Identification of Risk Factors in Patients with Recurrent Cystitis May Improve Individualized Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Design and Definitions
2.3. Study Population, History and Physical Examination
2.4. Urinalyses and Urine Culture
2.5. Study Rationale
2.6. Statistical Evaluation
3. Results
3.1. Clinical Assessment
3.1.1. Demographics
3.1.2. Symptoms
3.1.3. History and Risk Factors
3.2. Urinalysis and Microbiological Findings
3.2.1. Urinalysis
3.2.2. Urine Culture
3.3. Statistical Analysis
3.3.1. Weighting of Risk Factors Based on Relative Risk (RR)
3.3.2. Evaluation of Predicting Factors for Recurrent Cystitis Based on Odds Ratio (OR)
3.3.3. Performance and Validity of Predictive Models Based on Multivariate Logistic Regression
4. Discussion
4.1. Main Findings
4.2. Methodological Aspects
4.3. Context and Clinical Impact
4.4. Strengths and Limitations
4.5. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Acute (sporadic) cystitis |
| ACSS | Acute Cystitis Symptom Score |
| AI | Artificial intelligence |
| AUC | Area under the (receiver-operating characteristic) curve |
| BMI | Body mass index |
| CFU | Colony-forming units |
| CI | Confidence interval |
| eCRF | Electronic case report form |
| EAU | European Association of Urology |
| EQ-5D-3L | EuroQoL 5-Dimensions, 3-level version of the questionnaire |
| ESIU | EAU Section of Infections in Urology |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| GPIU.COM | Global Prevalence Study of Infections in Urinary tract in Community Setting |
| IQR | Interquartile range |
| LR | Logistic regression |
| LUTI | Lower urinary tract infection(s) |
| LUTIRE | Lower Urinary Tract Infection Recurrence Risk (nomogram) |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| OR | Odds ratio |
| ORENUC | Classification system of UTI risk factors |
| QoL | Quality of life |
| RC | Recurrent cystitis |
| RR | Relative risk |
| ROC | Receiver operating characteristic |
| SRF | Study report form |
| UTI(s) | Urinary tract infection(s) |
| VIF | Variance inflation factor |
| WBC | White blood cells |
References
- Bonkat, G.; Kranz, J.; Cai, T.; Greelings, S.E.; Köves, B.; Pilatz, A.; Medina-Polo, J.; Schneidewind, L.; Schubert, S.; Veeratterapillay, R.; et al. EAU Guidelines on Urological Infections. In EAU Guidelines. Edn., Proceedings of the EAU Annual Congress, Madrid, Spain, 21–24 March 2025; EAU Guidelines Office: Arnhem, The Netherlands, 2025. [Google Scholar]
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am. J. Med. 2002, 113 (Suppl. 1A), 5S–13S. [Google Scholar] [CrossRef]
- Ackerman, A.L.; Bradley, M.; D’Anci, K.E.; Hickling, D.; Kim, S.K.; Kirkby, E. Updates to Recurrent Uncomplicated Urinary Tract Infections in Women: AUA/CUA/SUFU Guideline (2025). J. Urol. 2025, 101097ju0000000000004723. [Google Scholar] [CrossRef]
- Ku, J.H.; Tartof, S.Y.; Contreras, R.; Ackerson, B.K.; Chen, L.H.; Reyes, I.A.C.; Pellegrini, M.; Schmidt, J.E.; Bruxvoort, K.J. Antibiotic Resistance of Urinary Tract Infection Recurrences in a Large Integrated US Healthcare System. J. Infect. Dis. 2024, 230, e1344–e1354. [Google Scholar] [CrossRef]
- Midby, J.S.; Miesner, A.R. Delayed and Non-Antibiotic Therapy for Urinary Tract Infections: A Literature Review. J. Pharm. Pract. Res. 2024, 37, 212–224. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.; Abramov-Sommariva, D.; Höller, M.; Steindl, H.; Naber, K.G. Non-Antibiotic Herbal Therapy (BNO 1045) versus Antibiotic Therapy (Fosfomycin Trometamol) for the Treatment of Acute Lower Uncomplicated Urinary Tract Infections in Women: A Double-Blind, Parallel-Group, Randomized, Multicentre, Non-Inferiority Phase III Trial. Urol. Int. 2018, 101, 327–336. [Google Scholar] [PubMed]
- Naber, K.G.; Alidjanov, J.F.; Fünfstück, R.; Strohmaier, W.L.; Kranz, J.; Cai, T.; Pilatz, A.; Wagenlehner, F.M. Therapeutic strategies for uncomplicated cystitis in women. GMS Infect. Dis. 2024, 12, Doc01. [Google Scholar] [PubMed]
