Abstract
Background/Objectives: Multimodal large language models (MLLMs) offer potential for automated fracture detection, yet their diagnostic stability under repeated inference remains underexplored. This study evaluates the diagnostic accuracy, stability, and intra-model consistency of four MLLMs in detecting hand fractures on plain radiographs. Methods: In total, images of hand radiographs of 65 adult patients with confirmed hand fractures (30 phalangeal, 30 metacarpal, 5 scaphoid) were evaluated by four models: GPT-5 Pro, Gemini 2.5 Pro, Claude Sonnet 4.5, and Mistral Medium 3.1. Each image was independently analyzed five times per model using identical zero-shot prompts (1300 total inferences). Diagnostic accuracy, inter-run reliability (Fleiss’ κ), case-level agreement profiles, subgroup performance, and exploratory demographic inference (age, sex) were assessed. Results: GPT-5 Pro achieved the highest accuracy (64.3%) and consistency (κ = 0.71), followed by Gemini 2.5 Pro (56.9%, κ = 0.57). Mistral Medium 3.1 exhibited high agreement (κ = 0.88) despite low accuracy (38.5%), indicating systematic error (“confident hallucination”). Claude Sonnet 4.5 showed low accuracy (33.8%) and consistency (κ = 0.33), reflecting instability. While phalangeal fractures were reliably detected by top models, scaphoid fractures remained challenging. Demographic analysis revealed poor capabilities, with age estimation errors exceeding 12 years and sex prediction accuracy near random chance. Conclusions: Diagnostic accuracy and consistency are distinct performance dimensions; high intra-model agreement does not imply correctness. While GPT-5 Pro demonstrated the most favorable balance of accuracy and stability, other models exhibited critical failure modes ranging from systematic bias to random instability. At present, MLLMs should be regarded as experimental diagnostic reasoning systems rather than reliable standalone tools for clinical fracture detection.
1. Introduction
Hand fractures represent one of the most frequent musculoskeletal injuries encountered in emergency departments and orthopedic clinics [1,2,3]. Accurate radiographic diagnosis is essential, as missed or delayed fracture detection may result in malunion, nonunion, prolonged immobilization, chronic pain, and long-term functional impairment. The diagnostic challenge is particularly pronounced in the hand due to its complex anatomy and the often subtle radiographic appearance of certain fracture types, such as scaphoid fractures [4,5,6,7,8,9].
In recent years, large language models (LLMs) equipped with vision capabilities have emerged as a new class of artificial intelligence (AI) systems capable of interpreting medical images while generating natural language explanations (i.e., through natural language processing, NLP). Such systems are commonly referred to as multimodal large language models (MLLMs) when they integrate textual information with one or more additional input modalities, including visual data. Unlike conventional convolutional neural networks (CNNs), which are typically trained for narrowly defined classification or detection tasks, these MLLMs operate as general-purpose reasoning systems, raising interest in their potential role as decision-support tools in radiology. Several recent studies have explored MLLM performance in fracture detection and musculoskeletal imaging tasks, reporting heterogeneous diagnostic accuracy [10,11,12,13,14,15,16,17,18].
However, the current literature is characterized by important methodological limitations. Most studies rely on single-run evaluations, implicitly assuming deterministic behavior of MLLMs. This approach neglects the probabilistic nature of generative models, in which identical inputs can yield different outputs across repeated inferences. As a result, intra-model stability and reproducibility, which are critical prerequisites for clinical reliability, remain largely unexamined. Moreover, diagnostic accuracy is frequently reported without explicit consideration of whether correct outputs are produced consistently or merely by chance [19,20,21,22,23,24,25,26].
Another unresolved issue is the relationship between accuracy and consistency. A model may demonstrate high agreement across repeated runs while remaining systematically incorrect, or conversely produce variable but occasionally correct outputs. From a clinical perspective, these behavioral patterns carry fundamentally different risks, yet they are rarely analyzed jointly [27,28].
The present study addresses these gaps by conducting a multi-model, multi-run evaluation of MLLM performance in hand fracture detection on plain radiographs. Rather than aiming to establish population-level diagnostic validity, this work focuses on model behavior under repeated inference conditions, emphasizing the dissociation between correctness and consistency. By combining run-wise accuracy analysis, intra-model reliability metrics, case-level agreement profiles, and descriptive subgroup analyses, this study provides a structured framework for assessing the clinical trustworthiness of MLLM-based diagnostic systems.
In addition, exploratory analyses were conducted to assess whether MLLMs implicitly infer demographic attributes such as patient age and sex from radiographic images. These analyses were performed to contextualize model behavior beyond diagnostic accuracy and are reported as secondary findings.
2. Materials and Methods
2.1. Study Design and Objectives
This observational study evaluated the diagnostic behavior of four MLLMs in hand fracture detection using a repeated-run framework. The overall study design and reporting structure adhere to the Checklist for Artificial Intelligence in Medical Imaging (CLAIM) guidelines [29], within the scope applicable to the evaluation of pre-trained foundation models. The study was designed to characterize accuracy, intra-model reliability, and response stability rather than to establish clinical validation or population-level diagnostic performance.
The study objectives were:
- To quantify diagnostic accuracy across multiple independent inference runs
- To assess intra-model reliability using Fleiss’ kappa (κ)
- To characterize the relationship between accuracy and consistency across models
2.2. Dataset and Case Selection
A total of 65 fracture-positive hand radiograph cases were selected from publicly available, curated educational radiology resources (https://radiopaedia.org, accessed on 25 December 2025) [30]. The cases were accessed and analyzed in compliance with the platform’s Terms of Use and are licensed under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License (CC BY-NC-SA 3.0). Case selection was performed manually by the authors and was guided by predefined qualitative criteria. No images were reproduced or redistributed as part of this study.
Inclusion criteria were:
- (1)
- availability of standardized radiographic projections, specifically comprising standard two-view protocols (anteroposterior/posteroanterior and oblique/lateral) for phalangeal and metacarpal fractures, and a dedicated scaphoid series (comprising at least three projections, preferably a four-view protocol) for scaphoid fractures [31];
- (2)
- absence of superimposed annotations (e.g., arrows, circles, or text overlays) that could serve as visual cues for the model, with the exception of standard anatomical side markers (L/R);
- (3)
- diagnostic-quality images in web-optimized formats (JPEG/PNG), with sufficient native resolution (e.g., on the order of ~600 × 600 pixels) to allow visual assessment of cortical margins, trabecular bone structure, and fracture lines;
- (4)
- availability of basic demographic metadata, including patient age and sex, as provided in the case description; and
- (5)
- representation of common, clinically typical fracture patterns without rare variants, syndromic associations, or atypical imaging presentations.
The final case set comprised 30 finger (phalangeal) fractures, 30 metacarpal fractures, and 5 scaphoid fractures, reflecting a representative distribution of frequently encountered hand fracture entities in routine clinical practice. Cases were limited to fractures that were clearly identifiable on plain radiographs and did not require secondary imaging for definitive diagnosis (e.g., CT or MRI).
The study design intentionally focused exclusively on fracture-positive cases to enable detailed analysis of model behavior under conditions of confirmed pathology. This approach prioritizes the assessment of diagnostic consistency and accuracy dissociation over population-level screening performance. Specificity and negative predictive value were not assessed due to the absence of non-fracture controls. The final dataset comprised 65 fracture-positive cases covering a broad age spectrum (range: 11–80 years) with a male predominance (69.2% male, 30.8% female).
