The Influence of the COVID-19 Pandemic on Patients Admitted with Pericardial Effusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Population
2.3. Endpoints and Terminology
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COVID-19 | Coronavirus disease 19 |
| CT | Computed tomography |
| ICD | International classification of disease |
| IQR | Interquartile range |
| PCR | Polymerase chain reaction |
| POCUS | Point-of-care ultrasound |
| SARS-CoV-2 | Severe acute respiratory syndrome Coronavirus 2 |
| SD | Standard deviation |
| SUMC | Soroka University Medical Center |
| USA | United States of America |
References
- Sagristà-Sauleda, J.; Mercé, A.S.; Soler-Soler, J. Diagnosis and management of pericardial effusion. World J. Cardiol. 2011, 3, 135–143. [Google Scholar] [CrossRef]
- Imazio, M.; Adler, Y. Management of pericardial effusion. Eur. Heart J. 2013, 34, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Sauer, F.; Dagrenat, C.; Couppie, P.; Jochum, G.; Leddet, P. Pericardial effusion in patients with COVID-19: Case series. Eur. Heart J. Case Rep. 2020, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.; Prokup, J.A.; Butson, K.; Jordan, K. Acute Effusive Pericarditis: A Late Complication of COVID-19. Cureus 2020, 12, e9074. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis with COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef]
