Abstract
Background/Objectives: Cefoperazone–sulbactam is frequently prescribed to older inpatients for severe infections but has been associated with coagulation abnormalities, particularly among individuals with malnutrition or hepatic dysfunction. Early identification of at-risk patients remains challenging. To develop and validate a clinically interpretable model for predicting cefoperazone–sulbactam-related coagulation abnormalities in elderly inpatients and to provide practical tools for bedside risk estimation. Methods: We conducted a retrospective, dual-center study of 485 patients aged ≥ 60 years treated at Fuxing Hospital and Xuanwu Hospital of Capital Medical University who received cefoperazone–sulbactam for ≥72 h. Baseline clinical and demographic variables were analyzed using univariate and multivariable logistic regression to identify independent risk factors. Ten supervised machine-learning models were trained and evaluated using area under the ROC curve (AUC), accuracy, sensitivity, specificity, precision, and F1-score. SHapley Additive exPlanations (SHAP) were applied to assess model interpretability. A nomogram was constructed from the final logistic regression model, and a web-based calculator was developed for clinical use. Results: Multivariable analysis identified age ≥ 75 years, hypoproteinemia, total parenteral nutrition, insomnia, and recent oral antibiotic use as independent predictors of coagulation abnormalities. Among machine-learning models, LightGBM achieved the best overall performance by AUC and balanced classification metrics. SHAP analyses provided individualized and global explanations of feature contributions, facilitating clinical interpretation. The nomogram and web calculator enable rapid, patient-specific risk estimation. Conclusions: An interpretable machine-learning approach, complemented by a nomogram and web calculator, accurately stratifies the risk of cefoperazone–sulbactam-induced coagulation abnormalities in elderly inpatients. These tools may support personalized risk evaluation and earlier preventive interventions in routine care. The web-based calculator facilitates rapid bedside risk estimation and may help guide earlier monitoring and preventive interventions in routine care.
1. Introduction
Cefoperazone–sulbactam, a commonly used β-lactam/β-lactamase inhibitor combination, is widely prescribed for severe infections in older inpatients, such as pneumonia, urinary tract infection, and intra-abdominal sepsis [1,2,3]. Due to its broad-spectrum antimicrobial activity and effectiveness against extended-spectrum β-lactamase-producing organisms, cefoperazone–sulbactam is a first-line empirical therapy in many tertiary care institutions. In older people who tend to have comorbidities, compromised immune systems, and prolonged hospitalizations, cefoperazone–sulbactam provides reliable coverage in cases of polymicrobial nosocomial infections [1,3,4]. Despite that, its wide application in older patients also calls for close surveillance of drug-associated adverse events, especially those that are insidious or not correctly estimated in the routine care [5,6,7].
Although clinically valuable, cefoperazone–sulbactam has been implicated in coagulation dysfunction, such as prolonged prothrombin time (PT) and activated partial thromboplastin time (APTT), as well as bleeding events. These complications often go underdiagnosed, especially in elderly patients with multiple comorbid conditions [8,9]. Possible mechanisms include suppression of synthesis of vitamin K–dependent clotting factors, possibly by the N-methylthiotetrazole (NMTT) side chain of cefoperazone, and alteration of intestinal flora, which diminishes the production of endogenous vitamin K. Older adults are at higher risk of being susceptible to these effects caused by age-related hepatic insufficiency, lowered dietary intake, reduced diversity of gut flora, and more commonly used broad-spectrum antibiotics, or antiplatelet drugs [2,9,10,11].
In a clinical setting, these alterations may be subtle or identified only in the setting of bleeding complications. Considering the high utilization rate of cefoperazone–sulbactam in geriatric medicine, it is clinically significant to take a proactive role in recognizing patients who carry a higher risk, which is currently lacking.
Although several case reports and pharmacovigilance studies have reported on coagulopathy related to cefoperazone, few attempts have been made to identify patients at the highest risk. Much of the literature to date has been univariate or has detected bleeds post hoc and, therefore, provides little indication for prospective clinical decision-making. However, there are no established methods for assessing coagulation risk before or while antimicrobial treatment is performed in older people [8,12,13].
This lack of risk stratification is particularly problematic in frail older patients, who frequently present with overlapping risk factors, i.e., malnutrition, polypharmacy, liver insufficiency, and insomnia, which together may enhance their vulnerability [14,15]. In the absence of predictive guidance, clinicians have to rely on their judgment, which could delay therapy or, conversely, result in continuation of treatment in low-risk subjects.
Because there were no established risk stratification systems for antibiotic-associated coagulopathy, the present study attempted to develop an easily applicable prediction model for detecting the development of coagulation abnormalities in elderly inpatients receiving treatment with cefoperazone–sulbactam. We first used multivariable logistic regression to identify independent clinical risk factors with retrospective data from two tertiary care hospitals. To further enhance predictive performance and explore algorithmic alternatives, we implemented and compared ten supervised machine learning models. Machine learning methods can model complex nonlinear relationships and interactions among clinical variables, which may improve risk stratification when multiple overlapping geriatric risk factors coexist. This complements regression-based tools by providing an algorithmic alternative when conventional models may be limited in capturing such complexity.
We also applied SHapley Additive exPlanations (SHAP) to enhance interpretability and provide individualized explanations of feature contributions. We constructed a nomogram for bedside use and developed a web-based tool to support real-time clinical decision-making. The aim of this integrative process was early identification of risk, therapeutic approach guide extension, belonging to the clinical admittance, and patient safety by antibiotics management.
