Abstract
Background: The purpose of this study was to examine the relationship between qualitative characteristics and quantitative parameters from contrast-enhanced ultrasound (CEUS) and microvessel density (MVD) in hepatoblastoma (HB), as well as to investigate whether CEUS could be utilized as a non-invasive method for predicting HB progression. Methods: This retrospective analysis was carried out in one medical center and included 34 children with histopathologically confirmed HB. Both grayscale ultrasound and CEUS results were reviewed. Lesions were evaluated using time–intensity curve (TIC) analysis software to extract quantitative parameters. Postoperative tissue specimens were stained with CD34 immunohistochemistry, and MVD was quantified as the reference standard. Statistical analyses were conducted to assess the correlation between CEUS findings and MVD. Results: Lesions were separated into high (n = 21, 61.76%; MVD ≥ 41) and low (n = 13, 38.24%; MVD < 41) MVD groups, using the median microvessel density of 41 vessels per high-power field (HPF) as the cutoff. High MVD lesions exhibited a significantly higher incidence of penetrating vessels compared with low MVD lesions (p < 0.05). Elevated MVD levels were significantly associated with increased Adler-grade blood flow (p < 0.05). Both TIC-derived and relative quantitative parameters exhibited significant intergroup differences. Among the relative parameters, the relative wash-out rate (rWoR) was significantly higher in the low MVD group (p < 0.05). Moreover, the Receiver Operating Characteristic (ROC) curve analysis indicated that an rWoR threshold of ≥1.36 could serve as a predictor for low MVD, resulting in 76.9% sensitivity and 81.0% specificity (AUC = 0.802; 95% CI: 0.634–0.970; p = 0.003). Conclusions: CEUS revealed an association with MVD, supporting its potential as a non-invasive tool to characterize tumor vascularity.
1. Introduction
Hepatoblastoma (HB) is recognized as the predominant malignant solid liver tumor in pediatric patients [1], with vascular-dependent characteristics. Tumor angiogenesis is key to sustaining cancer cell survival, driving progression, and promoting metastasis.
Microvessel density (MVD), a widely used histopathological marker of angiogenesis, reflects the degree of tumor microvascular proliferation [2]. In various solid tumors, high MVD has been consistently linked to aggressive biological behavior, therapeutic resistance, and unfavorable survival outcomes [3]. In HB, angiogenesis enhances tumor vascularization, thereby promoting rapid tumor growth, aggressiveness, and metastatic potential [4,5]. Quantitative analyses have further shown that stratification by the median MVD can identify prognostic groups, with higher MVD associated with significantly poorer overall survival [6]. Anti-angiogenic therapy has also been considered a promising strategy, particularly in combination with individualized chemotherapy [7].Therefore, these findings suggest that MVD may serve as a clinically relevant biomarker for prognostic assessment in pediatric HB.
However, MVD assessment relies on histological specimens, which are invasive, subject to sampling error, unable to fully capture intratumoral heterogeneity, and unsuitable for longitudinal monitoring in children. These limitations highlight the need for reproducible, non-invasive approaches to evaluate tumor vascularity. Contrast-enhanced ultrasound (CEUS) with microbubble agents, such as SonoVue, enables real-time visualization of hepatic microcirculation [8]. In 2016, the U.S. Food and Drug Administration (FDA) approved SonoVue for pediatric liver imaging, providing both theoretical and technical support for its wider clinical application in children, including those with HB [9,10,11]. In adult solid tumors, quantitative CEUS parameters derived from time–intensity curve (TIC) analysis have shown significant correlations with histological MVD [12,13].
To date, no study has examined the association between CEUS parameters and MVD in pediatric HB. The present study therefore aimed to explore this relationship and to evaluate whether CEUS could serve as a non-invasive surrogate for histological vascular assessment in this population, in line with the increasing adoption of CEUS for pediatric focal liver lesions as supported by international guidelines [14].
2. Materials and Methods
2.1. Study Design
This study was conducted as a retrospective exploratory pilot investigation. Given the rarity of pediatric HB and the absence of prior work directly correlating CEUS parameters with histological MVD, the cohort size was determined by the availability of eligible patients during the study period. A post hoc power analysis based on the observed AUC of 0.802 for rWoR indicated that a sample size of 34 provides approximately 80% power at an alpha of 0.05, supporting the findings as sufficiently powered for this pilot investigation.
2.2. General Information
The Ethics Committee of Chongqing Children’s Hospital, Chongqing Medical University, approved this study (Approval No. 34/2024 Ethics Committee [Research]) in accordance with the Declaration of Helsinki issued by the World Medical Association. The children’ parents or legal guardians provided informed consent. Data from HB patients who underwent CEUS prior to surgery at our institution during the period of May 2019 to December 2024 were retrospectively analyzed in this study.
Inclusion criteria were as follows: (i) age < 18 years; (ii) histologically confirmed HB; (iii) both conventional ultrasound and CEUS performed before surgery; (iv) availability of complete resection specimens for immunohistochemical staining and MVD quantification. Exclusion criteria were as follows: (i) incomplete clinical data; (ii) severe motion artifacts on CEUS that, despite software correction, prevented generation of complete TICs and calculation of quantitative parameters; (iii) patients with only biopsy specimens available; (iv) cases with failed CD34 immunohistochemical staining.
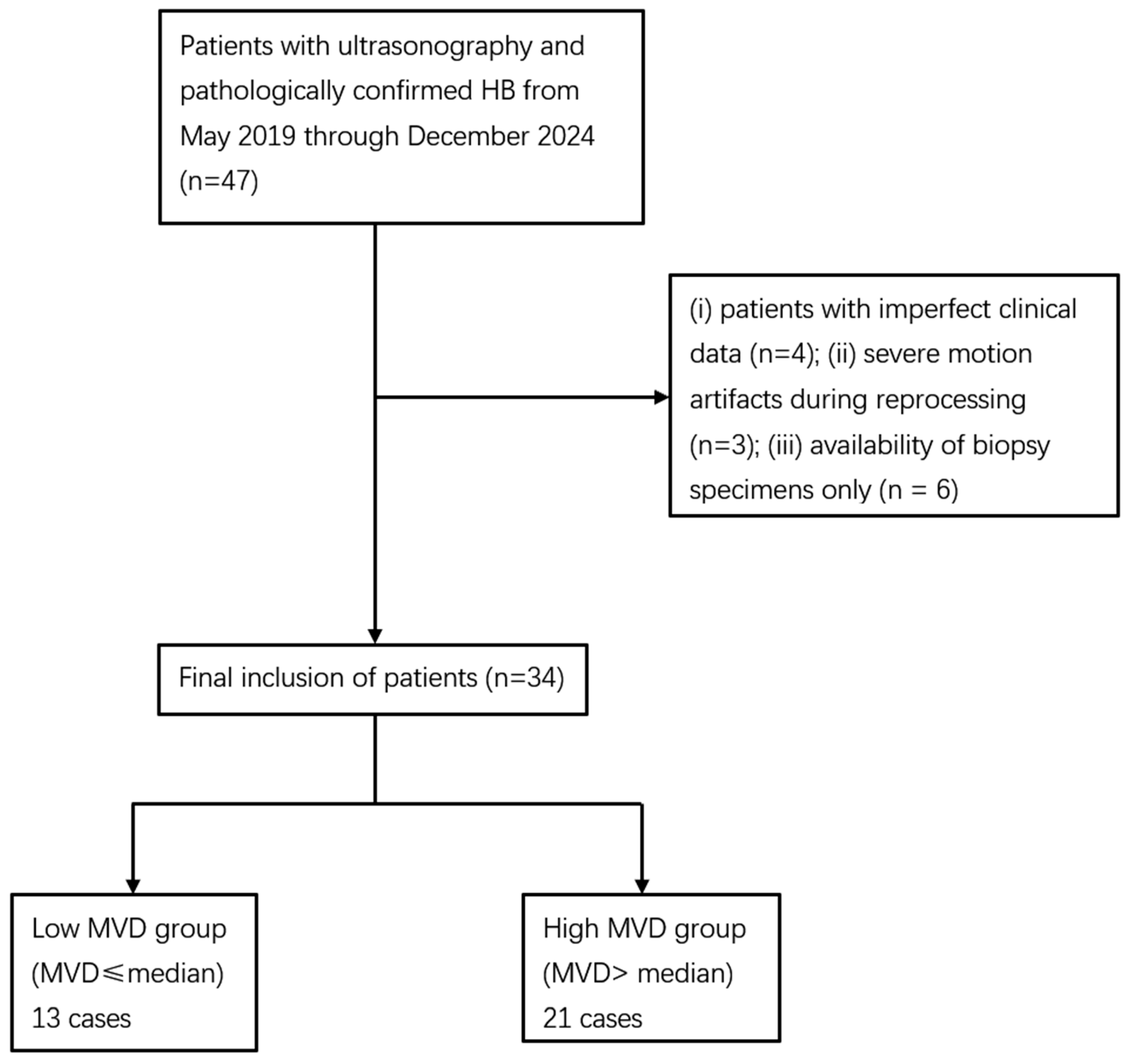
A total of 47 children were initially reviewed. Four cases were excluded due to incomplete clinical data, three because TIC analysis failed owing to severe motion artifacts, and six because only biopsy specimens were available, and no cases were excluded due to failed CD34 staining. Ultimately, 34 patients were included in the analysis. As no standardized guideline for MVD stratification has been established, patients in this cohort were divided according to the median MVD value (41 vessels per high-power field). This approach has been adopted in previous studies of HB [6] as well as in other tumor types [12]. Accordingly, they were categorized into a low MVD group (MVD < 41/HPF) and a high MVD group (MVD ≥ 41/HPF). The patient selection process is illustrated in Figure 1.