- Czeisler, M.É.; Marynak, K.; Clarke, K.E.N.; Salah, Z.; Shakya, I.; Thierry, J.M.; Ali, N.; McMillan, H.; Wiley, J.F.; Weaver, M.D.; et al. Delay or Avoidance of Medical Care Because of COVID-19–Related Concerns—United States, June 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 1250–1257. [Google Scholar] [CrossRef]
- Spodick, D.H. Acute Cardiac Tamponade. N. Engl. J. Med. 2003, 349, 684–690. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Bax, J.J.; Rademakers, F.E. The ESC Textbook of Cardiovascular Imaging, 2nd ed.; European Society of Cardiology: Sophia Antipolis Cedex, France, 2010; pp. 307–320. [Google Scholar]
- Imazio, M.; Lazaros, G.; Valenti, A.; De Carlini, C.C.; Maggiolini, S.; Pivetta, E.; Giustetto, C.; Tousoulis, D.; Adler, Y.; Rinaldi, M. Outcomes of idiopathic chronic large pericardial effusion. Heart 2019, 105, 477–481. [Google Scholar] [CrossRef]
- Diamantidis, C.J.; Cook, D.J.; Dunning, S.; Redelosa, C.K.; Bartolome, M.F.D.; Romero, R.A.A.; Vassalotti, J.A. Missing Care: The Initial Impact of the COVID-19 Pandemic on CKD Care Delivery. J. Gen. Intern. Med. 2022, 37, 4241–4247. [Google Scholar] [CrossRef]
- Mafi, J.N.; Craff, M.; Vangala, S.; Pu, T.; Skinner, D.; Tabatabai-Yazdi, C.; Nelson, A.; Reid, R.; Agniel, D.; Tseng, C.H.; et al. Trends in US Ambulatory Care Patterns During the COVID-19 Pandemic, 2019–2021. JAMA 2022, 327, 237–247. [Google Scholar] [CrossRef]
