kaya, M.; Kusbeci, T. Choroidal Thickness Changes in Rheumatoid Arthritis and the Effects of Short-term Hydroxychloroquine Treatment. Ocul. Immunol. Inflamm. 2017, 26, 770–775. [Google Scholar] [CrossRef]
- Braga, J.; Rothwell, R.; Oliveira, M.; Rodrigues, D.; Fonseca, S.; Varandas, R.; Ribeiro, L. Choroid thickness profile in patients with lupus nephritis. Lupus 2019, 28, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, J.; Yin, X.; Xiang, Z.; Qiu, W.; Huang, A.M.; Wang, L.; Lv, Q.; Liu, Z. Inter-eye asymmetry of microvascular density in patients on hydroxychloroquine therapy by optical coherence tomography angiography. Microvasc. Res. 2025, 157, 104747. [Google Scholar] [CrossRef]
- Bartol-Puyal, F.D.A.; González, M.C.; Arias-Peso, B.; Navarro, D.G.; Méndez-Martínez, S.; del Tiempo, M.P.R.; Comet, L.S.; Júlvez, L.P. Vision-Related Quality of Life in Patients with Systemic Lupus Erythematosus. Healthcare 2024, 12, 540. [Google Scholar] [CrossRef] [PubMed]
- Araújo, O.; Casaroli-Marano, R.P.; Hernández-Rodríguez, J.; Figueras-Roca, M.; Budi, V.; Morató, M.; Hernández-Negrín, H.; Ríos, J.; Adan, A.; Espinosa, G.; et al. New proposal for a multimodal imaging approach for the subclinical detection of hydroxychloroquine-induced retinal toxicity in patients with systemic lupus erythematosus. BMJ Open Ophthalmol. 2024, 9, e001608. [Google Scholar] [CrossRef] [PubMed]
- Leclaire, M.D.; Esser, E.L.; Dierse, S.; Koch, R.; Zimmermann, J.A.; Storp, J.J.; Gunnemann, M.-L.; Lahme, L.; Eter, N.; Mihailovic, N. Microvascular Density Analysis of Patients with Inactive Systemic Lupus Erythematosus—A Two-Year Follow-Up Optical Coherence Tomography Angiography Study. J. Clin. Med. 2024, 13, 2979. [Google Scholar] [CrossRef]
- Zhao, H.; Pan, M.; Liu, Y.; Cheng, F.; Shuai, Z. Evaluation of early retinal changes in patients on long-term hydroxychloroquine using optical coherence tomography angiography. Ther. Adv. Drug Saf. 2024, 15, 20420986231225851. [Google Scholar] [CrossRef]
- Liu, P.-C.; Luo, S.-L.; Lv, M.-N.; Wang, Y.; Li, J.-B.; Yu, S.-J.; Wu, R. Effect of hydroxychloroquine blood concentration on the efficacy and ocular toxicity of systemic lupus erythematosus. Sci. Rep. 2024, 14, 7674. [Google Scholar] [CrossRef]
- Vasilijević, J.B.; Kovačević, I.M.; Dijana, R.; Dačić, B.; Marić, G.; Stanojlović, S. Optical coherence tomography angiography parameters in patients taking hydroxychloroquine therapy. Indian J. Ophthalmol. 2023, 71, 3399–3405. [Google Scholar] [CrossRef] [PubMed]
- Esser, E.L.; Zimmermann, J.A.; Storp, J.J.; Eter, N.; Mihailovic, N. Retinal microvascular density analysis in patients with rheumatoid arthritis treated with hydroxychloroquine. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 261, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shi, W.; Chen, J.; Li, Q.J.; Wei, H.; Xu, S.H.; Kang, M.; Huang, H.; Chen, X.; Wang, Y.; et al. Retinal alterations in evaluation of rheumatoid arthritis with chloroquine treatment: A new approach. J. Biophotonics 2023, 16, e202300133. [Google Scholar] [CrossRef]
- Yu, C.; Zou, J.; Ge, Q.-M.; Liao, X.-L.; Pan, Y.-C.; Wu, J.-L.; Su, T.; Zhang, L.-J.; Liang, R.-B.; Shao, Y. Ocular microvascular alteration in Sjögren’s syndrome treated with hydroxychloroquine: An OCTA clinical study. Ther. Adv. Chronic Dis. 2023, 14, 20406223231164498. [Google Scholar] [CrossRef] [PubMed]
- Subasi, S.; Kucuk, K.D.; San, S.; Cefle, A.; Tokuc, E.O.; Balci, S.; Yazici, A. Macular and peripapillary vessel density alterations in a large series of patients with systemic lupus erythematosus without ocular involvement. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 3543–3552. [Google Scholar] [CrossRef]
