Abstract
Background: This retrospective cohort study evaluated the role of the PILE score as a prognostic biomarker for overall survival (OS) and progression-free survival (PFS) in patients with locally advanced esophageal squamous cell carcinoma (ESCC) who were treated with neoadjuvant chemoradiotherapy (nCRT). Methods: This study included 108 patients with ESCC treated with weekly paclitaxel-carboplatin and concurrent radiotherapy at Erzurum Atatürk University Faculty of Medicine Hospital between January 2018 and April 2024. Patients were categorized into low- (PILE score 0–1) and high-risk (PILE score 2–3) groups. Kaplan–Meier analysis and Cox regression models were used to evaluate the association between the PILE score and survival outcomes. Results: The results showed that the high-risk PILE group had significantly shorter median OS (18.6 months vs. not reached; p < 0.001) and PFS (12.4 months vs. not reached; p < 0.001) than the low-risk group. Multivariate analysis showed that the PILE risk classification [hazard ratio (HR) = 2.527; 95% confidence interval (CI): 1.380–4.629; p = 0.003] and surgical resection (HR = 0.249; 95% CI: 0.090–0.683; p = 0.007) were independent prognostic factors for OS, whereas the PILE risk classification (HR = 2.932; 95% CI: 1.525–5.639; p = 0.001) and surgical resection (HR = 0.131; 95% CI: 0.044–0.394; p < 0.001) were independent prognostic factors for PFS. Conclusions: The study concludes that the PILE score is a robust prognostic tool for OS and PFS in patients with ESCC undergoing nCRT, highlighting its potential for risk stratification and personalized treatment planning.
1. Introduction
Esophageal cancer is a significant global health concern and is among the leading causes of cancer-related deaths [1,2]. In 2020, there were an estimated 604,100 cases of this cancer, with 544,100 deaths, and this number is expected to increase [1]. Certain regions, particularly East Asia and Africa, experience high incidence and mortality rates, forming “the esophageal cancer belt” [3,4]. Within this belt, esophageal squamous cell carcinomas account for the vast majority of all esophageal cancer cases, often exceeding 90% [5,6]. Despite advancements in treatment methods, the prognosis remains poor for cases diagnosed at an advanced stage, highlighting the urgent need for enhanced strategies and techniques [7].
Currently, nCRT followed by esophagectomy is regarded as the standard approach for treating locally advanced resectable esophageal cancers, including ESCC [8,9]. The landmark CROSS trial provided strong evidence for the effectiveness of nCRT protocols that involve weekly doses of paclitaxel and carboplatin in combination with radiation, showing significant enhancements in overall survival and R0 resection rates, particularly in the ESCC group, compared to those who underwent only surgery [9,10]. Subsequent studies have reinforced the effectiveness and tolerability of paclitaxel-carboplatin regimens for nCRT [11,12].
Beyond traditional clinicopathological factors, systemic inflammation has emerged as a significant prognostic indicator in a range of cancers, including ESCC [13]. Blood tests, such as the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio, are recognized as biomarkers that reflect the host’s inflammatory response to tumors, influencing both tumor progression and treatment response [14,15,16]. Additionally, the Prognostic Inflammatory Index, which combines various inflammatory markers, is valuable for predicting patient outcomes in different cancers [17,18].
While certain inflammatory biomarkers offer valuable prognostic insights, their incorporation into more intricate scoring systems can enhance prognostic precision. The PILE score exemplifies this combined prognostic tool by integrating the Pan-Immune-Inflammation Value (PIV), lactate dehydrogenase (LDH) level, and Eastern Cooperative Oncology Group (ECOG) performance status [19]. Elevated LDH levels are indicative of a substantial tumor burden and aggressive disease, which correlates with poor prognostic outcomes [20,21]. Conversely, ECOG performance status represents the clinician’s subjective assessment of a patient’s overall functional status and is recognized as an independent prognostic factor of overall survival [19]. Despite the prognostic utility of the PILE score in other cancers, such as extensive-stage small-cell lung cancer, particularly concerning immunotherapy treatment, its prognostic value in the context of ESCC and nCRT remains underexplored [19]. There is a need to validate the PILE score in specific populations and contexts [22].
This study aimed to explore the prognostic value of the PILE score for OS and PFS in patients with locally advanced ESCC who underwent neoadjuvant chemoradiotherapy.
2. Materials and Methods
2.1. Study Design and Setting
This retrospective cohort study was conducted at the Erzurum Atatürk University Faculty of Medicine Hospital. This study included patients diagnosed with ESCC through histological examination, all of whom underwent nCRT between January 2018 and April 2024 at our hospital. Participants were required to be ≥18 years of age and have clinical stage II–IVA ESCC. The exclusion criteria were incomplete medical records, missing initial laboratory data, non-squamous histology, the presence of another active cancer, active infections, or known autoimmune or chronic inflammatory diseases, to prevent confounding of systemic inflammatory markers. A total of 145 patients were screened. Of these, 18 were excluded due to non-squamous histology, 13 due to incomplete medical or laboratory records, and 6 due to coexisting malignancy, acute infection, or autoimmune inflammatory conditions. The remaining 108 patients comprised the final study cohort. A detailed flowchart of patient selection is presented in Figure 1.
Figure 1.
Flow diagram of patient selection: A total of 145 patients were screened for eligibility. After exclusions for non-squamous histology (n = 18), incomplete clinical or laboratory records (n = 13), and coexisting malignancy, acute infection, or autoimmune inflammatory conditions (n = 6), 108 patients were included in the final analysis.
2.2. Treatment Protocol
All patients received a weekly regimen of neoadjuvant chemoradiotherapy based on paclitaxel and carboplatin. Chemotherapy involved administering paclitaxel at a dose of 50 mg/m2 and carboplatin with an area under the curve (AUC) of 2, administered weekly over five to six cycles. External beam radiotherapy was also administered, totaling 41.4 to 50.4 Gray (Gy), divided into 23 to 28 fractions, with each fraction being 1.8 to 2.0 Gy. Following the completion of nCRT, the patients were re-evaluated, and surgical resection was performed only for those considered suitable by the multidisciplinary tumor board.
2.3. Data Collection
Data on demographic, clinical, pathological, and treatment-related factors, including age, sex, smoking habits, existing health conditions, ECOG performance status, tumor site, clinical T/N stage, overall clinical stage, surgical status, and disease progression, were extracted from electronic hospital records. Laboratory values, such as neutrophil, lymphocyte, monocyte, platelet, and LDH levels, were measured within seven days before starting nCRT.
2.4. Inflammatory Indexes and PILE Score
The neutrophil-to-lymphocyte ratio (NLR) was calculated by dividing the absolute neutrophil count by the lymphocyte count, and the platelet-to-lymphocyte ratio (PLR) was calculated by dividing the platelet count by the lymphocyte count. The PIV was determined using the following formula: (neutrophils × platelets × monocytes)/lymphocytes. The PILE score comprises three factors assessed together: PIV of 413 or higher (median threshold), LDH levels exceeding the upper normal limit (ULN), and ECOG performance status of 2 or more, with each factor adding 1 point. At our institution, the ULN for LDH is 240 U/L, and values above this threshold are considered elevated. The PILE score ranges from 0 to 3, categorizing patients into low- (0–1 points) or high-risk (2–3 points) groups. A cohort-specific median PIV cut-off (413) was selected, which is consistent with previous studies on lymphoma, hepatocellular carcinoma, and small-cell lung cancer, where median-based thresholds were used owing to the absence of universally validated cut-offs for inflammation-based markers [19,23,24].