2.3. Models Under Evaluation
Four frontier MLLMs were evaluated, selected to represent the spectrum from proprietary US-based systems to the European open-weight paradigm:
- GPT-5 Pro (OpenAI, San Francisco, CA, USA; proprietary)
- Gemini 2.5 Pro (Google DeepMind, Mountain View, CA, USA; proprietary)
- Claude Sonnet 4.5 (Anthropic, San Francisco, CA, USA; proprietary)
- Mistral Medium 3.1 (Mistral AI, Paris, France; representing the open-source heritage)
All models were accessed via their official web interfaces using default inference settings. No API-level parameter modifications (e.g., temperature, top-p, or decoding strategies) were applied. Model versions reflect the state available in November 2024.
2.4. Prompting Strategy and Inference Protocol
Each model received five independent inference runs per case using an identical, standardized prompt (see Supplementary Material). The prompt instructed the model to analyze the uploaded radiograph and provide:
- A specific diagnostic classification (stating either “No fracture” or “Yes” followed by the specific fracture type, e.g., “metacarpal fracture”)
- An estimated patient age
- An inferred patient sex
To ensure independence across runs:
- Each run was conducted in a fresh session without conversational context
- No conversational memory, feedback, or adaptive prompting was permitted
Runs were treated as independent realizations of the model’s diagnostic response to identical input conditions. Evaluation was strictly stringent: Model outputs were scored as correct only if the predicted fracture type was concordant with the reference diagnosis. Consequently, misclassifications of the fracture type (e.g., correctly detecting the presence of a fracture but misidentifying a scaphoid fracture as a metacarpal fracture) were strictly scored as incorrect to rigorously penalize diagnostic imprecision and random guessing.
2.5. Outcome Definitions
Primary Outcomes
- Diagnostic accuracy, defined as the mean proportion of correct classifications across five runs
- Intra-model reliability, quantified using Fleiss’ kappa (κ) to assess agreement across repeated runs
Secondary Outcomes
- Case-level agreement profiles, describing how often a case was correctly classified across five runs (0/5 to 5/5 correct)
- Descriptive subgroup performance, stratified by fracture localization
2.6. Statistical Analysis
All statistical analyses were performed using Python 3.12 with standard statistical and data visualization libraries.
Diagnostic accuracy was summarized descriptively as the mean across five runs to characterize overall model performance, with the corresponding range (minimum–maximum) reported to illustrate intra-model variability. Intra-model reliability was assessed using Fleiss’ kappa, reflecting consistency across repeated model outputs rather than inter-observer agreement between independent raters. κ values were interpreted according to Landis and Koch [32,33,34]: <0.20 slight, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 substantial, and 0.81–1.00 almost perfect agreement.
Given the repeated-measures design and the absence of true negative cases, classical inferential testing and specificity-based metrics were not performed. Subgroup analyses were reported descriptively due to limited sample sizes, particularly for scaphoid fractures (n = 5).
2.7. Visualization Strategy
Results were visualized using four complementary approaches:
- Run-wise accuracy dot plots to assess diagnostic stability
- Accuracy–consistency dissociation matrix to relate correctness to reliability
- Heatmaps summarizing descriptive performance by fracture subtype
- Stacked bar charts illustrating case-level agreement profiles
This strategy was chosen to emphasize behavioral patterns and reliability characteristics rather than single-point performance estimates.
2.8. Exploratory Demographic Analyses
As an exploratory analysis, model outputs were additionally evaluated for implicit prediction of patient age and sex based solely on radiographic images. Age estimation accuracy was quantified using the Mean Absolute Error (MAE) between predicted and ground truth age values, averaged across five runs per model. Sex prediction accuracy was assessed as the percentage of correct classifications compared to recorded patient sex. Given the limited sample size and the lack of direct clinical relevance for fracture detection, these analyses were performed descriptively without formal hypothesis testing.
3. Results
3.1. Overall Diagnostic Accuracy and Stability
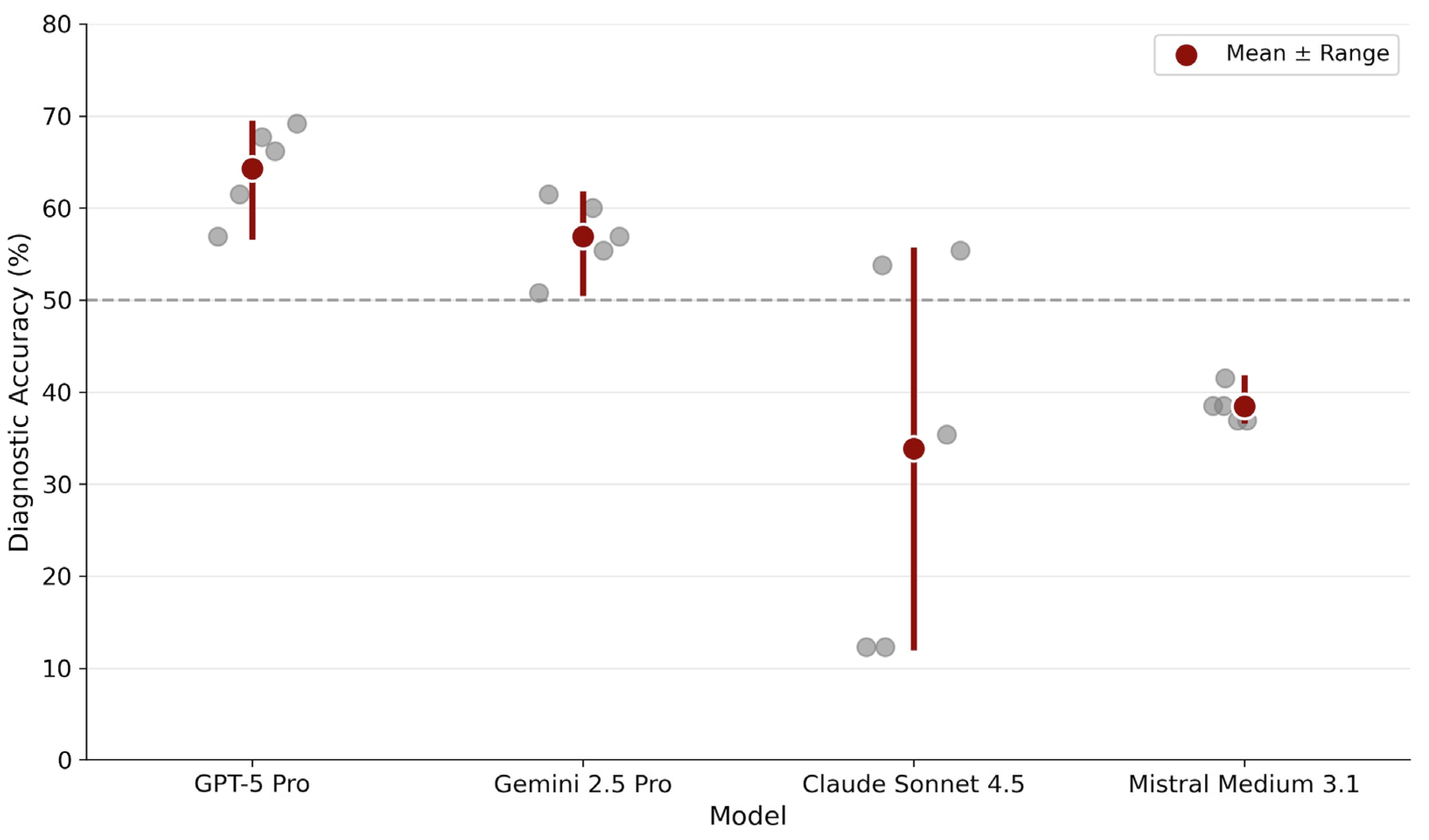
Overall diagnostic accuracy differed substantially between models (Table 1, Figure 1). GPT-5 Pro achieved the highest mean accuracy (64.3%), followed by Gemini 2.5 Pro (56.9%). Mistral Medium 3.1 (38.5%) and Claude Sonnet 4.5 (33.8%) demonstrated markedly lower performance.

