Abstract
Background: Endothelial dysfunction plays a critical role in ischemic stroke. The Endothelial Activation and Stress Index (EASIX), calculated from creatinine, lactate dehydrogenase (LDH), and platelet levels, reflects endothelial injury. This study aimed to investigate the relationship between EASIX and 90-day mortality in patients with posterior circulation ischemic stroke (PCIS) treated with mechanical thrombectomy. Methods: Fifty-eight patients with acute ischemic stroke who underwent mechanical thrombectomy (MT) or MT combined with intravenous thrombolysis (intravenous tissue plasminogen activator (tPA)) for posterior circulation ischemic stroke (PCIS) were included. EASIX was calculated using 24 h laboratory values of creatinine, LDH, and platelets. Its association with 90-day mortality, length of hospital stay, intubation, and parenchymal hemorrhage was analyzed. Results: In patients receiving reperfusion therapy, the Endothelial Activation and Stress Index (EASIX) showed modest ability to predict 90-day mortality (AUC = 0.583, 95% CI 0.428–0.739, p = 0.295). Higher EASIX values were linked to a 6.58-fold increase in mortality risk. Patients with elevated EASIX were generally older, had more frequent hyperlipidemia, had higher 24 h National Institutes of Health Stroke Scale (NIHSS) scores, had greater need for intubation, and had higher in-hospital mortality. Conclusions: EASIX is a simple, inexpensive, and non-invasive marker that may reflect endothelial dysfunction and help predict mortality in PCIS patients undergoing reperfusion therapy. Higher EASIX values are associated with poorer prognosis. Early identification of high-risk patients may support secondary prevention strategies.
1. Introduction
Ischemic stroke is one of the leading causes of death and disability worldwide, resulting in approximately 5 million deaths annually [1]. Approximately 20% of strokes occur in the vertebrobasilar system, known as the posterior circulation [2]. Despite improved recanalization rates with mechanical thrombectomy (MT) and tissue plasminogen activator (tPA), posterior circulation ischemic stroke (PCIS) continues to have a poor prognosis (68–90%) [3]. Although several studies have demonstrated that mechanical thrombectomy (MT) is safe and effective in posterior circulation ischemic stroke (PCIS), the BEST trial reported no significant superiority of MT over standard medical treatment [4,5].
Endothelial dysfunction, which contributes to vascular injury and reperfusion failure, plays a critical role in the pathophysiology of ischemic stroke [6]. Following acute ischemic stroke (AIS) and reperfusion therapy, the generation of reactive oxygen species (ROS) increases markedly, triggering inflammation and secondary vascular injury [7]. The vascular endothelium acts as a dynamic organ that maintains vascular homeostasis and regulates tone, permeability, and leukocyte adhesion [8]. Excessive ROS production may lead to endothelial dysfunction, a prothrombotic and proinflammatory condition characterized by vasoconstriction, inflammation, and vascular remodeling [9,10]. Several biomarkers, including integrins, selectins, cadherins, homocysteine, interleukin (IL)-8, IL-18, and C-reactive protein (CRP), have been proposed to reflect endothelial dysfunction. Among these, CRP has been consistently associated with endothelial injury and poor outcomes in both cardiovascular disease and ischemic stroke [11,12,13,14]. However, most studies have focused on anterior circulation infarctions, and the prognostic role of endothelial biomarkers in PCIS remains largely underexplored. This knowledge gap may stem from the relative rarity of PCIS, which limits the sample sizes and statistical power of prospective investigations.
Existing prognostic tools, such as the NIHSS and APACHE II scores, are valuable but have limited applicability to posterior circulation ischemic stroke (PCIS). Biomarkers like CRP and N-terminal pro-brain natriuretic peptide (NT-proBNP) have also demonstrated prognostic potential; however, their use in clinical practice is restricted by cost, limited specificity, and variable availability [15,16,17].
The Endothelial Activation and Stress Index (EASIX), defined by Luft et al., is calculated from creatinine, lactate dehydrogenase (LDH), and platelet count. Each parameter has a mechanistic association with endothelial injury: creatinine reflects renal and endothelial dysfunction, LDH indicates cellular stress and inflammation, and platelet count represents thrombotic activity. Together, these components provide an integrated estimate of endothelial stress. EASIX was initially developed in patients with hematologic malignancies and transplantation-related endothelial injury, where it demonstrated a strong correlation with survival [18]. More recently, Kleeberg et al. proposed that EASIX could serve as a prognostic biomarker in acute ischemic stroke [19]. Similarly, Huang et al., analyzing data from 43,853 individuals in the NHANES cohort, reported that elevated EASIX was positively associated with both stroke prevalence and all-cause mortality, even after adjustment for multiple covariates [20]. These findings highlight the potential role of EASIX in cerebrovascular disease; however, its prognostic value in posterior circulation ischemic stroke (PCIS), particularly in patients undergoing reperfusion therapy, has not yet been established. Therefore, this study aimed to evaluate the predictive value of EASIX for 90-day mortality in patients with PCIS treated with mechanical thrombectomy (MT) or MT combined with intravenous thrombolysis (tPA).