2. Materials and Methods
2.1. Study Population and Data Collection
This was a retrospective observational study performed at 2 tertiary hospitals in Beijing, namely Fuxing Hospital and Xuanwu Hospital of Capital Medical University. Elderly inpatients (aged ≥ 60 years) who received intravenous cefoperazone–sulbactam treatment for at least 72 h between August 2020 and August 2024 were considered for inclusion. We clarified that ≥72 h is an inclusion criterion (minimum exposure), while outcome ascertainment spans hospitalization after initiation. Patients with pre-existing coagulation defects, concomitant anticoagulant treatment, and incomplete medical records were excluded. Pre-existing coagulation defects were defined as any documented congenital or acquired coagulation disorder (e.g., hemophilia, von Willebrand disease, disseminated intravascular coagulation, or advanced liver disease–related coagulopathy), or abnormal baseline coagulation tests prior to cefoperazone–sulbactam exposure, defined as PT or APTT > 1.5× the upper limit of normal.
The main outcome was coagulation impairment, defined as prolonged PT, APTT, or evidence of bleeding during hospitalization. The primary outcome (coagulation abnormality) was defined as PT > 18 s or >1.5× the upper limit of normal, and/or APTT > 60 s or >1.5× the upper limit of normal, occurring after cefoperazone–sulbactam initiation during hospitalization; clinically evident bleeding events documented in the medical record were also counted as outcome events. A total of 485 patients fulfilled the inclusion criteria. Clinical and demographic information was obtained from electronic medical record systems and consisted of 18 potential variables categorized as demographics, comorbidities, medication history, nutrition status, and lifestyle.
2.2. Data Preprocessing and Cohort Splitting
All categorical variables were converted to binary variables or dummy variables, and continuous variables were checked for normal distribution and standardized, when necessary. Continuous variables (primarily BMI) were standardized using z-score normalization (mean = 0, standard deviation = 1) calculated from the training set and applied to the test set. Because BMI in our cohort was approximately normally distributed, no transformation was required; if substantial skewness had been present, a log-transformation would have been considered prior to standardization. Missing values were handled using complete-case analysis (listwise deletion). Because no predictor had >5% missingness, no imputation was performed; records with missing predictor values were excluded from model training and evaluation. The dependent variable, coagulation abnormality status, was dichotomous (present/absent).
The dataset was randomly divided into training (70%) and test (30%) datasets with stratified sampling to maintain the distribution of the positive outcome for the training and test sets. The following definitions were uniform: age (60–75, ≥75 y); feeding_state (normal diet, transnasal gastric tube feeding, and total parenteral nutrition); hypoproteinemia (serum albumin < 35 g/L); and lifestyle factors [smoking, drinking, and insomnia (present or absent)]. These feature sets were applied to all subsequent modeling and interpretability analyses.
2.3. Logistic Regression and Feature Selection
Logistic regression was used to identify independent predictors of cefoperazone–sulbactam-related coagulopathy. Univariate logistic regression was performed on each potential variable in the training set. Variables showing a potential significant relationship with the outcome (p < 0.05) were entered in a multivariable logistic regression model. A backward stepwise elimination method was used to iteratively remove nonsignificant variables, thereby refining the model to include predictors that were statistically and clinically relevant.
The multivariable logistic regression model was used to calculate adjusted odds ratios (ORs) with 95% confidence intervals (CIs) to assess the independent contribution of each variable in relation to confounding. This method provided insight into independent clinical and demographic variables that may predict the risks of coagulation dysfunction by cefoperazone–sulbactam exposure. The predictor variables in the final model were then chosen as input predictors for subsequent machine learning model building and interpretability analysis.
2.4. Model Training and Validation by Machine Learning Model
We used ten supervised machine learning methods for building the prediction models of coagulation abnormalities, including AdaBoost, CatBoost, Gradient Boosting Machine (GBM), k-Nearest Neighbors (KNN), Light Gradient Boosting Machine (LightGBM), logistic regression, Neural Network, Random Forest, Support Vector Machine (SVM), and eXtreme Gradient Boosting (XGBoost). Input features were those originated from the variables included in the multivariable logistic regression, and each model was trained in the training data.
For optimization of performance and overfitting, model training and internal validation were performed with ten-fold cross-validation. Hyperparameter tuning was performed using grid search when applicable. Comparisons were made between the training set and test set’s model performance based on a set of metrics, including AUC, accuracy, sensitivity, specificity, precision, and F1 score. Discriminative power was measured with ROC curves, model calibration was assessed using calibration plots, and net clinical benefit was evaluated using decision curve analysis (DCA).
2.5. Model Interpretation, Nomogram, and Web Deployment
To enhance the interpretability and clinical transparency of the best-performing machine learning model, SHapley Additive exPlanations (SHAP) analysis was performed. SHAP values quantified each feature’s contribution to the prediction at both the global and individual levels. Visualization outputs included summary plots, dependence plots, force plots, and waterfall charts, which offered insight into how specific variables influenced model outputs.
Additionally, a nomogram was constructed based on the final multivariable logistic regression model to provide an intuitive, point-based clinical tool for individualized risk estimation. Nomograms were developed using both the full dataset and the training subset to assess robustness and generalizability. Finally, an interactive web-based prediction tool was built using the Shiny framework. This tool allows clinicians to input patient-specific information and obtain real-time risk estimates for cefoperazone–sulbactam-associated coagulation abnormalities. All processes were performed using R software (version 4.5.0).