Table 1.
Diagnostic accuracy across five inference runs per model (n = 65 cases).
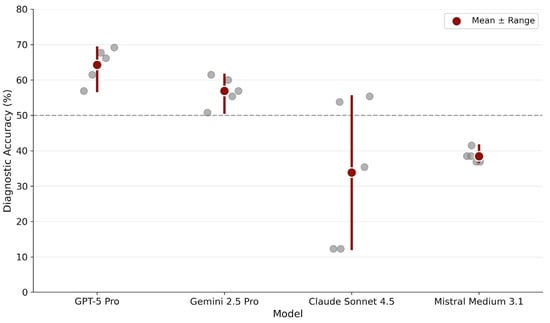
Figure 1.
Diagnostic stability across repeated runs. Each gray dot represents accuracy from one of five independent inference runs per model. Red circles indicate mean accuracy across runs. The horizontal dashed line marks 50% accuracy as a visual reference point.
Run-wise analysis revealed distinct stability profiles. GPT-5 Pro showed consistently high performance with minimal variability across runs (range: 56.9–69.2%). Gemini 2.5 Pro displayed a similarly stable profile, maintaining performance within a moderate band (range: 50.8–61.5%). In contrast, Claude Sonnet 4.5 exhibited pronounced run-to-run fluctuations (range: 12.3–55.4%), indicating probabilistic instability. Mistral Medium 3.1 displayed remarkably narrow variability (range: 36.9–41.5%) but at a substantially lower accuracy level.
3.2. Accuracy–Consistency Dissociation
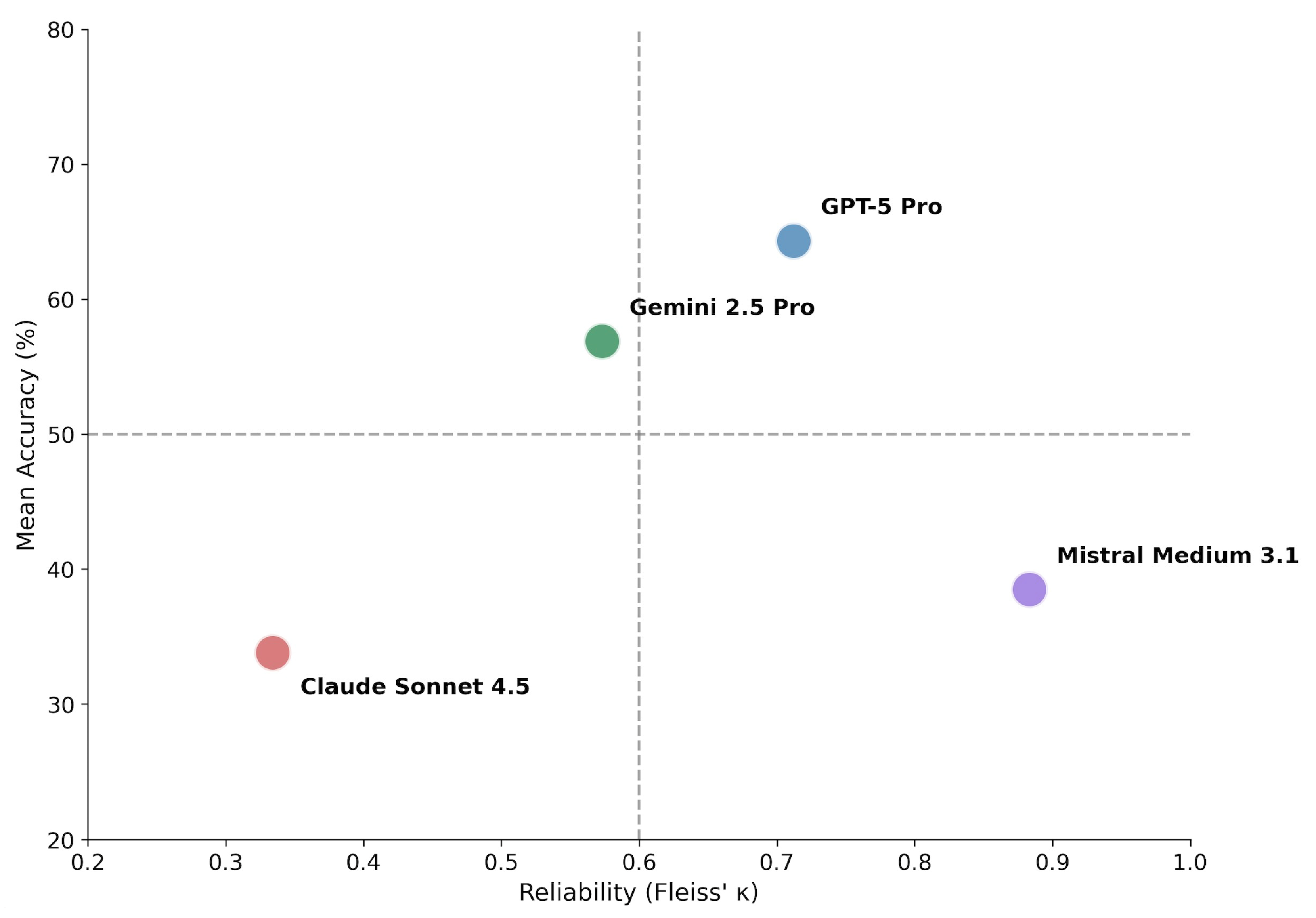
Fleiss’ kappa analysis revealed marked differences in inter-run consistency among models (Table 2, Figure 2). The relationship between diagnostic accuracy and intra-model reliability demonstrated a critical dissociation pattern.

Table 2.
Inter-run reliability (Fleiss’ κ) and summary statistics.
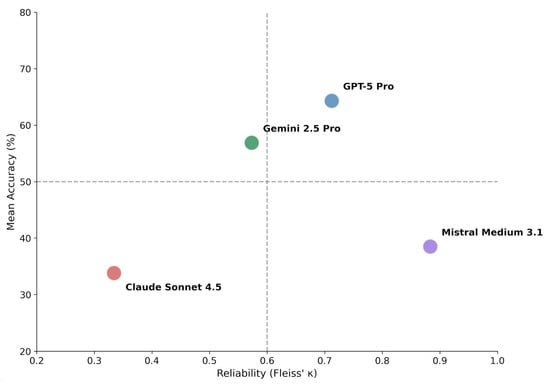
Figure 2.