2.5. Outcome Definitions
OS was measured as the duration from the time of diagnosis to death from any cause or until the most recent follow-up. PFS was defined as the period from treatment initiation to radiologically confirmed disease progression, recurrence or death.
2.6. Statistical Analysis
Continuous variables are expressed as mean ± standard deviation (SD), and categorical variables are expressed as counts and percentages. To compare the discriminative performance of the individual inflammatory markers (NLR, PLR, and PIV) with the composite PILE score, receiver operating characteristic (ROC) curve analysis was performed, and the AUC values were calculated. The Kaplan–Meier method was used to estimate survival outcomes, and comparisons were performed using the log-rank test. Univariate Cox proportional hazards analyses were conducted to identify possible prognostic factors for OS and PFS. Variables identified in the univariate analysis were included in the multivariate Cox regression model, and the results are presented as HRs and 95% CIs. Both variables with p < 0.10 in the univariate analysis and clinically relevant covariates were included in the multivariate model. To avoid collinearity, ECOG performance status and LDH level were excluded from the multivariate model once the composite PILE score was introduced, as these variables were already embedded within it. Therefore, the multivariate analysis assessed the prognostic effect of this composite index rather than its individual components. Model performance was assessed using the concordance index (C-index). The association between the PILE score and pathological complete response (pCR) was examined using Pearson Chi-square and Fisher’s exact test. Statistical analyses were performed using IBM SPSS Statistics version 31.0 (IBM Corp., Armonk, NY, USA), and a p-value of less than 0.05 was deemed statistically significant.
Additionally, patients were classified into two treatment cohorts: those who underwent esophagectomy after neoadjuvant chemoradiotherapy and those treated with definitive chemoradiotherapy (dCRT), defined as chemoradiation without subsequent surgery. Predefined subgroup Cox regression analyses were performed to evaluate whether the prognostic effect of the PILE score differed between the treatment groups. An interaction term (PILE × surgical status) was incorporated into the multivariable Cox regression models for both OS and PFS to formally assess the effect modification.
2.7. Language Editing Statement
AI-assisted language editing tools [Paperpal (version 4.4.1) and ChatGPT (OpenAI GPT-5.1, 2025 release)] were used only for grammar correction, paraphrasing, and translation support during the final proofreading stage.
3. Results
3.1. Patient Characteristics
This study analyzed 108 patients with ESCC who underwent neoadjuvant chemoradiotherapy consisting of weekly paclitaxel (50 mg/m2) and carboplatin (AUC 2) for five to six cycles, delivered concurrently with external beam radiotherapy totaling 41.4–50.4 Gy in 23–28 fractions (1.8–2.0 Gy per fraction). The average age of the participants was 62.3 ± 10.8 years (range: 37–89 years). The group comprised 63.9% females and 36.1% males. The majority of patients (66.7%) were non-smokers, 29.6% were current smokers, and 3.7% had quit smoking. The ECOG performance statuses were 0 (25.9%), 1 (53.7%), and 2 (20.4%), respectively. Comorbid conditions were found in 47.2% of the patients, with hypertension (20.4%), coronary artery disease (10.2%), diabetes mellitus (9.3%), and chronic obstructive pulmonary disease (COPD) (2.8%) being the most common. The tumors were primarily located in the lower third of the esophagus (75.0%), with the remaining 25.0% located in the middle third. Clinically, 37.0% of the patients had T2 tumors, whereas 63.0% had T3 tumors. Nodal involvement was categorized as N0 in 24.1%, N1 in 32.4%, N2 in 32.4%, and N3 in 11.1% of patients, corresponding to clinical stages II (37.0%), III (51.9%), and IVA (11.1%) disease. Post-neoadjuvant therapy and surgical resection were performed in 31.5% of patients. Among the surgically treated patients, pCR was achieved in 25 of 34 patients (73.5%). During follow-up, 39.8% of the patients experienced disease progression, and 14 patients developed distant metastases, primarily to the lungs (50%) and liver (21.4%). At the last follow-up, 57.4% of patients were alive. Laboratory tests showed a mean LDH level of 216.8 ± 51.5 U/L, lymphocyte count of 2.17 ± 0.68 × 109/L, neutrophil count of 5.35 ± 2.01 × 109/L, monocyte count of 0.61 ± 0.19 × 109/L, and platelet count of 296.4 ± 101.1 × 109/L. The mean PIV was 550.0 ± 577.6 (median PIV: 413), whereas the mean NLR and PLR values were 2.75 ± 1.68 (0.81–12.90) and 148.4 ± 66.9 (59.6–435.3), respectively. According to the PILE scoring system, 70.4% of patients were classified as low-risk (0–1), whereas 29.6% were considered high-risk (2–3). The mean OS was 26.34 ± 15.32 months and the mean PFS was 22.01 ± 15.39 months. The details of the study are summarized in Table 1.

Table 1.
Patient characteristics and laboratory findings.
3.2. ROC Analysis for Discriminatory Performance
To assess the prognostic capabilities of the PILE score against traditional inflammatory markers (NLR, PLR, and PIV), ROC curve analysis was conducted. The PILE score exhibited a higher ability to distinguish survival outcomes, achieving an AUC of 0.709, which surpassed that of PLR (0.631), NLR (0.572), and PIV (0.567) scores. When the PILE risk classification was categorized into low- and high-risk groups, the AUC increased to 0.715, demonstrating strong prognostic differentiation. These findings validate that the composite PILE score offers significantly enhanced prognostic accuracy compared with individual inflammatory markers.
3.3. Overall Survival Analysis
Kaplan–Meier analysis revealed no statistically significant difference in OS between males and females, with median OS of 41.4 months for males and 54.6 months for females (p = 0.336). The ECOG performance status has emerged as a significant prognostic factor for survival. Patients with an ECOG score of 0–1 experienced notably longer OS, whereas those with an ECOG score of 2 had significantly shorter survival times (median OS: ECOG 0 = 45.9 months, ECOG 1 = 54.6 months, ECOG 2 = 16.1 months; p = 0.006).
The presence of comorbidities did not lead to a notable difference in overall survival (median OS: no comorbidity = not reached, comorbidity present = 33.7 months; p = 0.463). Although survival rates appeared to decline with higher clinical stages, this variation was not statistically significant (median OS: stage II, 45.9 months; stage III, not reached; stage IVA, 18.6 months; p = 0.100).
Surgical resection is closely associated with enhanced OS. Patients who underwent surgery had a notably longer lifespan than those who did not undergo the procedure (median OS: 24.7 months for non-surgical patients versus not reached for surgical patients; p < 0.001).
The PILE risk classification was the most effective in distinguishing OS outcomes. Patients classified as low-risk had a notably longer survival period, whereas those in the high-risk category experienced significantly worse OS (median OS: low-risk = not reached, high-risk = 18.6 months; p < 0.001) (Figure 2).