- Baum, A.; Schwartz, M.D. Admissions to Veterans Affairs Hospitals for Emergency Conditions During the COVID-19 Pandemic. JAMA 2020, 324, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Kuzuu, K.; Misawa, N.; Ashikari, K.; Kessoku, T.; Kato, S.; Hosono, K.; Yoneda, M.; Nonaka, T.; Matsushima, S.; Komatsu, T.; et al. Gastrointestinal Cancer Stage at Diagnosis Before and During the COVID-19 Pandemic in Japan. JAMA Netw. Open 2021, 4, e2126334. [Google Scholar] [CrossRef] [PubMed]
- Alagoz, O.; Lowry, K.P.; Kurian, A.W.; Mandelblatt, J.S.; Ergun, M.A.; Huang, H.; Lee, S.J.; Schechter, C.B.; Tosteson, A.N.; Miglioretti, D.L.; et al. Impact of the COVID-19 Pandemic on Breast Cancer Mortality in the US: Estimates from Collaborative Simulation Modeling. JNCI J. Natl. Cancer Inst. 2021, 113, 1484–1494. [Google Scholar] [CrossRef] [PubMed]
- Popescu, A.; Craina, M.; Pantea, S.; Pirvu, C.; Chiriac, V.D.; Marincu, I.; Bratosin, F.; Bogdan, I.; Hosin, S.; Citu, C.; et al. COVID-19 Pandemic Effects on Cervical Cancer Diagnosis and Management: A Population-Based Study in Romania. Diagnostics 2022, 12, 907. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kang, M.; Cho, J.; Kang, D.; Min, K.H.; Suh, G.Y.; Shim, J.J.; Jeon, K.; Tuberculosis, T.K. Nationwide Social Distancing and the Epidemiology of Severe Acute Respiratory Infections. Yonsei Med. J. 2021, 62, 954. [Google Scholar] [CrossRef]