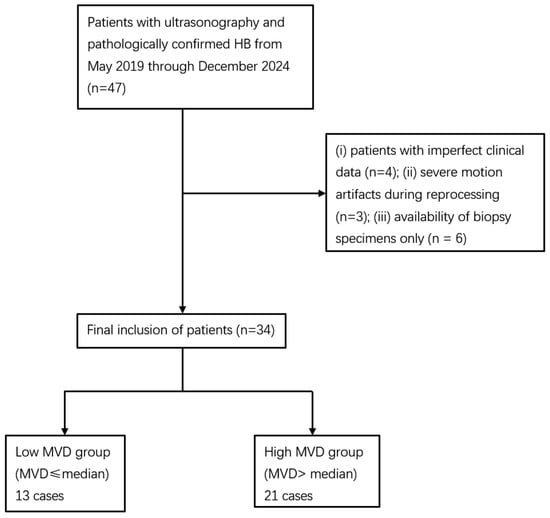
Figure 1.
Flowchart of the study population. HB: hepatoblastoma; CEUS: contrast-enhanced ultrasound; MVD: microvessel density.
2.3. Methods
2.3.1. Instrumentation
For the ultrasound examinations, an Acuson Sequoia unit (Siemens Medical Solutions USA, Mountain View, CA, USA) featuring a 1.0–4.0 MHz C251 convex array transducer was used. The contrast agent used in this study was SonoVue (sulphur hexafluoride microbubbles; Bracco Imaging, Milan, Italy), which is among the most widely used second-generation ultrasound contrast agents in pediatric practice. Other agents, such as Definity, Optison, and Sonazoid, are also clinically available [15,16]. SonoVue was administered at 0.03 mL/kg per patient according to FDA-recommended pediatric dosage guidelines, with a maximum volume not exceeding 2.4 mL per injection [14,17]. The excellent safety profile of SonoVue and other second-generation ultrasound contrast agents in the pediatric population is well-established [16].
2.3.2. Examination Method
The examinee was placed in the supine position with the examination site fully exposed. Standard ultrasound was first performed to evaluate the lesion, documenting its dimensions, number, margin, calcification, and echogenic features. The background liver condition was also assessed using grayscale ultrasound combined with clinical records. Subsequently, the intralesional vascular signal was assessed via Color Doppler Flow Imaging (CDFI), resulting in a four-tiered classification of its vascularity according to Adler’s criteria [18]: grade 0, no blood flow signals; grade I, a small amount of blood flow, with 1~2 short rods or dots of blood flow; grade II, a moderate vascular signal characterized by several small vessels or a single vessel surpassing the lesion’s radius; grade III, a rich perfusion pattern, showing over four vessels or complex vascular networks interwoven into a reticular structure with mixed-color flow. Subsequently, based on conventional B-mode ultrasound findings, the axial or longitudinal plane displaying the maximum diameter of the lesion was selected as the acquisition plane for CEUS.
This plane was required to include both the lesion and the adjacent normal liver parenchyma to allow comparative evaluation of perfusion characteristics. Following plane selection, the contrast medium was administered intravenously via the elbow vein. The video capture function was activated simultaneously to monitor perfusion in both the lesion and adjacent normal liver tissue in real time. Throughout the 3–4-min imaging window, echo intensity variations were recorded and stored for analysis. (i) The enhancement uniformity was categorized as uniform, defined as homogeneous and evenly distributed intralesional perfusion without regional variation, or non-uniform, defined as heterogeneous enhancement with irregular hypo-enhancing areas, including non-enhancing regions that persisted through the arterial and portal venous phases. (ii) The enhancement pattern was classified as centripetal, when enhancement began at the periphery and progressed toward the center or centrifugal when enhancement began centrally and extended toward the periphery. (iii) Penetrating vessels were operationally defined as thin, linear, or branching enhancing structures originating from adjacent hepatic branches and extending from the tumor margin into the lesion during the arterial phase [19,20,21]. Each ultrasound assessment was conducted by a sonographer boasting 20 years of thorough expertise.
2.3.3. Image Assessment
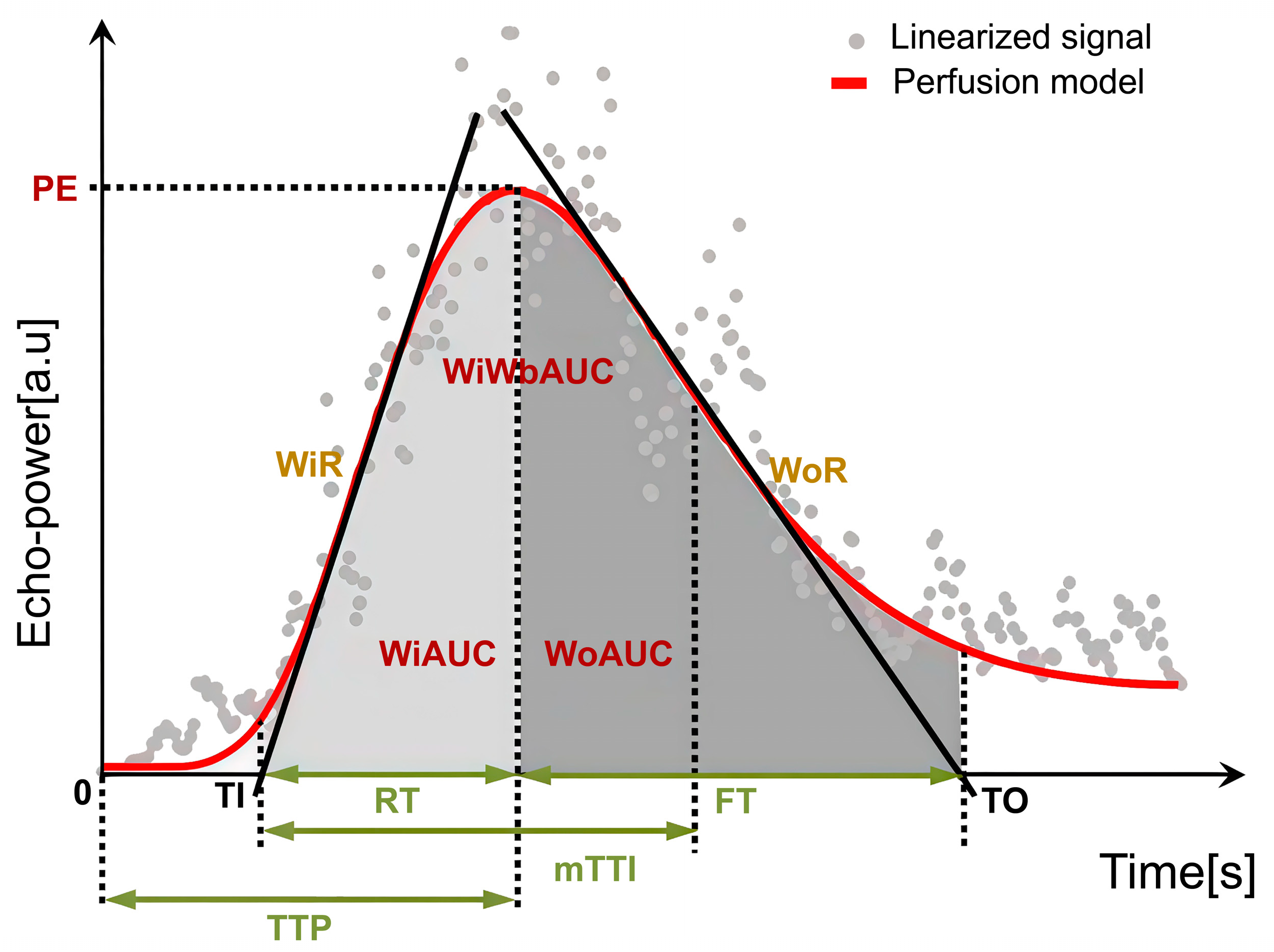
Regions of interest (ROIs) were manually delineated on CEUS images using dedicated analysis software. For tumor ROIs, representative solid areas were selected while carefully avoiding non-enhancing anechoic regions corresponding to necrosis, strongly echogenic foci with posterior acoustic shadowing indicative of calcification, or large intra-tumoral vessels. A control ROI was placed in adjacent normal liver parenchyma at the same depth (same skin-to-ROI center distance) to minimize the influence of acoustic attenuation. All ROIs were determined by a single radiologist with more than 2 years of experience in pediatric CEUS, who was blinded to the pathological results. To reduce variability, circular ROIs with a fixed diameter of 10 mm were used across cases [22,23]. Respiratory motion artifacts were corrected using motion compensation algorithms. Time–intensity curve (TIC) analysis was then performed, and perfusion parameters were calculated as ratios between tumor and parenchyma to reduce variability related to ROI depth. Accordingly, the following relative quantitative parameters were obtained: relative image maximum (rImax), relative time to peak (rTTP), relative rise time (rRT), relative fall time (rFT), relative fall half time (rFHT), relative mean transit time (rmTT), relative area under the curve (rAUC), relative area under the curve (rWiAUC), relative wash-in area under the curve (rWoAUC), relative wash-in rate (rWiR), and relative wash-out rate (rWoR) in Table 1 and Figure 2. All CEUS parameters were reported with their respective units (s, %, or %/s). Tumor-to-liver ratios were additionally calculated as semi-quantitative parameters and reported without units.