- Naber, K.G.; Alidjanov, J.F. Are there alternatives to antimicrobial therapy and prophylaxis of uncomplicated urinary tract infections? Urologiia 2014, 5–8, 10–13. [Google Scholar]
- Hayward, G.; Mort, S.; Hay, A.D.; Moore, M.; Thomas, N.P.B.; Cook, J.; Robinson, J.; Williams, N.; Maeder, N.; Edeson, R.; et al. d-Mannose for Prevention of Recurrent Urinary Tract Infection Among Women: A Randomized Clinical Trial. JAMA Intern. Med. 2024, 184, 619–628. [Google Scholar]
- Foxman, B.; Gillespie, B.; Koopman, J.; Zhang, L.; Palin, K.; Tallman, P.; Marsh, J.V.; Spear, S.; Sobel, J.D.; Marty, M.J.; et al. Risk factors for second urinary tract infection among college women. Am. J. Epidemiol. 2000, 151, 1194–1205. [Google Scholar]
- Cai, T.; Mazzoli, S.; Migno, S.; Malossini, G.; Lanzafame, P.; Mereu, L.; Tateo, S.; Wagenlehner, F.M.; Pickard, R.S.; Bartoletti, R. Development and validation of a nomogram predicting recurrence risk in women with symptomatic urinary tract infection. Int. J. Urol. 2014, 21, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Johansen, T.E.; Botto, H.; Cek, M.; Grabe, M.; Tenke, P.; Wagenlehner, F.M.; Naber, K.G. Critical review of current definitions of urinary tract infections and proposal of an EAU/ESIU classification system. Int. J. Antimicrob. Agents 2011, 38, 64–70. [Google Scholar] [CrossRef]
- Ackerson, B.K.; Tartof, S.Y.; Chen, L.H.; Contreras, R.; Reyes, I.A.C.; Ku, J.H.; Pellegrini, M.; Schmidt, J.E.; Bruxvoort, K.J. Risk Factors for Recurrent Urinary Tract Infections Among Women in a Large Integrated Health Care Organization in the United States. J. Infect. Dis. 2024, 230, e1101–e1111. [Google Scholar] [CrossRef]
- Soric Hosman, I.; Cvitkovic Roic, A.; Lamot, L. A Systematic Review of the (Un)known Host Immune Response Biomarkers for Predicting Recurrence of Urinary Tract Infection. Front. Med. 2022, 9, 931717. [Google Scholar] [CrossRef] [PubMed]
- Calin, R.; Hafner, J.; Ingersoll, M.A. Immunity to urinary tract infection: What the clinician should know. CMI Commun. 2024, 1, 105057. [Google Scholar] [CrossRef]
- Godaly, G.; Ambite, I.; Svanborg, C. Innate immunity and genetic determinants of urinary tract infection susceptibility. Curr. Opin. Infect. Dis. 2015, 28, 88–96. [Google Scholar] [CrossRef]
- Hou, Y.; Lv, Z.; Hu, Q.; Zhu, A.; Niu, H. The immune mechanisms of the urinary tract against infections. Front. Cell. Infect. Microbiol. 2025, 15, 1540149. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and Council of the European Union. Directive 2001/20/EC of 4 April 2001 on the Approximation of the Laws, Regulations and Administrative Provisions of the Member States Relating to the Implementation of Good Clinical Practice in the Conduct of Clinical Trials on Medicinal Products for Human Use; European Parliament and Council of the European Union: Strasbourg, France, 2001. [Google Scholar]
- Alidjanov, J.F.; Khudaybergenov, U.A.; Ayubov, B.A.; Pilatz, A.; Mohr, S.; Munst, J.C.; Ziviello Yuen, O.N.; Pilatz, S.; Christmann, C.; Dittmar, F.; et al. Linguistic and clinical validation of the acute cystitis symptom score in German-speaking Swiss women with acute cystitis. Int. Urogynecol. J. 2021, 32, 3275–3286. [Google Scholar] [CrossRef]
- Alidjanov, J.F.; Pilatz, A.; Abdufattaev, U.A.; Wiltink, J.; Weidner, W.; Naber, K.G.; Wagenlehner, F. German validation of the Acute Cystitis Symptom Score. Urologe A 2015, 54, 1269–1276. [Google Scholar] [CrossRef]
- Rabin, R.; de Charro, F. EQ-5D: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef]
- Tzimenatos, L.; Mahajan, P.; Dayan, P.S.; Vitale, M.; Linakis, J.G.; Blumberg, S.; Borgialli, D.; Ruddy, R.M.; Van Buren, J.; Ramilo, O.; et al. Accuracy of the Urinalysis for Urinary Tract Infections in Febrile Infants 60 Days and Younger. Pediatrics 2018, 141, e20173068. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST), Expert Rules and Expected Phenotypes. 2019. Available online: https://www.eucast.org/expert_rules_and_expected_phenotypes (accessed on 8 May 2023).