3. Results
3.1. Baseline Characteristics of the Study Population
A total of 485 elderly inpatients were included in the study, comprising 335 patients without coagulation abnormalities and 150 patients who developed coagulation abnormalities following the administration of cefoperazone–sulbactam. The baseline characteristics of the two groups are summarized in Table 1. Patients in the coagulation abnormalities group were significantly older, with 61.3% aged ≥ 75 years compared to 42.4% in the non-abnormal group (p < 0.001). A higher proportion of patients in the abnormal group had a history of intravenous (40.7% vs. 27.5%, p = 0.005) and oral (56.7% vs. 38.5%, p < 0.001) antibiotic use. Nutritional support methods also differed markedly, with total parenteral nutrition (18.0% vs. 8.4%) and transnasal gastric tube feeding (44.7% vs. 37.9%) more prevalent in the abnormal group (p < 0.001). Hypoproteinemia was significantly more frequent among patients with coagulation abnormalities (88.0% vs. 66.6%, p < 0.001), as was the use of antiplatelet medications (43.3% vs. 31.3%, p = 0.014). Insomnia was also more commonly reported in this group (82.7% vs. 69.0%, p = 0.002). No significant differences were observed between the two groups in terms of gender, marital status, smoking, drinking, hypertension, diabetes, cardiovascular disease, hepatic or renal insufficiency, hyperlipidemia, or body mass index (all p > 0.05). These variables were retained for further modeling to assess their potential contributions to the risk of coagulation abnormalities.

Table 1.
Characteristics of included patients.
3.2. Logistic Regression Analysis of Risk Factors
Univariate and multivariate logistic regression analyses were conducted to identify independent risk factors for cefoperazone–sulbactam-associated coagulation abnormalities in the training cohort. As shown in Table 2, several variables were significantly associated with coagulation risk in univariate models, including age ≥ 75 years (OR: 2.14, 95% CI: 1.34–3.43, p = 0.002), history of intravenous (OR: 2.14, p = 0.002) and oral (OR: 2.27, p < 0.001) antibiotic use, total parenteral nutrition (OR: 4.07, p < 0.001), antiplatelet use (OR: 1.99, p = 0.005), hypoproteinemia (OR: 2.33, p = 0.005), and insomnia (OR: 2.40, p = 0.003).

Table 2.
Training set univariate and multivariate logistic regression results.
Multivariate logistic regression confirmed five independent predictors: age ≥ 75 years (adjusted OR: 2.13, 95% CI: 1.27–3.56, p = 0.004), oral antibiotic use (adjusted OR: 1.90, 95% CI: 1.13–3.19, p = 0.015), total parenteral nutrition (adjusted OR: 3.82, 95% CI: 1.71–8.53, p = 0.001), hypoproteinemia (adjusted OR: 3.19, 95% CI: 1.64–6.19, p < 0.001), and insomnia (adjusted OR: 2.94, 95% CI: 1.56–5.52, p < 0.001). These variables were retained as inputs for subsequent machine learning modeling due to their robust statistical significance and clinical relevance. Other variables, such as gender, cardiovascular disease, and antiplatelet use, lost statistical significance in multivariate analysis and were excluded from model training.
3.3. Performance of Machine Learning Models
Ten machine learning algorithms were developed using predictors identified in the multivariate logistic regression model, and their performance was evaluated on both the training and test sets. As summarized in Table 3, the LightGBM model demonstrated the highest area under the receiver operating characteristic curve (AUC) in both datasets. In the test set, LightGBM achieved an AUC of 0.763 (95% CI: 0.685–0.841), followed closely by XGBoost (AUC: 0.747) and GBM (AUC: 0.743), as shown in Figure 1D. In the training set, LightGBM also achieved the highest AUC (0.771, 95% CI: 0.720–0.822), as illustrated in Figure 1A.

Table 3.
Evaluation metrics for training and test sets.
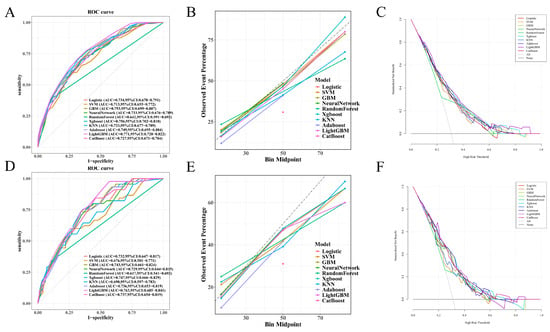
Figure 1.
Performance of machine learning models in training and test sets: (A–C) In the training set, LightGBM achieved the highest AUC (0.771, 95% CI: 0.720–0.822). Calibration curves showed good agreement between predicted and observed outcomes, particularly for LightGBM, GBM, and XGBoost. Decision curve analysis (DCA) demonstrated that LightGBM yielded the greatest net clinical benefit across a wide range of threshold probabilities. (D–F) In the independent test set, LightGBM also achieved the highest AUC (0.763, 95% CI: 0.685–0.841), followed by XGBoost and GBM. Calibration and DCA curves further supported the superior predictive performance and clinical utility of LightGBM.
Calibration curves (Figure 1B,E) demonstrated that most models exhibited good agreement between predicted and observed risks, particularly LightGBM, GBM, and XGBoost. Decision curve analysis (DCA) (Figure 1C,F) further confirmed the clinical utility of these models, with LightGBM yielding the highest standardized net benefit across a broad range of threshold probabilities in both datasets.
In terms of other metrics, LightGBM in the test set exhibited the highest sensitivity (97.8%) and F1-score (0.603), despite moderate specificity (43.0%), indicating its superior ability to identify true positives. Models like Random Forest showed high specificity but suffered from low sensitivity and F1-score. Overall, LightGBM demonstrated the most balanced and robust performance and was thus selected for further interpretation and model deployment.