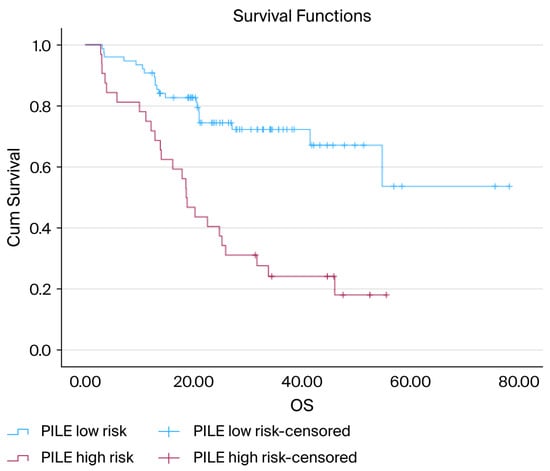
Figure 2.
Kaplan–Meier curves for OS according to the PILE risk groups: Kaplan–Meier OS curves stratified by the PILE risk classification. Patients in the low-risk group (PILE score 0–1) experienced significantly longer OS than those in the high-risk group (PILE score 2–3). The median OS was not reached in the low-risk group, but was 18.6 months in the high-risk group. The difference between the survival curves was statistically significant (log-rank, p < 0.001).
3.4. Cox Regression Analysis for OS
In the univariate Cox regression analysis, age was significantly correlated with the OS hazard ratio (HR = 1.032; 95% CI: 1.005–1.060; p = 0.021). However, sex, comorbidity status, and clinical stage were not significantly associated with overall survival (p = 0.338, 0.464, and 0.111, respectively). Nonetheless, in the categorical analysis of clinical stage, patients with stage IVa disease had a significantly higher risk than those with stage II disease (HR = 2.38; 95% CI: 1.02–5.52; p = 0.044). Surgical resection emerged as a strong protective factor, with patients who underwent surgery exhibiting notably improved survival rates (HR = 0.181; 95% CI: 0.071–0.460; p < 0.001). Additionally, PILE risk classification was significantly associated with OS, with high-risk patients experiencing considerably poorer survival outcomes (HR = 3.531; 95% CI: 1.974–6.318; p < 0.001).
In the multivariate Cox regression analysis, PILE risk classification (HR = 2.527; 95% CI: 1.380–4.629; p = 0.003) and surgical resection (HR = 0.249; 95% CI: 0.090–0.683; p = 0.007) were independent prognostic factors for OS. Factors such as age, sex, comorbidities, and clinical stage were not statistically significant in the adjusted model (p > 0.05). The C-index of the model was 0.469, reflecting a moderate discriminatory power (Table 2).

Table 2.
Univariate and multivariate cox regression analysis for OS.
Subgroup and Interaction Analysis for OS
Subgroup analyses were conducted to assess whether the prognostic significance of the PILE score varied between patients who underwent surgery and those who received dCRT. In the group that did not undergo surgery, the PILE score emerged as a robust and significant prognostic factor for overall survival (HR = 2.64; 95% CI: 1.42–4.91; p = 0.002). Conversely, in the group of patients who underwent surgery, the association between the PILE score and overall survival was not statistically significant (HR = 2.62; 95% CI: 0.41–16.81; p = 0.310), likely due to the small sample size and limited statistical power.
The interaction term between the PILE score and surgical status was non-significant (HR = 1.44; 95% CI: 0.22–9.62; p = 0.707), suggesting that the prognostic impact of the PILE score was not notably different across the two groups.
3.5. Progression-Free Survival Analysis
According to the Kaplan–Meier analysis, there was no notable difference in PFS between men and women, with the median PFS not being reached for men and standing at 27.3 months for women (p = 0.357). Patients without comorbid conditions experienced a longer PFS than those with comorbidities, with the median PFS not reached versus 24.7 months, respectively (p = 0.031).
ECOG performance status significantly influenced PFS. Patients with an ECOG score of 0–1 experienced notably longer PFS, whereas those with an ECOG score of 2 had the shortest survival duration (median PFS: ECOG 0 = not reached, ECOG 1 = not reached, ECOG 2 = 10.2 months; p < 0.001).
Although the clinical stage appeared to be associated with a trend toward reduced PFS as the disease progressed, this variation did not reach statistical significance (median PFS: stage II, 40.8 months; stage III, not reached; stage IVA, 19.8 months; p = 0.311).
Surgical resection was strongly associated with enhanced PFS. Patients who underwent surgery experienced a notably longer PFS than those who did not, with the median PFS not being reached versus 18.2 months (p < 0.001).
The PILE risk classification effectively distinguished between the different PFS outcomes. Patients classified as low-risk had notably longer PFS, whereas those in the high-risk category had significantly worse outcomes (median PFS not reached vs. 12.4 months; p < 0.001) (Figure 3).
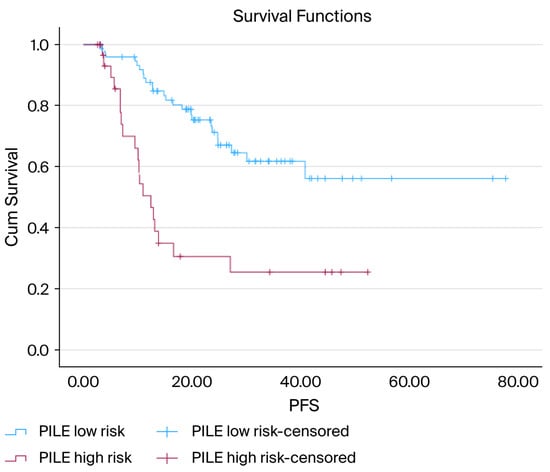
Figure 3.
Kaplan–Meier curves for PFS according to PILE risk groups: Kaplan–Meier PFS curves comparing low-risk (PILE score 0–1) and high-risk (PILE score 2–3) groups. The low-risk group demonstrated a markedly longer PFS, whereas the high-risk group showed substantially earlier progression. The median PFS was not reached in the low-risk group and was 12.4 months in the high-risk group. The difference in survival between the groups was statistically significant (log-rank, p < 0.001).
3.6. Cox Regression Analysis for PFS
In the univariate Cox regression analysis, age was significantly associated with reduced PFS, with an HR of 1.030 (95% CI: 1.002–1.060; p = 0.035). Sex was not associated with PFS (HR = 1.366; 95% CI: 0.701–2.663; p = 0.359). The presence of comorbidities significantly increased the risk of disease progression (HR = 1.938; 95% CI: 1.049–3.579; p = 0.034). Clinical stage was not significantly associated with PFS (p = 0.312). Surgical resection emerged as a significant protective factor, and patients who underwent surgery had significantly improved PFS (HR = 0.116; 95% CI: 0.041–0.328; p < 0.001). The PILE risk classification showed a strong prognostic value, as high-risk patients had notably shorter PFS (HR = 3.561; 95% CI: 1.936–6.551; p < 0.001).
In the multivariate Cox analysis, surgical resection and PILE risk classification were independent prognostic factors for PFS. Patients who underwent surgery had a significantly lower risk of disease progression (HR = 0.131; 95% CI: 0.044–0.394; p < 0.001). A high PILE risk was still associated with worse PFS outcomes (HR = 2.932; 95% CI: 1.525–5.639; p = 0.001). Factors such as age, sex, comorbidity status, and clinical stage were not significant in the adjusted model (p > 0.05). The model exhibited a strong discriminatory ability with a C-index of 0.786 (Table 3).

Table 3.
Univariate and multivariate cox regression analysis for PFS.
