Abstract
We describe a patient with recurrent, brief episodes of chest discomfort caused by a highly mobile papillary fibroelastoma originating from the aortic wall and intermittently encroaching on the right coronary artery ostium. Initial 2D and 3D transthoracic and 2D transesophageal echocardiography identified a highly mobile mass in the ascending aorta above the aortic valve; the exact site of attachment and its relationship to the coronary ostia could not be clearly defined. Three-dimensional transesophageal echocardiography enabled precise anatomical reconstruction of the lesion and surrounding structures, clearly demonstrating its pedicle and proximity to the right coronary ostium. This imaging modality clarified the pathophysiological mechanism of symptoms and facilitated optimal surgical planning without the need for additional complex imaging techniques.
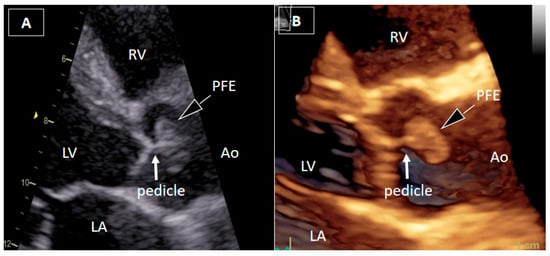
Figure 1.
Transthoracic echocardiography. A 54-year-old man with recurrent, brief episodes of chest discomfort and no cardiovascular risk factors was referred for echocardiographic evaluation. Two-dimensional transthoracic echocardiography (2DTTE) (A) and three-dimensional transthoracic echocardiography (3DTTE) (B) demonstrated a round, mobile mass in the ascending aorta just above the aortic valve, most likely attached to the downstream surface of the right valve leaflet by a short pedicle. The attachment site of the pedicle was not clearly identified. Other structural and functional echocardiographic parameters, including myocardial strain imaging, were within normal ranges.
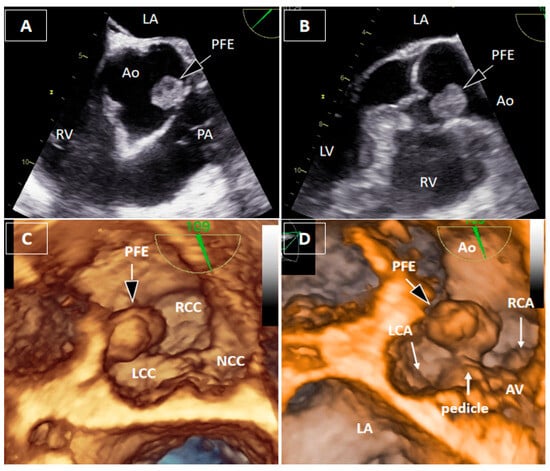
Figure 2.
Transesophageal echocardiography. Two-dimensional transesophageal echocardiography (2DTEE) localized the mass to the region of the right coronary sinus, between the left and right coronary cusps, without precise identification of the attachment site (A,B). Three-dimensional transesophageal echocardiography (3DTEE) provided detailed anatomical definition, clearly identifying a short pedicle originating from the aortic wall between the bases of the left and right coronary cusps (C,D). The mass measured 17 × 11 × 8 mm, with a pedicle length of approximately 4 mm, and demonstrated marked mobility toward the right coronary artery ostium during the cardiac cycle. Measurements were obtained from the 3DTEE.
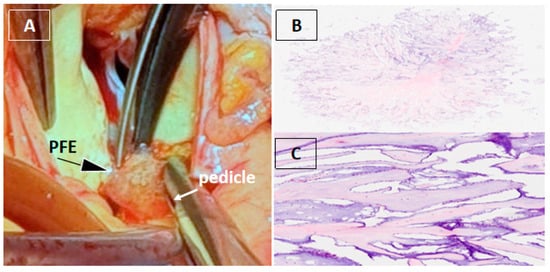
Figure 3.