- Pourhoseingholi, M.A.; Vahedi, M.; Rahimzadeh, M. Sample size calculation in medical studies. Gastroenterol. Hepatol. Bed Bench 2013, 6, 14–17. [Google Scholar] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Levene, H. In Contributions to Probability and Statistics; Essays in Honor of Harold Hotelling; Olkin, I., Hotelling, H., et al., Eds.; Robust tests for equality of variances. Stanford University Press: Stanford, CA, USA, 1960; pp. 278–292. [Google Scholar]
- Student, W.S.G. The Probable Error of a Mean. Biometrika 1908, 6, 1–25. [Google Scholar] [CrossRef]
- Welch, B.L. The generalisation of Student’s problems when several different population variances are involved. Biometrika 1947, 34, 28–35. [Google Scholar] [PubMed]
- Wilcoxon, F. Individual Comparisons by Ranking Methods. Biom. Bull. 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Fisher, R.A. On the Interpretation of χ2 from Contingency Tables, and the Calculation of P. J. R. Stat. Soc. 1922, 85, 87–94. [Google Scholar] [CrossRef]
- Pearson, K. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1900, 50, 157–175. [Google Scholar] [CrossRef]
- Fisher, R.A. The Correlation between Relatives on the Supposition of Mendelian Inheritance. Trans. R. Soc. Edinb. 1919, 52, 399–433. [Google Scholar] [CrossRef]
- Pearson, K. Note on Regression and Inheritance in the Case of Two Parents. Proc. R. Soc. Lond. 1895, 58, 240–242. [Google Scholar] [CrossRef]
- Weber, F.; Knapp, G.; Ickstadt, K.; Kundt, G.; Glass, Ä. Zero-cell corrections in random-effects meta-analyses. Res. Synth. Methods 2020, 11, 913–919. