Abstract
Background: Sarcoidosis is a multisystem inflammatory disorder characterized by non-caseating granulomas, most commonly affecting the lungs and intrathoracic lymph nodes. Angiotensin-converting enzyme (ACE) levels and calcium abnormalities are recognized biomarkers, while ^18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) is increasingly used to assess disease activity. However, neither provides sufficient diagnostic accuracy alone. Therefore, this study aimed to investigate the relationship between FDG-PET/CT metabolic findings and serum ACE and calcium (Ca2+) levels as surrogate indicators of inflammatory metabolic intensity in sarcoidosis. Methods: In this retrospective single-center study, 127 patients with pulmonary sarcoidosis who underwent PET/CT at diagnosis were evaluated. Demographic and clinical data, ACE, and Ca2+ levels were recorded. FDG uptake in mediastinal, pulmonary, and extrapulmonary sites was analyzed, and correlations with biomarkers were assessed. Results: The cohort included 89 females (70.1%) and 38 males (29.9%), mean age 51.3 ± 11.9 years. FDG uptake was most frequent in mediastinal lymph nodes (84.3%) and lung parenchyma (40.9%). ACE levels correlated weakly with total SUVmax (r = 0.214, p = 0.019). Calcium levels correlated with extrapulmonary SUVmax (r = 0.327, p = 0.001) and were higher in patients with extrapulmonary involvement (p = 0.045). No associations were found between symptom presence and biomarkers or SUVmax values. Conclusions: FDG-PET/CT metabolic parameters, particularly total and extrapulmonary SUVmax, demonstrated modest yet statistically significant associations with ACE and calcium levels. These findings suggest that a combined biomarker-imaging approach may provide complementary information regarding inflammatory metabolic intensity and systemic involvement; however, the results should be interpreted as exploratory and require validation in prospective studies.
1. Introduction
Sarcoidosis is a multisystemic inflammatory disease characterized by the formation of non-caseating granulomas in the affected organs, with pulmonary and/or thoracic lymph node involvement in more than 90% of cases. Although its etiology has not been fully elucidated, the interaction between genetic susceptibility and environmental exposures is thought to trigger granulomatous inflammation and sustain disease activity [1,2]. The pathogenesis of sarcoidosis involves an autoimmune inflammatory process involving T cells specific to autoantigens and antibody-producing B lymphocytes. Proinflammatory cytokines released by T helper (Th) cells and macrophages play an important role in initiating the inflammatory response, resulting in the development of granuloma formation, which is the fundamental pathological feature of the disease [3,4]. Clinical presentation is highly variable, ranging from asymptomatic cases detected incidentally to severe multisystemic involvement, which has led to sarcoidosis being called “the great imitator”. Diagnosis requires careful exclusion of other causes through medical history, radiological and pathological studies, and occupational/environmental assessment [5].
Currently, no standardized diagnostic method exists for sarcoidosis. Diagnosis generally relies on three main criteria: (i) a compatible clinical presentation, (ii) characteristic radiological findings, and (iii) histological confirmation of non-necrotizing granulomatous inflammation [6]. While pulmonary and intrathoracic lymph node involvement is observed in more than 90% of patients, extrathoracic sites such as the eyes, skin, and peripheral lymph nodes are affected in 20–40% of them, and clinically significant involvement of the central nervous system, heart, liver, spleen, bone, or kidneys occurs less frequently [7].
Although laboratory parameters in sarcoidosis are nonspecific, several biomarkers provide supportive diagnostic value. Serum angiotensin-converting enzyme (ACE) levels, secreted by activated mononuclear phagocytes, are elevated in approximately 50% of patients [8]. In addition, hypercalcaemia may occur due to excessive extrarenal production of 1,25-dihydroxyvitamin D by activated macrophages, which increases intestinal calcium absorption and may lead to complications such as hypercalciuria and nephrolithiasis [9]. Previous studies have reported hypercalcaemia in 3–12% of patients; in one large retrospective series, it was observed in 6% of 1606 sarcoidosis cases [10].