Accuracy–consistency dissociation matrix. Models are plotted according to mean diagnostic accuracy (y-axis) and inter-run reliability measured by Fleiss’ κ (x-axis). Dashed lines indicate proposed thresholds: 50% accuracy (horizontal) and κ = 0.60 corresponding to substantial agreement (vertical). The resulting quadrants characterize distinct behavioral profiles: upper-right (high accuracy, high consistency) represents ideal performance; upper-left (high accuracy, low consistency) suggests potential for improvement through consensus methods; lower-right (low accuracy, high consistency) indicates systematic error; lower-left (low accuracy, low consistency) reflects unstable diagnostic behavior.
GPT-5 Pro combined high accuracy with substantial agreement (κ = 0.712), consistent with robust diagnostic behavior. Gemini 2.5 Pro demonstrated intermediate performance across both dimensions (κ = 0.573, moderate agreement).
Notably, Mistral Medium 3.1 exhibited very high consistency (κ = 0.883, almost perfect agreement) despite low accuracy, indicating a pattern of consistently applied but incorrect diagnostic decisions (systematic error). Claude Sonnet 4.5 showed both low accuracy and low consistency (κ = 0.334, fair agreement), reflecting unstable and non-deterministic diagnostic behavior.
Using thresholds of 50% accuracy and κ = 0.60 to delineate quadrants (Figure 2), the four models distributed across distinct behavioral profiles: GPT-5 Pro occupied the upper-right quadrant (high accuracy, high consistency). Gemini 2.5 Pro positioned in the upper-left region with above-average accuracy but only moderate consistency. Mistral Medium 3.1 fell in the lower-right quadrant (low accuracy, high consistency), representing systematic error. Claude Sonnet 4.5 occupied the lower-left quadrant (low accuracy, low consistency), reflecting unstable diagnostic behavior.
3.3. Descriptive Subgroup Performance
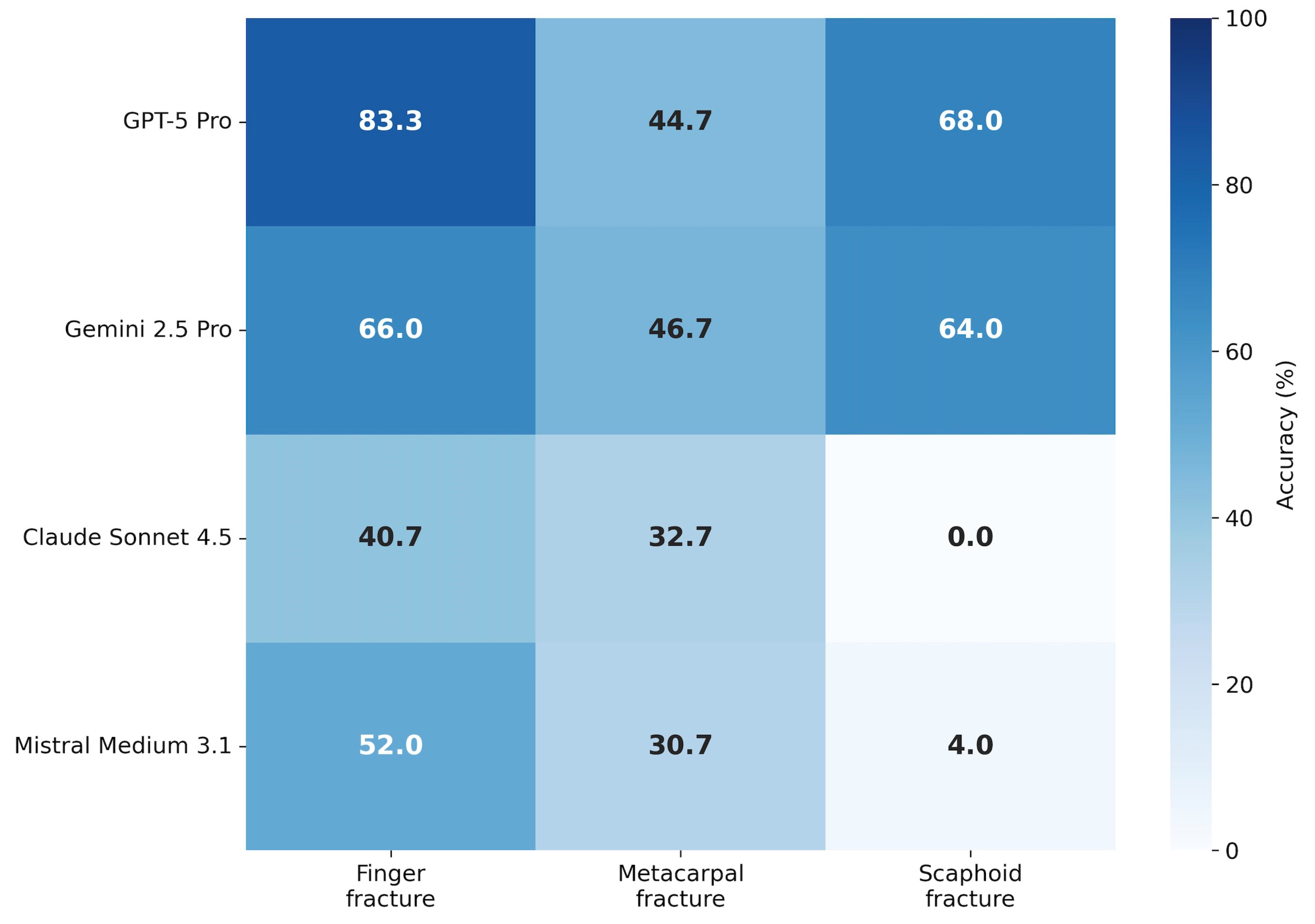
Model performance varied by fracture type (Table 3, Figure 3). Finger fractures were detected with the highest accuracy across most models, with GPT-5 Pro achieving 83.3% and Gemini 2.5 Pro reaching 66.0%. Metacarpal fractures proved more challenging, with accuracies ranging from 30.7% (Mistral Medium 3.1) to 46.7% (Gemini 2.5 Pro).

Table 3.
Diagnostic accuracy by fracture subtype (descriptive analysis).
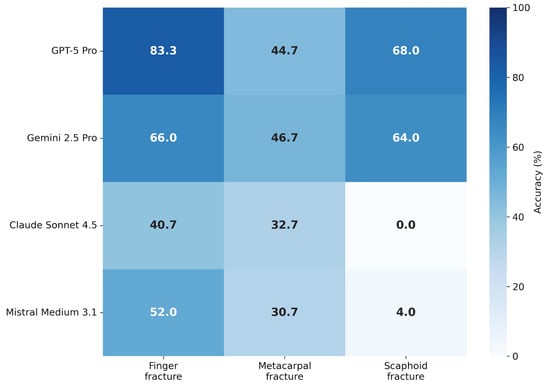
Figure 3.
Descriptive performance by fracture subtype. Heatmap displaying mean diagnostic accuracy stratified by fracture type (finger, metacarpal, scaphoid). Color intensity corresponds to accuracy percentage (darker = higher accuracy). Values are averaged across all five inference runs per model.
Scaphoid fractures (n = 5) represented the most difficult subgroup. While GPT-5 Pro and Gemini 2.5 Pro achieved moderate performance (68.0% and 64.0%, respectively), Claude Sonnet 4.5 failed entirely (0.0% accuracy across all runs), and Mistral Medium 3.1 achieved only 4.0%.