Surgical and histopathological findings. Surgical removal of a cardiac mass is recommended in both symptomatic and asymptomatic patients due to the risk of embolization, especially when the mass is mobile and larger than 10 mm [1,2,3]. Given the patient’s symptoms, the marked mobility of the lesion, and its immediate proximity to the right coronary ostium with suspected dynamic obstruction and high embolic risk, surgical excision was performed. Computed tomography coronary angiography, performed to rule out occult coronary disease as an alternative cause of chest discomfort, showed coronary arteries without atherosclerotic disease [4,5]. Cardiac magnetic resonance imaging can be useful for tissue characterization and defining the anatomical relationship of the mass to adjacent structures [3,4,5,6]. However, in our patient, 3DTEE provided sufficient anatomical definition of the mass and its relationship to the coronary ostium, obviating the need for additional imaging. Intraoperative inspection revealed a gelatinous mass attached by a small pedicle to the aortic wall immediately adjacent to the right coronary artery ostium, which was completely excised with preservation of the aortic valve and root (A). Histopathological analysis confirmed papillary fibroelastoma (PFE), characterized by avascular papillary fronds with a fibroelastic core lined by endothelium (B,C) [7].
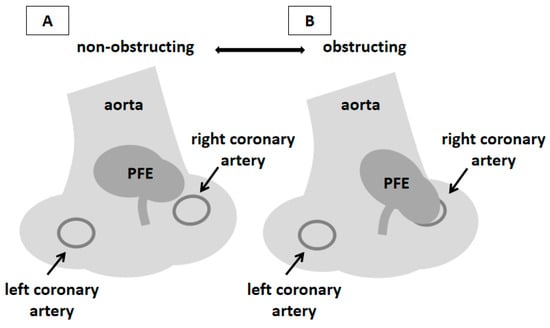
Figure 4.
Schematic representation of the localization of PFE and the pathophysiological mechanism of the patient’s symptoms. PFE typically arises from the downstream surface of left-sided valves, most frequently aortic; localization in the aortic wall is extremely rare [6,8,9]. In the presented patient, the onset of symptoms was closely related to the mobility of the mass and its occasional contact with the ostium of the right coronary artery. Similar cases have already been described in the literature [10,11,12,13,14]. 3DTEE enabled direct visualization of this interaction, providing pathophysiological insight and facilitating tailored surgical planning. This schematic illustration (prepared from Figure 2D) summarizes the hypothesized mechanism of intermittent ostial obstruction of the right coronary artery. During the cardiac cycle, a mobile PFE arising from the aortic wall adjacent to the right coronary artery ostium moves toward and away from the ostium, occasionally resulting in its transient occlusion and consequent myocardial ischemia (A,B). During the four-year follow-up, the patient remained asymptomatic with normal echocardiographic findings. Therefore, the resolution of symptoms after the PFE excision points to this mechanism as the most likely cause of the symptoms in our patient.
Author Contributions
Conceptualization, T.B., R.P.-A., and D.F.; methodology, T.B. and R.P.-A.; resources, D.F.; writing—original draft preparation, T.B. and R.P.-A.; writing—review and editing, D.F.; supervision, D.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University Hospital of Split, Split, Croatia (approval number: No. 2181-147/01-06/LJ.Z.-25-02; approval date: 19 December 2025).