Table 1.
Quantitative parameter definitions.
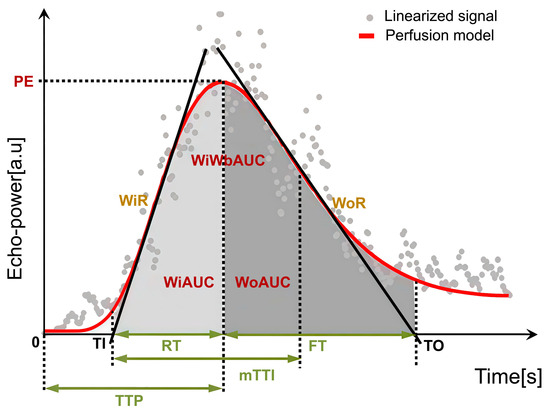
Figure 2.
Schematic illustration of CEUS quantitative perfusion parameters.
2.3.4. MVD Analysis
Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded sections using a rabbit polyclonal antibody against CD34 (ZenBio, Chengdu, China; dilution 1:100). Antigen retrieval was performed using standard heat-induced epitope retrieval according to the manufacturer’s instructions. The primary antibody was incubated for 15 min, followed by incubation with a horseradish peroxidase–conjugated goat anti-mouse/rabbit IgG polymer III (Maixin, Fuzhou, China) for 30 min. Staining was visualized with a DAB kit (ZSGB-BIO, Beijing, China), and sections were counterstained with hematoxylin. Endothelial cells in non-tumoral vessels within the same sections served as the internal positive reference, and omission of the primary antibody served as the negative control.
Vascular “hot spots” were localized by initially scanning the entire slide under a 40× light microscope, following Weidner’s criteria [24], a well-established approach for MVD quantification in oncologic pathology [25]. Three areas exhibiting high vascular density were selected, and subsequently, the number of positively stained vessels within these hot spots was counted using a 200× light microscope. Neoplastic tumor blood vessels were identified as endothelial cells or cell clusters within the tumor tissue that stained brown or tan, distinctly separate from both tumor cells and neighboring microvessels. A single microvessel was enumerated at each point, and the MVD was determined as the mean of three such measurements. Each pathological interpretation was performed by a pathologist with 15 years of dedicated experience at our institution, who was blinded to patient sonographic information.
2.3.5. Statistical Methods
The entirety of statistical analyses was performed using the SPSS software package (version 25.0, IBM Corporation, Armonk, NY, USA). The distribution of continuous variables was assessed using the Shapiro–Wilk test. Normally distributed data were expressed as mean ± SD and compared using the independent-samples t-test, while non-normally distributed data were expressed as median (IQR) and compared using the Mann–Whitney U test. Categorical variables were presented as counts and percentages, and group differences were assessed with the chi-square test. To evaluate the ability of CEUS quantitative parameters to differentiate varying degrees of MVD, receiver operating characteristic (ROC) curve analysis was performed, and the area under the curve (AUC), sensitivity, and specificity were calculated. Given the relatively small sample size and the exploratory nature of this study, no formal adjustment for multiple comparisons was applied. Statistical significance was defined as a two-sided p < 0.05.
3. Results
3.1. Patient Information and Safety
A total of 34 children with HB were included in the study. According to the median MVD value (41 vessels/HPF), 13 patients were classified into the low MVD group and 21 into the high MVD group. Baseline clinical characteristics, including demographic data, height, body weight, body mass index (BMI), serum alpha-fetoprotein (AFP), and liver function indices, are summarized in Table 2. Most patients presented with elevated AFP and increased transaminases, including alanine aminotransferase (ALT) and aspartate aminotransferase (AST), whereas albumin and total bilirubin were largely within normal ranges. BMI values were within the normal range for age, and all patients had a normal background liver without evidence of fatty infiltration, chronic hepatitis, or cirrhosis. No significant differences were observed between the high and low MVD groups. Age showed no significant association with CEUS parameters (p > 0.05), and subgroup analyses are provided in Appendix A Table A1.

Table 2.
Baseline demographic and biochemical characteristics of pediatric HB patients stratified by MVD status.
For safety monitoring, key physiological indicators, including blood pressure, pulse rate, and oxygen levels, were recorded prior to, throughout, and half an hour following the ultrasound procedure. All children maintained stable vital signs—including heart rate, respiratory rate, oxygen saturation, and blood pressure—throughout the CEUS examination. No adverse events such as nausea, vomiting, fever, or rash were reported.
3.2. Ultrasound Features of HB Lesions: High and Low MVD Groups
The two groups showed no significant differences in dimensions, number, echogenicity, margin, or calcification (p > 0.05). Nonetheless, a statistically significant variation in CDFI blood-flow grades was observed between groups (p < 0.05), as shown in Table 3. In the low-MVD group, 4/13 (30.77%) tumors presented Adler grade 0–I, while 9/13 (69.23%) showed Adler grade II–III. In contrast, all 21/21 (100%) tumors in the high-MVD group demonstrated Adler grade II–III.

Table 3.
Ultrasound features of HB lesions: high and Low MVD groups.
3.3. Qualitative CEUS Performance of HB Lesions: High and Low MVD Groups
Penetrating vessels were significantly more frequent in the high MVD group (p < 0.05). In contrast, no significant differences were observed between the two groups regarding enhancement uniformity or enhancement order (p > 0.05). Representative CEUS features are illustrated in Figure 3 and Figure 4, and the comparative results are summarized in Table 4.