2. Materials and Methods
This study included 58 patients who presented to the emergency department of Bursa City Hospital with acute ischemic stroke (AIS) between March 2020 and November 2024. Posterior circulation occlusion was confirmed using carotid computed tomography (CT) angiography and cranial CT angiography. All patients underwent mechanical thrombectomy (MT) within 6 h or MT combined with intravenous thrombolysis (tPA) within 4.5 h after symptom onset and were subsequently monitored in the intensive care unit. In accordance with our institutional protocol for posterior circulation ischemic stroke, only patients treated with MT or MT + tPA were included to achieve a homogeneous cohort. Patients receiving other treatment modalities were excluded. There were no missing laboratory data for creatinine, LDH, or platelet count in the analyzed cohort.
Demographic and clinical characteristics, laboratory data, and radiological imaging of the patients were retrospectively reviewed from patient files. The following variables were recorded: age, sex, smoking and alcohol use, hypertension (HT), diabetes mellitus, cardiac disease, history of prior stroke or transient ischemic attack (TIA), and presence of atrial fibrillation. Stroke severity at hospital admission was assessed using the National Institutes of Health Stroke Scale (NIHSS) (0 h NIHSS). Furthermore, stroke etiology was classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Symptom onset time, hospital arrival time (symptom-to-door time), presence of wake-up stroke, and time from symptom onset to reperfusion therapy initiation (symptom-to-groin time) were also documented. Recanalization success during mechanical thrombectomy (MT) was classified using the Thrombolysis in Cerebral Infarction (TICI) scale. Successful revascularization was defined as TICI 2b–3.
Post-procedure 24 h NIHSS (24 h NIHSS), Acute Physiology and Chronic Health Evaluation (APACHE) II score, hospital stay duration, intubation status (yes/no), duration of intubation (days), post-MT decompression, hemorrhagic transformation (symptomatic or asymptomatic) on 24 h post-MT/MT + tPA brain CT, and 90-day modified Rankin Scale (mRS) scores were recorded. The 90-day mRS was determined during outpatient clinic visits. Poor prognosis was defined as death within 90 days. Patients with mRS 0–2 were classified as independent, mRS 3–5 as dependent, and mRS 6 as deceased. For deceased patients, outcome information was obtained through telephone interviews with relatives.
Platelet count and creatinine levels were obtained at the time of emergency department admission, prior to any reperfusion therapy. In our institution, serum LDH is routinely measured after the endovascular procedure, during the patient’s initial admission to the intensive care unit (ICU). In the original version of the manuscript, these parameters were collectively described as “within 24 h,” and this has now been clarified to accurately reflect the timing of each laboratory measurement.
The Endothelial Activation and Stress Index (EASIX) was calculated using the following formula:
EASIX = LDH [U/L] × creatinine [mg/dL]/platelet count [109/L].
Patients with systemic inflammatory or hematologic diseases, immunosuppressant therapy, active malignancy, recent major surgery or trauma (within the past 3 months), or those lost to follow-up within 90 days after acute ischemic stroke (AIS) were excluded from the analysis.
This study was conducted retrospectively using anonymized clinical data. Because of the retrospective design and the nature of the data, individual informed consent was not required according to local ethical standards and institutional policies.
Statistical Analysis
Descriptive statistics for continuous variables were presented as median and interquartile range (IQR), while categorical variables were given as numbers and percentages. The normality of the data was checked with the Shapiro–Wilk test. Comparisons between independent groups without normal distribution were performed using the Mann–Whitney U test, and relationships between categorical variables were analyzed with Pearson’s chi-square or Fisher’s exact test. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the ability of EASIX to predict mortality and to determine the optimal cut-off value, defined as the point with the shortest distance to the upper-left corner of the curve. The area under the curve (AUC), sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were reported. Variables such as age, sex, clinical and laboratory findings, and treatment types, as well as other possible factors affecting mortality, were first examined using univariate logistic regression. Variables that were statistically significant in the univariate analysis were included in a multivariate logistic regression model (Enter method). A two-tailed p value < 0.05 was considered statistically significant. All analyses were carried out using SPSS for Windows, version 25 (SPSS Inc., Chicago, IL, USA).
3. Results
The study included 58 patients diagnosed with PCIS who underwent reperfusion therapy. Of the patients, 74.1% were male and 25.9% female. The median age was 70 (Q1–Q3: 57–76). Other demographic data are presented in Table 1.

Table 1.
Distribution of demographic and clinical characteristics of the participants.
The optimal EASIX cut-off value for predicting 90-day mortality (mRS 6) was ≥ 1.63, with an AUC of 0.583 (95% CI: 0.428–0.739, p = 0.295). At this threshold, sensitivity was 29.2%, specificity was 94.2%, positive predictive value (PPV) was 77.8%, and negative predictive value (NPV) was 65.3%. These findings indicate that EASIX has high specificity but limited sensitivity in predicting 90-day mortality. (Table 2; Figure 1).