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper. The patient’s identity is protected.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Ao | Aorta |
| AV | Aortic valve |
| LA | Left atrium |
| LCA | Left coronary artery |
| LCC | Left coronary cusp |
| LV | Left ventricle |
| NCC | Non coronary cusp |
| PFE | Papillary fibroelastoma |
| PA | Pulmonary artery |
| RCA | Right coronary artery |
| RCC | Right coronary cusp |
| RV | Right ventricle |
| 2DTTE | Two-dimensional transthoracic echocardiography |
| 3DTTE | Three-dimensional transthoracic echocardiography |
| 2DTEE | Two-dimensional transesophageal echocardiography |
| 3DTEE | Three-dimensional transesophageal echocardiography |
References
- Gowda, R.M.; Khan, I.A.; Nair, C.K.; Mehta, N.J.; Vasavada, B.C.; Sacchi, T. Cardiac papillary fibroelastoma: A comprehensive analysis of 725 cases. J. Am. Heart J. 2003, 146, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, H.; Andrei, A.C.; Li, Z.; McCarthy, P.M.; Malaisrie, S.C. Papillary fibroelastoma of the aortic valve: Analysis of 21 cases, including a presentation with cardiac arrest. Tex. Heart Inst. J. 2015, 42, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Alozie, A.; Zimpfer, A.; Erbersdobler, A.; Neßelmann, C.; Öner, A.; Dohmen, P.M. Surgery for Valvular and Nonvalvular Papillary Fibroelastomas. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Aggeli, C.; Dimitroglou, Y.; Raftopoulos, L.; Sarri, G.; Mavrogeni, S.; Wong, J.; Tsiamis, E.; Tsioufis, C. Cardiac Masses: The Role of Cardiovascular Imaging in the Differential Diagnosis. Diagnostics 2020, 10, 1088. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, F.B.; Gnan, E.; Pandolfi, A.; Iacuzio, L.; Kim, J.K.; Canetta, C.; Rizzuto, A.S.; Ruscica, M.; Carugo, S. Diagnostic Algorithm Using Multimodal Imaging for the Differential Diagnosis of Intra-Cardiac Masses. J. Clin. Med. 2025, 14, 508. [Google Scholar] [CrossRef] [PubMed]
- Tyebally, S.; Chen, D.; Bhattacharyya, S.; Mughrabi, A.; Hussainm, Z.; Manisty, C.; Westwood, M.; Ghosh, A.K.; Guha, A. Cardiac Tumors. J. Am. Coll. Cardiol. Cardio Oncol. 2020, 2, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Kurmann, R.; El-Am, E.; Ahmad, A.; Abbasi, M.A.; Mazur, P.; Akiki, E.; Anand, V.; Herrmann, J.; Casanegra, A.I.; Young, P.; et al. Cardiac Masses Discovered by Echocardiogram; What to Do Next? Struct. Heart. 2023, 7, 100154. [Google Scholar] [CrossRef] [PubMed]
- Al-Azizi, K.M.; Hamandi, M.; Baxter, R.; Krueger, A.; Crawford, A.W.; William, M.; Good, C.; Mead, N.J. Papillary Fibroelastoma of the Ascending Aorta. Investig. Med. High Impact. Case Rep. 2019, 7, 2324709619840377. [Google Scholar] [CrossRef] [PubMed]
- Tadic, S.; Ilic, A.; Stefanovic, M.; Stojsic-Milosavljevic, A.; Popov, T.; Bjelobrk, M.; Milovancev, A.; Maksimovic, N.; Drid, P. Case Report: Multimodality Imaging as a lifeline for fatal localization of Valsalva sinus fibroelastoma. Front. Cardiovasc. Med. 2021, 8, 683534. [Google Scholar] [CrossRef] [PubMed]
- Erdoes, G.; Stalder, M.; Basciani, R.; Gugger, M.; Carrel, T.; Eberle, B. An uncommon cause of coronary artery ostial obstruction: Papillary fibroelastoma. Echocardiography 2010, 27, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Masiello, P.; Catalano, A.; Mastrogiovanni, G.; Eusebio, G.; De Roberto, A.M.; Chivasso, P.; Ciancia, G.; Iesu, I.; Triggiani, D.; Iesu, S. Surgical removal of an exceedingly rare papillary fibroelastoma of the aortic wall causing unstable angina. Clin. Case Rep. 2021, 9, e04688. [Google Scholar] [CrossRef] [PubMed]
- Huenges, K.; Hartmann, F.; Panholzer, B.; Puehler, T. Successful interdisciplinary treatment of a rare cause of acute myocardial ischaemia from intermittent tumour-associated obstruction of the left main coronary artery: A case report. Eur. Heart J. Case Rep. 2021, 5, ytab292. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.Y.; Kashuba-Shanahan, K.M.; Cavallaro, J.A.; Fentanes, E. Mobile Papillary Fibroelastoma Leading to Severe Chest Pain via Left Main Occlusion. JACC Case Rep. 2025, 30, 103535. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, I.; Bertrand, P.B.; Vrolix, M.; Ferdinande, B.; Cruysberghs, Y. Papillary fibro-elastoma as a rare cause of rate-dependent angina: Importance of diastolic coronary perfusion. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 632. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.