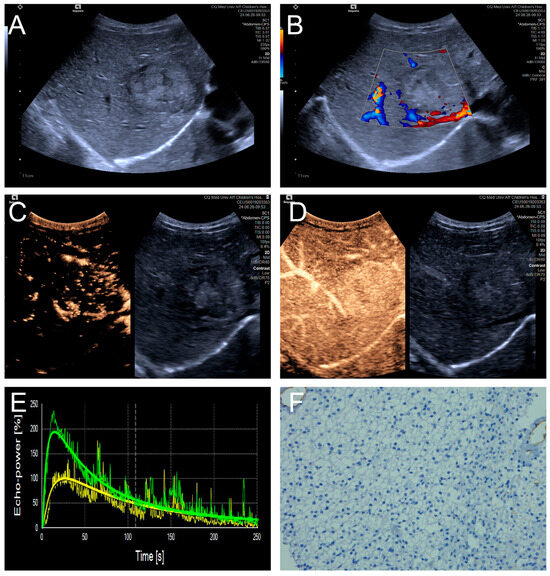
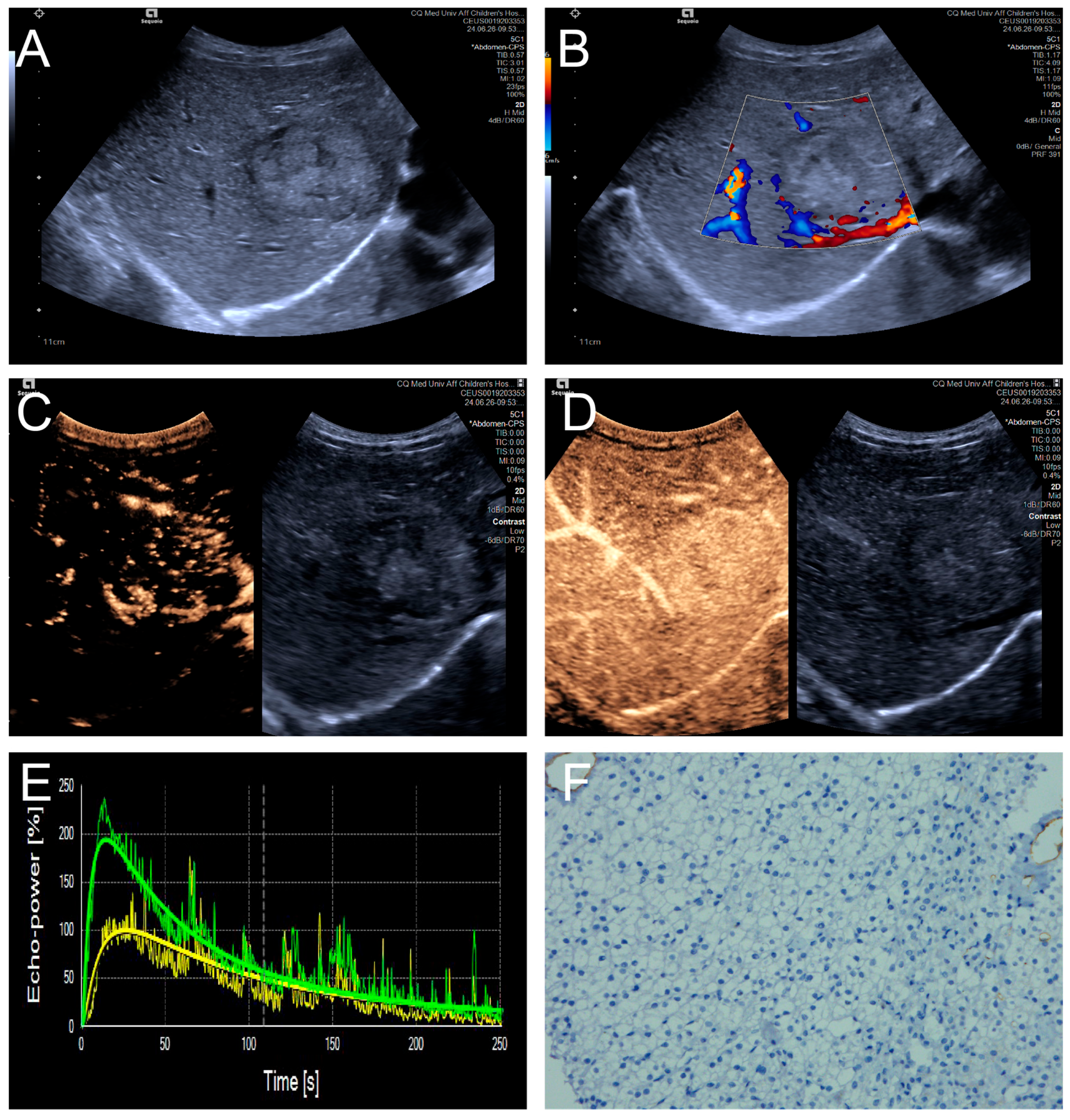
Figure 3.
(A–F) A male patient aged 4 years and 4 months with HB and low MVD. (A) The imaging revealed a well-defined mass with clear margins and hyperechoic echogenicity in the right hepatic lobe; (B) The tumor’s color Doppler flow imaging revealed distinct blood flow patterns and Adler grade I blood flow; (C,D) The CEUS exhibited uniform internal enhancement from periphery to center without any peripheral penetrating vessels; (E) TIC curves showed contrast enhancement of the lesion (green) and perfusion of the adjacent liver parenchyma (yellow). Jagged lines indicated raw signal data, and smooth lines indicated the fitted curves; (F) The biopsy specimen’s cytological immunohistochemical staining revealed few microvessels (CD34 staining × 200), and the MVD measured 21 lines/HPF.
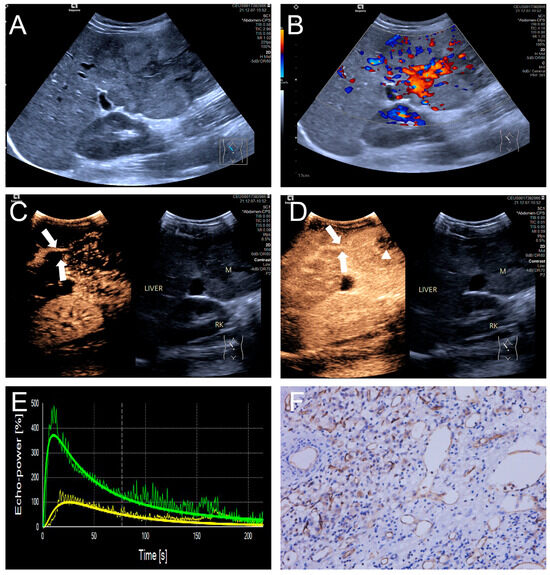
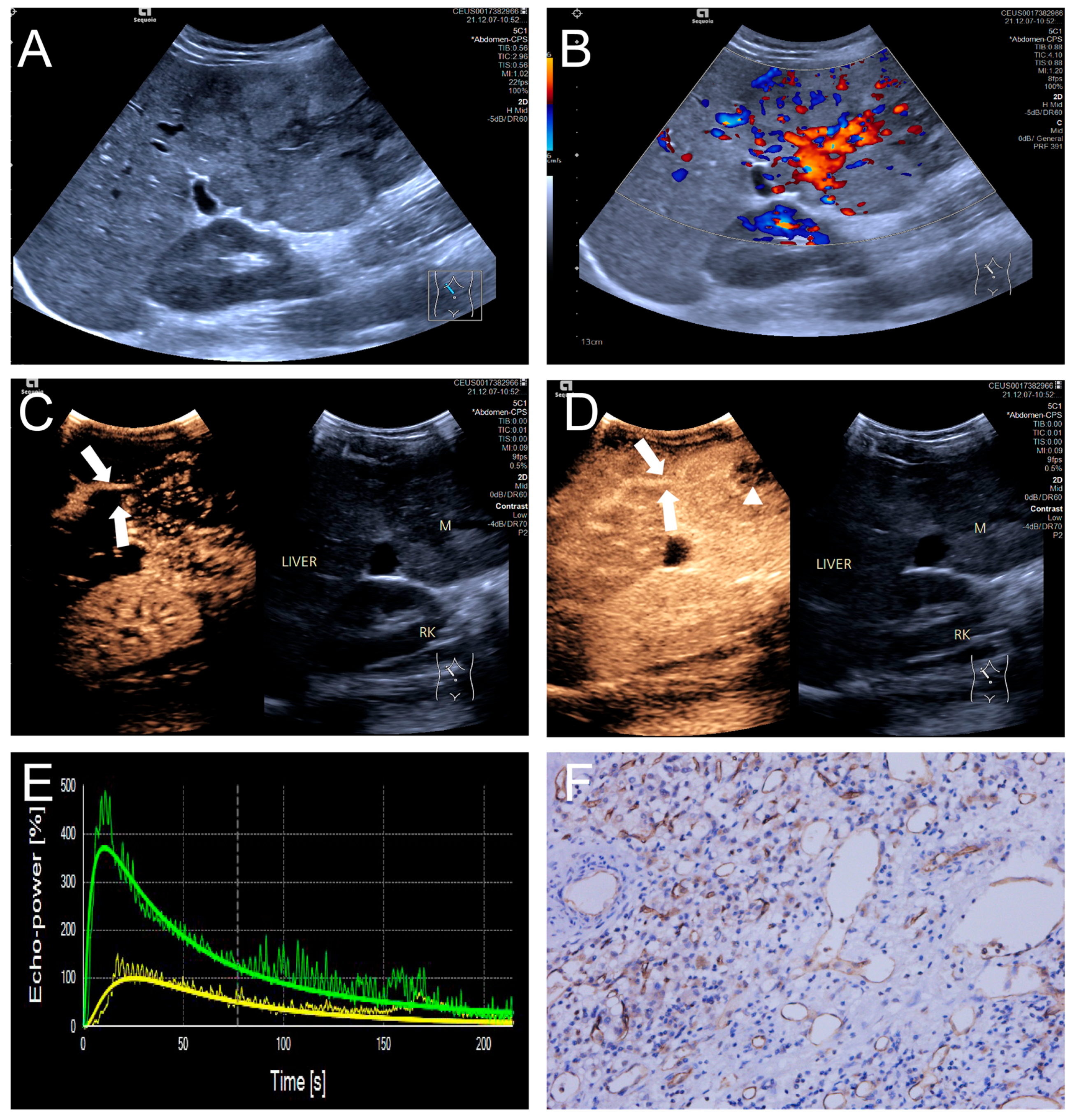
Figure 4.