- Edwards, K.M. The Impact of Social Distancing for Severe Acute Respiratory Syndrome Coronavirus 2 on Respiratory Syncytial Virus and Influenza Burden. Clin. Infect. Dis. 2021, 72, 2076–2078. [Google Scholar] [CrossRef]
- Gracia-Ramos, A.E.; Martin-Nares, E.; Hernández-Molina, G. New Onset of Autoimmune Diseases Following COVID-19 Diagnosis. Cells 2021, 10, 3592. [Google Scholar] [CrossRef]
- Jamal, M.; Bangash, H.I.; Habiba, M.; Lei, Y.; Xie, T.; Sun, J.; Wei, Z.; Honf, Z.; Shao, L.; Zhang, Q. Immune Dysregulation and System Pathology in COVID-19. Virulence; Bellwether Publishing, Ltd.: Columbia, MD, USA, 2021; Volume 12, pp. 918–936. [Google Scholar]
- Labovitz, A.J.; Noble, V.E.; Bierig, M.; Goldstein, S.A.; Jones, R.; Kort, S.; Porter, T.R.; Spencer, K.T.; Tayal, V.S.; Wei, K. Focused Cardiac Ultrasound in the Emergent Setting: A Consensus Statement of the American Society of Echocardiography and American College of Emergency Physicians. J. Am. Soc. Echocardiogr. 2010, 23, 1225–1230. [Google Scholar] [CrossRef]
- Alpert, E.A.; Amit, U.; Guranda, L.; Mahagna, R.; Grossman, S.A.; Bentancur, A. Emergency department point-of-care ultrasonography improves time to Pericardiocentesis for clinically significant effusions. Clin. Exp. Emerg. Med. 2017, 4, 128–132. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Raman, B.; Bluemke, D.A.; Lüscher, T.F.; Neubauer, S. Long COVID: Post-Acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 2022, 43, 1157–1172. [Google Scholar] [CrossRef]
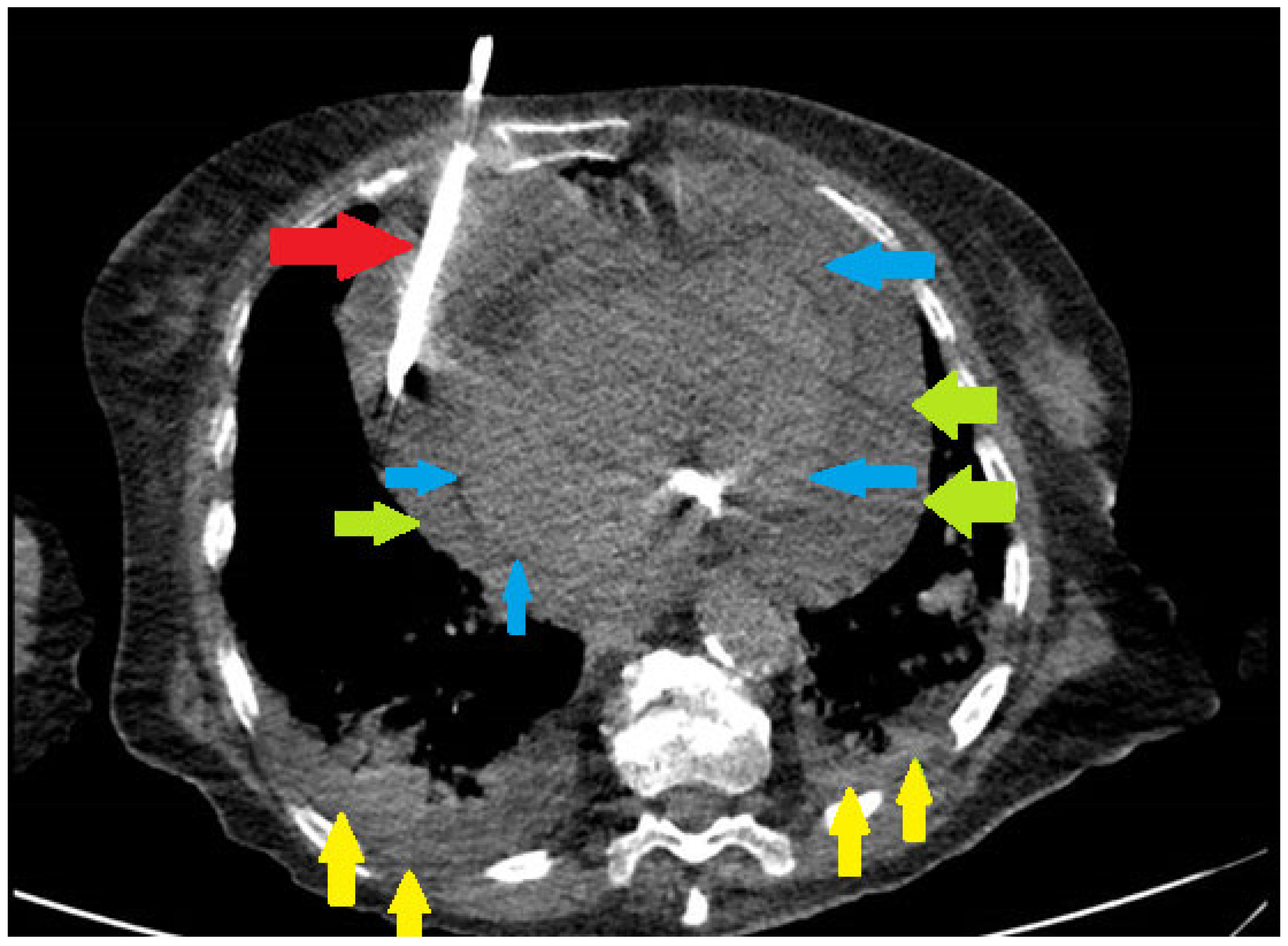
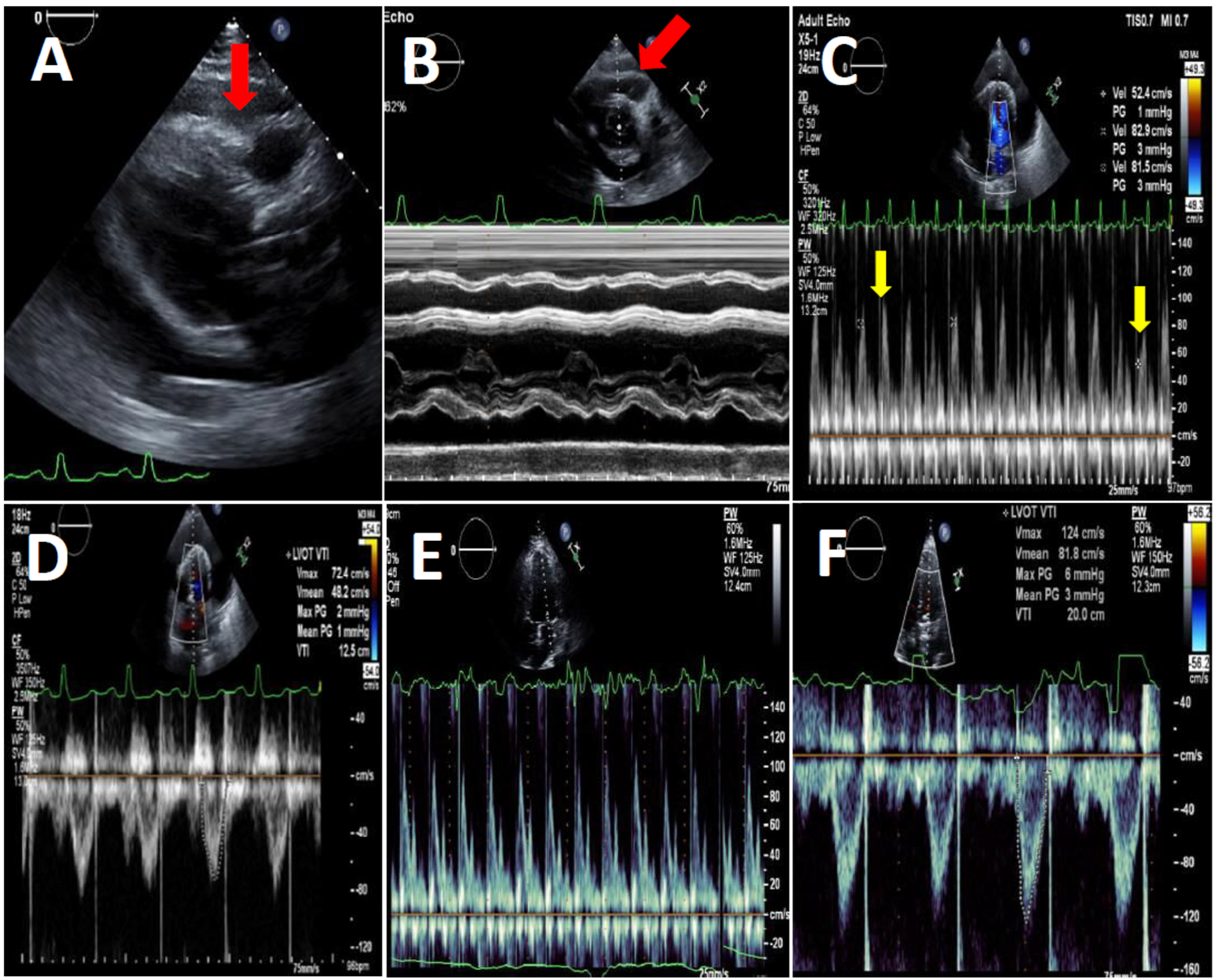
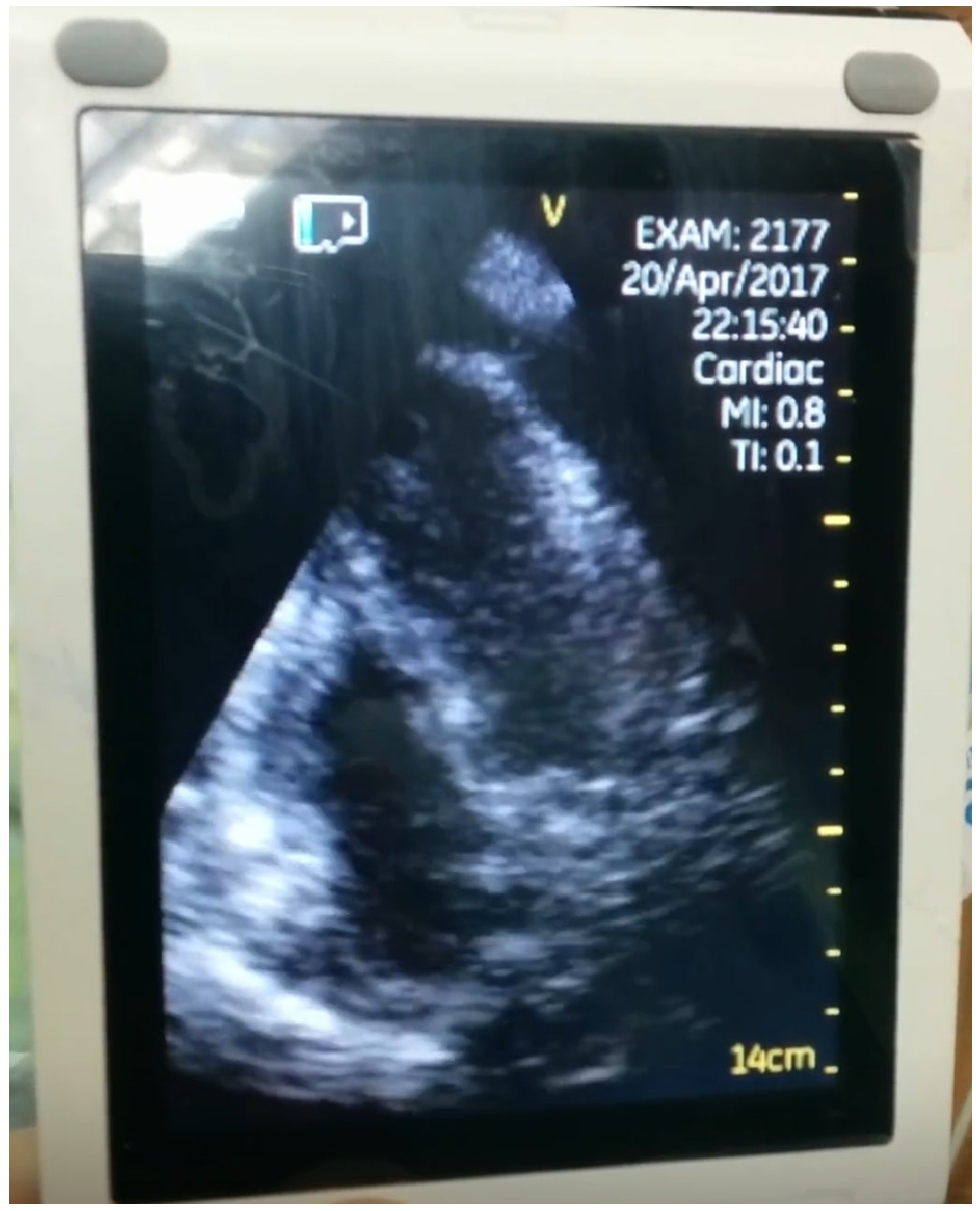
| Variable | Pre-COVID-19 Period (n = 65) | COVID-19 Period (n = 97) | p Value |
|---|---|---|---|
| Age (mean ± SD) | 62.3 (2.0) | 65.4 (1.6) | 0.23 |
| Females (n,%) | 35 (53.8) | 40 (41.2) | 0.14 |
| Jews (n,%) | 52 (80.0) | 81 (83.5) | 0.67 |
| Socio-economic status (n,%) | |||
| Low | 27 (45.8) | 29 (34.9) | 0.220 |
| Medium | 23 (39.0) | 32 (38.6) | |
| High | 9 (15.3) | 22 (26.5) | |
| Smokers (n,%) | 22 (34.9) | 38 (41.3) | 0.50 |
| Heart failure(n,%) | 10 (15.4) | 23 (23.7) | 0.23 |
| Ischemic heart disease (n,%) | 10 (15.4) | 19 (19.6) | 0.53 |
| Diabetes (n,%) | 31 (47.7) | 37 (38.1) | 0.25 |
| Chronic kidney disease (n,%) | 20 (30.8) | 28 (28.9) | 0.86 |
| Connective tissue disease (n,%) | 6 (9.2) | 5 (5.2) | 0.35 |
| Malignancy (n,%) | 22 (33.8) | 32 (33.0) | 1.00 |
| Dementia (n,%) | 2 (3.1) | 2 (2.1) | 1.00 |
| Charlson comorbidity index (median, interquartile range) | 6.0 (3.0–9.0) | 6.0 (4.0–9.0) | 0.71 |