Table 2.
Predicting mortality in patients according to EASIX cut-offs.
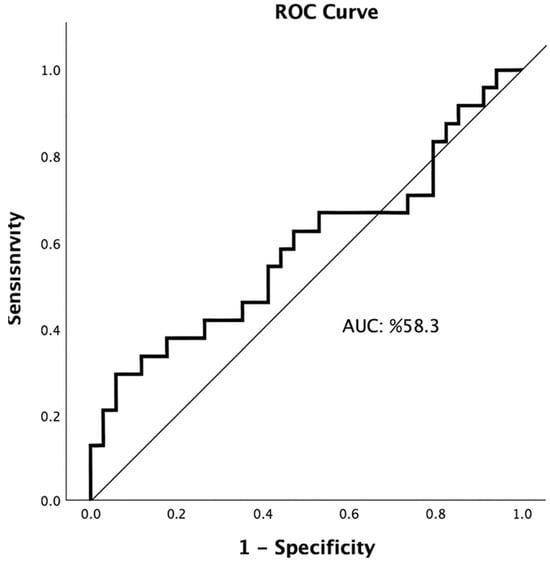
Figure 1.
Receiver operating characteristic (ROC) curve illustrating the predictive performance of the Endothelial Activation and Stress Index (EASIX) for 90-day mortality (AUC = 0.583, 95% CI: 0.428–0.739).
Patients were stratified into two groups based on EASIX values (<1.63 vs. ≥1.63). Significant differences between the groups were observed in age, presence of hyperlipidemia, intubation, in-hospital mortality, 24 h NIHSS, discharge mRS, and 90-day mRS (all p < 0.05). The high-EASIX group had a significantly higher proportion of patients aged > 70 years (p = 0.011), hyperlipidemia (p = 0.009), intubation (p = 0.035), in-hospital mortality (p = 0.016), 24 h NIHSS (p = 0.049), and both discharge and 90-day mRS (p = 0.048 for each) (Table 3).

Table 3.
Comparison of basic clinical features of cases between low- and high-EASIX groups.
Other variables, including smoking and heart disease, did not differ significantly between groups. This lack of statistical significance is most likely due to the limited sample size and heterogeneity of the study population. These factors may have reduced statistical power and obscured potential associations.
Univariate and multivariate logistic regression analyses were performed to evaluate the clinical parameters affecting mortality. In the univariate analysis, a high EASIX value was significantly associated with mortality (p = 0.028). Patients with high EASIX had a 6.588-fold increased risk of death (95% CI: 1.230–35.277). Intubation status (yes/no) was also a strong predictor, being associated with a 12.833-fold increased risk of mortality. Additionally, an intubation duration of >3 days was associated with a 6.133-fold higher mortality risk. The presence of hemorrhage (11.875-fold), APACHE II > 13 (7.381-fold), NIHSS > 12 on admission (3.510-fold), 24 h NIHSS > 15 (6.571-fold), and discharge mRS > 5 (125.4-fold) were all associated with substantially increased mortality risk. No independent predictors were identified in the multivariate logistic regression model (Table 4).

Table 4.
Univariate and multivariate logistic regression analysis for mortality.
4. Discussion
In this retrospective study, we found that a high Endothelial Activation and Stress Index (EASIX)—reflecting endothelial dysfunction and systemic stress—was significantly associated with both in-hospital and 90-day mortality in patients with posterior circulation ischemic stroke (PCIS) who underwent reperfusion therapy. Specifically, patients with high EASIX values had a 6.58-fold higher risk of 90-day mortality. In addition, elevated EASIX was correlated with older age, hyperlipidemia, higher 24 h NIHSS scores, and a greater need for intubation.
EASIX has been recognized as a valuable biomarker reflecting endothelial dysfunction and clinical prognosis across various patient populations. Elevated EASIX levels have been linked to poor survival outcomes in multiple myeloma and diffuse large B-cell lymphoma [21,22]. In a prospective study of allogeneic stem cell transplant recipients, a pre-transplant EASIX value ≥ 3 was associated with a twofold increase in treatment-related mortality [23]. Similarly, in critically ill patients with COVID-19, high EASIX values were shown to predict in-hospital mortality [24].
Several studies have explored the relationship between coronary artery disease and EASIX. In a study involving 1934 patients with acute coronary syndrome, EASIX was identified as a prognostic marker for overall survival before and after cardiac catheterization [25]. Sang et al. reported that EASIX outperformed its individual components (creatinine, LDH, and platelet count) in predicting 30-day mortality following acute myocardial infarction (AMI) [26]. In line with these findings, our study also demonstrated a significant association between elevated EASIX values and mortality in patients with posterior circulation ischemic stroke (PCIS) who underwent reperfusion therapy.