(A–F) A male patient aged 10 years and 6 months with HB and high MVD. (A) The imaging revealed a lesion with unclear margins and hyperechoic echogenicity in the liver’s left lobe; (B) During the enhancement phase, CDFI revealed abundant vascular signals within the tumor tissue, classified as grade III by Adler; (C,D) The CEUS exhibited non-uniform enhancement progressing from periphery to center, with non-enhancing regions (triangles) within the lesion and peripheral penetrating vessels (arrows); (E) TIC curves showed contrast enhancement of the lesion (green) and perfusion of the adjacent liver parenchyma (yellow). Jagged lines indicated raw signal data, and smooth lines indicated the fitted curves; (F) The biopsy specimen’s cytological immunohistochemical staining revealed dense microvessels (CD34 staining × 200), and the MVD measured 42 lines/HPF.

Table 4.
Qualitative CEUS performance of HB lesions: high and low MVD groups.
3.4. CEUS Perfusion Parameters of HB Lesion Tissues: High and Low MVD Groups
The low MVD group exhibited significant differences in TTP (s), RT (s), FHT (s), Imax (%), and WoR (%) when compared to the adjacent healthy hepatic tissue (p < 0.05). Compared to adjacent non-tumorous hepatic tissue, high MVD lesions showed distinct characteristics in TTP (s), RT (s), and FHT (s) (p < 0.05); the other evaluated parameters did not exhibit meaningful variations between these tissue types (p > 0.05), as listed in Table 5. To minimize variability related to ROI depth, relative parameters were calculated as tumor-to-liver ratios for intergroup comparisons. The analysis indicated that rImax, rTTP, rRT, rFHT, rAUC, rWoAUC, and rWoR showed significant intergroup differences (p < 0.05), while no such significance was found for rFT, rmTT, rWiAUC, or rWiR, as detailed in Table 6.

Table 5.
Quantitative CEUS parameters of HB lesion tissues: high and low MVD groups.

Table 6.
Relative CEUS parameters of HB lesion tissues: high and low MVD groups.
3.5. ROC Curve and Box Plot Analysis of Relative CEUS Parameters of HB Lesion Tissues: High and Low MVD Groups
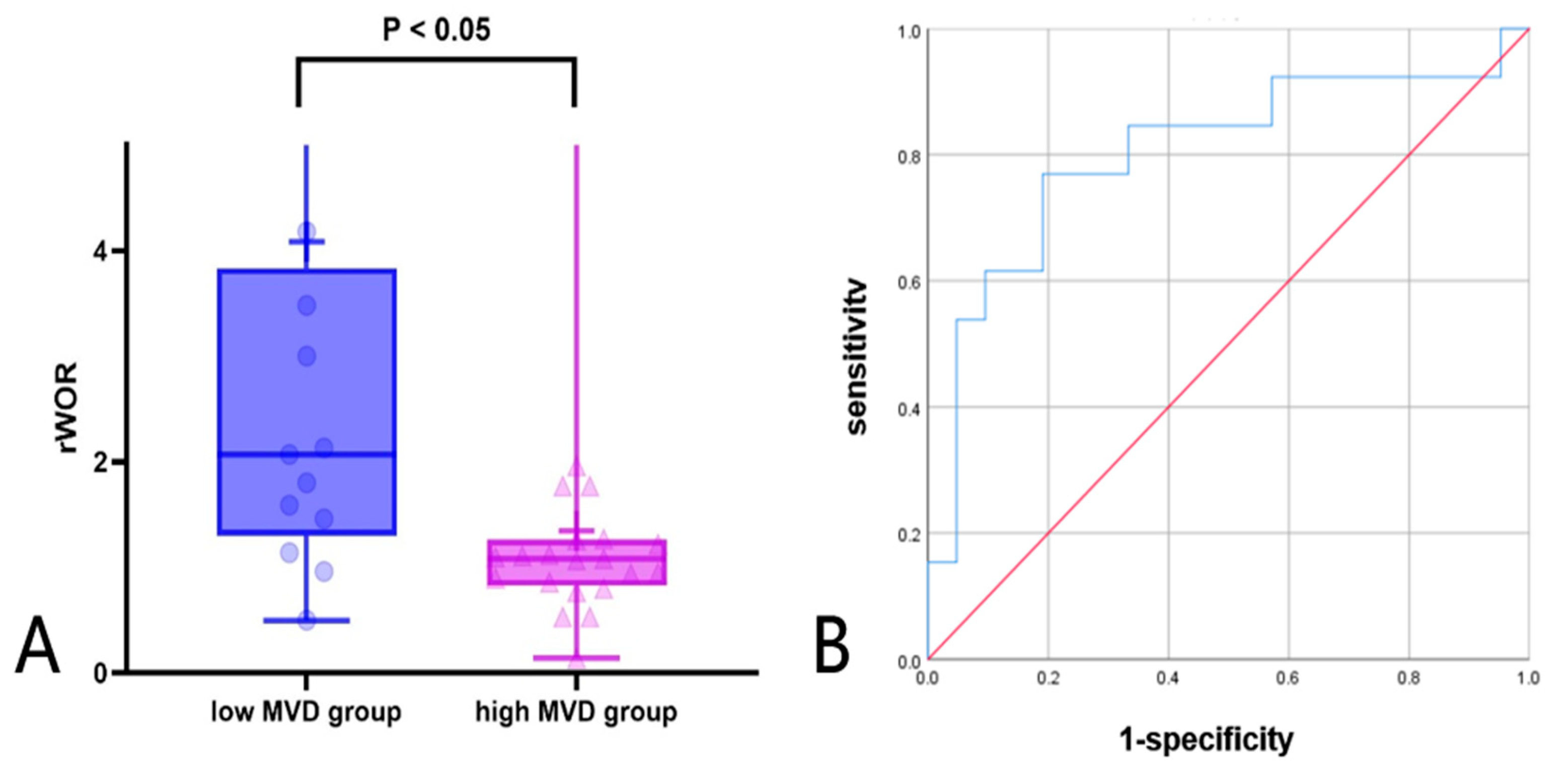
Further ROC analysis was conducted to evaluate the diagnostic performance of CEUS relative parameters. Among these semi-quantitative parameters, rWoR showed the best performance in differentiating high and low MVD groups. The AUC of rWoR was 0.802, with a cutoff value of 1.36, a diagnostic sensitivity of 76.9%, and a specificity of 81.0% in Figure 5A and Table 7. To further visualize the distribution of rWoR across different MVD groups, a box plot was constructed, which showed that the rWoR values in the low MVD group were significantly higher than those in the high MVD group (p < 0.05) in Figure 5B.
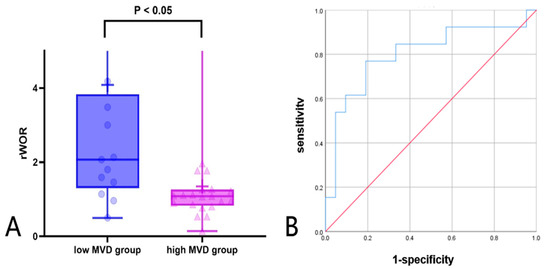
Figure 5.