Subgroup and Interaction Analysis for PFS
Subgroup analyses were performed to assess whether the prognostic impact of the PILE score on PFS differed between patients who underwent surgery and those who received dCRT. In the group that did not undergo surgery, the PILE score emerged as a robust and statistically significant prognostic indicator of PFS (HR = 2.50, 95% CI: 1.31–4.76; p = 0.005). For patients who underwent surgical treatment, the PILE score also showed a significant correlation with PFS (HR = 7.30, 95% CI: 1.02–52.07; p = 0.047), although the broad confidence interval indicated the small sample size and reduced statistical power of this subgroup analysis.
The interaction term between the PILE score and surgical status was not statistically significant (HR = 3.15, 95% CI: 0.40–24.82; p = 0.275), suggesting that the prognostic impact of the PILE score on PFS was similar for patients who underwent surgery and those who underwent dCRT.
3.7. Association Between PILE Score and pCR
Of the 34 patients who underwent surgery, 25 (73.5%) achieved pCR. In the low PILE-risk category, 22 of 30 patients (73.3%) reached pCR, whereas in the high-risk category, three of four patients (75.0%) did so. Both the Pearson’s chi-square and Fisher’s exact tests indicated no significant difference in pCR rates between the two PILE risk categories (p = 0.943 and p = 1.000, respectively).
3.8. Model Performance
The ability of the Cox regression models to differentiate was assessed using the C-index. For OS, the multivariate model showed limited discrimination, with a C-index of 0.469, indicating a restricted capacity to differentiate between patients with varying survival risks. Conversely, the PFS model exhibited a strong discriminatory capability, achieving a C-index of 0.786, which signifies excellent prognostic accuracy for progression risk.
4. Discussion
In this study, we demonstrated that the PILE score serves as a robust and significant prognostic indicator for both overall survival and progression-free survival in patients with esophageal squamous cell carcinoma who underwent nCRT. The critical influence of the PILE score on patient outcomes, along with the substantial variation in survival rates among patients who underwent surgery after nCRT based on their PILE score, highlights the importance of the PILE score in forecasting outcomes for this challenging patient group.
Our findings align with those of earlier studies highlighting the prognostic significance of individual inflammatory biomarkers and performance status across various cancers, including ESCC. For instance, pan-immune inflammation is recognized as a prognostic factor for survival in ESCC and other solid tumors [17,18,19]. Additionally, elevated lactate dehydrogenase levels are strongly associated with worse outcomes in patients with ESCC receiving different treatments [20,21]. The ECOG performance status is a well-established prognostic biomarker that indicates a patient’s overall health and functional status [19,25]. Furthermore, studies have validated the prognostic value of other systemic inflammation markers, such as the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios, as prognostic factors for the survival of patients with ESCC undergoing concurrent chemoradiotherapy [25,26]. Although numerous studies have examined other components or combinations, such as the Gustave Roussy Immune Score [27] or CALLY Index [28], the PILE score integrates all three elements: PIV, LDH, and ECOG. To our knowledge, this is the first study to thoroughly evaluate the PILE score in patients with ESCC undergoing nCRT, thereby addressing a significant gap in understanding the role of comprehensive biomarker prognostic tools in this clinical context.
The prognostic significance of the PILE score is derived from the interconnected roles of the component biomarkers in tumor progression. A high PIV index indicates an increased level of systemic inflammation, characterized by elevated neutrophil, platelet, and monocyte counts along with reduced lymphocyte [29]. Neutrophils contribute to tumor growth, angiogenesis, and invasion by releasing factors that promote tumors, and their adaptability allows them to support tumors within the tumor microenvironment [30,31,32,33]. Platelets play a role in protecting against immune destruction and facilitating metastasis by aiding extravasation and microembolus formation, as well as contributing to tumor vascular integrity and immune evasion [34,35,36,37,38,39,40]. Monocytes transform into tumor-associated macrophages, promoting tumor growth, angiogenesis, invasion, and metastasis, which are strongly linked to poor patient outcomes [41,42,43,44,45,46,47,48,49,50]. Conversely, low lymphocyte levels are associated with an ineffective antitumor immune response, allowing tumor escape and correlating with reduced overall survival [29,51,52,53,54]. Elevated LDH levels often indicate a high tumor burden, anaerobic glycolysis, and hypoxia, all of which are associated with aggressive tumor types, resistance, and poor prognosis [20,21,55,56]. Lastly, a poor ECOG Performance Status reflects diminished general physical functioning and health, often indicating a negative prognosis and serving as a measure of functional status in patients with cancer [19,57,58,59,60,61]. By integrating these distinct yet complementary biomarkers, the PILE score enhances prognostic accuracy by providing a comprehensive view of the interaction between the host and tumor, as well as metabolic stress. Consistent with this biological rationale, our ROC analysis showed that the PILE score demonstrated superior prognostic discrimination compared with individual inflammatory markers such as NLR, PLR, and PIV, further supporting the advantage of composite inflammation-based indices over single-ratio biomarkers in predicting prognosis.
The PILE score plays a vital role in the clinical management of patients receiving nCRT. It is an effective prognostic factor for both OS and PFS, making it valuable for risk stratification in the context of nCRT. Patients with ESCC identified as high-risk by the PILE score may require special attention and follow-up, potentially involving frequent evaluations through imaging or endoscopic procedures. Furthermore, these high-risk patients with ESCC might benefit from alternative treatment strategies, such as exploring novel agents in combination with surgery as an adjunctive treatment or participating in clinical trials assessing the effectiveness of immune checkpoint inhibitors and targeted therapies, which could enhance their prognosis. The PILE score can be a significant tool in team discussions, aiding in tailoring and improving treatment decisions for physicians and, consequently, their patients. Moreover, the prognostic performance of the model was markedly stronger for PFS than for OS, as demonstrated by the substantially higher C-index (0.786 vs. 0.469), indicating that the PILE score more accurately discriminated the progression risk than the long-term mortality risk.
Surgical removal after nCRT continues to be a fundamental component of curative treatment for locally advanced ESCC, with strong evidence indicating notable enhancements in both overall survival and progression-free survival compared to nCRT alone or initial surgery [62,63,64,65]. The crucial CROSS trial and subsequent research have conclusively demonstrated the survival benefit of esophagectomy following nCRT in this group [66]. Our research further revealed that prognostic variations based on the PILE score remained evident even among patients who underwent surgical removal, highlighting its significant prognostic value. A high PILE score likely indicates not only aggressive tumor characteristics but also increased systemic inflammation and diminished physiological capacity, which may hinder the ability to endure major surgery and lead to postoperative complications and poorer long-term outcomes [67,68,69]. Moreover, an inadequate pathological response to nCRT, often linked to elevated inflammatory markers, may further worsen the surgical outcomes [70,71,72]. Consistent with its primarily prognostic nature, the PILE score did not show a significant association with pCR to nCRT in our cohort. Consequently, the PILE score could serve as a crucial perioperative risk assessment tool, assisting multidisciplinary teams in evaluating surgical eligibility, enhancing patient counseling, and identifying patients who might benefit from intensified preoperative preparation or alternative treatment approaches to minimize surgical risk and boost overall survival [73,74].