EASIX combines three well-established mortality predictors in AIS—creatinine, LDH, and platelet count—into a single index. Serum creatinine reflects renal function, and elevated creatinine levels indicate renal dysfunction, which is closely linked to cerebral small-vessel disease [27]. The kidney and brain share delicate vascular structures exposed to high pulsatile flow and pressure with low resistance [28]. Advanced age, HT, diabetes, smoking, and dyslipidemia are common risk factors for ischemic stroke and renal dysfunction. Oxidative stress, inflammation, and accumulation of uremic toxins further impair renal function, aggravating endothelial dysfunction and atherosclerosis [29]. Elevated serum creatinine is a strong independent predictor of post-stroke survival in the elderly [30].
Dehydration is considered to play a multifactorial role in the pathophysiology of ischemic stroke. It increases blood viscosity and may reduce cerebral perfusion by decreasing intravascular volume. Elevated hematocrit levels have been associated with larger infarct volumes, supporting the link between dehydration and impaired cerebral hemodynamics. Moreover, dehydration has been correlated with thrombotic complications, such as recurrent embolic stroke and venous thromboembolism following acute stroke [31]. In patients treated with intravenous thrombolysis (tPA), dehydration was associated with unfavorable outcomes, particularly among those with NIHSS scores greater than 6 [32]. Consistently, Dong et al. reported that patients with a glomerular filtration rate (GFR) below 60 mL/min/1.73 m2 had a fourfold higher risk of death at 3 months compared to those with GFR values above 90 mL/min/1.73 m2 [33]
In acute ischemic stroke (AIS), endothelial injury triggers platelet activation and aggregation at the site of vascular damage, promoting thrombosis and increased platelet consumption. Activated platelets contribute to thromboinflammation, amplifying tissue injury and the inflammatory cascade. A reduced platelet count may result from enhanced platelet consumption or excessive destruction, both of which are observed in severe systemic inflammation and disseminated intravascular coagulation. Platelet count has been linked to mortality, stroke recurrence, and poor functional outcomes in several studies [34,35].
LDH is a glycolytic enzyme commonly found in human tissues and is released from cells when the cell membrane is damaged [36]. In AIS, disruption of cerebral blood flow leads to hypoxia and necrosis. As a result of tissue damage, LDH is released. Being an important marker of inflammation, elevated LDH levels may be attributed to endothelial and circulating cells such as platelets and leukocytes [37]. In AIS, inflammation affects the development, progression, and prognosis of the stroke [38]. Several studies have linked LDH to stroke prognosis. For example, elevated LDH levels in cerebrospinal fluid have been proposed as a marker of early brain injury in subarachnoid hemorrhage-related cerebral ischemia [39]. Jin et al. reported that AIS patients with high LDH levels had a higher risk of stroke recurrence and death during an 18-month follow-up, demonstrating its association with stroke severity and poor prognosis [40].
Each of these three parameters holds prognostic value in acute ischemic stroke (AIS). We believe that EASIX, by integrating these variables into a single index, provides improved prognostic utility. The findings of the present study demonstrate a significant association between EASIX and patient survival.
Treatment and follow-up of ischemic stroke vary according to the etiology. There was insufficient data to support a strong distinction between lacunar and non-lacunar stroke for other biomarkers. However, endothelial biomarker expression varies by vessel size (e.g., capillary vs. arteriole/large artery endothelium), which may obscure distinctions between non-lacunar and lacunar strokes [6,41]. Kleeberg et al. suggested that EASIX, reflecting systemic endothelial dysfunction independent of tissue type, could address this limitation [19]. In a study of acute myocardial infarction (AMI) patients, a relationship between EASIX and MI types (ST-MI, non-ST-MI), as well as gender, was reported [26]. In our study, no significant relationship was found between EASIX and gender or TOAST subtypes (embolic, lacunar, and other etiologies). This finding may be related to the relatively small sample size, limited to patients with large-vessel occlusion, or to the fact that EASIX represents a tissue-independent biomarker.
Experimental studies in rodents have demonstrated that hyperlipidemia exacerbates ischemic injury by promoting endothelial cell damage, oxidative stress, inflammation, and neuronal death [42]. Collectively, these parameters reflect endothelial stress, which contributes to reperfusion injury, thromboinflammation, and ultimately poor clinical outcomes in patients with PCIS.
Hyperlipidemia has also been shown to negatively influence outcomes in patients undergoing reperfusion therapies such as intravenous thrombolysis (tPA) and mechanical thrombectomy (MT) [43]. The higher frequency of hyperlipidemia among patients with elevated EASIX values may reflect its contribution to endothelial dysfunction. However, data on hyperlipidemia in relation to EASIX remain limited, underscoring the need for further investigation.
Aging is another key factor linked to endothelial dysfunction in both cardiovascular disease and stroke. With advancing age, endothelial and vascular smooth muscle cells develop functional impairments, and alterations occur in intracellular signaling and endothelial–smooth muscle communication [44]. The higher EASIX values observed in patients aged over 70 years in our study further support the relationship between age-related endothelial dysfunction and poor stroke prognosis [45].