- Halouani, S.; Le, H.M.; Cohen, S.Y.; Terkmane, N.; Herda, N.; Souied, E.H.; Miere, A. Choriocapillaris Flow Deficits Quantification in Hydroxychloroquine Retinopathy Using Swept-Source Optical Coherence Tomography Angiography. J. Pers. Med. 2022, 12, 1445. [Google Scholar] [CrossRef]
- Forte, R.; Haulani, H.; Dyrda, A.; Jürgens, I. Swept source optical coherence tomography angiography in patients treated with hydroxychloroquine: Correlation with morphological and functional tests. Br. J. Ophthalmol. 2019, 105, 1297–1301. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Kianersi, F.; Radmehr, H.; Dehghani, A.; Beni, A.N.; Noorshargh, P. Evaluation of optical coherence tomography angiography parameters in patients treated with Hydroxychloroquine. BMC Ophthalmol. 2021, 21, 209. [Google Scholar] [CrossRef]
- Cinar, E.; Yuce, B.; Aslan, F. Evaluation of retinal and choroidal microvascular changes in patients who received hydroxychloroquine by optical coherence tomography angiography. Arq. Bras. Oftalmol. 2021, 84, 2–10. [Google Scholar] [CrossRef]
- Tarakcioglu, H.N.; Ozkaya, A.; Yigit, U. Is optical coherence tomography angiography a useful tool in the screening of hydroxychloroquine retinopathy? Int. Ophthalmol. 2020, 41, 27–33. [Google Scholar] [CrossRef]
- Lenfant, T.; Salah, S.; Leroux, G.; Bousquet, E.; Le Guern, V.; Chasset, F.; Francès, C.; Morel, N.; Chezel, J.; Papo, T.; et al. Risk factors for hydroxychloroquine retinopathy in systemic lupus erythematosus: A case–control study with hydroxychloroquine blood-level analysis. Rheumatology 2020, 59, 3807–3816. [Google Scholar] [CrossRef]
- Mihailovic, N.; Leclaire, M.D.; Eter, N.; Brücher, V.C. Altered microvascular density in patients with systemic lupus erythematosus treated with hydroxychloroquine—An optical coherence tomography angiography study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2263–2269. [Google Scholar] [CrossRef]
- Ozek, D.; Onen, M.; Karaca, E.E.; Omma, A.; Kemer, O.E.; Coskun, C. The optical coherence tomography angiography findings of rheumatoid arthritis patients taking hydroxychloroquine. Eur. J. Ophthalmol. 2018, 29, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Conigliaro, P.; Cesareo, M.; Chimenti, M.S.; Triggianese, P.; Canofari, C.; Aloe, G.; Nucci, C.; Perricone, R. Evaluation of retinal microvascular density in patients affected by systemic lupus erythematosus: An optical coherence tomography angiography study. Ann. Rheum. Dis. 2019, 78, 287–288. [Google Scholar] [CrossRef]
- Goker, Y.S.; Atılgan, C.U.; Tekin, K.; Kızıltoprak, H.; Yetkin, E.; Karahan, N.Y.; Koc, M.; Kosekahya, P. The Validity of Optical Coherence Tomography Angiography as a Screening Test for the Early Detection of Retinal Changes in Patients with Hydroxychloroquine Therapy. Curr. Eye Res. 2018, 44, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Bulut, M.; Akıdan, M.; Gözkaya, O.; Erol, M.K.; Cengiz, A.; Çay, H.F. Optical coherence tomography angiography for screening of hydroxychloroquine-induced retinal alterations. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 2075–2081. [Google Scholar] [CrossRef]
- Marmor, M.F.; Kellner, U.; Lai, T.Y.Y.; Melles, R.B.; Mieler, W.F. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology 2016, 123, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Marmor, M.F.; Ahn, S.J.; Ehlers, J.P.; Melles, R.B.; Mieler, W.F.; Sarraf, D.; Yusuf, I.H. Special AAO Report: Recommendations on Screening for Hydroxychloroquine Retinopathy (2025 Revision). Ophthalmology, 2025; in press. [Google Scholar]
- Yusuf, I.H.; Latheef, F.; Ardern-Jones, M.; Lotery, A.J. New recommendations for retinal monitoring in hydroxychloroquine users: Baseline testing is no longer supported. Br. J. Dermatol. 2021, 185, 435–438. [Google Scholar] [CrossRef]