(A,B) Diagnostic performance of rWOR in high and low MVD groups (A) box plot; (B) ROC curve: receiver operating characteristic curve.

Table 7.
ROC curve analysis of relative CEUS parameters of HB lesion tissues: high and low MVD group.
4. Discussion
Hepatoblastoma, a common pediatric malignancy, accounts for approximately 90% of hepatic malignancies below five years old [26,27]. Currently, grayscale ultrasound struggles to identify microscopic blood flow and liquefied necrosis tissue. Contrast-Enhanced Computed Tomography (CECT) and Magnetic Resonance Imaging (MRI) are alternative imaging methods that can offer additional information about the surrounding tissues. Nevertheless, CT exposes children to potentially harmful radiation, and MRI is limited by its lengthy procedure and poor patient compliance. In contrast, CEUS avoids radiation exposure and permits continuous real-time visualization of microvasculature, making it uniquely advantageous for children. This aligns with the WFUMB-EFSUMB guidelines, which highlight CEUS as a valuable, real-time, and safe modality for characterizing focal liver lesions [28].
MVD serves as a crucial indicator for evaluating tissue angiogenesis, effectively indicating the proliferation and invasiveness of tumor cells. This retrospective analysis explored the association of CEUS with MVD expression in HB. The findings indicated that the low MVD group showed a lower incidence of penetrating vessels while also exhibiting more quantitative parameters that differed significantly from the surrounding normal parenchyma. In addition, the high and low MVD groups differed in rImax, rTTP, rRT, rFHT, rAUC, rWoAUC, and rWoR. Specifically, rWoR was significantly higher in the low MVD group than in the high MVD group, suggesting its potential advantage in reflecting generation. Further analysis revealed that rWoR ≥1.36 could be a valid parameter for predicting low MVD expression in HB preoperatively.
The growth, invasion, and metastasis of cancer cells are closely dependent on their microvascular supply [29,30]. As a histological marker of angiogenesis, MVD reflects tissue microvasculature and has been correlated with tumor growth, recurrence, and prognosis in multiple malignancies. For example, Goyal et al. [31] reported that breast tumors with higher MVD exhibited greater proliferative capacity, while Zvrko [32] showed that elevated MVD in laryngeal cancer was associated with recurrence, advanced stage, and lymph node metastasis. Collectively, these findings indicate that high MVD generally corresponds to more aggressive tumor biology and poorer prognosis. In HB, patient stratification by the median MVD has also demonstrated prognostic differences [6]. So we adopted the cohort median as the cutoff in the present study to assess its association with CEUS parameters.
Assessment of MVD currently relies on tumor tissues collected via surgical excision or needle biopsy. However, the invasiveness and heavy reliance on operator expertise of these techniques limit their broader clinical application. Therefore, it is crucial to adopt a non-invasive imaging method that effectively and rapidly predicts MVD counts. CEUS is becoming a recognized and prospective way to assess MVD in hepatic neoplasms. Faccia et al. [33] reported that CEUS quantitatively evaluates capillary flow and reliably supervises antiangiogenic therapy outcomes, with a diagnostic accuracy comparable to CT and MRI. Also, Mostafa et al. [11] observed that CEUS, by imaging tumor vasculature before surgery, facilitated early diagnosis, personalized therapy, and medical outcomes.
All tumors in the high MVD group showed Adler grade II–III blood flow, and 9 of 13 tumors in the low MVD group also demonstrated grade II–III signals (69.23%). Adler grading is semi-quantitative and dependent on machine parameters and acquisition settings, and Doppler is preferentially sensitive to larger-caliber vessels or higher-velocity flow while relatively insensitive to slow capillary perfusion. Consistent with these properties, the frequent grade II–III signals in the low MVD group indicate that Doppler is not a reliable surrogate for capillary-level perfusion, despite a group-level association with MVD. By contrast, CEUS provides not only quantitative parameters from TIC analysis but also information on the dynamic characteristics of microvascular perfusion at the capillary level, thereby offering incremental value beyond Doppler, which is limited to depicting larger, faster-flowing vessels [34].
Currently, no study has been found to apply CEUS to predict MVD counts in pediatric HB. Using CEUS, this study offered a real-time, dynamic visualization of microcirculatory perfusion within lesions. A significant association was observed between the presence of penetrating vessels and MVD. In the high MVD group, peripheral penetrating vessels were frequently observed. This observation is consistent with previous findings. Li et al. [35] reported that the peripheral regions of tumors typically have significantly higher MVD than the central areas, and the denser peripheral vasculature may facilitate the formation of penetrating vessels [36]. Tumor morphology may change before angiogenesis commences, and grayscale sonography cannot discern the extent of tissue infiltration. By contrast, microbubble agents enable the visualization of blood flow, specifically revealing penetrating and irregular vessels [37].
To minimize visual bias and subjectivity, quantitative analysis software was applied to objectively characterize perfusion distribution in the form of quantitative parameters [38]. Differences between high MVD lesions and the surrounding liver parenchyma were mainly reflected in TTP (s), RT (s), and FHT (s). In contrast, low MVD lesions demonstrated additional significant discrepancies, including Imax (%) and WoR (%). Imax (%) reflects the vascularity and blood inflow of the lesion, being closely related to its perfusion status. In the low MVD group, Imax (%) was significantly higher than that of the surrounding parenchyma, indicating stronger arterial enhancement. Such a pattern may be attributed to the relatively preserved vascular integrity in low MVD tumors, which facilitates microbubble inflow, increases the effective intravascular blood pool volume, and generates more homogeneous peak enhancement, consistent with the findings of Li et al. [39]. WoR (%) represents the washout rate of the contrast agent [34]. In low MVD lesions, WoR (%) was also significantly higher than that of the background liver, suggesting more rapid clearance of microbubbles. This likely reflects that, although relatively preserved vascular integrity allows higher Imax through rapid arterial inflow, the sparse vascular network limits perfusion sustainability, leading to insufficient retention and consequently faster washout.
To further reduce potential confounding, we compared relative parameters between the high and low MVD groups. Significant differences were observed in rImax, rTTP, rRT, rFHT, rAUC, rWOAUC, and rWoR. Specifically, the high MVD group exhibited elevated rTTP, rRT, and rFHT, indicating delayed peak enhancement and prolonged washout. This may be explained by the fact that, although containing more neovessels, their abnormal, tortuous structure and high permeability limit effective vascular volume and perfusion efficiency, leading to slower inflow and delayed clearance [40]. In contrast, the low MVD group showed higher rImax, rAUC, rWOAUC, and rWoR, reflecting stronger arterial enhancement and greater overall perfusion. With fewer but relatively intact vessels, these lesions might achieve more efficient perfusion, resulting in rapid inflow, accelerated clearance, and a larger effective blood volume.
Among these parameters, rWoR yielded the largest area under the ROC curve, indicating superior diagnostic performance in distinguishing between high and low MVD. rWoR expresses the clearance rate as the ratio of lesion to background liver, providing a more objective measure of their relative difference [41]. In low MVD lesions, the limited number of vessels shortened intratumoral transit, leading to more rapid enhancement decline compared with the background liver. This amplified the lesion-to-background difference, leading to elevated rWoR. In contrast, high MVD tumors, with abundant neovessels, arteriovenous shunts, and increased permeability, also showed accelerated clearance. However, the surrounding liver parenchyma, with its rich perfusion, cleared at a similarly rapid pace, thereby attenuating the relative difference and resulting in lower rWoR [34,42].
Therefore, the quantitative CEUS parameters were associated with intratumoral MVD. On this basis, rWoR may be further explored as a non-invasive indicator to assess intratumoral vascularity, thereby potentially reducing reliance on invasive biopsy and enabling longitudinal monitoring of microvascular changes for evaluating responses to anti-angiogenic therapy. Moreover, since higher MVD has been linked to more aggressive tumor biology and poorer prognosis, CEUS perfusion parameters may also provide supportive information for risk stratification. These potential implications remain exploratory and warrant further investigation in future clinical research.
5. Limitations
This study has several limitations. First, this was a retrospective, single-center pilot study with a limited sample size and no formal sample size calculation. Larger, prospective multicenter studies are required to validate these findings. Second, the median value of MVD was used for grouping, following the previous literature, but standardized thresholds should be established in future work. Third, interobserver variability in CEUS quantification was not assessed. The ROI analysis was performed by a single reader, which may introduce bias and limits the generalizability of the absolute parameter values. Future studies assessing reproducibility across observers are needed to confirm the reliability and generalizability of CEUS quantification [38]. Finally, this study did not include direct comparison with MRI perfusion. CEUS was evaluated as an independent modality with potential utility for real-time, radiation-free assessment of tumor vascularization. Future studies integrating CEUS with MRI or other modalities would be valuable for broader clinical validation.