The NIHSS is a validated and widely used tool for evaluating stroke severity in patients with acute ischemic stroke (AIS). Higher NIHSS scores indicate more severe neurological impairment. In our study, patients with elevated EASIX values also exhibited higher 24 h NIHSS scores, supporting the prognostic significance of EASIX. Although intubation (particularly lasting > 3 days), post-reperfusion hemorrhage, APACHE II > 13, and admission NIHSS > 12 were each associated with increased mortality risk, no direct relationship was observed between these variables and EASIX. Future multicenter studies with larger cohorts may further clarify the predictive role of EASIX in determining intubation needs, ventilation duration, and length of intensive care unit stay.
In a previous study evaluating endothelial dysfunction biomarkers (e.g., von Willebrand factor [vWF], E-selectin, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1) in patients undergoing mechanical thrombectomy (MT), elevated levels of these markers were correlated with the occurrence of parenchymal hematoma [46]. In contrast, our study did not demonstrate a significant association between EASIX and post-MT parenchymal hemorrhage, likely due to the limited sample size and the small number of hemorrhagic cases. Further research is warranted to investigate the potential relationship between EASIX and parenchymal hematoma following reperfusion therapy.
Our findings indicate that higher EASIX values may predict poorer prognosis in patients with posterior circulation ischemic stroke. This association may be mechanistically explained by the components of EASIX: creatinine as a marker of renal and endothelial dysfunction, LDH as an indicator of cellular injury and systemic inflammation, and platelet count as a measure of thrombotic activity and consumption. Collectively, these parameters reflect the degree of endothelial dysfunction, which plays a central role in stroke evolution and clinical outcomes.
Numerous biomarkers have been explored for their prognostic significance in acute ischemic stroke (AIS). Shen et al. demonstrated that elevated NT-proBNP levels were associated with larger ischemic core volumes on computed tomography perfusion (CTP) imaging and poorer 90-day functional outcomes [15]. However, NT-proBNP is limited in clinical practice by issues of cost, specificity, and availability. Similarly, multiple meta-analyses have demonstrated that elevated CRP, including high-sensitivity CRP, is strongly associated with mortality, recurrent stroke, and poor functional outcomes. Chen et al. found that high hs-CRP levels at admission were associated with nearly a four-fold increase in mortality and higher risk of recurrent stroke in AIS patients [47] while Yu et al. showed that CRP elevation independently predicted all-cause mortality after stroke [48].
Although these biomarkers provide valuable prognostic insights, their use in clinical practice is limited by factors such as high cost, restricted availability, and suboptimal specificity. In contrast, the EASIX represents a composite score based on routine laboratory parameters, combining markers of endothelial stress into a single, low-cost, and easily obtainable prognostic tool.
5. Conclusions
In this retrospective study, we found that higher EASIX values were significantly associated with increased 90-day mortality in patients with posterior circulation ischemic stroke undergoing reperfusion therapy. These findings suggest that EASIX, calculated from routine laboratory parameters, may serve as a simple, inexpensive, and readily available tool for early risk stratification in this high-risk patient population.
To the best of our knowledge, this is the first study to investigate EASIX in reperfusion-treated posterior circulation ischemic stroke, thereby addressing an important gap in the current literature. From a clinical perspective, patients with elevated EASIX values may warrant closer monitoring and, potentially, more intensive management strategies.Future research should aim to include larger, multicenter, prospective cohorts, incorporate serial EASIX measurements, and evaluate its prognostic relevance not only in posterior circulation stroke but also in anterior circulation and lacunar subtypes. Moreover, the relationship between EASIX and long-term outcomes—such as functional recovery, disability, and recurrent stroke—requires further investigation.
This study has several limitations. Its retrospective, single-center design inherently limits the generalizability of the findings. The relatively small sample size—particularly the limited number of patients in the high-EASIX group (n = 9) and the small proportion of patients with good functional outcomes (mRS 0–2, n = 21)—reduced the statistical power of both the univariate and multivariable analyses. Although the EASIX showed a significant association with 90-day mortality in the univariate analysis, the low number of events likely limited the ability of the multivariable model to demonstrate an independent predictive effect. Thrombolytic therapy was not associated with mortality in the univariate analysis and was therefore not included in the multivariable logistic regression, minimizing the potential for confounding.
Additionally, although all EASIX components were collected as part of routine clinical care, platelet and creatinine values were obtained at emergency department admission, LDH was measured after the endovascular procedure during intensive care unit (ICU) admission. This non-uniform sampling may have affected the comparability of the parameters.
Furthermore, EASIX was measured only once, preventing the evaluation of temporal changes. The exclusion of patients with systemic inflammatory diseases, malignancy, or hematologic disorders may have improved internal validity by reducing confounding, but may limit external validity. Taken together, these findings should be considered preliminary and hypothesis-generating, and validation in larger, prospective, multicenter studies is warranted.
Author Contributions
Conceptualization, D.K.S. and C.H.; Methodology, D.K.S. and S.K.; Data Curation, D.K.S., G.K.Y., F.N.K. and K.S.; Writing—Original Draft Preparation, D.K.S.; Writing—Review and Editing, D.K.S., C.H. and S.K.; Supervision, S.K. and K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Bursa City Hospital Ethics Committee (approval date: 5 February 2025; decision no: 2025-3/14).