| Symptoms to pericardiocentesis, days (median, interquartile range) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 0.88 |
| Chief complaint (n,%) | |||
| Chest pain | 8 (22.9) | 9 (15.0) | 0.11 |
| Dyspnea | 9 (25.7) | 26 (43.3) | |
| Other | 6 (17.1) | 12 (20.0) | |
| Could not determine | 12 (34.3) | 13 (21.7) | |
| Length of hospitalization (median, interquartile range) | 7.0 (4.0–10.5) | 6.0 (4.0–9.7) | 0.55 |
| ICU admission (n,%) | 51 (78.5) | 69 (71.1) | 0.36 |
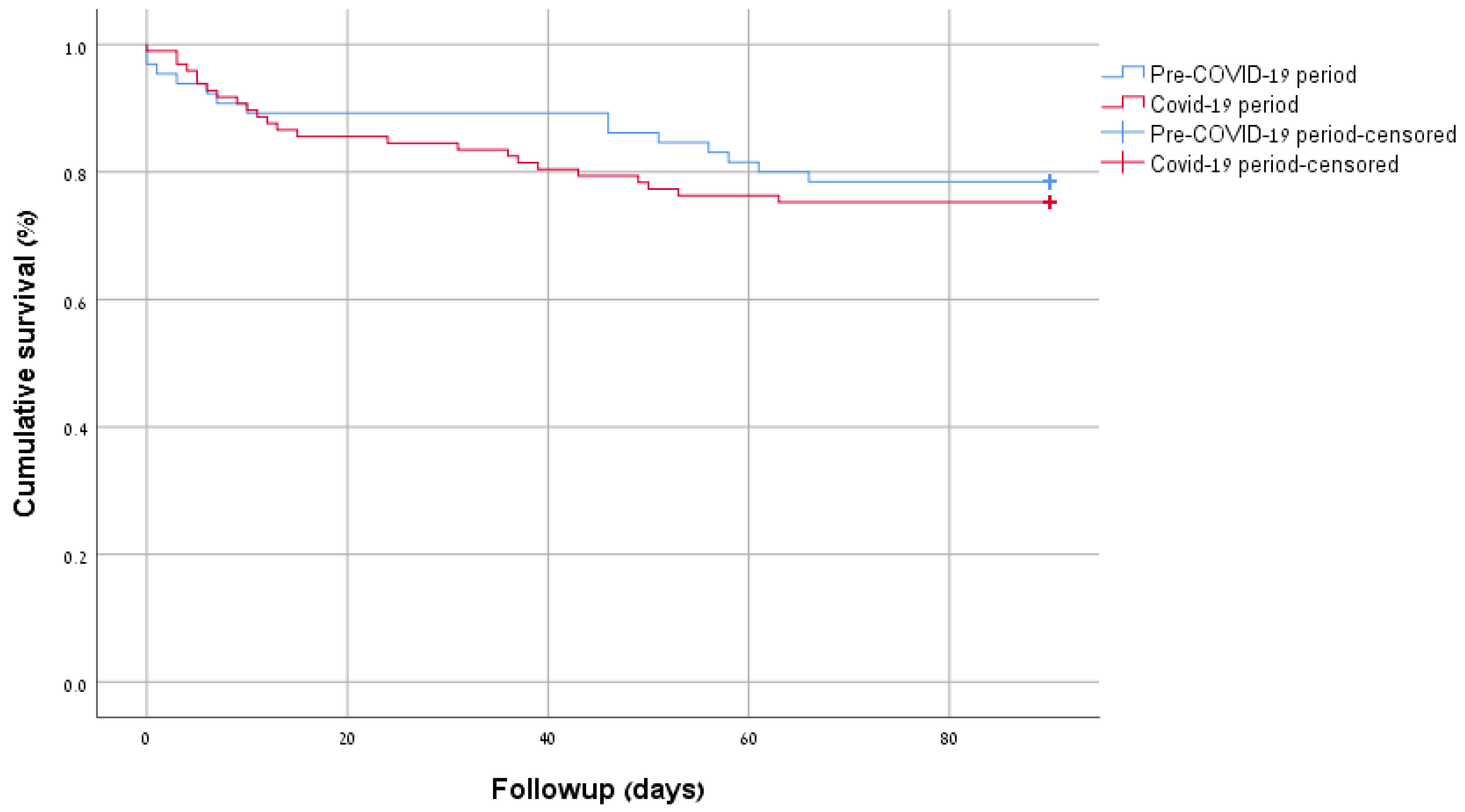
| In-hospital mortality (n,%) | 5 (7.7) | 14 (14.4) | 0.22 |
| 90-day mortality (n,%) | 14 (21.5) | 24 (24.7) | 0.70 |
| Variable | Pre-COVID-19 Period (n = 65) | COVID-19 Period (n = 97) | p Value |
|---|---|---|---|
| Large effusion size (n,%) | 52 (81.3) | 73 (75.3) | 0.44 |
| Tamponade n (%) | 35 (54.7) | 63 (64.9) | 0.24 |
| Reduced left ventricular systolic function n (%) | 16 (25.0) | 19 (19.6) | 0.44 |
| Reduced right ventricular systolic function n (%) | 12 (25.0) | 12 (16.0) | 0.24 |
| Fluid pathology n (%) | |||
| Inflammatory | 27 (42.2) | 40 (41.2) | 0.22 |
| Bloody | 3 (4.7) | 13 (13.4) | |
| Neoplastic | 10 (15.6) | 16 (16.5) | |
| Pyogenic | 0 (0.0) | 2 (2.1) | |
| POCUS done n (%) | 25 (39.7) | 48 (49.5) | 0.25 |
| Tamponade on POCUS n (%) | 6 (9.5) | 9 (9.3) | 1.00 |
| POCUS to diagnosis, days (median, interquartile range) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.88 |