The present study had several strengths. The chief among them is that all participants were administered an identical nCRT regimen, specifically paclitaxel-carboplatin, on a weekly basis, which minimizes potential biases stemming from different nCRT treatments. Furthermore, this study leveraged real-world data to enhance the applicability of our findings to actual clinical environments. Notably, this is the first study to assess the prognostic significance of PILE in a cohort of patients with ESCC post-nCRT, marking the first effort to illuminate this complex and challenging scenario. However, this study had some limitations. The primary concern is the inherent risk of selection and unspecified biases typical of retrospective analyses. Additionally, the dataset was somewhat constrained in size, as the study was conducted at a single center, which may limit its generalizability and necessitate validation in other contexts. Inaccuracies may also exist in our PIV and LDH measurements because their levels can fluctuate under different conditions. The decision to perform elective surgery following nCRT may also be influenced by factors beyond response rates, potentially leading to inconsistencies in management practices. Another important limitation is the heterogeneity between patients who underwent surgery after nCRT and those who received dCRT. These groups differ biologically and prognostically, and although subgroup analyses were performed, residual confounding related to treatment selection could not be fully excluded. Furthermore, the use of a median PIV cutoff, rather than an optimized threshold derived from ROC analysis using the Youden Index or X-tile software, may limit the generalizability of our findings. Although median-based thresholds are widely used in inflammation-based indices, external validation in larger, independent cohorts is needed to establish a more robust and universally applicable cutoff.
Clinical Implications and Future Research
The findings of this study indicate that the PILE score holds significant clinical value, serving not only as a prognostic biomarker before treatment but also as a potential instrument for tailoring personalized treatment plans in ESCC. By combining the PILE score with established prognostic indicators, such as tumor regression grade, pathological lymph node status, and new markers such as circulating tumor DNA, risk stratification can be improved, enhancing the prediction of treatment outcomes. Furthermore, because surgical resection was found to be an independent factor for both OS and PFS, the PILE score might aid in surgical decision-making, especially for patients who are borderline operable or have high systemic inflammation, by pinpointing those who might benefit from prehabilitation or increased monitoring. Nevertheless, as this study was conducted at a single institution using a specific weekly paclitaxel–carboplatin regimen, wider external validation is crucial. Future multicenter prospective studies across various geographic areas and treatment protocols, including different chemoradiotherapy regimens and combinations with immunotherapy, are necessary to verify the general applicability of the PILE score and define its role in modern ESCC management.
5. Conclusions
In summary, the PILE score is a straightforward, accessible, and efficient prognostic tool for forecasting both OS and PFS in patients with locally advanced esophageal squamous cell carcinoma undergoing neoadjuvant chemoradiotherapy. Notably, the prognostic performance of the PILE-based model was stronger for PFS than for OS, as reflected by its superior C-index, indicating better discriminatory ability for early disease progression. Incorporating essential inflammatory and performance metrics offers a thorough evaluation of the patient’s risk. We suggest that these findings be validated in larger multicenter prospective studies to further confirm the significance of the PILE score in guiding risk stratification and personalized treatment plans for patients with ESCC.
Author Contributions
Conceptualization, A.T.; methodology, A.T. and M.E.B.; validation, A.T. and Z.H.; formal analysis, A.T. and A.A.Ç.; investigation, A.T. and M.E.B.; resources, Z.H. and M.E.B.; data curation, A.T. and A.A.Ç.; writing—original draft preparation, A.T.; writing—review and editing, M.E.B. and Z.H.; visualization, M.B. and S.B.T.; supervision, M.B. and S.B.T.; project administration, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Institutional Review Board Statement
The Atatürk University Non-Interventional Clinical Research Ethics Committee approved this study (Approval No: B.30.2.ATA.0.01.00/528, Date: 27 September 2024). All procedures were performed in accordance with the principles of the Declaration of Helsinki.
Informed Consent Statement
The requirement for informed consent was waived because this was a retrospective study.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available because of patient privacy and institutional data protection policies but are available from the corresponding author upon reasonable requests.
Acknowledgments
The authors thank the staff of the Oncology Department, Faculty of Medicine, Atatürk University, for their valuable assistance with data collection and patient management throughout the study period. The authors also acknowledge the contribution of a professional biostatistician in performing statistical analyses. During the preparation of this manuscript, we used ChatGPT (OpenAI GPT-5.1, 2025 release) for grammatical corrections, paraphrasing, and translation support. All AI-assisted outputs were reviewed, edited, and verified by the authors responsible for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript.
| AUC | Area Under the Curve |
| CAD | Coronary Artery Disease |
| C-index | Concordance Index |
| CALLY Index | CRP–Albumin–Lymphocyte Index |
| CI | Confidence Interval |
| COPD | Chronic Obstructive Pulmonary Disease |
| dCRT | Definitive Chemoradiotherapy |
| DM | Diabetes Mellitus |
| ECOG | Eastern Cooperative Oncology Group |
| ESCC | Esophageal Squamous Cell Carcinoma |
| GRIm Score | Gustave Roussy Immune Score |
| Gy | Gray |
| HR | Hazard Ratio |
| HT | Hypertension |
| LDH | Lactate Dehydrogenase |
| nCRT | Neoadjuvant Chemoradiotherapy |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| OS | Overall Survival |