3.4. Confusion Matrix Analysis of Classification Performance
Confusion matrices for each of the ten machine learning models were generated to evaluate classification performance in both the training (Figure 2) and test (Figure 3) sets. In this setting, sensitivity was prioritized because failing to identify a truly high-risk patient may delay monitoring or preventive measures and increase the likelihood of clinically significant bleeding, while false-positive alerts primarily prompt closer surveillance with relatively low harm. In the training set, LightGBM (Figure 2E) demonstrated a favorable balance between true positives (TP) and true negatives (TN), with minimal false negatives (FN), highlighting its sensitivity in identifying patients at risk for coagulation abnormalities. GBM (Figure 2C) and XGBoost (Figure 2J) also showed high TP counts but had slightly increased false positive (FP) rates compared to LightGBM. Conversely, Random Forest (Figure 2H) and SVM (Figure 2I) exhibited more conservative behavior with higher TNs but lower TPs, suggesting reduced sensitivity.
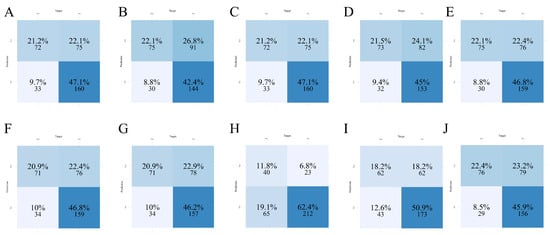
Figure 2.
Confusion matrices of ten machine learning models in the training set. (A) AdaBoost; (B) CatBoost; (C) GBM; (D) KNN; (E) LightGBM; (F) Logistic Regression; (G) Neural Network; (H) Random Forest; (I) SVM; (J) XGBoost.
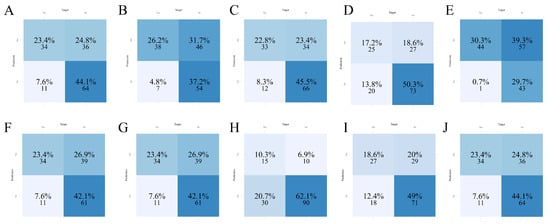
Figure 3.
Confusion matrices of ten machine learning models in the test set. (A) AdaBoost; (B) CatBoost; (C) GBM; (D) KNN; (E) LightGBM; (F) Logistic Regression; (G) Neural Network; (H) Random Forest; (I) SVM; (J) XGBoost.
In the test set, LightGBM again demonstrated dominant classification performance (Figure 3E), with the highest sensitivity as previously supported by Table 3, but at the expense of increased false positives. This trade-off suggests a prioritization of detecting abnormal cases over avoiding unnecessary alerts, which may be clinically acceptable in high-risk settings. Logistic Regression (Figure 3F) and Neural Network (Figure 3G) models yielded moderate classification balance, whereas models such as KNN (Figure 3D) and Random Forest (Figure 3H) underperformed in identifying positive cases. Overall, the matrix-based evaluations visually confirmed the superior detection capability of LightGBM, justifying its further exploration for interpretability and deployment.
3.5. Feature Importance and SHAP-Based Model Interpretation
To further elucidate the decision-making mechanisms across different models, feature importance analyses were conducted for all machine learning algorithms, with SHAP (SHapley Additive exPlanations) interpretation performed specifically for the best-performing model, LightGBM. In SHAP plots, positive values indicate that a feature increases the predicted risk relative to the baseline, whereas negative values indicate a decrease; larger absolute values reflect a stronger influence on the prediction. As shown in Figure 4, the top five most influential features included feeding_state, age, hypoproteinemia, insomnia, and history_of_oral_antibiotics. This ranking was further supported by the mean absolute SHAP values (Figure 5A), which quantified each variable’s contribution to model output.
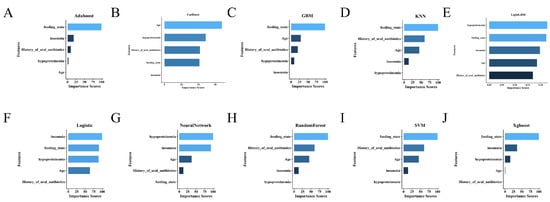
Figure 4.
Comparison of feature importance across ten machine learning models. (A) AdaBoost; (B) CatBoost; (C) GBM; (D) KNN; (E) LightGBM; (F) Logistic Regression; (G) Neural Network; (H) Random Forest; (I) SVM; (J) XGBoost.
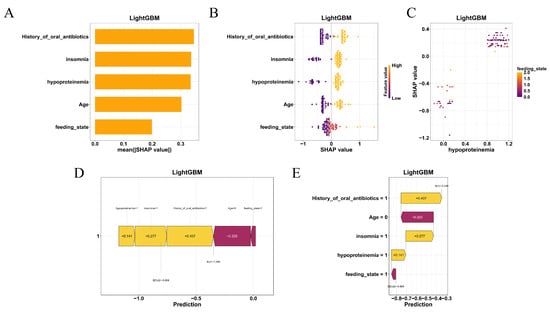
Figure 5.
SHAP-based interpretability of the LightGBM model. (A) Mean absolute SHAP values showing the global impact of each feature. (B) SHAP beeswarm plot illustrating the direction and magnitude of individual feature effects. (C) SHAP dependence plots showing nonlinear interactions, between feeding state and hypoproteinemia. (D) SHAP force plot and (E) waterfall chart explaining individual patient predictions by visualizing how risk factors contribute to the model’s output.
The SHAP beeswarm plot (Figure 5B) illustrated the direction and magnitude of individual feature impacts on the predicted risk. Notably, hypoproteinemia and enteral feeding (tube or parenteral nutrition) consistently contributed to a higher probability of coagulation abnormalities, while younger age and absence of insomnia were associated with decreased risk. SHAP dependence plots (Figure 5C) revealed nonlinear interactions, particularly between feeding_state and hypoproteinemia, further emphasizing the model’s sensitivity to combinations of nutritional and clinical factors.