| POCUS to pericardiocentesis, days (median, interquartile range) | 1.0 (1.0–2.0) | 1.0 (1.0–1.0) | 0.10 |
| Final etiology | |||
| Malignant | 16 (24.6) | 17 (17.5) | 0.04 |
| Peri-procedural | 11 (16.9) | 14 (14.4) | |
| Infectious | 12 (18.5) | 17 (17.5) | |
| COVID-19 related | 0 (0) | 15 (15.5) | |
| Idiopathic | 18 (27.7) | 24 (24.7) | |
| Other | 8 (12.3) | 10 (10.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Shabtay, A.; Sagy, I.; Rabaev, E.; Shmueli, H.; Barski, L. The Influence of the COVID-19 Pandemic on Patients Admitted with Pericardial Effusion. Diagnostics 2026, 16, 464. https://doi.org/10.3390/diagnostics16030464
Shabtay A, Sagy I, Rabaev E, Shmueli H, Barski L. The Influence of the COVID-19 Pandemic on Patients Admitted with Pericardial Effusion. Diagnostics. 2026; 16(3):464. https://doi.org/10.3390/diagnostics16030464
Chicago/Turabian StyleShabtay, Amir, Iftach Sagy, Elizaveta Rabaev, Hezzy Shmueli, and Leonid Barski. 2026. "The Influence of the COVID-19 Pandemic on Patients Admitted with Pericardial Effusion" Diagnostics 16, no. 3: 464. https://doi.org/10.3390/diagnostics16030464
APA StyleShabtay, A., Sagy, I., Rabaev, E., Shmueli, H., & Barski, L. (2026). The Influence of the COVID-19 Pandemic on Patients Admitted with Pericardial Effusion. Diagnostics, 16(3), 464. https://doi.org/10.3390/diagnostics16030464