| pCR | Pathological complete response |
| PFS | Progression-Free Survival |
| PILE | Composite inflammation–laboratory evaluation score derived from PIV, LDH, and ECOG |
| PIV | Pan-Immune-Inflammation Value |
| PLR | Platelet-to-Lymphocyte Ratio |
| R0 | Microscopically margin-negative resection |
| ROC | Receiver Operating Characteristic |
| SD | Standard Deviation |
| ULN | Upper Limit of Normal |
References
- Morgan, E.; Soerjomataram, I.; Rumgay, H.; Coleman, H.G.; Thrift, A.P.; Vignat, J.; Laversanne, M.; Ferlay, J.; Arnold, M. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates from Globocan 2020. Gastroenterology 2022, 163, 649–658.e2. [Google Scholar] [CrossRef] [PubMed]
- Santucci, C.; Mignozzi, S.; Malvezzi, M.; Collatuzzo, G.; Levi, F.; La Vecchia, C.; Negri, E. Global Trends in Esophageal Cancer Mortality with Predictions to 2025, and in Incidence by Histotype. Cancer Epidemiol. 2023, 87, 102486. [Google Scholar] [CrossRef] [PubMed]
- Klingelhofer, D.; Zhu, Y.; Braun, M.; Bruggmann, D.; Schoffel, N.; Groneberg, D.A. A World Map of Esophagus Cancer Research: A Critical Accounting. J. Transl. Med. 2019, 17, 150. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Xia, C.; Cao, M.; Yang, F.; Yan, X.; He, S.; Cao, M.; Zhang, S.; Li, Q.; Tan, N.; et al. Esophageal Cancer Global Burden Profiles, Trends, and Contributors. Cancer Biol. Med. 2024, 21, 656–666. [Google Scholar] [CrossRef]
- Li, J.; Xu, J.; Zheng, Y.; Gao, Y.; He, S.; Li, H.; Zou, K.; Li, N.; Tian, J.; Chen, W.; et al. Esophageal Cancer: Epidemiology, Risk Factors and Screening. Chin. J. Cancer Res. 2021, 33, 535–547. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, L.; Xiu, Y.; Zhang, H.; Zhang, Y.; Ying, W. Burden of Esophageal Cancer in Global, Regional and National Regions from 1990 to 2021 and Its Projection until 2050: Results from the Gbd Study 2021. Front. Oncol. 2025, 14, 1518567. [Google Scholar] [CrossRef]
- Christodoulidis, G.; Agko, S.E.; Koumarelas, K.E.; Kouliou, M.N.; Zacharoulis, D. Advancements and Challenges in the Treatment of Esophageal Cancer: A Comprehensive Review. World J. Clin. Oncol. 2024, 15, 1463–1467. [Google Scholar] [CrossRef]
- Hou, S.; Pan, Z.; Hao, X.; Hang, Q.; Ding, Y. Recent Progress in the Neoadjuvant Treatment Strategy for Locally Advanced Esophageal Cancer. Cancers 2021, 13, 5162. [Google Scholar] [CrossRef]
- Lorenz, E.; Weitz, A.; Reinstaller, T.; Hass, P.; Croner, R.S.; Benedix, F. Neoadjuvant Radiochemotherapy with Cisplatin/5-Flourouracil or Carboplatin/Paclitaxel in Patients with Resectable Cancer of the Esophagus and the Gastroesophageal Junction—Comparison of Postoperative Mortality and Complications, Toxicity, and Pathological Tumor Response. Langenbecks Arch. Surg. 2023, 408, 429. [Google Scholar]
- Logarajah, S.; Jeyarajah, P.; Darwish, M.; Moslim, M.; Jureller, M.; Osman, H.; Jeyarajah, D.R. Does Chemotherapy Regimen Matter in the Neoadjuvant Treatment of Esophageal Cancer? J. Gastrointest. Oncol. 2022, 13, 2713–2720. [Google Scholar] [CrossRef]
- Munch, S.; Pigorsch, S.U.; Feith, M.; Slotta-Huspenina, J.; Weichert, W.; Friess, H.; Combs, S.E.; Habermehl, D. Comparison of Neoadjuvant Chemoradiation with Carboplatin/Paclitaxel or Cisplatin/5-Fluoruracil in Patients with Squamous Cell Carcinoma of the Esophagus. Radiat. Oncol. 2017, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- van Meerten, E.; Muller, K.; Tilanus, H.W.; Siersema, P.D.; Eijkenboom, W.M.; van Dekken, H.; Tran, T.C.; van der Gaast, A. Neoadjuvant Concurrent Chemoradiation with Weekly Paclitaxel and Carboplatin for Patients with Oesophageal Cancer: A Phase Ii Study. Br. J. Cancer 2006, 94, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Lee, C.T.; Tsai, Y.N.; Tseng, C.M.; Chen, T.H.; Hsu, M.H.; Wang, C.C.; Wang, W.L. Prognostic Significance of Systemic Inflammatory Response Markers in Patients with Superficial Esophageal Squamous Cell Carcinomas. Sci. Rep. 2022, 12, 18241. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, T.; Dai, Y.; Li, J. Preoperative Systemic Inflammation Score (Sis) Is Superior to Neutrophil to Lymphocyte Ratio (Nlr) as a Predicting Indicator in Patients with Esophageal Squamous Cell Carcinoma. BMC Cancer 2019, 19, 721. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, W.; Cai, S.; Zhang, F.; Shao, F.; Zhang, G.; Liu, T.; Tan, F.; Li, N.; Xue, Q.; et al. Systemic Immune-Inflammation Index (Sii) Is Useful to Predict Survival Outcomes in Patients with Surgically Resected Esophageal Squamous Cell Carcinoma. J. Cancer 2019, 10, 3188–3196. [Google Scholar] [CrossRef]
- Xu, X.; Jing, J. Inflammation-Related Parameter Serve as Prognostic Biomarker in Esophageal Squamous Cell Carcinoma. Front. Oncol. 2022, 12, 900305. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Yang, X.; Chen, Q.; Cheng, X. Pretreatment Pan-Immune-Inflammation Value (Piv) in Predicting Therapeutic Response and Clinical Outcomes of Neoadjuvant Immunochemotherapy for Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2024, 31, 272–283. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, L.; Che, G. Could Pretreatment Pan-Immune-Inflammation Value Predict Survival in Esophageal Cancer? Ann. Surg. Oncol. 2024, 31, 3868–3869. [Google Scholar] [CrossRef]
- Zeng, R.; Liu, F.; Fang, C.; Yang, J.; Luo, L.; Yue, P.; Gao, B.; Dong, Y.; Xiang, Y. Piv and Pile Score at Baseline Predict Clinical Outcome of Anti-Pd-1/Pd-L1 Inhibitor Combined with Chemotherapy in Extensive-Stage Small Cell Lung Cancer Patients. Front. Immunol. 2021, 12, 724443. [Google Scholar] [CrossRef]
- Li, Y.; Wang, K.; Zhao, E.; Li, B.; Li, S.; Dong, X.; Yuan, L.; Yang, H. Prognostic Value of Lactate Dehydrogenase in Second-Line Immunotherapy for Advanced Esophageal Squamous Cell Carcinoma. Pathol. Oncol. Res. 2022, 28, 1610245. [Google Scholar] [CrossRef]
- Liu, C.; Han, J.; Han, D.; Huang, W.; Li, B. A New Risk Score Model Based on Lactate Dehydrogenase for Predicting Prognosis in Esophageal Squamous Cell Carcinoma Treated with Chemoradiotherapy. J. Thorac. Dis. 2023, 15, 2116–2128. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Chen, Z.; Gao, Y.; He, J. Immunohistochemical Prognostic Markers of Esophageal Squamous Cell Carcinoma: A Systematic Review. Chin. J. Cancer 2017, 36, 65. [Google Scholar] [CrossRef] [PubMed]