Radiological imaging plays a central role in diagnosis. While conventional chest radiography and computed thorax tomography (CTT) remain standard tools, they may be limited in assessing the inflammatory burden and predicting prognosis. In recent years, ^18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography (^18F-FDG PET/CT) has gained importance in sarcoidosis because of its ability to detect increased glucose metabolism in activated neutrophils, macrophages, and lymphocytes, thus reflecting active inflammation [11]. Several studies have suggested that PET/CT may provide valuable information for determining disease severity and guiding treatment decisions [12]. Despite these advances, both ACE levels and PET/CT have limitations when used alone. ACE lacks sufficient sensitivity and specificity, while PET/CT, although highly sensitive, may yield nonspecific uptake in other granulomatous or inflammatory conditions. Therefore, there is a need for studies evaluating whether combining these modalities can enhance diagnostic accuracy. In this study, we aimed to investigate the correlations between PET/CT findings and ACE and calcium levels in patients with sarcoidosis, as well as to evaluate their potential value as complementary tools in reflecting inflammatory intensity rather than simply improving diagnostic accuracy.
2. Materials and Methods
2.1. Study Design and Population
This retrospective observational study was conducted at Yedikule Training and Research Hospital. Between 1 January 2020 and 30 September 2024, the medical records of 210 adult patients (>18 years) diagnosed with pulmonary sarcoidosis through systematic evaluation, including histological confirmation by endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), were reviewed. Patients without pulmonary function tests (PFTs) or PET/CT imaging at the time of diagnosis, or with comorbidities that could affect inflammatory markers (such as autoimmune diseases or active malignancy), were excluded. Patients receiving medications affecting the renin–angiotensin–aldosterone system (ACE inhibitors or angiotensin II receptor blockers) were also excluded. After applying these criteria, 127 patients were included in the final analysis.
2.2. Data Collection
Demographic characteristics, clinical features (disease duration, radiological stage, and treatments administered), laboratory results (serum ACE levels, serum biochemical parameters, and complete blood count), and pulmonary function test values were obtained from the electronic medical record system. None of the patients were receiving drugs known to interfere with ACE activity. 18F-FDG PET/CT images were retrieved from the institution’s data repository and retrospectively reviewed using the same image review workstation (Advantage Workstation 4.7, GE Healthcare, Chicago, IL, USA). The maximum standardized uptake value (SUVmax) was measured for mediastinal, pulmonary, and extrapulmonary lesions using a three-dimensional region of interest (ROI). In cases where more than one area of FDG uptake was detected (i.e., pulmonary plus extrapulmonary, lymph node plus pulmonary, lymph node plus extrapulmonary, or all three areas), total SUVmax values were calculated by adding the SUVmax values of each region. In cases where more than one FDG-positive lymph node or lesion was present within an area, the lesion with the highest SUVmax was selected. No background correction and/or normalization was applied to raw SUVmax values. We aimed to assess inflammatory metabolic intensity using SUVmax, which represents the highest focal FDG uptake within metabolically active lesions. As TLG and MTV are not routinely assessed, we considered SUVmax measurement, which reflects inflammatory metabolic intensity, to be a practical and widely used parameter in routine PET/CT assessment rather than a measure of total granuloma burden.
2.3. PET/CT Protocol
All patients underwent PET/CT scanning after at least six hours of fasting, provided that fasting blood glucose levels were <150 mg/dL. Imaging was performed using a dedicated PET/CT scanner (Discovery 600, GE Medical Systems, Milwaukee, WI, USA) approximately 60 min after intravenous administration of 3.7 MBq/kg (~0.1 mCi/kg) of ^18F-FDG. A low-dose non-contrast CT scan was acquired (tube voltage: 120 kV; tube current: 100–210 mAs), followed by a three-dimensional PET acquisition from the skull base to mid-thigh with an acquisition time of 1.75 min per bed position. Semi-quantitative analysis was performed by calculating SUVmax for mediastinal and abdominal lymph nodes, as well as pulmonary lesions. SUVmax is known to be scanner-dependent and noise-sensitive; therefore results should be interpreted cautiously.
2.4. Statistical Analysis
Statistical analyses were performed using SPSS software, version 26.0 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation or median (minimum–maximum), and categorical variables as frequencies and percentages. The normality of distribution was assessed using the Shapiro–Wilk test and visual inspection of histograms and box plots. For group comparisons, Student’s t-test was used for normally distributed variables, and the Mann–Whitney U test for non-normally distributed variables. Categorical variables were compared using the Chi-square test or Fisher’s exact test, where appropriate. Correlations between continuous variables were evaluated using Spearman’s rank correlation coefficient. A two-tailed p-value < 0.05 was considered statistically significant, and results were reported with 95% confidence intervals (Table 1).

Table 1.