Informed Consent Statement
This study was conducted retrospectively using anonymized clinical data. Because of the retrospective design and the nature of the data, individual informed consent was not required according to local ethical standards and institutional policies.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Linfante, I.; Walker, G.R.; Castonguay, A.C.; Dabus, G.; Starosciak, A.K.; Yoo, A.J.; Abou-Chebl, A.; Britz, G.W.; Marden, F.A.; Alvarez, A.; et al. Predictors of mortality in acute ischemic stroke intervention: Analysis of the North American Solitaire Acute Stroke Registry. Stroke 2015, 46, 2305–2308. [Google Scholar] [CrossRef]
- Salerno, A.; Strambo, D.; Nannoni, S.; Dunet, V.; Michel, P. Patterns of ischemic posterior circulation strokes: A clinical, anatomical, and radiological review. Int. J. Stroke 2022, 17, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Gramegna, L.L.; Requena, M.; Dinia, L.; Melendez, F.; Hernández, D.; Coscojuela, P.; Quintana, M.; Vert, C.; Rubiera, M.; Ribò, M.; et al. Predictors of response to endovascular treatment of posterior circulation stroke. Eur. J. Radiol. 2019, 116, 219–224. [Google Scholar] [CrossRef]
- Yoshimura, S.; Sakai, N.; Okada, Y.; Kitagawa, K.; Kimura, K.; Tanahashi, N.; Hyogo, T.; Yamagami, H.; Egashira, Y. Efficacy of endovascular treatment for acute cerebral large-vessel occlusion: Analysis of nationwide prospective registry. J. Stroke Cerebrovasc. Dis. 2014, 23, 1183–1190. [Google Scholar] [CrossRef]
- Liu, X.; Dai, Q.; Ye, R.; Zi, W.; Liu, Y.; Wang, H.; Zhu, W.; Ma, M.; Yin, Q.; Li, M.; et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): An open-label, randomised controlled trial. Lancet Neurol. 2020, 19, 115–122. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Daidone, M.; Pinto, A. Endothelial dysfunction and inflammation in ischemic stroke pathogenesis. Curr. Pharm. Des. 2020, 26, 4209–4219. [Google Scholar] [CrossRef] [PubMed]
- Živančević, M.K.; Lović, D.; Radenovic, P.L. Neuroinflammation in post-ischemic brain. In Cerebral Ischemia [Internet]; Exon Publications: Brisbane, Australia, 2021; pp. 87–109. [Google Scholar]
- Shah, W.; Zhao, Q.; Wang, S.; Zhang, M.; Ma, H.; Guan, Y.; Zhang, Y.; Liu, Y.; Zhu, C.; Wang, S.; et al. Polydatin improves vascular endothelial function by maintaining mitochondrial homeostasis under high glucose conditions. Sci. Rep. 2023, 13, 16550. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, Y.; Chen, S.; Li, X.; Dong, J.; Chen, W.; Tao, S.; Yang, W.; Zhang, Y. The role of vascular endothelial growth factor in ischemic stroke. Pharmazie 2021, 76, 127–131. [Google Scholar] [PubMed]
- Wang, X.; He, B. Endothelial dysfunction: Molecular mechanisms and clinical implications. MedComm 2024, 5, e651. [Google Scholar] [CrossRef]
- Piccardi, B.; Giralt, D.; Bustamante, A.; Llombart, V.; García-Berrocoso, T.; Inzitari, D.; Montaner, J. Blood markers of inflammation and endothelial dysfunction in cardioembolic stroke: Systematic review and meta-analysis. Biomarkers 2017, 22, 200–209. [Google Scholar] [CrossRef]
- Poggesi, A.; Pasi, M.; Pescini, F.; Pantoni, L.; Inzitari, D. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: A review. J. Cereb. Blood Flow. Metab. 2016, 36, 72–94. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, S.; Marlborough, F.; Doubal, F.; Webb, D.J.; Wardlaw, J. Blood markers of coagulation, fibrinolysis, endothelial dysfunction and inflammation in lacunar stroke versus non-lacunar stroke and non-stroke: Systematic review and meta-analysis. Cerebrovasc. Dis. 2014, 37, 64–75. [Google Scholar] [CrossRef]
- Esse, R.; Barroso, M.; Almeida, I.T.; Castro, R. The contribution of homocysteine metabolism disruption to endothelial dysfunction: State-of-the-art. Int. J. Mol. Sci. 2019, 20, 867. [Google Scholar] [CrossRef]
- Shen, X.; Liao, J.; Jiang, Y.; Xu, Y.; Liu, M.; Zhang, X.; Dong, N.; Yu, L.; Chen, Q.; Fang, Q. Elevated NT-proBNP levels are associated with CTP ischemic volume and 90-day functional outcomes in acute ischemic stroke: A retrospective cohort study. BMC Cardiovasc. Disord. 2022, 22, 431. [Google Scholar] [CrossRef]