- Karadag, I.; Karakaya, S.; Yilmaz, M.E.; Oksuzoglu, O.B.C. The Potential Prognostic Novel Markers Piv and Pile Score to Predict Survival Outcomes at Hepatocellular Cancer. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 7679–7686. [Google Scholar] [PubMed]
- Duan, L.; Li, W.; Chen, F. The Candidate Novel Markers Piv and Pile Score to Predict Survival Outcomes and Therapeutic Response in Patients with Primary Central Nervous System Lymphoma. J. Clin. Oncol. 2024, 42, 24. [Google Scholar] [CrossRef]
- Tseng, R.H.; Lai, K.M.; Tsai, C.Y.; Yan, S.L. Elevated Platelet-to-Lymphocyte Ratio and Neutrophil-to-Lymphocyte Ratio after First Cycle of Chemotherapy and Better Survival in Esophageal Cancer Patients Receiving Concurrent Chemoradiotherapy. Curr. Oncol. 2022, 29, 8825–8834. [Google Scholar] [CrossRef]
- Da, L.; Qu, Z.; Zhang, C.; Shen, Y.; Huang, W.; Zhang, Y.; Gu, K. Prognostic Value of Inflammatory Markers and Clinical Features for Survival in Advanced or Metastatic Esophageal Squamous Cell Carcinoma Patients Receiving Anti-Programmed Death 1 Treatment. Front. Oncol. 2023, 13, 1144875. [Google Scholar] [CrossRef]
- Feng, J.F.; Wang, L.; Yang, X.; Chen, S. Gustave Roussy Immune Score (Grim-Score) Is a Prognostic Marker in Patients with Resectable Esophageal Squamous Cell Carcinoma. J. Cancer 2020, 11, 1334–1340. [Google Scholar] [CrossRef]
- Wu, B.; Liu, J.; Shao, C.; Yu, D.; Liao, J. Integrating Inflammation, Nutrition, and Immunity: The Cally Index as a Prognostic Tool in Digestive System Cancers—A Systematic Review and Meta-Analysis. BMC Cancer 2025, 25, 672. [Google Scholar] [CrossRef]
- Guven, D.C.; Sahin, T.K.; Erul, E.; Kilickap, S.; Gambichler, T.; Aksoy, S. The Association between the Pan-Immune-Inflammation Value and Cancer Prognosis: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 2675. [Google Scholar] [CrossRef]
- Di Ceglie, I.; Carnevale, S.; Rigatelli, A.; Grieco, G.; Molisso, P.; Jaillon, S. Immune Cell Networking in Solid Tumors: Focus on Macrophages and Neutrophils. Front. Immunol. 2024, 15, 1341390. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, H.; Bao, Y.; Pang, L.; Yang, C. Neutrophils in Cancer: From Immune Defense to Tumor Promotion. Cancer Biol. Med. 2025, 22, 598–617. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Dong, L.; Cheng, L. Neutrophils in Cancer Carcinogenesis and Metastasis. J. Hematol. Oncol. 2021, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Saxena, S.; Awaji, M.; Singh, R.K. Tumor-Associated Neutrophils in Cancer: Going Pro. Cancers 2019, 11, 564. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Huang, A.; Zeller, J.; Peter, K.; McFadyen, J.D. Decoding the Role of Platelets in Tumour Metastasis: Enigmatic Accomplices and Intricate Targets for Anticancer Treatments. Front. Immunol. 2023, 14, 1256129. [Google Scholar] [CrossRef]
- Garcia-Leon, M.J.; Liboni, C.; Mittelheisser, V.; Bochler, L.; Follain, G.; Mouriaux, C.; Busnelli, I.; Larnicol, A.; Colin, F.; Peralta, M.; et al. Platelets Favor the Outgrowth of Established Metastases. Nat. Commun. 2024, 15, 3297. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, J.; Bao, S.; Zhang, B.; Li, X.; Wang, H.; Cheng, Y.; Zhang, H.; Zu, L.; Xu, X.; et al. Cancer Progression and Tumor Hypercoagulability: A Platelet Perspective. J. Thromb. Thrombolysis 2024, 57, 959–972. [Google Scholar] [CrossRef]
- Obermann, W.M.J.; Brockhaus, K.; Eble, J.A. Platelets, Constant and Cooperative Companions of Sessile and Disseminating Tumor Cells, Crucially Contribute to the Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 674553. [Google Scholar] [CrossRef]
- Schmied, L.; Hoglund, P.; Meinke, S. Platelet-Mediated Protection of Cancer Cells from Immune Surveillance—Possible Implications for Cancer Immunotherapy. Front. Immunol. 2021, 12, 640578. [Google Scholar] [CrossRef]
- Dovizio, M.; Ballerini, P.; Fullone, R.; Tacconelli, S.; Contursi, A.; Patrignani, P. Multifaceted Functions of Platelets in Cancer: From Tumorigenesis to Liquid Biopsy Tool and Drug Delivery System. Int. J. Mol. Sci. 2020, 21, 9585. [Google Scholar] [CrossRef]
- Cai, C.; Liu, Y.; Lu, R.; Fan, X.; Zeng, S.; Gan, P. Platelets in Cancer and Immunotherapy: Functional Dynamics and Therapeutic Opportunities. Exp. Hematol. Oncol. 2025, 14, 83. [Google Scholar] [CrossRef]
- Salmaninejad, A.; Layeghi, S.M.; Falakian, Z.; Golestani, S.; Kobravi, S.; Talebi, S.; Yousefi, M. An Update to Experimental and Clinical Aspects of Tumor-Associated Macrophages in Cancer Development: Hopes and Pitfalls. Clin. Exp. Med. 2024, 24, 156. [Google Scholar] [CrossRef] [PubMed]
- Jahandideh, A.; Yarizadeh, M.; Masjedi, M.N.-K.; Fatehnejad, M.; Jahandideh, R.; Soheili, R.; Eslami, Y.; Zokaei, M.; Ahmadvand, A.; Ghalamkarpour, N.; et al. Macrophage’s Role in Solid Tumors: Two Edges of a Sword. Cancer Cell Int. 2023, 23, 150. [Google Scholar] [CrossRef]
- Cendrowicz, E.; Sas, Z.; Bremer, E.; Rygiel, T.P. The Role of Macrophages in Cancer Development and Therapy. Cancers 2021, 13, 1946. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as Tools and Targets in Cancer Therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Y.; Xia, H.; Chen, Y.H. Monocytes in Tumorigenesis and Tumor Immunotherapy. Cells 2023, 12, 1673. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-Associated Macrophages: An Accomplice in Solid Tumor Progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-Associated Macrophages as Major Players in the Tumor Microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef]
- Nasrollahzadeh, E.; Razi, S.; Keshavarz-Fathi, M.; Mazzone, M.; Rezaei, N. Pro-Tumorigenic Functions of Macrophages at the Primary, Invasive and Metastatic Tumor Site. Cancer Immunol. Immunother. 2020, 69, 1673–1697. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, M.; Zhang, Y.; Ge, S.; Zhong, F.; Xia, G.; Sun, C. Tumor-Associated Macrophages: A Potential Target for Cancer Therapy. Front. Oncol. 2021, 11, 693517. [Google Scholar] [CrossRef]
- Kusmartsev, S.; Serafini, P.; Bharadwaj, S.N.; Kortylewski, M. Editorial: Roles of Tumor-Recruited Myeloid Cells in Immune Evasion in Cancer. Front. Immunol. 2021, 12, 749605. [Google Scholar] [CrossRef]
- Jain, A.; Bobdey, S.; Sathwara, J.; Ganesh, B.; Saoba, S.; Khan, A. Role of Monocyte and Lymphocyte Counts in Prognosis of Cervical Cancer. Int. J. Reprod. Contracept. Obstet. Gynecol. 2016, 5, 2243–2249. [Google Scholar] [CrossRef]