3.4. Case-Level Agreement Profiles
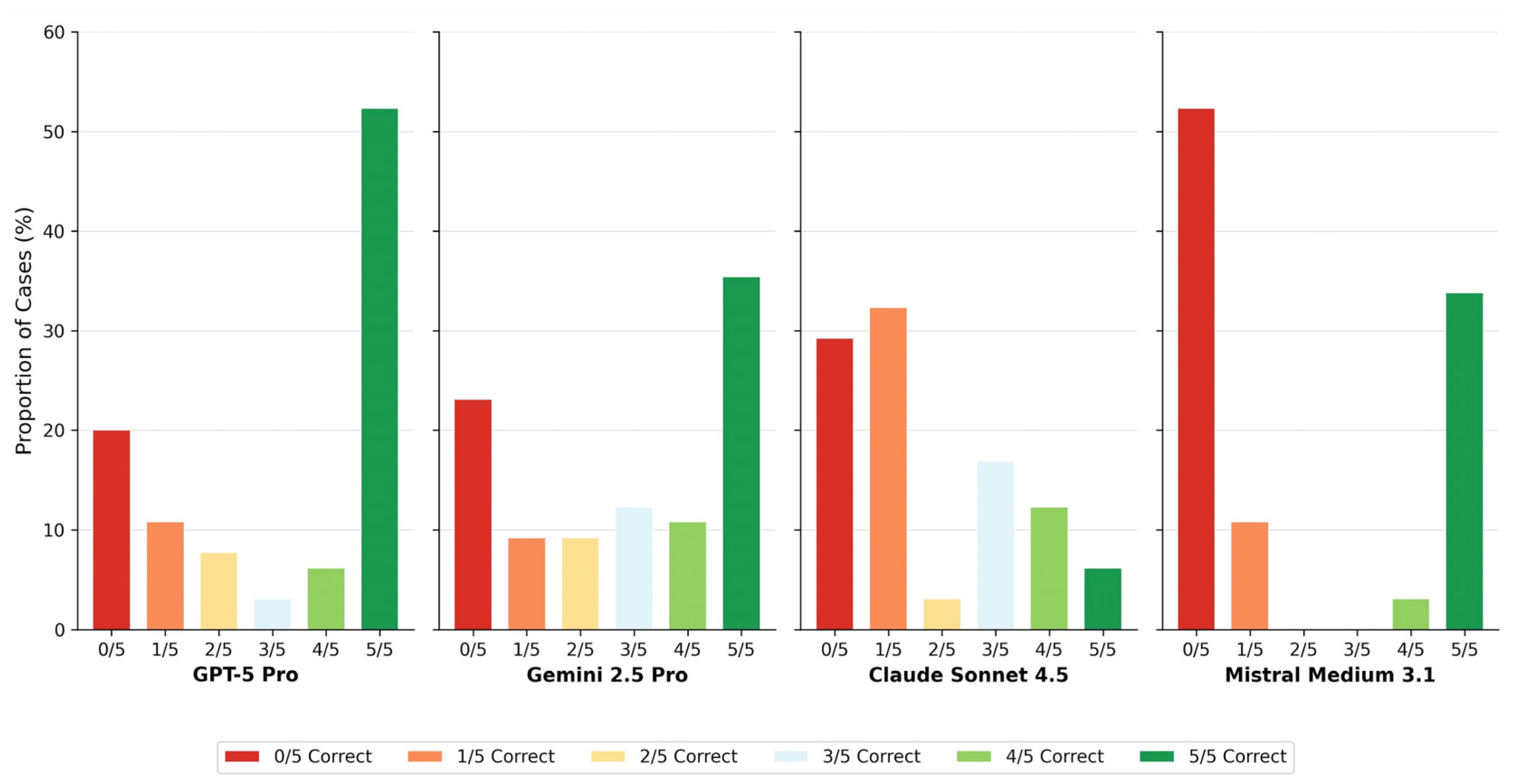
Claude Sonnet 4.5 showed predominantly unstable case-level behavior across runs, whereas Mistral Medium 3.1 demonstrated a polarized pattern characterized by consistently correct or consistently incorrect classifications.
3.5. Exploratory Demographic Attribute Inference
As a secondary analysis, model outputs were examined for implicit inference of patient age and sex across all five runs (n = 325 observations per model). Performance was generally poor.
For age estimation, GPT-5 Pro achieved the lowest Mean Absolute Error (MAE) of 12.98 ± 9.81 years, whereas other models exhibited larger deviations: Gemini 2.5 Pro (16.00 ± 12.02 years), Mistral Medium 3.1 (17.72 ± 14.07 years), and Claude Sonnet 4.5 (18.28 ± 12.98 years). Mean age estimation errors exceeded one decade across all evaluated models.
Sex prediction accuracy was remarkably low, ranging from 48.9% (Gemini 2.5 Pro) to 62.8% (GPT-5 Pro). Notably, given the male predominance in the study cohort (69.2%), a naive classifier predicting “male” for every case would have achieved higher accuracy than any of the evaluated models. Claude Sonnet 4.5 (49.2%) and Mistral Medium 3.1 (49.2%) approximated chance-level performance.
4. Discussion
4.1. Accuracy–Consistency Dissociation as a Core Behavioral Property
The central finding of this study is the marked dissociation between diagnostic accuracy and intra-model consistency, as illustrated in the Accuracy–Consistency Dissociation Matrix (Figure 2). This observation challenges a common implicit assumption in current AI evaluation paradigms, namely that higher diagnostic accuracy or reproducibility necessarily implies more reliable or clinically trustworthy behavior [35].
GPT-5 Pro occupied the quadrant of both high accuracy and substantial inter-run agreement (κ = 0.71), representing the most favorable behavioral profile among the evaluated systems. Gemini 2.5 Pro demonstrated intermediate performance across both dimensions (κ = 0.57), suggesting partially stable but not fully robust diagnostic behavior. In contrast, Mistral Medium 3.1 exhibited a clinically concerning pattern: despite achieving almost perfect intra-model agreement (κ = 0.88), its diagnostic accuracy remained low (~38%). This constellation reflects a form of systematic error characterized by highly reproducible incorrect predictions, a pattern commonly referred to in the MLLM/LLM literature as confident hallucination. In a clinical context, such high reproducibility may be misinterpreted as confidence or validity, thereby posing substantial risk [11,36].
Claude Sonnet 4.5 demonstrated the opposite failure mode, with low overall accuracy combined with pronounced run-to-run variability (accuracy range: 12.3–55.4%; κ = 0.33; Figure 1). This probabilistic instability undermines the reliability of individual outputs and precludes dependable clinical use, even when occasional correct predictions are observed.
4.2. Diagnostic Stability Beyond Single-Run Accuracy
Run-wise accuracy analysis (Figure 1) and case-level agreement profiles (Figure 4) underscore that single-point accuracy metrics provide an incomplete characterization of MLLM diagnostic performance. Traditional AI benchmarks often rely on aggregate metrics derived from a single inference pass, implicitly assuming deterministic model behavior. This assumption does not hold for generative models, where repeated inferences from identical inputs may yield divergent outputs [19,20,21,22,23,24,25,26].