Interpretation of Cohen’s Effect Size [13].
3. Results
The study was conducted with a total of 127 patients, of whom n = 89 (70.1%) were female and n = 38 (29.9%) were male. The ages of the patients ranged between 27 and 82 years and the mean age was 51.35 ± 11.95 years.
Comorbidity was observed in 54.3% (n = 69) of the participants. The most common comorbidity was DM in 24.6% (n = 17).
Symptoms were present in 66.9% (n = 85) of the cases. The most common symptoms were shortness of breath (36.5%) and cough (40%). Demographic characteristics, symptoms and comorbidities of the patients are given in Table 2.

Table 2.
Distribution of Descriptive Characteristics.
When the radiological stages were examined, it was found that 2.4% (n = 3) of the patients were stage 0, 51.2% (n = 65) were stage 1, 45.2% (n = 54) were stage 2, 3.1% (n = 4) were stage 3, and 0.8% (n = 1) were stage 4.
PET CT was performed in all participants (n = 127). When FDG uptake was analyzed, the most common site of uptake was the mediastinal lymph nodes with 84.3% and lung parenchyma with 40.9%. 13.4% (n = 17) had no FDG uptake (Table 3).

Table 3.
Distribution of PET-CT, ACE, Calcium Findings.
ACE measurements of the participants ranged between 3 and 191 ıu/mL, with a mean value of 58.61 ± 35.91 U/L.
Serum calcium values of the participants ranged between 7.9 and 14, and the mean value was 9.62 ± 0.67 mg/dL.
There was a statistically significant weak positive correlation between ACE measurements and Total SUV (max) measurements (as ACE measurement increased, Total SUV value increased) (r = 0.214; p = 0.019; p < 0.05) (Table 4, Figure 1).

Table 4.
Correlation between ACE, serum calcium levels and SUVmax values.
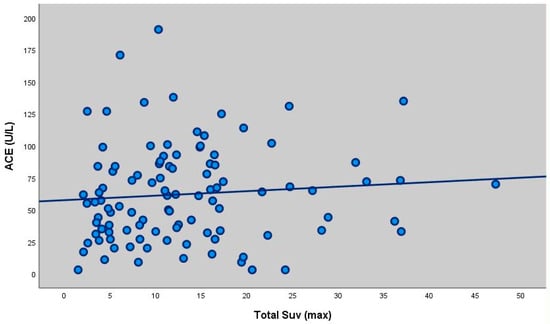
Figure 1.
Scatter plot showing the correlation between ACE levels and total SUVmax (r = 0.214, p = 0.019). There was no statistically significant correlation between ACE measurements and LAP SUV (max) and Parenchymal SUV (max) measurements of the participants (p > 0.05). There was no statistically significant correlation between extrapulmonary SUVmax values and ACE levels of the participants (p > 0.05). There was no statistically significant correlation between calcium levels and Total SUV (max), LAP SUV (max) and Parenchymal SUV (max) measurements of the participants (p > 0.05). There was a statistically significant weak positive correlation between extrapulmonary FDG measurements and calcium values (extrapulmonary FDG value increased as calcium value increased) (r = 0.327; p = 0.001; p < 0.01).
Accompanying disease, presence of symptoms, smoking status, age and gender did not show statistically significant differences in LAP SUV (max), total SUV (max), parenchymal SUV (max) and extrapulmonary SUV (max) values (all p > 0.05).
No statistically significant difference was found between the ACE measurements of the patients according to the presence of extrapulmonary involvement (p < 0.05) (Table 5).

Table 5.
Comparison of ACE and serum calcium levels according to extrapulmonary involvement.
Serum calcium measurements of patients with extrapulmonary involvement were statistically significantly higher (p = 0.045; p < 0.05) (Table 5, Figure 2 and Figure 3).
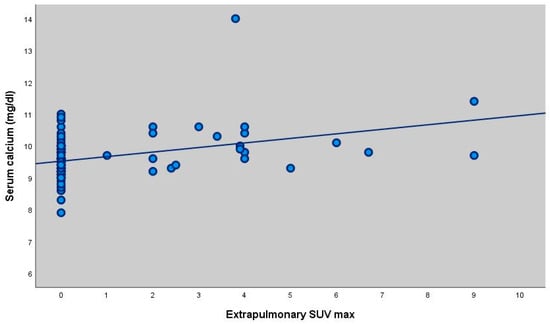
Figure 2.