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, A.G.; Bruins, H.M.; Carrion, A.; Cathomas, R.; Compérat, E.M.; Dimitropoulos, K.; Efstathiou, J.A.; Fietkau, R.; Lorch, A.; Mariappan, P.; et al. EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. In EAU Guidelines. Edn., Proceedings of the EAU Annual Congress, Madrid, Spain, 21–24 March 2025; EAU Guidelines Office: Arnhem, The Netherlands, 2025. [Google Scholar]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. Resampling Methods. In An Introduction to Statistical Learning: With Applications in R, Springer Texts in Statistics; Springer: New York, NY, USA, 2013; pp. 175–197. [Google Scholar]
- Alidjanov, J.F.; Naber, K.G.; Pilatz, A.; Wagenlehner, F.M. Validation of the American English Acute Cystitis Symptom Score. Antibiotics 2020, 9, 929. [Google Scholar] [CrossRef] [PubMed]
- Radzhabov, A.; Zamuddinov, M.; Alidjanov, J.F.; Pilatz, A.; Wagenlehner, F.M.; Naber, K.G. Linguistic and Clinical Validation of the Tajik Acute Cystitis Symptom Score for Diagnosis and Patient-Reported Outcome in Acute Uncomplicated Cystitis. Medicina 2023, 59, 1549. [Google Scholar] [CrossRef] [PubMed]
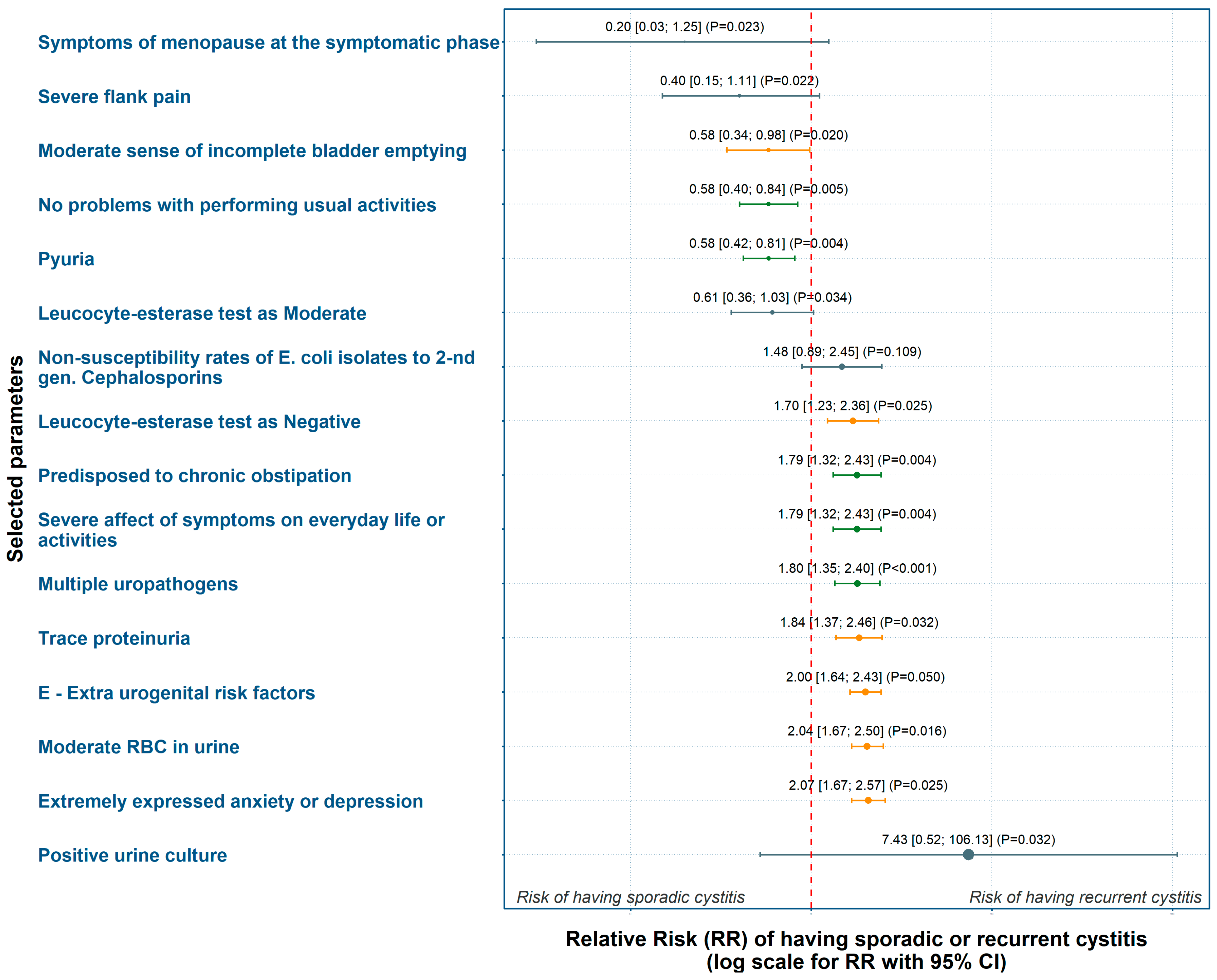
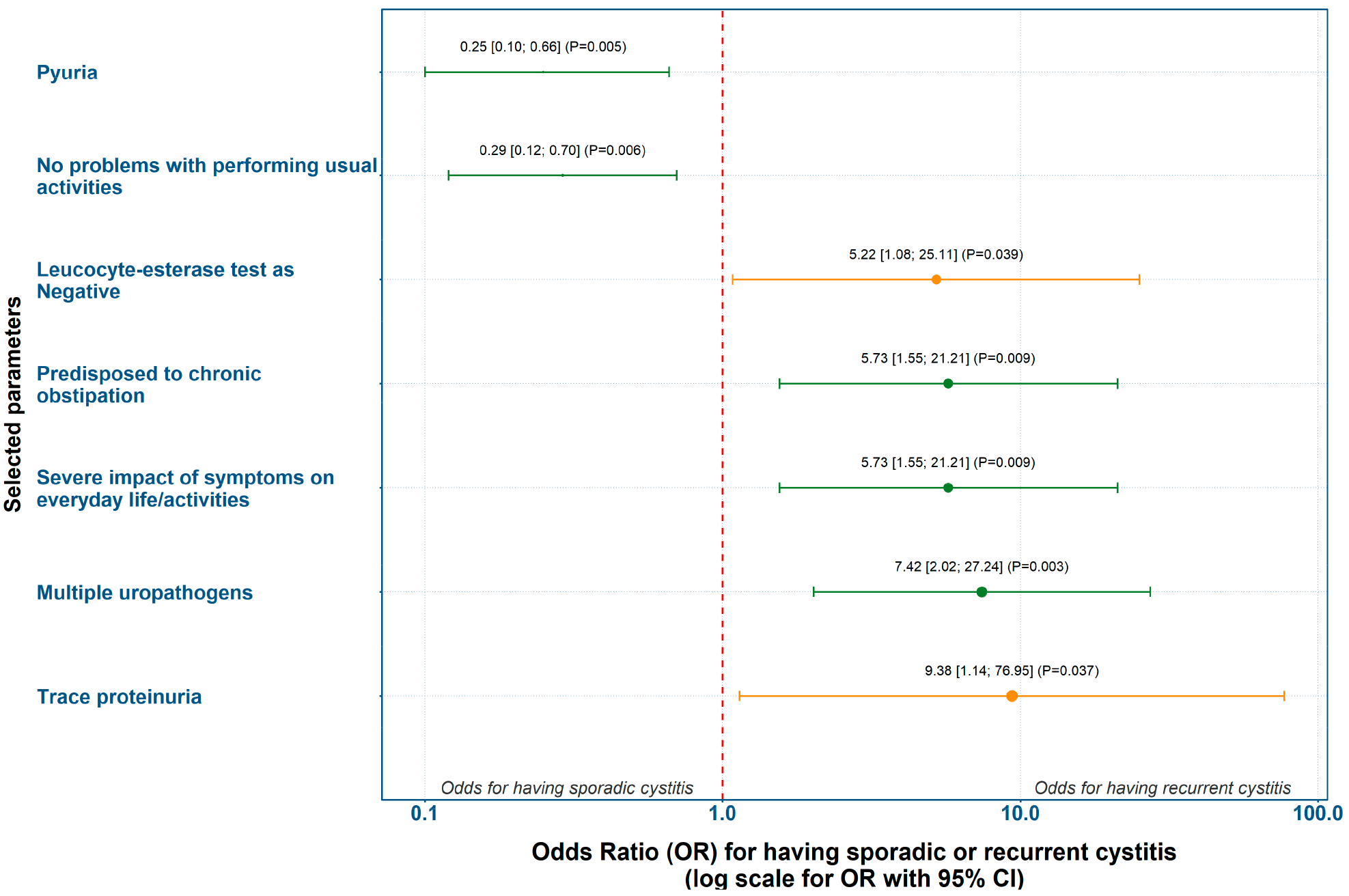
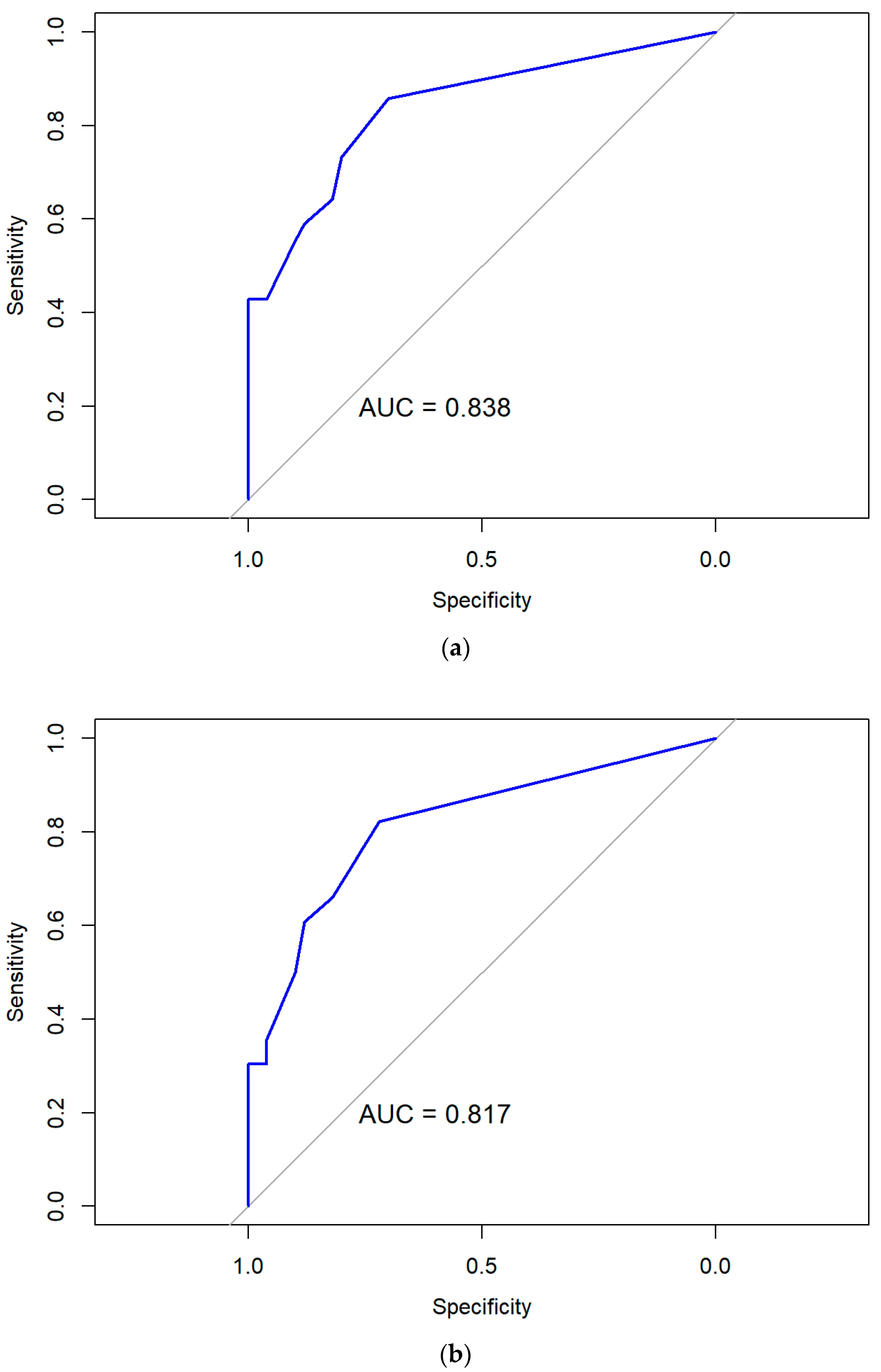
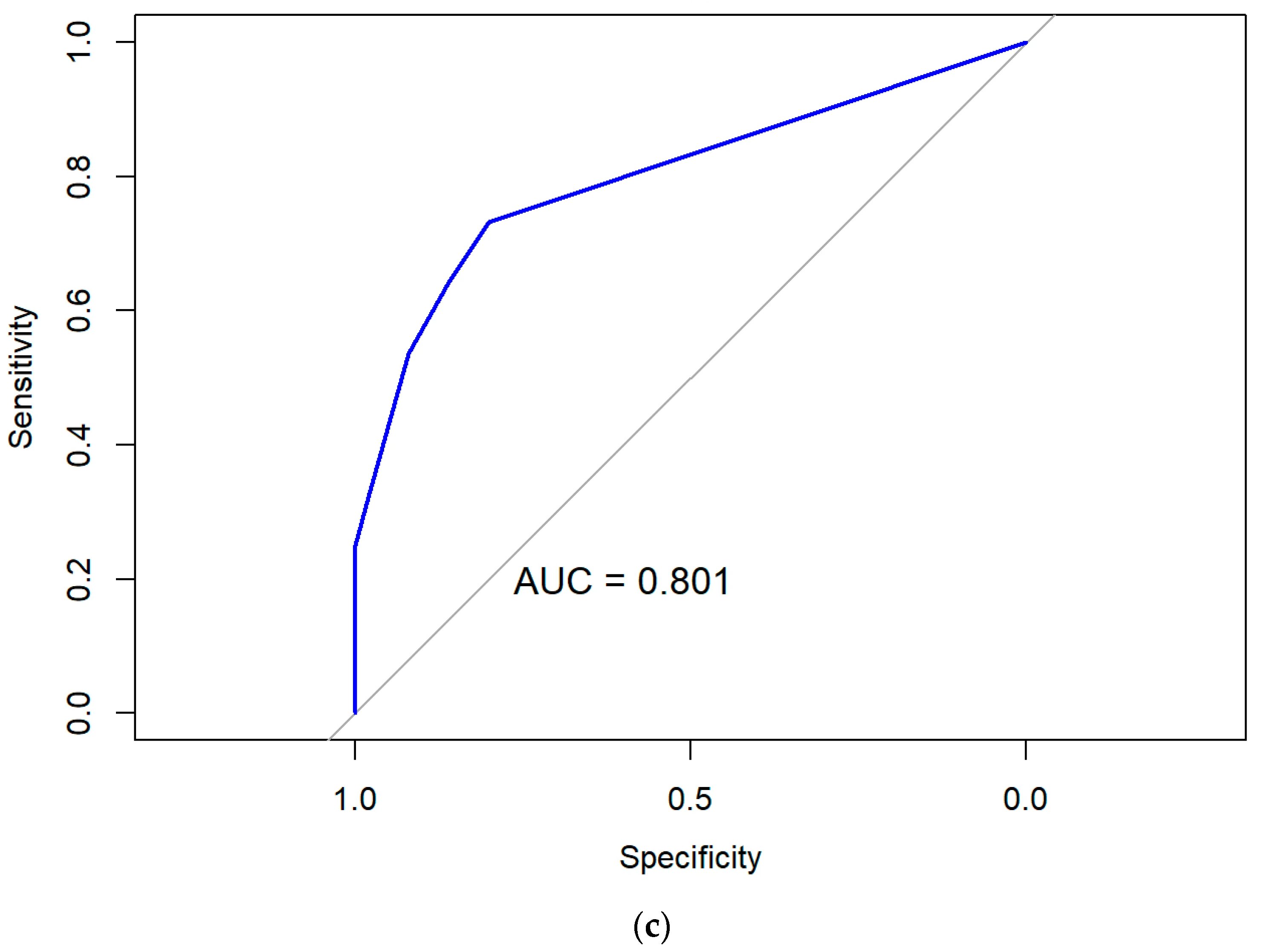
| Step | Statistical Method | Purpose | Input Variables | Outcome/Value |
|---|---|---|---|---|
| 1 | Distribution and variance testing | Assess normality and homogeneity of variance | All continuous variables | Suitability of variables for parametric vs. non-parametric tests |
| 2 | Descriptive statistics | Characterize the study population and variables | Demographics, symptoms, lab data, ACSS, ORENUC, LUTIRE | Central tendency and distribution |