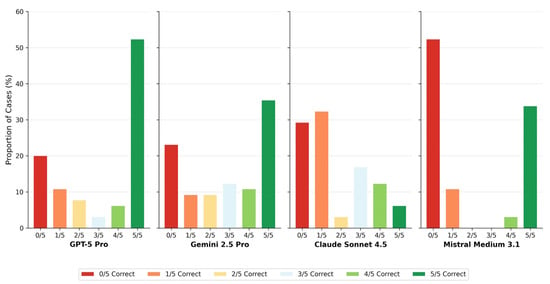
Figure 4.
Case-level agreement profiles. Bar chart showing the distribution of consensus categories for each model. Colors range from red (0/5 runs correct) through yellow (2–3/5) to green (5/5 runs correct). The proportion of cases achieving full consensus (5/5 or 0/5) versus mixed results (1–4/5) characterizes model decision stability.
Case-level agreement profiles further elucidate these behavioral differences. GPT-5 Pro achieved full consensus (5/5 correct runs) in 46.2% of cases, whereas Claude Sonnet 4.5 reached this level in only 15.4%. Conversely, complete diagnostic failure (0/5 correct runs) occurred in over half of all cases for Claude Sonnet 4.5 (53.8%), compared with 21.5% for GPT-5 Pro (Table 4, Figure 4). The distribution of intermediate agreement levels differed markedly across models, revealing fundamentally different decision stability profiles that are obscured when performance is summarized solely by mean accuracy.

Table 4.
Case-level agreement distribution of correct agreement across five runs.
4.3. Anatomical Complexity and Task-Specific Limitations
Descriptive subgroup analysis demonstrated pronounced performance differences across fracture localizations (Table 3, Figure 3). While phalangeal fractures were detected relatively reliably by the top-performing model (>80% accuracy for GPT-5 Pro), scaphoid fractures represented a major challenge. Despite the small subgroup size (n = 5), which necessitates cautious interpretation, the near-complete failure of Claude Sonnet 4.5 (0.0% accuracy) and Mistral Medium 3.1 (4.0% accuracy) suggests a fundamental limitation in integrating complex, overlapping carpal anatomy across multiple two-dimensional radiographic projections.
These findings are consistent with known challenges in automated scaphoid fracture detection and highlight the current performance gap between general-purpose MLLMs and specialized CNNs optimized for musculoskeletal imaging. While MLLMs offer flexibility and broad reasoning capabilities, they currently lack the task-specific inductive biases required for reliable detection of subtle orthopedic pathologies [37].
4.4. Demographic Attribute Inference and Diagnostic Performance
Exploratory analyses revealed that all evaluated models performed poorly in inferring patient age and sex from hand radiographs (Table 5). MAE in age estimation exceeded one decade across all systems, and sex prediction accuracy approximated chance level for most models. Importantly, demographic inference performance did not correlate with diagnostic accuracy in fracture detection tasks.

Table 5.
Exploratory results for demographic attribute inference.
These findings serve as a negative control rather than a performance benchmark. The inability of MLLMs to reliably infer demographic attributes from hand radiographs suggests that fracture detection is not driven by incidental correlations with age- or sex-related visual cues. Accordingly, correct fracture detection in this study does not appear to depend on implicit demographic inference, and demographic attribute prediction should not be interpreted as a proxy for diagnostic competence or clinical reasoning capability.
4.5. Limitations and Future Directions
This study has several methodological limitations that can be grouped into four main domains: data heterogeneity and cohort composition, scalability and evaluation constraints, benchmarking scope, and model-related sources of variability. These limitations primarily reflect current practical and conceptual constraints in evaluating MLLMs under real-world conditions.
- 1.
- The radiographic cases were derived from heterogeneous sources [30] and were not acquired under standardized imaging protocols. Differences in imaging devices, acquisition parameters, projection angles, and post-processing settings reflect real-world variability but limit strict control over image quality and comparability. Consequently, model performance may be influenced by uncontrolled technical factors inherent to the source material.
- 2.
- The study design exclusively included fracture-positive cases. While this approach was intentionally chosen to enable focused analysis of diagnostic behavior, consistency, and error patterns under conditions of confirmed pathology, it necessarily limits the scope of inference. In particular, specificity, false-positive rates, and population-level screening performance could not be assessed. Future investigations should therefore incorporate substantially larger and balanced datasets that include both fracture-positive and fracture-negative cases.
However, performing such large-scale, balanced evaluations with repeated inference runs is not feasible through standard web-based user interfaces. Repeated manual uploading of thousands of radiographic images across multiple runs would be impractical. Consequently, reproducible large-scale repeated-run analyses require programmatic access to MLLMs via application programming interfaces (APIs). Such API-based evaluation deviates from typical end-user interaction paradigms and introduces additional constraints related to token-based billing, scalability, and comparability with real-world clinical usage. Accordingly, the present study intentionally reflects real-world web-interface usage rather than optimized API-based deployments, prioritizing ecological validity over scalability.
- 3.
- The sample size, particularly for anatomically complex subgroups such as scaphoid fractures, was limited. As a result, subgroup analyses were descriptive in nature and should not be interpreted as definitive performance estimates. Larger, pathology-stratified cohorts will be required to robustly assess model behavior across less frequent but clinically critical fracture types.
- 4.
- This study evaluated MLLMs in isolation and did not include direct comparisons with human experts or with specialized CNN–based fracture detection systems. Benchmarking against experienced radiologists and task-specific CNN tools represents an important next step to contextualize the observed MLLM behavior within established clinical workflows. Comparative evaluations involving human experts, CNN-based systems, and MLLMs would allow assessment of complementary strengths, failure modes, and potential hybrid decision-support strategies.
- 5.
- The present analysis focused on diagnostic accuracy and intra-model consistency across repeated inference runs. While these metrics capture important aspects of reliability, they do not fully reflect clinical utility. Future benchmarking frameworks should incorporate additional dimensions, including time-to-decision, workflow integration, cognitive load reduction, and robustness under realistic deployment constraints. In particular, comparisons between human experts, CNN-based systems, and MLLMs should consider diagnostic performance in relation to time expenditure and operational efficiency rather than accuracy alone.
- 6.
- Generative models introduce unique sources of variability related to stochastic decoding processes. Parameters such as temperature, sampling strategies, and system-level nondeterminism may substantially influence output stability. In many real-world deployments, especially web-based interfaces, such parameters are not fully transparent or controllable by the user. This limits reproducibility and complicates fair benchmarking across systems. Future studies should therefore prioritize controlled evaluation environments that allow systematic regulation of stochastic inference settings.
- 7.
- Binary fracture detection alone captures only a limited aspect of clinical decision-making. From a clinical perspective, additional descriptive elements, including fracture classification, displacement, extent, and contextual interpretation, would likely be required before such systems could be considered for meaningful decision support.
- 8.
- Model performance represents a temporal snapshot tied to specific model versions and deployment configurations. Given the rapid evolution of multimodal architectures, frequent updates, and opaque system changes, longitudinal assessments will be necessary to determine whether observed behavioral patterns remain stable over time or shift with subsequent model iterations.
- 9.
- In addition, evolving model-level safety and moderation policies introduce an external source of variability that may affect the reproducibility of diagnostic evaluations across model versions and time.