At the individual level, SHAP force plots (Figure 5D) and waterfall charts (Figure 5E) provided transparent visualizations of the prediction for specific patients. These interpretive visualizations delineated how combinations of high-risk features—such as older age, nutritional compromise, and prior antibiotic use—aggregated to shift the model’s output toward a positive prediction.
Together, the SHAP-based interpretability framework enhanced the clinical transparency of the LightGBM model and supported its applicability in individualized risk prediction.
3.6. Nomogram Construction for Individualized Risk Estimation
To facilitate clinical application, nomograms were constructed based on the final logistic regression model using the top predictors identified through multivariate analysis. As shown in Figure 6A, the nomogram incorporating the entire dataset provides a quantitative scoring tool for estimating the probability of cefoperazone–sulbactam-associated coagulation abnormalities in elderly patients. Each predictor—such as age, hypoproteinemia, insomnia, oral antibiotic use, and nutritional support—was assigned a weighted point scale reflecting its relative contribution to risk.
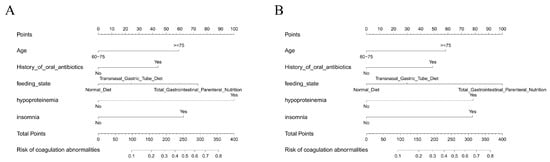
Figure 6.
Nomograms for individualized risk prediction: (A) Nomogram based on the full dataset for estimating the risk of cefoperazone–sulbactam-associated coagulation abnormalities. (B) Nomogram derived from the training set, showing similar predictor structure and stability.
An additional nomogram was generated using only the training dataset (Figure 6B) to assess consistency and internal validity. The structure and weighting of predictors in both nomograms were largely concordant, indicating model stability across sampling subsets. These nomograms allow clinicians to compute individualized risk scores and visually interpret how combinations of patient factors translate into predicted risk.
3.7. Web-Based Risk Prediction Tool Deployment
To enhance accessibility and promote clinical translation, a web-based risk prediction tool was developed using the Shiny platform. As illustrated in Figure 7, the interface allows users to input patient-specific variables—such as age group, nutritional status, presence of hypoproteinemia, insomnia, and recent oral antibiotic use—and instantly receive an individualized risk estimate for developing cefoperazone–sulbactam-associated coagulation abnormalities. The predictive engine is powered by the optimized LightGBM model, and the tool provides a user-friendly visualization of both probability output and contributing factors.
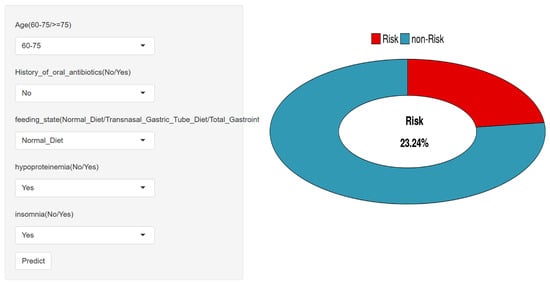
Figure 7.
Web-based risk prediction tool interface for clinical use.
The application is publicly accessible at https://cacsriskmodel.shinyapps.io/make_web-1/ (29 November 2025), supporting shared decision-making and real-time clinical use. This tool serves as a practical extension of the study’s findings and reinforces the feasibility of integrating machine learning into bedside risk assessment.
4. Discussion
In this work, a predictive model was established and validated to identify the risk of coagulation abnormalities in elderly inpatients treated with cefoperazone–sulbactam. We initially employed multivariable logistic regression to determine potential independent risk factors based on routinely collected clinical variables. We then trained and tested ten machine learning models, and LightGBM achieved better discrimination in both the training and test sets. Additional predictive power beyond the model was provided by SHapley Additive exPlanations (SHAP) analysis, which provided interpretable and transparent observations of feature importance at the population and per-individual level. We further established a nomogram using logistic regression results in order to establish a simple, point-based risk prediction tool. For clinical relevance, a web-based application was created that enabled real-time personalized risk prediction. These results also imply that machine learning, especially LightGBM, may serve as a powerful tool for early alerting of cefoperazone–sulbactam-related coagulation risks in elderly patients.
A number of clinical factors were found to be independent risk factors for cefoperazone–sulbactam-associated coagulation abnormalities in older people, all appropriate to their pathophysiological mechanism. Older age (age ≥ 75 years) was also significantly associated with risk, which is likely to have been mediated by age-related hepatic metabolic dysfunction, reduced absorption of vitamin K, and the higher prevalence of subclinical nutritional deficiencies, all common among older adults and leading to coagulopathy [16,17].
Hypoproteinemia was also significantly correlated with abnormal coagulation. Serum albumin is responsible for the maintenance of oncotic pressure and transport of coagulation factors, and a decrease in serum albumin levels may be associated with malnutrition and impaired hepatic synthetic function, which can lead to increased bleeding tendency [18,19,20,21]. Additionally, total parenteral nutrition was identified as a risk factor, likely related to low enteral vitamin K intake and alterations in the gut microbiota, both of which may lead to derangement in vitamin K-dependent coagulation pathways [22,23].
Insomnia, being less traditionally associated with hemostatic outcomes, might be a marker of frailty, inflammation, or neuroendocrine dysregulation in older people, all of which have been previously associated with changes in platelet function and clotting cascades [24,25,26]. Insomnia should be interpreted cautiously as a clinical marker rather than a confirmed causal driver of antibiotic-associated coagulopathy. Sleep disturbance in older inpatients may reflect frailty, systemic inflammation, neuroendocrine dysregulation, or higher illness severity, each of which may correlate with hemostatic vulnerability. In addition, residual confounding is possible because detailed exposure to sedative–hypnotics, over-the-counter agents, and herbal supplements with potential antiplatelet effects (e.g., Ginkgo preparations) was not consistently captured in this retrospective dataset. Therefore, the observed association may partly represent unmeasured co-medication use or documentation bias, and future prospective studies should collect structured sleep and medication/supplement data to clarify directionality and mechanisms. Finally, recent use of oral antibiotics that potentially influence coagulation by reducing the gut flora responsible for endogenous vitamin K production can be relevant, particularly in the case of β-lactam antibiotics such as cefoperazone [27,28].