- Alemseged, F.; Rocco, A.; Arba, F.; Schwabova, J.P.; Wu, T.; Cavicchia, L.; Ng, F.; Ng, J.L.; Zhao, H.; Williams, C.; et al. Posterior National Institutes of Health Stroke Scale Improves Prognostic Accuracy in Posterior Circulation Stroke. Stroke 2022, 53, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, P.; Fakhri, S.; Heidarizadeh, K.; Kordestani-Moghadam, P.; Mokhayeri, Y. The relationship between APACHE II score and mortality among patients with stroke. Interdiscip. J. Acute Care 2022, 3, 64–68. [Google Scholar]
- Luft, T.; Benner, A.; Jodele, S.; Dandoy, C.E.; Storb, R.; Gooley, T.; Sandmaier, B.M.; Becker, N.; Radujkovic, A.; Dreger, P.; et al. EASIX in patients with acute graft-versus-host disease: A retrospective cohort analysis. Lancet Haematol. 2017, 4, e414–e423. [Google Scholar] [CrossRef]
- Kleeberg, A.; Luft, T.; Golkowski, D.; Purrucker, J.C. Endothelial dysfunction in acute ischemic stroke: A review. J. Neurol. 2025, 272, 143–150. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Z.; Wang, J.; Wang, D.; Yin, X. Endothelial activation and stress index is a reliable predictor for the prevalence and mortality outcomes of stroke. Sci. Rep. 2025, 15, 23285. [Google Scholar] [CrossRef] [PubMed]
- Song, G.Y.; Jung, S.H.; Kim, K.; Kim, S.J.; Yoon, S.E.; Lee, H.S.; Kim, M.; Ahn, S.-Y.; Ahn, J.-S.; Yang, D.-H.; et al. Endothelial activation and stress index (EASIX) is a reliable predictor for overall survival in patients with multiple myeloma. BMC Cancer 2020, 20, 803. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Go, S.I.; Lee, G.W. The Endothelial Activation and Stress Index (EASIX) score is an independent prognostic factor in patients with diffuse large B-cell lymphoma. BMC Cancer 2022, 22, 816. [Google Scholar] [CrossRef] [PubMed]
- Penack, O.; Luft, T.; Peczynski, C.; Benner, A.; Sica, S.; Arat, M.; Itäla-Remes, M.; Corral, L.L.; Schaap, N.P.M.; Karas, M.; et al. Endothelial Activation and Stress Index (EASIX) to predict mortality after allogeneic stem cell transplantation: A prospective study. J. Immunother. Cancer 2024, 12, e007635. [Google Scholar] [CrossRef]
- Xu, H.B.; Ye, Y.; Xue, F.; Wu, J.; Suo, Z.; Zhang, H. Association Between Endothelial Activation and Stress Index and 28-Day Mortality in Septic ICU patients: A Retrospective Cohort Study. Int. J. Med. Sci. 2023, 20, 1165–1173. [Google Scholar] [CrossRef]
- Finke, D.; Hund, H.; Frey, N.; Luft, T.; Lehmann, L.H. Endothelial activation and stress index (EASIX) in coronary artery disease: A simplified measure as a promising biomarker. Clin. Res. Cardiol. 2024, 113, 1775–1777. [Google Scholar] [CrossRef]
- Sang, M.; Ma, X.; Zhu, F.; Zhu, C.; Ying, Z. Association between endothelial activation and stress index and 30-day mortality risk in acute myocardial infarction patients: A study based on the medical information mart for intensive care-IV database. BMC Cardiovasc. Disord. 2024, 24, 699. [Google Scholar] [CrossRef]
- Oksala, N.K.J.; Salonen, T.; Strandberg, T.; Oksala, A.; Pohjasvaara, T.; Kaste, M.; Karhunen, P.J.; Erkinjuntti, T. Cerebral small vessel disease and kidney function predict long-term survival in patients with acute stroke. Stroke 2010, 41, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, M.F.; Safar, M.E. Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension 2005, 46, 200–204. [Google Scholar] [CrossRef]
- McCullough, P.A.; Jurkovitz, C.T.; Pergola, P.E.; McGill, J.B.; Brown, W.W.; Collins, A.J.; Chen, S.-C.; Li, S.; Singh, A.; Norris, K.C.; et al. Independent components of chronic kidney disease as a cardiovascular risk state: Results from the Kidney Early Evaluation Program (KEEP). Arch. Intern. Med. 2007, 167, 1122–1129. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Shaper, A.G.; Perry, I.J. Serum creatinine concentration and risk of cardiovascular disease: A possible marker for increased risk of stroke. Stroke 1997, 28, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Rowat, A.; Graham, C.; Dennis, M. Dehydration in hospital-admitted stroke patients: Detection, frequency, and association. Stroke 2012, 43, 857–859. [Google Scholar] [CrossRef]