| 3 | Between-group comparative analysis | Identify differences between AC and RC | Each candidate variable | Variables with p < 0.05 are selected for the next step |
| 4 | Correlation and redundancy analysis, manual exclusion | Remove redundant or collinear predictors | Significant variables from Step 3 | Reduced set of potential predictors |
| 5 | Association measures | Assess direction and magnitude of association | Selected set of potential predictors | Relative risk (RR) with 95% CI |
| 6 | Logistic regression (binomial, stepwise) | Build the final predictive model | Independent predictors from Steps 4 and 5 | Odds ratios with 95% CI, model equation |
| 7 | Assessment of model performance and internal validation | Evaluate discrimination, calibration, and multicollinearity; verify stability of predictive performance | Final model output | AUC > 0.8, p > 0.05 (Hosmer–Lemeshow), VIF < 5, bootstrap resampling |
| Demographic Characteristics | Total | Sporadic Acute Cystitis (AC) | Recurrent Cystitis (RC) | p-Value (Significance) |
|---|---|---|---|---|
| Number of patients, n (%) | 106 (100.0) | 50 (47.2) | 56 (52.8) | n.a. |
| Age, median (IQR) | 36.5 (26.0–58.5) | 36.0 (27.2–51.0) | 37.5 (24.0–66.5) | 0.219 (ns) * |
| Weight in kg, median (IQR) | 66.0 (57.0–74.8) | 63.0 (56.0–70.0) | 67.5 (60.0–75.0) | 0.191 (ns) * |
| Height in m, median (IQR) | 1.6 (1.6–1.7) | 1.6 (1.6–1.7) | 1.7 (1.6–1.7) | 0.054 (ns) * |
| Body mass index, median (IQR) | 23.4 (21.3–27.5) | 23.0 (21.3–27.2) | 23.4 (21.4–27.7) | 0.477 (ns) * |
| Pregnancy, n (%) | 2 (1.9) | 1 (2.0) | 1 (1.8) | 1.000 (ns) ‡ |
| Data from the Medical History | Total | Sporadic Acute Cystitis (AC, n = 50) | Recurrent Cystitis (RC, n = 56) | p-Value (Significance) | |