4.6. Practical Considerations: Terms of Use, Cost Structure, and Workflow Constraints
All evaluated MLLMs are offered under provider-specific terms of use and are available in both free and multiple paid access tiers. Basic personal usage is typically covered by low-cost subscription models with strict limitations on image uploads and token consumption, whereas professional or high-volume usage is associated with substantially higher costs and, in some cases, adaptive pricing models. Precise cost comparisons were not performed, as pricing structures are dynamic, subject to frequent changes, and strongly dependent on the user’s region.
From a workflow perspective, access via web-based interfaces requires manual image upload and prompt submission for each inference, which is not suitable for frequent or large-scale use. Such interfaces are not designed for batch processing or systematic image evaluation, as required in research settings or clinical workflows. Programmatic access via APIs would therefore be a prerequisite for scalable deployment. In addition, current MLLMs increasingly restrict responses to medical and diagnostic queries, frequently issuing safety-related refusals or deferring to human clinicians, which represents a further practical limitation for reproducible medical image evaluation.
5. Conclusions
In this multi-run evaluation of MLLMs for hand fracture detection, diagnostic accuracy and intra-model consistency emerged as distinct and partially dissociated performance dimensions. GPT-5 Pro demonstrated the most favorable balance of accuracy (64.3%) and stability (κ = 0.71) among the evaluated systems, whereas other models exhibited critical failure modes ranging from systematic error with high reproducibility (Mistral Medium 3.1) to pronounced probabilistic instability (Claude Sonnet 4.5).
Key Diagnostic Insights
- Dissociation of Accuracy and Consistency: High intra-model agreement must not be equated with diagnostic correctness, as reproducibility may conceal consistently erroneous reasoning or unstable probabilistic behavior.
- Task-Dependent Performance: Model performance varied substantially by anatomical complexity, with relatively robust detection of phalangeal fractures but persistent limitations in complex carpal anatomy such as scaphoid fractures.
- Demographic Independence: Diagnostic performance was independent of implicit age or sex inference, indicating that fracture detection was not driven by incidental demographic visual cues.
Research Roadmap for Future Evaluation
- Reliability-Aware Evaluation: Future studies should routinely incorporate repeated-run analyses and reliability metrics alongside single-run accuracy to distinguish robust reasoning from stable or unstable error patterns.
- Dataset Expansion: Larger and balanced datasets including fracture-negative cases are required to assess specificity and population-level screening performance.
- Clinical Benchmarking: Systematic comparisons with task-specific CNN-based systems and human experts, ideally using controlled API-based evaluation environments, are necessary to contextualize MLLM behavior within real-world clinical workflows.
- Longitudinal Stability Monitoring: Given the rapid evolution of multimodal architectures, longitudinal assessments are necessary to determine if diagnostic behavior remains stable over subsequent model iterations and changing safety policies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics16030424/s1, Supplementary Material: Full standardized zero-shot prompt used for all inference runs.
Author Contributions
Conceptualization, I.G. and H.S.; methodology, I.G. and H.S.; software, I.G. and H.S.; validation, I.G., H.S., G.G., A.K. and M.L.; formal analysis, I.G. and H.S.; investigation, I.G. and H.S.; resources, G.G., A.K. and M.L.; data curation, I.G. and H.S.; writing—original draft preparation, I.G. and H.S.; writing—review and editing, I.G., H.S., G.G., A.K. and M.L.; visualization, I.G. and H.S.; supervision, G.G., A.K. and M.L.; project administration, I.G. and H.S.; funding acquisition, none. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval was not required because this study did not involve prospective data collection, human participants, or animals. All radiographic images were obtained from publicly available, fully anonymized educational resources.
Informed Consent Statement
Patient consent was not required because no new patient data were generated, collected, or analyzed, and no individual subjects could be identified.
Data Availability Statement
The radiographic images used in this study were obtained from publicly available educational resources and were used solely as input stimuli for model evaluation. In accordance with applicable content licenses and third-party copyright, the original image data are not redistributed. Derived data, including aggregated performance metrics and model outputs, are available from the corresponding author upon request.
Acknowledgments
This study is situated within the field of generative artificial intelligence and investigates multimodal large language models, with model architectures and versions reported in the Materials and Methods section, as both the subject and methodological context of investigation. Generative AI–based tools (Claude Sonnet 4.5) were used during manuscript preparation to support the authors in auxiliary tasks, including language editing, grammar and stylistic refinement, as well as assistance with code generation. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AI | Artificial Intelligence |
| API | Application Programming Interface |
| CNN | Convolutional Neural Network |
| κ | Fleiss’s Kappa (inter-rater reliability coefficient) |
| LLM | Large Language Model |
| MAE | Mean Absolute Error |
| MLLM | Multimodal Large Language Model |
| NLP | Natural Language Processing |
| SD | Standard Deviation |
References
- Cheah, A.E.; Yao, J. Hand Fractures: Indications, the Tried and True and New Innovations. J. Hand Surg. Am. 2016, 41, 712–722. [Google Scholar] [CrossRef]
- Vasdeki, D.; Barmpitsioti, A.; De Leo, A.; Dailiana, Z. How to Prevent Hand Injuries—Review of Epidemiological Data is the First Step in Health Care Management. Injury 2024, 55, 111327. [Google Scholar] [CrossRef]
- Karl, J.W.; Olson, P.R.; Rosenwasser, M.P. The Epidemiology of Upper Extremity Fractures in the United States, 2009. J. Orthop. Trauma 2015, 29, e242–e244. [Google Scholar] [CrossRef]
- Flores, D.V.; Gorbachova, T.; Mistry, M.R.; Gammon, B.; Rakhra, K.S. From Diagnosis to Treatment: Challenges in Scaphoid Imaging. Radiographics 2026, 46, e250050. [Google Scholar] [CrossRef]
- Krastman, P.; Mathijssen, N.M.; Bierma-Zeinstra, S.M.A.; Kraan, G.; Runhaar, J. Diagnostic accuracy of history taking, physical examination and imaging for phalangeal, metacarpal and carpal fractures: A systematic review update. BMC Musculoskelet. Disord. 2020, 21, 12. [Google Scholar] [CrossRef]