Scatter plot showing the correlation between serum calcium levels and extrapulmonary SUVmax (r = 0.327, p = 0.001).
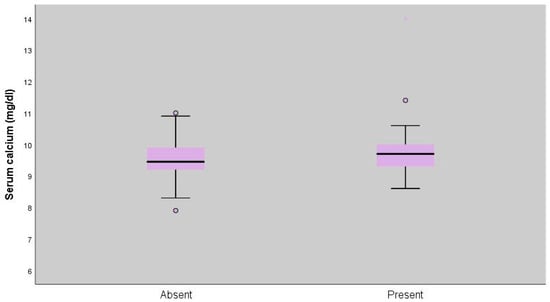
Figure 3.
Distribution of serum calcium levels according to extrapulmonary involvement (p = 0.045).
Table 6 shows that there was no statistically significant difference between ACE, calcium and total SUV max measurements of the patients according to the presence of symptoms (p < 0.05).

Table 6.
Comparison of ACE, calcium, and total SUVmax according to the presence of symptoms.
4. Discussion
In this study, we investigated the relationship between FDG-PET/CT metabolic parameters and serum ACE and calcium levels as surrogate indicators of inflammatory metabolic intensity in patients with biopsy-proven sarcoidosis. The main findings were a modest but statistically significant correlation between total SUVmax and ACE levels, and a stronger association between extrapulmonary metabolic involvement and serum calcium levels. In contrast, no significant associations were observed between PET/CT findings, biochemical markers, and the presence of clinical symptoms. These results suggest that PET/CT-derived metabolic intensity and conventional serum biomarkers may reflect different and complementary aspects of the inflammatory process in sarcoidosis, rather than directly indicating clinical disease severity or diagnostic performance.
Epidemiological studies have reported that the incidence of sarcoidosis may be higher in women than in men, although some findings are conflicting [14]. In our cohort, female predominance was evident (70.1%), which is consistent with most of the literature.
18F-fluoro-2-deoxyglucose positron emission tomography combined with computed tomography (18F-FDG PET/CT) is a highly sensitive imaging modality capable of identifying sites of active inflammation, often before morphological changes are visible on conventional imaging [15]. Several studies have demonstrated its usefulness in reliably diagnosing suspected pulmonary sarcoidosis [16]. Furthermore, whole-body FDG- PET has been shown to reflect metabolically active granulomatous involvement in patients with biopsy-proven sarcoidosis [15].
Since the 1930s, sarcoidosis has been recognized as a cause of hypercalcaemia [17]. Sarcoidosis-related hypercalcaemia is clinically important because it may result in serious morbidity and often requires long-term treatment. Identifying patients at higher risk could enable earlier intervention and closer follow-up [18]. In our study, no significant correlation was found between SUVmax values and serum calcium levels. However, calcium levels were significantly higher in patients with extrapulmonary involvement. This finding suggests that extrapulmonary disease may be a risk factor for hypercalcaemia, underlining the need for closer monitoring in such cases. Although serum calcium levels were statistically higher in patients with extrapulmonary involvement, the effect size was small to moderate (Cohen’s d = 0.39). This finding suggests that while extrapulmonary metabolic involvement may be associated with calcium dysregulation, the magnitude of this difference is modest and may not translate into a strong clinical discriminator at the individual patient level. Therefore, this association should be interpreted as biologically relevant but clinically limited.
Serum ACE levels are elevated in many patients with sarcoidosis and were initially thought to parallel disease activity [19]. Despite its proposed role in disease monitoring, the utility of ACE as a reliable biomarker remains controversial due to inconsistent findings across studies [20]. In our study, the mean ACE value was above the reference range, in line with reports suggesting frequent ACE elevation in sarcoidosis.
When ACE levels were compared with PET/CT results, no significant correlation was found with parenchymal or lymph node SUVmax values. However, a weak but significant positive correlation was observed between total SUVmax and ACE levels (p = 0.019), suggesting that higher ACE levels may be associated with greater overall inflammatory metabolic intensity. This finding contrasts with previous reports [12,21] that reported no association between SUVmax values and biochemical markers of disease activity.
Another notable result was the absence of significant differences in SUVmax, ACE, or calcium levels between symptomatic and asymptomatic patients. This finding highlights that elevations in biochemical markers and PET/CT-derived metabolic activity may not always correlate with clinical presentation. The absence of statistically significant differences between symptomatic and asymptomatic patients was accompanied by very small effect sizes across ACE, calcium, and total SUVmax measurements, indicating a negligible association between clinical symptom burden and both biochemical markers and PET-derived metabolic intensity.