These results emphasize the multifactorial nature of antibiotic-associated coagulation derangement and the relevance of nutritional, microbiological, hepatotoxic, and geriatric comorbidities in clinical risk evaluations. Our results are in line with the prior literature, which recommended cautious use of the cefoperazone–sulbactam combination in patients with advanced age and poor nutrition due to the higher risk of coagulopathy [2,10,29]. Data from previous pharmacovigilance studies and case series have reported the potential for cefoperazone to inhibit the synthesis of vitamin K–dependent clotting factors, possibly associated with inhibition via the N-methylthiotetrazole (NMTT) side chain or inhibition of intestinal microbiota. However, most previous studies lacked structured modeling or did not provide quantified risk in different clinical subsets [2,30].
To the best of our knowledge, the present study is the most systematic and extensive exploration of different ML models for coagulation dysfunction specifically caused by cefoperazone–sulbactam in the geriatric inpatient population. Although machine learning has been used to predict surgical or anticoagulant-related bleeding risk in previous studies, our study examines a more specific, medication-related adverse event in a real-world, non-interventional setting. Furthermore, the application of SHAP values allows interpretability beyond conventional statistical models, thus permitting clinicians to gain insights into the contribution of each variable to risk profiles on an individual level.
The recognition of insomnia as a possible predictor is also congruent with a growing body of literature supporting an association between sleep disturbance, systemic inflammation, and vascular endothelial dysfunction, albeit a bridge still to be strategically crossed within the backdrop of antibiotic-associated coagulopathy [31,32]. Therefore, our findings add to the existing knowledge that combines typical clinical judgment with algorithmic expertise. Our model is of great value in preventing cefoperazone–sulbactam therapy-related injury in older people. Through the incorporation of readily available clinical variables into a predictive model, clinicians can identify patients at increased risk of coagulopathy to intervene before the development of clinically apparent hemorrhage. This is especially important in geriatric medicine, where minor laboratory alterations may signify early stages of clinical deterioration and where early intervention could avoid cascades ending in major morbidity.
Crucially, the LightGBM model was not only more accurate but also remained sensitive for ward-based screening of high-risk inpatients (e.g., geriatric and intensive care settings). Associated SHAP-based explanations deliver actionable insight by identifying which patient attributes drive the risk the most so that clinicians can build trust and use it for shared decision-making [33,34,35].
While logistic regression supports a simple, transparent nomogram suitable for bedside scoring, LightGBM achieved improved discrimination and sensitivity. To preserve clinical usability, we combined LightGBM with SHAP-based explanations and a web-based calculator, providing both performance and accessibility. More importantly, the construction of an easy-to-use nomogram and the establishment of a web-based calculator based on Shiny directly convert this model to an easily applied clinical reference. They can be used for bedside risk assessment and to assist in management decisions, including early vitamin K supplementation, closer monitoring of hemostasis, and the selection of a different antibiotic in susceptible patients. As the clinical implementation of machine learning methods continues to expand, such interpretability and accessibility of models will be important for their real-world use.
There are several notable strengths to this study. It is one of the first papers to compare a large number of machine learning algorithms with traditional logistic regression to predict cefoperazone–sulbactam-associated coagulopathy in elderly inpatients. The two-center design (Fuxing Hospital and Xuanwu Hospital of Capital Medical University) increases the heterogeneity and generalizability of the data. The incorporation of real-world collected variables increases the generalizability of the model, and the SHAP analysis and nomogram development increase interpretability. Finally, the introduction of a web-based risk calculator highlights the translation of the study to clinical practice.
Nonetheless, limitations remain. Although this is a multi-institutional study, it was conducted at one metropolitan area and was performed in a retrospective fashion that can introduce selection and documentation biases. Some possibly related predictors, such as serum concentrations of vitamin K, more detailed liver function tests, or the composition of gut microbiota, were not measured or could not be included in the model. Some laboratory parameters with high biological relevance—particularly coagulation factor VII (Factor VII) and other vitamin K–dependent factors—were not routinely available in this retrospective dataset and therefore could not be incorporated. Given the short half-life of Factor VII, future prospective work should prioritize serial assessment of Factor VII (and related factors) to better characterize early functional changes. In contrast, vitamin K concentration alone may not fully reflect downstream coagulation factor activity and may provide limited mechanistic resolution without concurrent functional factor measurements. Although internal validation was performed, the absence of external validation limits generalizability; external testing in independent cohorts is a key priority for future work.
Based on the current study, future research should include validation of the proposed model in a prospective, multicenter design to ensure its applicability across different patient populations and healthcare systems. The addition of time-dependent data, such as time-variant coagulation parameters or nutritional markers, might increase predictive granularity and responsiveness of the model. Moreover, inclusion of laboratory biomarkers not measured in the present study, such as serum levels of vitamin K, liver function indices (i.e., factor V, INR), and inflammation-induced cytokines, would increase biological specificity.
An important direction is to implement the model into an electronic health record (EHR) system for real-time clinical decision support. This could enable automated risk alerts at the time of prescription or antibiotic administration for timely preventive interventions (e.g., coagulation monitoring, vitamin K supplementation, or reconsideration of antibiotic choice). Lastly, it would be interesting to investigate an adaptive or ensemble modeling approach with clinician feedback that could support iterative learning and continuous improvement of the models in practice.