- Soyfer, V.; Lugovoy, E.; Nikolaevski-Berlin, A.; Korzets, Y.; Schlocker, A.; Gutfeld, O.; Ospovat, I.; Amit, U.; Rabin, T.; Natan-Oz, Y.F.; et al. The Effect of Long-Standing Lymphopenia after Radiation Therapy on Survival in Rectal Cancer. Surg. Oncol. 2024, 56, 102119. [Google Scholar] [CrossRef] [PubMed]
- Conroy, M.R.; O’Sullivan, H.; Collins, D.C.; Bambury, R.M.; Power, D.; Grossman, S.; O’Reilly, S. Exploring the Prognostic Impact of Absolute Lymphocyte Count in Patients Treated with Immune-Checkpoint Inhibitors. BJC Rep. 2024, 2, 31. [Google Scholar] [CrossRef] [PubMed]
- Gianni, C.; Palleschi, M.; Schepisi, G.; Casadei, C.; Bleve, S.; Merloni, F.; Sirico, M.; Sarti, S.; Cecconetto, L.; Di Menna, G.; et al. Circulating Inflammatory Cells in Patients with Metastatic Breast Cancer: Implications for Treatment. Front. Oncol. 2022, 12, 882896. [Google Scholar] [CrossRef]
- Claps, G.; Faouzi, S.; Quidville, V.; Chehade, F.; Shen, S.; Vagner, S.; Robert, C. The Multiple Roles of Ldh in Cancer. Nat. Rev. Clin. Oncol. 2022, 19, 749–762. [Google Scholar] [CrossRef]
- Shang, S.; Wang, M.Z.; Xing, Z.; He, N.; Li, S. Lactate Regulators Contribute to Tumor Microenvironment and Predict Prognosis in Lung Adenocarcinoma. Front. Immunol. 2022, 13, 1024925. [Google Scholar] [CrossRef]
- Jang, R.W.; Caraiscos, V.B.; Swami, N.; Banerjee, S.; Mak, E.; Kaya, E.; Rodin, G.; Bryson, J.; Ridley, J.Z.; Le, L.W.; et al. Simple Prognostic Model for Patients with Advanced Cancer Based on Performance Status. J. Oncol. Pr. 2014, 10, e335–e341. [Google Scholar] [CrossRef]
- Simcock, R.; Wright, J. Beyond Performance Status. Clin. Oncol. R. Coll. Radiol. 2020, 32, 553–561. [Google Scholar] [CrossRef]
- Hess, L.M.; Smith, D.; Cui, Z.L.; Montejano, L.; Liepa, A.M.; Schelman, W.; Bowman, L. The Relationship between Eastern Cooperative Oncology Group Performance Status and Healthcare Resource Utilization among Patients with Advanced or Metastatic Colorectal, Lung or Gastric Cancer. J. Drug Assess. 2020, 10, 10–17. [Google Scholar] [CrossRef]
- Kumar, D.; Neeman, E.; Zhu, S.; Sun, H.; Kotak, D.; Liu, R. Revisiting the Association of Ecog Performance Status with Clinical Outcomes in Diverse Patients with Cancer. J. Natl. Compr. Cancer Netw. 2024, 22, e237111. [Google Scholar] [CrossRef]
- Sorensen, J.B.; Klee, M.; Palshof, T.; Hansen, H.H. Performance Status Assessment in Cancer Patients. An Inter-Observer Variability Study. Br. J. Cancer 1993, 67, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Yue, J.; Wang, S.; Zhao, F.; Zhang, W.; Qie, S.; Jiang, H. Prognostic Role of the Pathological Status Following Neoadjuvant Chemoradiotherapy and Surgery in Esophageal Squamous Cell Carcinoma. BMC Cancer 2025, 25, 61. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Tan, L.; Shen, Y.; Wang, H.; Lin, M.; Feng, M.; Xu, S.; Guo, W.; Qian, C.; Liu, T.; et al. Cmisg1701: A Multicenter Prospective Randomized Phase Iii Clinical Trial Comparing Neoadjuvant Chemoradiotherapy to Neoadjuvant Chemotherapy Followed by Minimally Invasive Esophagectomy in Patients with Locally Advanced Resectable Esophageal Squamous Cell Carcinoma (Ct3–4aN0–1M0) (Nct03001596). BMC Cancer 2017, 17, 450. [Google Scholar]
- Chen, C.Y.; Li, C.C.; Chien, C.R. Neoadjuvant Vs Definitive Concurrent Chemoradiotherapy in Locally Advanced Esophageal Squamous Cell Carcinoma Patients. World J. Surg. Oncol. 2018, 16, 141. [Google Scholar] [CrossRef]
- Yang, H.; Liu, H.; Chen, Y.; Zhu, C.; Fang, W.; Yu, Z.; Mao, W.; Xiang, J.; Han, Y.; Chen, Z.; et al. Long-Term Efficacy of Neoadjuvant Chemoradiotherapy Plus Surgery for the Treatment of Locally Advanced Esophageal Squamous Cell Carcinoma: The Neocrtec5010 Randomized Clinical Trial. JAMA Surg. 2021, 156, 721–729. [Google Scholar]
- Eyck, B.M.; van Lanschot, J.J.B.; Hulshof, M.C.C.M.; van der Wilk, B.J.; Shapiro, J.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; et al. Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled Cross Trial. J. Clin. Oncol. 2021, 39, 1995–2004. [Google Scholar] [CrossRef]
- Toyokawa, T.; Tamura, T.; Sakurai, K.; Kubo, N.; Tanaka, H.; Muguruma, K.; Yashiro, M.; Ohira, M. Postoperative Inflammation Is an Independent Prognostic Factor in Patients with Thoracic Esophageal Squamous Cell Carcinoma. Anticancer. Res. 2019, 39, 2777–2784. [Google Scholar] [CrossRef]
- Booka, E.; Kikuchi, H.; Hiramatsu, Y.; Takeuchi, H. The Impact of Infectious Complications after Esophagectomy for Esophageal Cancer on Cancer Prognosis and Treatment Strategy. J. Clin. Med. 2021, 10, 4614. [Google Scholar] [CrossRef]
- Aoyama, T.; Ju, M.; Komori, K.; Tamagawa, H.; Tamagawa, A.; Maezawa, Y.; Hashimoto, I.; Kano, K.; Hara, K.; Cho, H.; et al. The Systemic Inflammation Score Is an Independent Prognostic Factor for Esophageal Cancer Patients Who Receive Curative Treatment. Anticancer. Res. 2022, 42, 2711–2717. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Y.; Lei, M.; He, H.; Zhang, D.; Lin, J.; Lin, H.; Wei, W.; Chen, P.; Zhuang, F.; et al. Comparison of Neoadjuvant Chemoimmunotherapy and Neoadjuvant Chemotherapy for Resectable Esophageal Squamous Cell Carcinoma: A Retrospective Study with 3-Year Survival Analysis. J. Cancer Res. Clin. Oncol. 2024, 150, 477. [Google Scholar] [CrossRef]
- Li, K.; Hao, W.; Liu, X.; Li, Y.; Sun, H.; Liu, S.; Xing, W.; Zheng, Y. The Role of Adjuvant Chemotherapy in the Treatment of Esophageal Squamous Cell Carcinoma after Neoadjuvant Chemotherapy. J. Cancer 2023, 14, 3130–3138. [Google Scholar] [CrossRef]
- Fan, L.; Yang, Z.; Chang, M.; Chen, Z.; Wen, Q. Ct-Based Delta-Radiomics Nomogram to Predict Pathological Complete Response after Neoadjuvant Chemoradiotherapy in Esophageal Squamous Cell Carcinoma Patients. J. Transl. Med. 2024, 22, 579. [Google Scholar] [CrossRef]
- Li, N.; Liu, Y.; Zhou, J.; Li, X.; Lian, S.; Yang, Y. Comprehensive Risk Score of the E-Pass Scoring System Serves a Prognostic Indicator for Patients after Neoadjuvant Therapy and Curative Esophageal Cancer Surgery: A Multicenter Retrospective Study. Front. Oncol. 2025, 15, 1617683. [Google Scholar] [CrossRef]
- Li, C.; Lin, J.W.; Yeh, H.L.; Chuang, C.Y.; Chen, C.C. Good Prediction of Treatment Responses to Neoadjuvant Chemoradiotherapy for Esophageal Cancer Based on Preoperative Inflammatory Status and Tumor Glucose Metabolism. Sci. Rep. 2021, 11, 11626. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).