- Rosenbaum, J.T.; Costenbader, K.H.; Desmarais, J.; Ginzler, E.M.; Fett, N.; Goodman, S.; O’Dell, J.R.; Schmajuk, G.; Werth, V.P.; Melles, R.B.; et al. American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and American Academy of Ophthalmology 2020 Joint Statement on Hydroxychloroquine Use With Respect to Retinal Toxicity. Arthritis Amp; Rheumatol. 2021, 73, 908–911. [Google Scholar] [CrossRef]
- Guven, T.K.; Alexander, A.; Smith, G.T. Hydroxychloroquine retinopathy screening guidelines: A false positive. BMJ Case Rep. 2023, 16, e249052. [Google Scholar] [CrossRef]
- Stern, E.; Johnson, J.; Mazzulla, D. Highly Accelerated Onset of Hydroxychloroquine Macular Retinopathy. Ochsner J. 2017, 17, 280–283. [Google Scholar]
- Bel, L.H.; Adsuara, C.M.; Garfella, M.H.; Taulet, E.C. Toxicidad macular precoz tras 2 meses de tratamiento con hidroxicloroquina. Arch. Soc. Española Oftalmol. 2018, 93, e20–e21. [Google Scholar]
- Pasaoglu, I.; Onmez, F. Macular toxicity after short-term hydroxychloroquine therapy. Indian J. Ophthalmol. 2019, 67, 289. [Google Scholar] [CrossRef]
- Lian, Y.-Y.; Ma, Y.-C.; Cheng, C.-K. Unusual Presentation of Acute Hydroxychloroquine Retinopathy. Ocul. Immunol. Inflamm. 2022, 31, 1720–1723. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.; Gupta, P.; Ratra, D. Accelerated hydroxychloroquine toxic retinopathy. Doc. Ophthalmol. 2023, 148, 37–45. [Google Scholar] [CrossRef]
- Sheth, N.; Schaab, T.; Mirza, R.G. Advanced Pericentral Hydroxychloroquine Retinopathy in an Asian Woman. J. Vitreoretin. Dis. 2019, 4, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.A.; Nair, A.A.; Mehta, N.; Lee, G.D.; Freund, K.B.; Modi, Y.S. Delayed Detection of Predominantly Pericentral Hydroxychloroquine Toxicity in a Dominican Patient. J. Vitreoretin. Dis. 2021, 6, 324–328. [Google Scholar] [CrossRef]
- Mack, H.G.; Fuzzard, D.R.W.; Symons, R.C.A.; Heriot, W.J. Asymmetric Hydroxychloroquine Macular Toxicity with Aphakic Fellow Eye. Retin. Cases Brief Rep. 2021, 15, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Hallali, G.; Seyed, Z.; Maillard, A.-V.; Drine, K.; Lamour, L.; Faure, C.; Audo, I. Extreme Interocular Asymmetry in an Atypical Case of a Hydroxychloroquine-Related Retinopathy. Medicina 2022, 58, 967. [Google Scholar] [CrossRef]
- Jeltsch, B.M.; Sarraf, D.; Madjdpour, D.; Hanson, J.V.M.; Pfiffner, F.K.; Koller, S.; Berger, W.; Barthelmes, D.; Al-Sheikh, M. Rapid Onset Hydroxychloroquine Toxicity. Retin. Cases Brief Rep. 2023, 18, 351–354. [Google Scholar] [CrossRef]
- Ozawa, H.; Ueno, S.; Ohno-Tanaka, A.; Sakai, T.; Hashiguchi, M.; Shimizu, M.; Fujinami, K.; Ahn, S.J.; Kondo, M.; Browning, D.J.; et al. Ocular findings in Japanese patients with hydroxychloroquine retinopathy developing within 3 years of treatment. Jpn. J. Ophthalmol. 2021, 65, 472–481. [Google Scholar] [CrossRef]
- Lee, J.M.; Kwon, H.Y.; Ahn, S.J. Atypical Presentations of Hydroxychloroquine Retinopathy: A Case Series Study. J. Clin. Med. 2024, 13, 3411. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Vinayagamoorthy, N.; Han, K.; Kwok, S.K.; Ju, J.H.; Park, K.S.; Jung, S.; Park, S.; Chung, Y.; Park, S. Association of Polymorphisms of Cytochrome P450 2D6 With Blood Hydroxychloroquine Levels in Patients With Systemic Lupus Erythematosus. Arthritis Rheumatol. 2015, 68, 184–190. [Google Scholar] [CrossRef]
- Petri, M.; Elkhalifa, M.; Li, J.; Magder, L.S.; Goldman, D.W. Hydroxychloroquine Blood Levels Predict Hydroxychloroquine Retinopathy. Arthritis Rheumatol. 2020, 72, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Marshall, E.; Robertson, M.; Kam, S.; Penwarden, A.; Riga, P.; Davies, N. Correction: Prevalence of hydroxychloroquine retinopathy using 2018 Royal College of Ophthalmologists diagnostic criteria. Eye 2020, 35, 358. [Google Scholar] [CrossRef]