- Torabi, M.; Lenchik, L.; Beaman, F.D.; Wessell, D.E.; Bussell, J.K.; Cassidy, R.C.; Czuczman, G.J.; Demertzis, J.L.; Khurana, B.; Klitzke, A.; et al. ACR Appropriateness Criteria® Acute Hand and Wrist Trauma. J. Am. Coll. Radiol. 2019, 16, S7–S17. [Google Scholar] [CrossRef]
- Amrami, K.K.; Frick, M.A.; Matsumoto, J.M. Imaging for Acute and Chronic Scaphoid Fractures. Hand Clin. 2019, 35, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Catalano, L.W., 3rd; Minhas, S.V.; Kirby, D.J. Evaluation and Management of Carpal Fractures Other Than the Scaphoid. J. Am. Acad. Orthop. Surg. 2020, 28, e651–e661. [Google Scholar] [CrossRef]
- Pinto, A.; Berritto, D.; Russo, A.; Riccitiello, F.; Caruso, M.; Belfiore, M.P.; Papapietro, V.R.; Carotti, M.; Pinto, F.; Giovagnoni, A.; et al. Traumatic fractures in adults: Missed diagnosis on plain radiographs in the Emergency Department. Acta Biomed. 2018, 89, 111–123. [Google Scholar] [CrossRef]
- Zhang, A.; Zhao, E.; Wang, R.; Zhang, X.; Wang, J.; Chen, E. Multimodal large language models for medical image diagnosis: Challenges and opportunities. J. Biomed. Inform. 2025, 169, 104895. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.; Kim, D.Y.; Kyung, S.; Seo, J.; Song, J.M.; Kwon, J.; Kim, J.; Jo, W.; Park, H.; Sung, J.; et al. Multimodal Large Language Models in Medical Imaging: Current State and Future Directions. Korean J. Radiol. 2025, 26, 900–923. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, T.J.; Tie, X.; Warner, J.; Hu, J.; Li, Q.; Li, X. Large Language Models and Large Multimodal Models in Medical Imaging: A Primer for Physicians. J. Nucl. Med. 2025, 66, 173–182. [Google Scholar] [CrossRef]
- Fang, M.; Wang, Z.; Pan, S.; Feng, X.; Zhao, Y.; Hou, D.; Wu, L.; Xie, X.; Zhang, X.Y.; Tian, J.; et al. Large models in medical imaging: Advances and prospects. Chin. Med. J. 2025, 138, 1647–1664. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Adams, L.C.; Nebelung, S.; Kather, J.N.; Bressem, K.K.; Truhn, D. Multimodal large language models are generalist medical image interpreters. medRxiv 2023. [Google Scholar] [CrossRef]
- Urooj, B.; Fayaz, M.; Ali, S.; Dang, L.M.; Kim, K.W. Large Language Models in Medical Image Analysis: A Systematic Survey and Future Directions. Bioengineering 2025, 12, 818. [Google Scholar] [CrossRef]
- Reith, T.P.; D’Alessandro, D.M.; D’Alessandro, M.P. Capability of multimodal large language models to interpret pediatric radiological images. Pediatr. Radiol. 2024, 54, 1729–1737. [Google Scholar] [CrossRef]
- Gosai, A.; Kavishwar, A.; McNamara, S.L.; Samineni, S.; Umeton, R.; Chowdhury, A.; Lotter, W. Beyond diagnosis: Evaluating multimodal LLMs for pathology localization in chest radiographs. arXiv 2025. [Google Scholar] [CrossRef]
- da Silva, N.B.; Harrison, J.; Minetto, R.; Delgado, M.R.; Nassu, B.T.; SilvaTH. Multimodal LLMs see sentiment. arXiv 2025. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Shin, J.; Cho, B. Analyzing evaluation methods for large language models in the medical field: A scoping review. BMC Med. Inform. Decis. Mak. 2024, 24, 366. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, X.; Wang, J.; Wu, Y.; Yang, L.; Zhu, K.; Chen, H.; Yi, X.; Wang, C.; Wang, Y.; et al. A survey on evaluation of large language models. ACM Trans. Intell. Syst. Technol. 2024, 15, 39:1–39:45. [Google Scholar] [CrossRef]
- Shool, S.; Adimi, S.; Saboori Amleshi, R.; Bitaraf, E.; Golpira, R.; Tara, M. A systematic review of large language model (LLM) evaluations in clinical medicine. BMC Med. Inform. Decis. Mak. 2025, 25, 117. [Google Scholar] [CrossRef]
- Lieberum, J.L.; Toews, M.; Metzendorf, M.I.; Heilmeyer, F.; Siemens, W.; Haverkamp, C.; Böhringer, D.; Meerpohl, J.J.; Eisele-Metzger, A. Large language models for conducting systematic reviews: On the rise, but not yet ready for use-a scoping review. J. Clin. Epidemiol. 2025, 181, 111746. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, Y.; Ma, J.; Rui, W.; Zhao, C.; Heacock, L.; Huang, C. Multi-modal large language models in radiology: Principles, applications, and potential. Abdom. Radiol. 2025, 50, 2745–2757. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Xiao, T.; Albert, M.V. A survey on multimodal large language models in radiology for report generation and visual question answering. Information 2025, 16, 136. [Google Scholar] [CrossRef]
- Nakaura, T.; Ito, R.; Ueda, D.; Nozaki, T.; Fushimi, Y.; Matsui, Y.; Yanagawa, M.; Yamada, A.; Tsuboyama, T.; Fujima, N.; et al. The impact of large language models on radiology: A guide for radiologists on the latest innovations in AI. Jpn. J. Radiol. 2024, 42, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Hwang, J.; Kim, P.H.; Shim, W.H.; Seo, M.J.; Kim, D.; Shin, J.I.; Kim, I.H.; Heo, H.; Suh, C.H. Accuracy of Large Language Models in Detecting Cases Requiring Immediate Reporting in Pediatric Radiology: A Feasibility Study Using Publicly Available Clinical Vignettes. Korean J. Radiol. 2025, 26, 855–866. [Google Scholar] [CrossRef]
- Lacaita, P.G.; Galijasevic, M.; Swoboda, M.; Gruber, L.; Scharll, Y.; Barbieri, F.; Widmann, G.; Feuchtner, G.M. The Accuracy of ChatGPT-4o in Interpreting Chest and Abdominal X-Ray Images. J. Pers. Med. 2025, 15, 194. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Datta, S.; Swarup, M.S.; Panda, S.; Nayak, D.S.K.; Malik, A.; Datta, A.; Mondal, H. Radiologic Decision-Making for Imaging in Pulmonary Embolism: Accuracy and Reliability of Large Language Models-Bing, Claude, ChatGPT, and Perplexity. Indian. J. Radiol. Imaging 2024, 34, 653–660. [Google Scholar] [CrossRef]
- Mongan, J.; Moy, L.; Kahn, C.E., Jr. Checklist for Artificial Intelligence in Medical Imaging (CLAIM): A Guide for Authors and Reviewers. Radiol. Artif. Intell. 2020, 2, e200029. [Google Scholar] [CrossRef]
- Radiopaedia.org. License. Available online: https://radiopaedia.org/licence (accessed on 25 December 2025).
- Han, S.M.; Cao, L.; Yang, C.; Yang, H.-H.; Wen, J.-X.; Guo, Z.; Wu, H.-Z.; Wu, W.-J.; Gao, B.-L. Value of the 45-degree reverse oblique view of the carpal palm in diagnosing scaphoid waist fractures. Injury 2022, 53, 1049–1056. [Google Scholar] [CrossRef]
- Schober, P.; Mascha, E.J.; Vetter, T.R. Statistics from A (Agreement) to Z (z Score): A Guide to Interpreting Common Measures of Association, Agreement, Diagnostic Accuracy, Effect Size, Heterogeneity, and Reliability in Medical Research. Anesth. Analg. 2021, 133, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Aydin, C.; Duygu, O.B.; Karakas, A.B.; Er, E.; Gokmen, G.; Ozturk, A.M.; Govsa, F. Clinical Failure of General-Purpose AI in Photographic Scoliosis Assessment: A Diagnostic Accuracy Study. Medicina 2025, 61, 1342. [Google Scholar] [CrossRef] [PubMed]
- Das, A.B.; Sakib, S.K.; Ahmed, S. Trustworthy medical imaging with large language models: A study of hallucinations across modalities. arXiv 2025. [Google Scholar] [CrossRef]
- Ahmed, S.; Sakib, S.K.; Das, A.B. Can large language models challenge CNNs in medical image analysis? arXiv 2025. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.