6. Conclusions
In conclusion, as a non-invasive approach to vascular assessment, CEUS showed significant differences in TIC-derived parameters and relative parameters between high and low MVD groups in pediatric HB. Among the assessed parameters, rWoR was most helpful for group discrimination.
Author Contributions
Conceptualization, Y.Y. and Y.T. (Yi Tang); methodology, Y.Y.; software, Y.T. (Yuxin Tang); validation, Y.Y., L.Z. and H.Z.; formal analysis, L.Z.; investigation, H.Z.; resources, J.C.; data curation, Y.T. (Yuxin Tang); writing—original draft preparation, Y.Y.; writing—review and editing, Y.T. (Yi Tang); visualization, J.C.; supervision, Y.T. (Yi Tang); project administration, Y.T. (Yi Tang). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Chongqing Children’s Hospital, Chongqing Medical University (approval code: No. 34/2024 Ethics Committee [Research]; approval date: 19 December 2024).
Informed Consent Statement
The children’ parents or legal guardians provided informed consent.
Data Availability Statement
The original contributions presented in this study are included in the article/Appendix A. Further inquiries can be directed at the corresponding author.
Acknowledgments
We thank all medical staff and technicians who agreed to participate in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CEUS | Contrast-Enhanced Ultrasound |
| MVD | Microvessel Density |
| HB | Hepatoblastoma |
| TIC | Time–Intensity Curve |
| CDFI | Color Doppler Flow Imaging |
| ROC | Receiver Operating Characteristic |
| ROI | Region Of Interest |
| Imax | Image Maximum |
| TTP | Time to Peak |
| RT | Rise Time |
| FT | Fall Time |
| FHT | Fall Half Time |
| mTT | Mean Transit Time |
| AUC | Area Under The Curve |
| WiAUC | Wash-Out Area Under The Curve |
| WoAUC | Wash-In Area Under The Curve |
| WiR | Wash-In Rate |
| WoR | Wash-Out Rate |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
Appendix A

Table A1.
Association between CEUS quantitative parameters and age subgroups in pediatric HB.
Table A1.
Association between CEUS quantitative parameters and age subgroups in pediatric HB.
| Quantitative Parameter | <3 Years (n = 22) | ≥3 Years (n = 12) | p Value |
|---|---|---|---|
| Imax (%) | 115.99 (92.36, 207.13) | 121.91 (108.74, 205.71) | 0.582 |
| TTP (s) | 11.97 ± 4.25 | 23.30 ± 8.53 | 0.783 |
| RT (s) | 10.91 ± 3.75 | 20.83 ± 8.14 | 0.792 |
| FT (s) | 62.35 (40.57, 102.73) | 59.61 (49.89, 77.43) | 0.958 |
| FHT (s) | 28.06 (18.20, 44.91) | 27.97 (21.84, 33.39) | 0.901 |
| mTT (s) | 58.23 (36.03, 97.33) | 50.91 (41.78, 82.87) | 0.790 |
| AUC (%/s) | 45,047.21 (25,833.10, 75,984.30) | 44,040.51 (17,668.64, 82,750.17) | 0.709 |
| WiAUC (%/s) | 759.82 (248.85, 1714.97) | 421.88 (198.85, 779.55) | 0.292 |
| WoAUC (%/s) | 26,214.10 (14,609.24, 52151.01) | 26,417.45 (9708.47, 65,472.60) | 0.736 |
| WiR (%) | 59.98 (29.00, 120.61) | 40.95 (16.33, 59.14) | 0.292 |
| WoR (%) | 467.45 (260.68, 732.62) | 417.67 (189.96, 579.68) | 0.683 |
Imax: image maximum; TTP: time to peak; RT: rise time; FT: fall time; FHT: fall half time; mTT: mean transit time; AUC: area under the curve; WiAUC: wash-in area under the curve; WoAUC: wash-out area under the curve; WiR: wash-in rate; WoR: wash-out rate.
References
- Kahla, J.A.; Siegel, D.A.; Dai, S.; Lupo, P.J.; Foster, J.H.; Scheurer, M.E.; Heczey, A.A. Incidence and 5-year survival of children and adolescents with hepatoblastoma in the United States. Pediatr. Blood Cancer 2022, 69, e29763. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Liu, Z.; Li, C. Correlation of lymph node metastasis with contrast-enhanced ultrasound features, microvessel density and microvessel area in patients with papillary thyroid carcinoma. Clin. Hemorheol. Microcirc. 2022, 82, 361–370. [Google Scholar] [CrossRef]
- Muench, M.O.; Fomin, M.E.; Gutierrez, A.G.; López-Terrada, D.; Gilfanova, R.; Nosworthy, C.; Beyer, A.I.; Ostolaza, G.; Kats, D.; Matlock, K.L.; et al. CD203c is expressed by human fetal hepatoblasts and distinguishes subsets of hepatoblastoma. Front. Oncol. 2023, 13, 927852. [Google Scholar] [CrossRef]
- Kong, M.; Zhai, Y.; Liu, H.; Zhang, S.; Chen, S.; Li, W.; Ma, X.; Ji, Y. Insights into the mechanisms of angiogenesis in hepatoblastoma. Front. Cell Dev. Biol. 2025, 13, 1535339. [Google Scholar] [CrossRef]
- Gong, W.; Han, Z.; Fang, F.; Chen, L. Yap Expression Is Closely Related to Tumor Angiogenesis and Poor Prognosis in Hepatoblastoma. Fetal Pediatr. Pathol. 2022, 41, 929–939. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, W.; Zhang, Y.; Gao, Y.; Zhi, T.; Li, F.; Li, J.; Gu, H.; Liao, R.; Wu, R.; et al. Individualized chemotherapy and efficacy analysis of hepatoblastoma in children. Pediatr. Blood Cancer 2024, 71, e30693. [Google Scholar] [CrossRef]
- Uz, Z.; Shen, L.; Milstein, D.M.J.; van Lienden, K.P.; Swijnenburg, R.-J.; Ince, C.; van Gulik, T.M. Intraoperative Imaging Techniques to Visualize Hepatic (Micro)Perfusion: An Overview. Eur. Surg. Res. 2020, 61, 2–13. [Google Scholar] [CrossRef]
- Seitz, K.; Strobel, D. A Milestone: Approval of CEUS for Diagnostic Liver Imaging in Adults and Children in the USA. Ultraschall Med. 2016, 37, 229–232. [Google Scholar] [CrossRef]
- Torres, A.; Koskinen, S.K.; Gjertsen, H.; Fischler, B. Contrast-enhanced ultrasound is useful for the evaluation of focal liver lesions in children. Australas. J. Ultrasound Med. 2021, 24, 143–150. [Google Scholar] [CrossRef]
- Mostafa, A.; Abramson, Z.; Ghbrial, M.; Biswas, S.; Chan, S.; Darji, H.; Gartrell, J.; Karol, S.E.; Li, Y.; Mulrooney, D.A.; et al. Contrast enhanced ultrasound of liver lesions in patients treated for childhood malignancies. Cancer Imaging 2024, 24, 115. [Google Scholar] [CrossRef]
- Battistella, A.; Partelli, S.; Andreasi, V.; Marinoni, I.; Palumbo, D.; Tacelli, M.; Lena, M.S.; Mushtaq, J.; Muffatti, F.; Capurso, G.; et al. Preoperative assessment of microvessel density in nonfunctioning pancreatic neuroendocrine tumors (NF-PanNETs). Surgery 2022, 172, 1236–1244. [Google Scholar] [CrossRef]
- Deng, S.; Jiang, Q.; Wang, Y.; Lu, X.; Zhang, Y. Relationship between quantitative contrast-enhanced ultrasonography parameters and angiogenesis in primary small hepatocellular carcinoma A retrospective study. Medicine 2021, 100, e26489. [Google Scholar] [CrossRef]
- Fang, C.; Anupindi, S.A.; Back, S.J.; Franke, D.; Green, T.G.; Harkanyi, Z.; Jungert, J.; Kwon, J.K.; Paltiel, H.J.; Squires, J.H.; et al. Contrast-enhanced ultrasound of benign and malignant liver lesions in children. Pediatr. Radiol. 2021, 51, 2181–2197. [Google Scholar] [CrossRef]
- Frinking, P.; Segers, T.; Luan, Y.; Tranquart, F. Three Decades of Ultrasound Contrast Agents: A Review of the Past, Present and Future Improvements. Ultrasound Med. Biol. 2020, 46, 892–908. [Google Scholar] [CrossRef]