5. Conclusions
This research effectively established and verified a machine learning–powered predictive model for early detection of coagulation disorders among elderly inpatients undergoing treatment with cefoperazone–sulbactam. Of the ten algorithms compared, the predictive capability and clinical utility of the LightGBM model were the best. Responsibility of main risk factors, including older age, hypoalbuminemia, parenteral alimentation, impaired sleep, and oral antibiotic medication, was determined on the results of logistic regression analysis and explained using SHAP analysis.
Between the model accuracy, interpretability, and accessibility via the web-based instrument, this work provides an applicable method for early risk stratification and personalized clinical decision support. These results emphasize the potential of machine learning to improve monitoring of antibiotic safety in vulnerable populations, and suggest that future model refinement and external validation will be required in wider healthcare contexts.
Author Contributions
All authors (Y.L., H.D. and Y.G.) made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Key Research and Development Program of China [2021YFC2500500].
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Fuxing Hospital, Capital Medical University (protocol code 2025FXHEC-KSP043, approval date 3 September 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The de-identified patient-level data and the codes used in the current study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy and ethical restrictions related to patient clinical information.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PT | prothrombin time |
| APTT | activated partial thromboplastin time |
| SHAP | SHapley Additive exPlanations |
| ORs | odds ratios |
| CIs | confidence intervals |
| DCA | decision curve analysis |
| AUC | area under the curve |
References
- Lan, S.H.; Chao, C.M.; Chang, S.P.; Lu, L.C.; Lai, C.C. Clinical Efficacy and Safety of Cefoperazone-Sulbactam in Treatment of Intra-Abdominal Infections: A Systematic Review and Meta-Analysis. Surg. Infect. 2021, 22, 763–770. [Google Scholar] [CrossRef]
- Miao, W.; Guo, J.; Cheng, H.; Zhao, Q. Risk Factors for Cefoperazone/Sulbactam-Induced Coagulation Disorder. Infect. Drug Resist. 2023, 16, 6277–6284. [Google Scholar] [CrossRef]
- Wu, R.; Wang, X.; Cui, P.; Chen, W.; Yang, W.; Lai, Y. Efficacy and safety of generic cefoperazone/sulbactam versus branded cefoperazone/sulbactam in the treatment of bacterial infections: A systematic review and meta-analysis. J. Chemother. 2024, 36, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Song, X.; Liu, Y.; Zheng, X. Clostridium difficile Bacteremia in an Elderly Patient with Multiple Comorbidities: A Case Report. Surg. Infect. 2025, 26, 112–115. [Google Scholar] [CrossRef]
- Yao, H.; Wang, Y.; Peng, Y.; Huang, Z.; Gan, G.; Wang, Z. A Real-World Pharmacovigilance Study of Ceftazidime/Avibactam: Data Mining of the Food and Drug Administration Adverse Event Reporting System Database. J. Clin. Pharmacol. 2024, 64, 820–827. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, L.; Chen, P.; Liu, W.; Huang, X.; Li, X. Risk-factor analysis and predictive-model development of acute kidney injury in inpatients administered cefoperazone-sulbactam sodium and mezlocillin-sulbactam sodium: A single-center retrospective study. Front. Pharmacol. 2023, 14, 1170987. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, J.; Xiao, M.; Shan, H.; Liu, M.; Lu, Y.; Zou, Y.; Wu, B. Severe cutaneous adverse reactions due to antibiotics therapy: A pharmacovigilance analysis of FDA adverse event reporting system events. Expert Opin. Drug Saf. 2023, 1–8. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Yu, C.; Tan, J.; Xiong, W.; Dong, D.; Li, S.; Zhang, R.; Li, J.; Wu, Y.; et al. Cefoperazone-sulbactam and risk of coagulation disorders or bleeding: A retrospective cohort study. Expert Opin. Drug Saf. 2020, 19, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Jin, P.; Li, J.; Zhu, Y. Retrospective Analysis of Risk Factors for Cefoperazone/Sulbactam-Induced Thrombocytopenia in Adult Chinese Patients: A Six-Year Real-World Study. Infect. Drug Resist. 2024, 17, 3901–3911. [Google Scholar] [CrossRef]
- Ebid, A.I.; Abdeen, H.A.; Muhammed Maher, R.; Mohamed-Abdel-Motaleb, S.M. Cefoperazone-Sulbactam-Induced Coagulopathy in Critically Ill Egyptian Patients: Role of Vitamin K Prophylactic Doses. Hosp. Pharm. 2024, 59, 575–583. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, J.; Wu, H.; Ye, J.; Li, Y.; Zou, B.; Li, Y. A retrospective cohort study of coagulation function in patients with liver cirrhosis receiving cefoperazone/sulbactam with and without vitamin K1 supplementation. Int. J. Clin. Pharm. 2024, 46, 1492–1499. [Google Scholar] [CrossRef]
- Li, X.; Lei, W.; Wang, M.; Xu, L. Risk Stratification for Cephalosporin-Induced Thrombocytopenia: Development and Validation of a Multidimensional Predictive Model in Older Adults. Risk Manag. Healthc. Policy 2025, 18, 2107–2120. [Google Scholar] [CrossRef]
- Stöllberger, C. Drug interactions with new oral anticoagulants in elderly patients. Expert Rev. Clin. Pharmacol. 2017, 10, 1191–1202. [Google Scholar] [CrossRef]