- Li, S.; Yin, M.; Zhou, Z.; Chen, H.-S. Dehydration is a strong predictor of long-term prognosis of thrombolysed patients with acute ischemic stroke. Brain Behav. 2017, 7, e00849. [Google Scholar] [CrossRef]
- Dong, K.; Huang, X.; Zhang, Q.; Yu, Z.; Ding, J.; Song, H. A lower baseline glomerular filtration rate predicts high mortality and newly cerebrovascular accidents in acute ischemic stroke patients. Medicine 2017, 96, e5868. [Google Scholar] [CrossRef]
- Yang, M.; Pan, Y.; Li, Z.; Yan, H.; Zhao, X.; Liu, L.; Jing, J.; Meng, X.; Wang, Y.; Wang, Y. Platelet Count Predicts Adverse Clinical Outcomes After Ischemic Stroke or TIA: Subgroup Analysis of CNSR II. Front. Neurol. 2019, 10, 370. [Google Scholar] [CrossRef]
- Gauer, R.L.; Whitaker, D.J. Thrombocytopenia: Evaluation and management. Am. Fam. Physician 2022, 106, 288–298. [Google Scholar] [PubMed]
- Han, Y.; Zhang, H.; Mu, S.; Wei, W.; Jin, C.; Tong, C.; Song, Z.; Zha, Y.; Xue, Y.; Gu, G. Lactate dehydrogenase, an independent risk factor of severe COVID-19 patients: A retrospective and observational study. Aging 2020, 12, 11245–11254. [Google Scholar] [CrossRef]
- Coppo, P.; Schwarzinger, M.; Buffet, M.; Wynckel, A.; Clabault, K.; Presne, C.; Poullin, P.; Malot, S.; Vanhille, P.; Azoulay, E.; et al. Predictive Features of Severe Acquired ADAMTS13 Deficiency in Idiopathic Thrombotic Microangiopathies: The French TMA Reference Center Experience. PLoS ONE 2010, 5, e10208. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Cunningham, M.W.; Pabbidi, M.R.; Wang, S.; Booz, G.W.; Fan, F. Targeting vascular inflammation in ischemic stroke: Recent developments on novel immunomodulatory approaches. Eur. J. Pharmacol. 2018, 833, 531–544. [Google Scholar] [CrossRef]
- Anan, M.; Nagai, Y.; Fudaba, H.; Fujiki, M. Lactate and Lactate Dehydrogenase in Cistern as Biomarkers of Early Brain Injury and Delayed Cerebral Ischemia of Subarachnoid Hemorrhage. J. Stroke Cerebrovasc. Dis. 2020, 29, 104765. [Google Scholar] [CrossRef]
- Jin, X.X.; Fang, M.D.; Hu, L.L.; Yuan, Y.; Xu, J.-F.; Lu, G.-G.; Li, T. Elevated lactate dehydrogenase predicts poor prognosis of acute ischemic stroke. PLoS ONE 2022, 17, e0275651. [Google Scholar] [CrossRef]
- Aird, W.C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ. Res. 2007, 100, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.L.; Du, J.; Zhang, Y.; Yan, J.-T.; Hu, X.-M. Hyperlipidemia exacerbates cerebral injury through oxidative stress, inflammation and neuronal apoptosis in MCAO/reperfusion rats. Exp. Brain Res. 2015, 233, 2753–2765. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, L.; Bang, O.Y.; Ovbiagele, B.; Ali, L.; Kim, D.; Liebeskind, D.S.; Starkman, S.; Vinuela, F.; Duckwiler, G.R.; Jahan, R.; et al. Impact of Hyperlipidemia and Statins on Ischemic Stroke Outcomes after Intra-Arterial Fibrinolysis and Percutaneous Mechanical Embolectomy. Cerebrovasc. Dis. 2009, 28, 384–390. [Google Scholar] [CrossRef]
- Yildiz, O. Vascular smooth muscle and endothelial functions in aging. Ann. N. Y. Acad. Sci. 2007, 1100, 353–360. [Google Scholar] [CrossRef]
- Soriano-Tárraga, C.; Lazcano, U.; Jiménez-Conde, J.; Ois, A.; Cuadrado-Godia, E.; Giralt-Steinhauer, E.; Rodríguez-Campello, A.; Gomez-Gonzalez, A.; Avellaneda-Gómez, C.; Vivanco-Hidalgo, R.M.; et al. Biological age is a novel biomarker to predict stroke recurrence. J. Neurol. 2021, 268, 285–292. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, P.; Chen, S.; Wan, T.; Wang, X.; Wang, M.; Zhou, J.; Xie, Y.; Jiang, T. Endothelial Dysfunction and Parenchymal Hematoma in Ischemic Stroke Patients after Endovascular Thrombectomy. Cerebrovasc. Dis. 2023, 52, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, M.; Yang, C.; Wang, Y.; Hou, B. The role of high-sensitivity C-reactive protein serum levels in the prognosis for patients with stroke: A meta-analysis. Front. Neurol. 2023, 14, 1199814. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yang, P.; Xu, X.; Shao, L. C-reactive protein for predicting all-cause mortality in patients with acute ischemic stroke: A meta-analysis. Biosci. Rep. 2019, 39, BSR20181135. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).