- Ntoulia, A.; Anupindi, S.A.; Back, S.J.; Didier, R.A.; Hwang, M.; Johnson, A.M.; McCarville, M.B.; Papadopoulou, F.; Piskunowicz, M.; Sellars, M.E.; et al. Contrast-enhanced ultrasound: A comprehensive review of safety in children. Pediatr. Radiol. 2021, 51, 2161–2180. [Google Scholar] [CrossRef]
- Han, D.; Wang, T.; Wang, R.; Chen, J.; Tang, Y. Application of Quantitative Parameters of Contrast-Enhanced Ultrasound in Common Benign and Malignant Lesions in Pediatric Livers: A Preliminary Study. Diagnostics 2023, 13, 3443. [Google Scholar] [CrossRef]
- Che, D.; Yang, Z.; Wei, H.; Wang, X.; Gao, J. The Adler grade by Doppler ultrasound is associated with clinical pathology of cervical cancer: Implication for clinical management. PLoS ONE 2020, 15, e0236725. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Dong, T.; Li, Y.; Yin, C.; Nie, F. Pancreatic Ductal Adenocarcinoma: The Characteristics of Contrast-Enhanced Ultrasound Are Correlated with the Hypoxic Microenvironment. Diagnostics 2023, 13, 3270. [Google Scholar] [CrossRef]
- Cai, W.-J.; Ying, M.; Zheng, R.-Q.; Liao, J.; Luo, B.; Tang, L.; Cheng, W.; Yang, H.; Wei, A.; Yang, Y.; et al. Contrast-Enhanced Ultrasound Liver Imaging Reporting and Data System in Hepatocellular Carcinoma ≤5 cm: Biological Characteristics and Patient Outcomes. Liver Cancer 2023, 12, 356–371. [Google Scholar] [CrossRef]
- Wang, T.; Han, D.; Xiao, H.; Yang, H.; Chen, J.Y.; Tang, Y. A Preliminary Study on the Application of Contrast-Enhanced Ultrasonography in Children With Peripheral Neuroblastic Tumors. Ultrasound Med. Biol. 2024, 50, 954–960. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Correas, J.M.; Cui, X.W.; Dong, Y.; Havre, R.F.; Jenssen, C.; Jung, E.M.; Krix, M.; Lim, A.; Lassau, N.; et al. EFSUMB Technical Review—Update 2023: Dynamic Contrast-Enhanced Ultrasound (DCE-CEUS) for the Quantification of Tumor Perfusion. Ultraschall Med. 2024, 45, 36–46. [Google Scholar] [CrossRef]
- Guan, X.; Li, X.; Yang, D.; Zhao, C.; Lu, D.; Zhang, Y.; Sun, Y.; Zhou, B.; Chen, Z.; Hu, X.; et al. Dynamic contrast-enhanced ultrasound perfusion analysis for preoperative prediction of aggressive hepatocellular carcinoma subtypes. Insights Imaging 2025, 16, 202. [Google Scholar] [CrossRef]
- Weidner, N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res. Treat. 1995, 36, 169–180. [Google Scholar] [CrossRef]
- Hu, X.; Liu, H.; Ye, M.; Zhu, X. Prognostic value of microvessel density in cervical cancer. Cancer Cell Int. 2018, 18, 152. [Google Scholar] [CrossRef]
- Dasgupta, P.; Henshaw, C.; Youlden, D.R.; Aitken, J.F.; Sullivan, A.; Irving, H.; Baade, P.D. Global trends in incidence rates of childhood liver cancers: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2020, 34, 609–617. [Google Scholar] [CrossRef]
- Zadeh, E.S.; Huber, K.P.; Goerg, C.; Moeller, K.; Jenssen, C.; Lim, A.; Taut, H.; Dong, Y.; Cui, X.W.; Dietrich, C.F. Comments on and illustrations of the WFUMB CEUS liver guidelines: Rare malignant neuroendocrine and predominant epithelioid liver lesions. Med. Ultrason. 2024, 26, 391–397. [Google Scholar] [CrossRef]
- Claudon, M.; Dietrich, C.F.; Choi, B.I.; Cosgrove, D.O.; Kudo, M.; Nolsoe, C.P.; Piscaglia, F.; Wilson, S.R.; Barr, R.G.; Chammas, M.C.; et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver--update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013, 34, 11–29. [Google Scholar] [CrossRef]
- Lawler, J. Counter regulation of tumor angiogenesis by vascular endothelial growth factor and thrombospondin-1. Semin. Cancer Biol. 2022, 86, 126–135. [Google Scholar] [CrossRef]
- Kane, K.; Edwards, D.; Chen, J. The influence of endothelial metabolic reprogramming on the tumor microenvironment. Oncogene 2025, 44, 51–63. [Google Scholar] [CrossRef]
- Goyal, A.V.; Shukla, S.; Acharya, S.; Vagha, S.; Jajoo, S. Correlation of microvessel density with histopathological parameters of carcinoma breast. Indian J. Med. Res. 2023, 158, 417–422. [Google Scholar] [CrossRef]
- Zvrko, E.; Vuckovic, L. Expression of CD105 but not of E-cadherin is associated with malignancy recurrence and disease-free interval in laryngeal cancer in men. Folia Histochem. Cytobiol. 2023, 61, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Faccia, M.; Garcovich, M.; Ainora, M.E.; Riccardi, L.; Pompili, M.; Gasbarrini, A.; Zocco, M.A. Contrast-Enhanced Ultrasound for Monitoring Treatment Response in Different Stages of Hepatocellular Carcinoma. Cancers 2022, 14, 481. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.K.; Burns, P.N.; Jang, H.-J.; Kono, Y.; Khalili, K.; Wilson, S.R.; Kim, T.K. Contrast-enhanced ultrasound approach to the diagnosis of focal liver lesions: The importance of washout. Ultrasonography 2019, 38, 289–301. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhu, Y.; Fu, L.; Liu, P. Association between enhancement patterns and parameters of contrast-enhanced ultrasound and microvessel distribution in breast cancer. Oncol. Lett. 2018, 15, 5643–5649. [Google Scholar] [CrossRef] [PubMed]
- Kasherman, L.; Siu, D.H.W.; Woodford, R.; Harris, C.A. Angiogenesis Inhibitors and Immunomodulation in Renal Cell Cancers: The Past, Present, and Future. Cancers 2022, 14, 1406. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Guo, R. Application value of color doppler ultrasound and ultrasound contrast in the differential diagnosis of ovarian tumor. Pak. J. Med. Sci. 2020, 36, 80–84. [Google Scholar] [CrossRef]
- Averkiou, M.A.; Juang, E.K.; Gallagher, M.K.; Cuevas, M.A.; Wilson, S.R.; Barr, R.G.; Carson, P.L. Evaluation of the Reproducibility of Bolus Transit Quantification with Contrast-Enhanced Ultrasound Across Multiple Scanners and Analysis Software Packages—A Quantitative Imaging Biomarker Alliance Study. Investig. Radiol. 2020, 55, 643–656. [Google Scholar] [CrossRef]
- Li, L.-L.; Su, Q.-L.; Deng, Y.-X.; Guo, W.-W.; Lun, H.-M.; Hu, Q. Contrast-enhanced ultrasound for the preoperative prediction of pathological characteristics in breast cancer. Front. Oncol. 2024, 14, 1320714. [Google Scholar] [CrossRef]
- Newport, E.L.; Pedrosa, A.R.; Njegic, A.; Hodivala-Dilke, K.M.; Muñoz-Félix, J.M. Improved Immunotherapy Efficacy by Vascular Modulation. Cancers 2021, 13, 5207. [Google Scholar] [CrossRef]
- Badiu, M.S.; Gheorghe, E.C.; Nicolau, C.; Saftoiu, A. Quantitative time intensity curve analysis of contrast-enhanced ultrasound (CEUS) examinations for the assessment of focal liver lesions. Med. Ultrason. 2024, 26, 63–71. [Google Scholar] [CrossRef]
- Bartolotta, T.V.; Terranova, M.C.; Gagliardo, C.; Taibbi, A. CEUS LI-RADS: A pictorial review. Insights Imaging 2020, 11, 9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).