- Aydin, A.E.; Ates Bulut, E.; Kocyigit, S.E.; Dost, F.S.; Mutlay, F.; Altunkalem Seydi, K.; Esenkaya, F.; Isik, A.T. An Instant Relationship Between Hyponatremia, Geriatric Syndromes, and Drugs in Older Adults: A Cross-Sectional Analysis from a Single Geriatric Clinic. Diagnostics 2025, 15, 744. [Google Scholar] [CrossRef]
- Dewar, S. A geriatrician’s approach to managing the complex older adult with obesity. Obesity 2025, 33, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Bultynck, C.; Munim, N.; Harrington, D.J.; Judd, L.; Ataklte, F.; Shah, Z.; Dockery, F. Prevalence of vitamin K deficiency in older people with hip fracture. Acta Clin. Belg. 2020, 75, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Popa, D.-S.; Bigman, G.; Rusu, M.E. The Role of Vitamin K in Humans: Implication in Aging and Age-Associated Diseases. Antioxidants 2021, 10, 566. [Google Scholar] [CrossRef]
- Kim, S.; McClave, S.A.; Martindale, R.G.; Miller, K.R.; Hurt, R.T. Hypoalbuminemia and Clinical Outcomes: What is the Mechanism behind the Relationship? Am. Surg. 2017, 83, 1220–1227. [Google Scholar] [CrossRef]
- Levitt, D.G.; Levitt, M.D. Human serum albumin homeostasis: A new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int. J. Gen. Med. 2016, 9, 229–255. [Google Scholar] [CrossRef]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. JPEN J. Parenter Enter. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Chen, X.; Wei, S.; Ma, X.; Zhao, Y. Research Progress and Treatment Status of Liver Cirrhosis with Hypoproteinemia. Evid. Based Complement Altern. Med. 2022, 2022, 2245491. [Google Scholar] [CrossRef]
- Camelo-Castillo, A.; Rivera-Caravaca, J.M.; Orenes-Piñero, E.; Ramírez-Macías, I.; Roldán, V.; Lip, G.Y.H.; Marín, F. Gut Microbiota and the Quality of Oral Anticoagulation in Vitamin K Antagonists Users: A Review of Potential Implications. J. Clin. Med. 2021, 10, 715. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Singla, R.K.; Qin, Q.; Ding, Y.; Liu, L.; Ding, X.; Qu, W.; Huang, C.; Shen, Z.; Shen, B.; et al. Exploring the complex relationship between vitamin K, gut microbiota, and warfarin variability in cardiac surgery patients. Int. J. Surg. 2023, 109, 3861–3871. [Google Scholar] [CrossRef]
- Konieczyńska, M.; Natorska, J.; Undas, A. Thrombosis and Aging: Fibrin Clot Properties and Oxidative Stress. Antioxid. Redox. Signal. 2024, 41, 233–254. [Google Scholar] [CrossRef]
- Pan, Y.; Yuan, Y.; Yang, J.; Feng, Z.Q.; Tang, X.Y.; Jiang, Y.; Hu, G.M.; Luo, D.L.; Jiang, Y.; Dong, J.C. Bidirectional Communication Between Circadian Rhythm Disruption and Frailty: A Hypothesis From Gut Microbiota Metabolites. Nat. Sci. Sleep 2025, 17, 701–708. [Google Scholar] [CrossRef]
- Wang, J.; Maxwell, C.A.; Yu, F. Biological Processes and Biomarkers Related to Frailty in Older Adults: A State-of-the-Science Literature Review. Biol. Res. Nurs. 2019, 21, 80–106. [Google Scholar] [CrossRef]
- Gryszczyńska, B.; Budzyń, M.; Grupińska, J.; Kasprzak, M.P.; Gryszczyńska, A. Nutritional Behaviors, Vitamin Supplementation and Physical Activity among Polish Adults during the COVID-19 Pandemic. Nutrients 2022, 14, 331. [Google Scholar] [CrossRef]
- Roberts, K.; Thakkar, R.; Autor, D.; Bisordi, F.; Fitton, H.; Garner, C.; Garvin, M.; Honig, P.; Hukkelhoven, M.; Kowalski, R.; et al. Creating E-Labeling Platforms: An Industry Vision. Clin. Pharmacol. Ther. 2020, 108, 716–718. [Google Scholar] [CrossRef]
- Min, M.; Zeng, J.; Zou, M.; Peng, Y. Construction and Validation of a Nomogram Prediction Model for the Risk of Cefoperazone Sodium/Sulbactam Sodium-Related Coagulation Disorders. Infect. Drug Resist. 2025, 18, 3859–3866. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhu, J.; Tu, K.; Tang, H.; Zhou, X.; Li, Q.; Chen, K.; Yang, X.; Huang, Y. The clinical features and risk factors of coagulopathy associated with cefoperazone/sulbactam: A nomogram prediction model. Front. Pharmacol. 2024, 15, 1505653. [Google Scholar] [CrossRef] [PubMed]
- Feuth, T. Interactions between sleep, inflammation, immunity and infections: A narrative review. Immun. Inflamm. Dis. 2024, 12, e70046. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Lee, D.-B.; Yoon, D.-W.; Yoo, S.-L.; Kim, J. The Effect of Sleep Disruption on Cardiometabolic Health. Life 2025, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Rafferty, A.R.; McAuliffe, F.M.; Wei, L.; Mooney, C. An explainable machine learning-based clinical decision support system for prediction of gestational diabetes mellitus. Sci. Rep. 2022, 12, 1170. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, D.; Xu, J.; Pang, H.; Hu, M.; Li, J.; Zhou, S.; Guo, L.; Yi, F. Explainable machine learning model reveals its decision-making process in identifying patients with paroxysmal atrial fibrillation at high risk for recurrence after catheter ablation. BMC Cardiovasc. Disord. 2023, 23, 91. [Google Scholar] [CrossRef]
- Zihni, E.; Madai, V.I.; Livne, M.; Galinovic, I.; Khalil, A.A.; Fiebach, J.B.; Frey, D. Opening the black box of artificial intelligence for clinical decision support: A study predicting stroke outcome. PLoS ONE 2020, 15, e0231166. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.