In conclusion, our study demonstrated a significant correlation between total SUVmax and ACE levels, as well as higher calcium levels in patients with extrapulmonary involvement. These results suggest that combined evaluation of PET/CT findings and biochemical markers such as ACE and calcium may provide additional insights into inflammatory metabolic intensity and systemic involvement in sarcoidosis.
Strengths and Limitations
The main strength of our study is that it evaluates both PET/CT parameters and biochemical markers (ACE and calcium) together in a relatively large cohort of patients with biopsy-proven sarcoidosis. The combined analysis of imaging and laboratory data provides a more comprehensive assessment of disease activity and systemic involvement. Additionally, all patients underwent PET/CT with a standardized imaging protocol, which increases the reliability of the metabolic measurements.
However, this study also has limitations. Its retrospective and single-center design may limit the generalizability of the findings. The cross-sectional nature of the data does not allow for longitudinal evaluation of changes in biomarkers or PET/CT parameters during follow-up. Furthermore, although serum ACE and calcium levels were evaluated, other potentially relevant biomarkers of inflammation were not included, which may have provided additional insight. Finally, the absence of histopathological correlation with PET/CT findings for all extrapulmonary sites is another limitation.
5. Conclusions
In this study, we demonstrated that serum ACE levels showed a positive correlation with total SUVmax values obtained from PET/CT, while calcium levels were higher in patients with extrapulmonary involvement. These findings suggest that PET/CT metabolic parameters and biochemical markers such as ACE and calcium may provide complementary information regarding inflammatory metabolic intensity and systemic involvement in sarcoidosis. However, the results should be considered exploratory and hypothesis-generating, and require confirmation in prospective multicenter studies before any clinical application can be considered.
Author Contributions
Conceptualization, Y.I.; methodology, Y.I. and E.C.; software, Y.I.; validation, Y.I. and R.E.; formal analysis, Y.I. and E.C.; investigation, Y.I. and E.C.; resources, Y.I.; data curation, Y.I. and R.A.; writing—original draft preparation, Y.I.; writing—review and editing, Y.I. and M.C.; visualization, M.C.; supervision, Y.I.; project administration, Y.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the principles of the Declaration of Helsinki. Ethical approval was obtained from the Karamanoğlu Mehmetbey University Faculty of Medicine Local Scientific Medical Research Ethics Committee (decision number 15-2025/05, dated 15 January 2025).
Informed Consent Statement
This study was designed retrospectively, patient identity information was kept confidential and no consent form was obtained since no identifying data were used.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors confirm that they have no relevant financial or personal relationships that could influence the content of this manuscript.
References
- Rossides, M.; Darlington, P.; Kullberg, S.; Arkema, E.V. Sarcoidosis: Epidemiology and clinical insights. J. Intern. Med. 2023, 293, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, J.; Grutters, J.C.; Arkema, E.V.; Saketkoo, L.A.; Moller, D.R. Sarcoidosis. Nat. Rev. Dis. Primers 2019, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- d’Alessandro, M.; Bergantini, L.; Gangi, S.; Conticini, E.; Cavallaro, D.; Cameli, P.; Mezzasalma, F.; Cantarini, L.; Frediani, B.; Bargagli, E. Immunological Pathways in Sarcoidosis and Autoimmune Rheumatic Disorders—Similarities and Differences in an Italian Prospective Real-Life Preliminary Study. Biomedicines 2023, 11, 1532. [Google Scholar] [CrossRef]