- Dadhaniya, N.V.; Sood, I.; Patil, A.; Mallaiah, U.; Upadhyaya, S.; Handa, R.; Gupta, S.J. Screening for Hydroxychloroquine Retinal Toxicity in Indian Patients. JCR: J. Clin. Rheumatol. 2020, 27, e395–e398. [Google Scholar] [CrossRef] [PubMed]
- Browning, D.J.; Lee, C. Somatotype, the risk of hydroxychloroquine retinopathy, and safe daily dosing guidelines. Clin. Ophthalmol. 2018, 12, 811–818. [Google Scholar] [CrossRef]
- Zirtiloglu, S.; Alikma, M.S.; Acar, O.P.A.; Güven, F.; Icacan, O.C.; Yigit, F.U. Evaluation of the Optic Nerve Head Using Optical Coherence Tomography Angiography in Systemic Sclerosis Patients. Klin. Monatsblätter Augenheilkd. 2022, 240, 1277–1283. [Google Scholar] [CrossRef]
- Melles, R.B.; Marmor, M.F. Pericentral Retinopathy and Racial Differences in Hydroxychloroquine Toxicity. Ophthalmology 2015, 122, 110–116. [Google Scholar] [CrossRef]
- Bousquet, E.; Santina, A.; Somisetty, S.; Morales, V.R.; Holland, G.N.; Sarraf, D. Hydroxychloroquine retinopathy in a 23-year-old male. Retin. Cases Brief Rep. 2024, 20, 1–4. [Google Scholar] [CrossRef]
- Lu, J.; Huang, Y.; Ye, Q.; Shang, F.; Ming, M.; Xu, H.; Li, Z. Low-dose oral hydroxychloroquine led to impaired vision in a child with renal failure: Case report and literature review. Medicine 2021, 100, e24919. [Google Scholar] [CrossRef]
- AlAhmed, O.; Way, A.; Akoghlanian, S.; Barbar-Smiley, F.; Lemle, S.; MacDonald, D.; Frost, E.; Wise, K.; Lee, L.; Ardoin, S.P.; et al. Improving eye screening practice among pediatric rheumatology patients receiving hydroxychloroquine. Lupus 2020, 30, 269–279. [Google Scholar] [CrossRef]
- Donica, V.C.; Donica, A.L.; Pavel, I.A.; Danielescu, C.; Alexa, A.I.; Bogdănici, C.M. Variabilities in Retinal Hemodynamics Across the Menstrual Cycle in Healthy Women Identified Using Optical Coherence Tomography Angiography. Life 2024, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Sheu, S.-J.; Chang, C.-Y. Macular edema might be a rare presentation of hydroxychloroquine-induced retinal toxicity. Taiwan J. Ophthalmol. 2017, 7, 56. [Google Scholar] [CrossRef]
- Elhusseiny, A.M.; Relhan, N.; Smiddy, W.E. Docetaxel-induced maculopathy possibly potentiated by concurrent hydroxychloroquine use. Am. J. Ophthalmol. Case Rep. 2019, 16, 100560. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.H.; Ahn, S.J.; Lim, H.W.; Lee, B.R. The effect of oral acetazolamide on cystoid macular edema in hydroxychloroquine retinopathy: A case report. BMC Ophthalmol. 2017, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Mathai, M.; Zeleny, A.; Jacobsen, B.H.; Garfinkel, R.A.; Katira, R.; Fein, J.G. Intravitreal Dexamethasone for the Treatment of Macular Edema Secondary to Hydroxychloroquine Toxicity. Retin. Cases Brief Rep. 2022, 18, 346–350. [Google Scholar] [CrossRef]
- Han, X.; Shi, W.; Yang, Y. The efficacy and safety of hydroxychloroquine for COVID-19 prophylaxis and clinical assessment: An updated meta-analysis of randomized trials. J. Thorac. Dis. 2024, 16, 2983–2993. [Google Scholar] [CrossRef]
- Nti, A.A.; Serrano, L.W.; Sandhu, H.S.; Uyhazi, K.E.; Edelstein, I.D.; Zhou, E.J.; Bowman, S.; Song, D.; Gangadhar, T.C.; Schuchter, L.M.; et al. Frequent Subclinical Macular Changes in Combined Braf/Mek Inhibition with High-Dose Hydroxychloroquine as Treatment for Advanced Metastatic Braf Mutant Melanoma: Preliminary Results From a Phase I/II Clinical Treatment Trial. Retina 2019, 39, 502–513. [Google Scholar] [CrossRef]
- Danielescu, C.; Dabija, M.G.; Nedelcu, A.H.; Lupu, V.V.; Lupu, A.; Ioniuc, I.; Gîlcă-Blanariu, G.-E.; Donica, V.-C.; Anton, M.-L.; Musat, O. Automated Retinal Vessel Analysis Based on Fundus Photographs as a Predictor for Non-Ophthalmic Diseases—Evolution and Perspectives. J. Pers. Med. 2023, 14, 45. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.