|---|---|---|---|---|---|
| Anamnestic Data | At least one prior symptomatic episode of UTIs in the past 6 months, n (%) | 38 (35.8) | 7 (14.0) | 31 (55.4) | <0.001 (****) † |
| At least one prior symptomatic episode of UTIs in the past 12 months, n (%) | 64 (60.4) | 8 (16.0) | 56 (100.0) | <0.001 (****) † | |
| Number of prior symptomatic episodes UTIs in the past 6 months, median (IQR) | 2.0 (1.0–5.0) | 1.0 (0.0–1.0) | 3.0 (2.0–5.0) | <0.001 (****) ‡ | |
| Number of prior symptomatic episodes UTIs in the past 12 months, median (IQR) | 3.0 (0.0–12.0) | 0.0 (0.0–0.0) | 11.0 (5.0–40.0) | <0.001 (****) ‡ | |
| No prophylactic measure in the past 12 months, n (%) | 55 (51.9) | 37 (74.0) | 18 (32.1) | <0.001 (****) † | |
| Multiple prophylactic measures in the past 12 months, n (%) | 43 (40.6) | 10 (20.0) | 33 (58.9) | <0.001 (***) † | |
| ACSS | Symptoms of menopause, n (%) | 9 (8.5) | 8 (16.0) | 1 (1.8) | 0.012 (*) ‡ |
| Moderate sense of incomplete bladder emptying, n (%) | 29 (27.4) | 19 (38.0) | 10 (17.9) | 0.035 (*) † | |
| Severe flank pain, n (%) | 13 (12.3) | 10 (20.0) | 3 (5.4) | 0.035 (*) ‡ | |
| Severe impact of UTI symptoms on everyday life/activities, (n%) | 18 (17.0) | 3 (6.0) | 15 (26.8) | 0.005 (**) ‡ | |
| EQ-5D-3L | No problems with performing usual activities, n (%) | 59 (55.7) | 35 (70.0) | 24 (42.9) | 0.009 (**) † |
| Extremely anxious or depressed, n (%) | 7 (6.6) | 0 (0.0) | 7 (12.5) | 0.014 (*) ‡ | |
| LUTIRE | Predisposed to chronic constipation, according to the LUTIRE nomogram, n (%) | 18 (17.0) | 3 (6.0) | 15 (26.8) | 0.005 (**) ‡ |
| Known Gram-negative uropathogen isolated at the last acute episode, according to the LUTIRE nomogram, n (%) | 25 (23.6) | 4 (8.0) | 21 (37.5) | <0.001 (***) ‡ | |