- Pozzan, R.; Salton, F.; Confalonieri, P.; Trotta, L.; Barbieri, M.; Reccardini, N.; Torregiani, C.; Screm, G.; Hughes, M.; Baratella, E.; et al. Autoantibodies in sarcoidosis: Innocent bystander or promising biomarker for organ involvement? Sarcoidosis Vasc. Diffus. Lung Dis. 2024, 41, e2024056. [Google Scholar] [CrossRef]
- Llanos, O.; Hamzeh, N. Sarcoidosis. Med. Clin. N. Am. 2019, 103, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Maier, L.A.; Wilson, K.C.; Bonham, C.A.; Morgenthau, A.S.; Patterson, K.C.; Abston, E.; Bernstein, R.C.; Blankstein, R.; Chen, E.S.; et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 201, e26–e51. [Google Scholar] [CrossRef] [PubMed]
- Belperio, J.A.; Fishbein, M.C.; Abtin, F.; Channick, J.; Balasubramanian, S.A.; Lynch, J.P., III. Pulmonary sarcoidosis: A comprehensive review: Past to present. J. Autoimmun. 2024, 149, 103107. [Google Scholar] [CrossRef] [PubMed]
- Sejdic, A.; Graudal, N.; Baslund, B. Clinical and biochemical presentation of sarcoidosis with high and normal serum angiotensin-converting enzyme. Scand. J. Rheumatol. 2018, 47, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Donovan, P.J.; Sundac, L.; Pretorius, C.J.; d’Emden, M.C.; McLeod, D.S. Calcitriol-mediated hypercalcemia: Causes and course in 101 patients. J. Clin. Endocrinol. Metab. 2013, 98, 4023–4029. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.P.; Janovcik, J.; Ray, M.; Sweiss, N.; Lower, E.E. Calcium and vitamin D metabolism in sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2013, 30, 113–120. [Google Scholar]
- Régis, C.; Benali, K.; Rouzet, F. FDG PET/CT Imaging of Sarcoidosis. Semin. Nucl. Med. 2023, 53, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Sahin Ozdemirel, T.; Akıncı Özyürek, B.; Tatci, E.; Ertan, O.; Akkurt, E.S.; Senturk, A.; Ozmen, O. Relationships Between Systemic Inflammatory Markers and 18F-FDG PET/CT Imaging and Clinical Findings in Pulmonary Sarcoidosis. Cureus 2023, 15, e36521. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Cozier, Y.C.; Berman, J.S.; Palmer, J.R.; Boggs, D.A.; Wise, L.A.; Rosenberg, L. Reproductive and hormonal factors in relation to incidence of sarcoidosis in US Black women: The Black Women’s Health Study. Am. J. Epidemiol. 2012, 176, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Popević, S.; Šumarac, Z.; Jovanović, D.; Babić, D.; Stjepanović, M.; Jovičić, S.; Šobić-Šaranović, D.; Filipović, S.; Gvozdenović, B.; Omčikus, M.; et al. Verifying Sarcoidosis Activity: Chitotriosidase versus ACE in Sarcoidosis—A Case-control Study. J. Med. Biochem. 2016, 35, 390–400. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donnelly, R.; McDermott, M.; McManus, G.; Franciosi, A.N.; Keane, M.P.; McGrath, E.E.; McCarthy, C.; Murphy, D.J. Meta-analysis of [18F] FDG-PET/CT in pulmonary sarcoidosis. Eur. Radiol. 2025, 35, 2222–2232. [Google Scholar] [CrossRef] [PubMed]
- Harrell, G.T.; Fisher, S. Blood chemical changes in Boeck’s sarcoid, with special reference to protein, calcium, and phosphatase values. J. Clin. Investig. 1939, 18, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.; Rivera, N.; Grunewald, J.; Eklund, A.; Iseda, T.; Darlington, P.; Kullberg, S. HLA-DRB1 alleles associate with hypercalcemia in sarcoidosis. Respir. Med. 2021, 187, 106537. [Google Scholar] [CrossRef] [PubMed]
- Danilov, S.M.; Kurilova, O.V.; Sinitsyn, V.E.; Kamalov, A.A.; Garcia, J.G.N.; Dudek, S.M. Predictive potential of ACE phenotyping in extrapulmonary sarcoidosis. Respir. Res. 2022, 23, 211. [Google Scholar] [CrossRef]
- McGrath, D.S.; Foley, P.J.; Petrek, M.; Izakovicova-Holla, L.; Kolek, V.; Veeraraghavan, S.; Lympany, P.A.; Pantelidis, P.; Vasku, A.; Wells, A.U.; et al. Ace gene I/D polymorphism and sarcoidosis pulmonary disease severity. Am. J. Respir. Crit. Care Med. 2001, 164, 197–201. [Google Scholar] [CrossRef]
- Milojevic, I.G.; Sobic-Saranovic, D.; Petrovic, N.; Beatovic, S.; Tadic, M.; Artiko, V.M. Hybrid Imaging in Evaluation of Abdominal Sarcoidosis. Curr. Med. Imaging Rev. 2019, 15, 26–31. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.