| No known uropathogen in the past, according to the LUTIRE nomogram, n (%) | 73 (68.9) | 44 (88.0) | 29 (51.8) | <0.001 (***) † | |
| Up to 2 acute episodes per year, according to the LUTIRE nomogram, n (%) | 52 (49.1) | 50 (100.0) | 2 (3.6) | <0.001 (****) ‡ | |
| Three or more acute episodes per year, according to the LUTIRE nomogram, n (%) | 54 (50.9) | 0 (0.0) | 54 (96.4) | <0.001 (****) ‡ | |
| Probability of recurrence according to the LUTIRE nomogram, median (IQR) | 0.30 (0.20–0.40) | 0.20 (0.20–0.30) | 0.40 (0.30–0.50) | <0.001 (****) • | |
| ORENUC | O—No known risk factor, n (%) | 56 (52.8) | 37 (74.0) | 19 (33.9) | <0.001 (****) † |
| R—Risk factors for recurrent UTIs, but no risk of more severe outcome, n (%) | 32 (30.2) | 9 (18.0) | 23 (41.1) | 0.018 (*) † | |
| E—Extra-urogenital risk factors with risk of more severe outcome, n (%) | 6 (5.7) | 0 (0.0) | 6 (10.7) | 0.028 (*) | |
| Results of Urine Tests at Baseline Visit | Total | Sporadic Acute Cystitis (AC, n = 50) | Recurrent Cystitis (RC, n = 56) | p-Value (Significance) |
|---|---|---|---|---|
| Negative leucocyte esterase test, n (%) | 12 (11.3) | 2 (4.0) | 10 (17.9) | 0.032 (*) ‡ |
| Moderate leucocyte esterase test, n (%) | 28 (26.4) | 18 (36.0) | 10 (17.9) | 0.001 (**) † |
| Pyuria *, n (%) | 77 (72.6) | 43 (86.0) | 34 (60.7) | 0.007 (*) † |
| Moderate erythrocyturia, n (%) | 8 (7.5) | 0 (0.0) | 8 (14.3) | 0.006 (**) ‡ |
| Trace proteinuria, n (%) | 10 (9.4) | 1 (2.0) | 9 (16.1) | 0.018 (*) ‡ |
| Positive urine culture (CFU ≥ 103/mL), n (%) | 87 (82.1) | 33 (66.0) | 54 (96.4) | <0.001 (***) † |
| Multiple uropathogens, n (%) | 24 (22.6) | 3 (6.0) | 21 (37.5) | <0.001 (***) ‡ |
| Non-susceptibility rates of E. coli isolates to 2-nd gen. Cephalosporins, n (%) | 28 (28.9) | 9 (17.6) | 19 (41.3) | 0.019 (*) † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alidjanov, J.F.; Khudaybergenov, U.A.; Khudayberdiev, K.B.; Kranz, J.; Schneidewind, L.; Stangl, F.P.; Medina-Polo, J.; Pilatz, A.; Cai, T.; Naber, K.G.; et al. Identification of Risk Factors in Patients with Recurrent Cystitis May Improve Individualized Management. Diagnostics 2025, 15, 2885. https://doi.org/10.3390/diagnostics15222885
Alidjanov JF, Khudaybergenov UA, Khudayberdiev KB, Kranz J, Schneidewind L, Stangl FP, Medina-Polo J, Pilatz A, Cai T, Naber KG, et al. Identification of Risk Factors in Patients with Recurrent Cystitis May Improve Individualized Management. Diagnostics. 2025; 15(22):2885. https://doi.org/10.3390/diagnostics15222885
Chicago/Turabian StyleAlidjanov, Jakhongir F., Ulugbek A. Khudaybergenov, Khurshid B. Khudayberdiev, Jennifer Kranz, Laila Schneidewind, Fabian P. Stangl, José Medina-Polo, Adrian Pilatz, Tommaso Cai, Kurt G. Naber, and et al. 2025. "Identification of Risk Factors in Patients with Recurrent Cystitis May Improve Individualized Management" Diagnostics 15, no. 22: 2885. https://doi.org/10.3390/diagnostics15222885
APA StyleAlidjanov, J. F., Khudaybergenov, U. A., Khudayberdiev, K. B., Kranz, J., Schneidewind, L., Stangl, F. P., Medina-Polo, J., Pilatz, A., Cai, T., Naber, K. G., Wagenlehner, F. M., & Bjerklund Johansen, T. E., on behalf of the ESIU. (2025). Identification of Risk Factors in Patients with Recurrent Cystitis May Improve Individualized Management. Diagnostics, 15(22), 2885. https://doi.org/10.3390/diagnostics15222885







