Abstract
The oral cavity harbors one of the most diverse microbial ecosystems in the human body, second only to the gut. Periodontitis, a chronic inflammatory disease arising from oral microbiota dysbiosis, has been increasingly associated with systemic disorders such as diabetes mellitus, atherosclerosis, rheumatoid arthritis, inflammatory bowel disease, and neurodegenerative conditions. Although hematogenous dissemination of oral pathogens and inflammatory mediators has long been proposed as a mechanistic link, emerging evidence identifies the oral–gut axis as a novel bidirectional pathway. Swallowed oral pathobionts, such as Porphyromonas gingivalis and Fusobacterium nucleatum, can colonize the gut, disrupt the intestinal barrier, and induce dysbiosis, immune imbalance, and metabolic alterations that aggravate systemic inflammation and disease progression. In contrast, gut dysbiosis, especially in obesity or high-fat-diet models, can exacerbate periodontal tissue destruction through hyperuricemia, altered bone metabolism, and Th17/Treg immune imbalance. Experimental and clinical studies further support this reciprocal relationship, implicating microbial, metabolic, and immune crosstalk in both oral and systemic pathology. Understanding this oral–gut–systemic axis offers a paradigm shift in diagnostics and therapeutics, focusing on precision interventions such as microbiome modulation, probiotics, and integrated oral care to mitigate systemic inflammatory burden and improve overall health outcomes.
1. Introduction
The oral cavity, the initial site of the digestive tract, hosts one of the most diverse microbial ecosystems in the human body, second only to the gut [1]. This community plays a crucial role in maintaining mucosal and systemic homeostasis [2]. Recently, the term “oralome” has been introduced to comprehensively describe the dynamic and multidimensional interactions between the oral microbiome and its host, encompassing microbial, molecular, and immunological cross-talk that collectively influence oral and systemic health [3]. Periodontitis is a chronic inflammatory disease caused by oral microbiosis, which disrupts immune balance and barrier integrity [4]. Periodontitis arises from a shift in the oral microbiome from a balanced (eubiotic) to a dysbiotic state, progressing from early “plaque-based” theories to modern ecological and polymicrobial models that emphasize microbial interactions and host response. The current Polymicrobial Synergy and Dysbiosis model recognizes periodontitis as a community-driven disease that involves various bacterial, fungal and viral species that collectively sustain chronic inflammation and tissue destruction [3]. Epidemiological evidence has suggested an association between periodontitis and multiple systemic diseases such as cardiovascular disease, diabetes mellitus, rheumatoid arthritis, Inflammatory Bowel Disease (IBD), and even neurodegenerative disorders [4,5,6].
Traditionally, multiple lines of evidence, such as the detection of periodontal bacteria’s DNA after periodontal treatment, suggested that the hematogenous spread of oral bacteria occurs through inflamed tissues and pro-inflammatory mediators from inflamed periodontal tissues. However, the number of oral bacteria detected in the bloodstream differs greatly depending on the testing approach and the individual’s oral condition, and a unified conclusion has not yet been reached [4,5,6].
Furthermore, recent advances in sequencing technologies and microbiome research have shed light on an alternative pathway that links oral and systemic health: the oral–gut axis. In this model, oral pathobionts such as Porphyromonas gingivalis (P. gingivalis) and Fusobacterium nucleatum (F. nucleatum) are continuously swallowed with saliva and can survive gastric acidity to reach the intestinal tract [7,8,9]. Once established, these microorganisms may disrupt the balance of the gut microbiota, leading to dysbiosis characterized by reduced microbial diversity, altered metabolite production, and impaired epithelial barrier function [9,10,11]. Such changes facilitate systemic dissemination of bacterial products, promote endotoxemia, and shift host immune responses, particularly through activation of Th17 pathways and suppression of regulatory T cells [12,13].
This oral–gut axis not only provides a mechanistic explanation for the observed association between periodontitis and conditions such as diabetes, atherosclerosis, and IBD, but also highlights the bidirectional nature of this relationship, where intestinal dysbiosis can in turn exacerbate periodontal inflammation. As a result, the oral–gut connection represents a paradigm shift in understanding the systemic impact of oral diseases and has emerged as a promising focus for both mechanistic studies and translational therapeutic strategies.
This review will discuss recent reports on the interaction between the oral and intestinal microbiota and the causes of systemic pathological changes, including periodontal tissue.
2. Oral Dysbiosis, Gut Colonization, and Their Interplay in Systemic Diseases
A substantial body of evidence indicates that oral pathogens can survive transit through the gastrointestinal tract and subsequently colonize the gut, a phenomenon particularly prominent in individuals with compromised intestinal microbiota. The oral cavity, especially in the context of severe periodontitis, serves as a significant reservoir for this microbial translocation, with patients’ ingestion of a continuous high-volume bolus of oral bacteria. Estimates suggest that patients with periodontitis may swallow as many as \( 10^ {910^ {10}\) bacterial cells daily, a massive microbial load that challenges the gut’s normal “colonization resistance”. This phenomenon has been mechanistically validated in animal models, where oral administration of pathogens such as P. gingivalis has been shown to result in their successful persistence within the gastrointestinal tract. Intestinal colonization by these oral pathobionts subsequently alters the composition of the native gut microbiota, disrupts the integrity of the intestinal barrier, and contributes to a systemic inflammatory state. This evidence highlights a dynamic and critical oral–gut axis in which oral pathogens exploit a disrupted gut microenvironment to influence systemic health [9,14,15,16,17].
Among these translocating species, F. nucleatum has been one of the most consistently associated with gastrointestinal pathology. It has been frequently detected in colorectal carcinoma tissue and fecal samples from patients with colorectal cancer. Mechanically, F. nucleatum expresses FadA adhesin, which binds to E-cadherin in colonic epithelial cells, activating β-catenin signaling pathways that drive tumor cell proliferation. In addition, its Fap2 protein enables immune evasion by binding to the inhibitory receptor TIGIT on natural killer cells and T cells, thus dampening anti-tumor immunity. Beyond cancer, F. nucleatum has been implicated in exacerbating colitis by inducing pro-inflammatory cytokines such as IL-17 and TNF-α, further supporting its pathogenic role in the oral–gut axis (Table 1) [14,15,16,17,18].

Table 1.
Oral Pathogens, their Mechanisms in the Gut/Immune Axis, and Associated Systemic Effects.
Similarly, P. gingivalis has been identified as a key modulator of gut physiology. Experimental oral inoculation of mice with P. gingivalis results in decreased expression of tight junction proteins such as Tjp1 (ZO-1) and occludin (Ocln) in the intestinal epithelium, thus altering intestinal barrier function and leading to systemic endotoxemia [19]. Moreover, Oral administration of P. gingivalis has been shown to alter the gut microbiota, resulting in entero-hepatic metabolic disturbances that exacerbate hyperglycemia in obese murine models of type 2 diabetes [20]. Neurodegenerative models have demonstrated that oral P. gingivalis exacerbates Parkinsonian pathology and induces cognitive impairment through gut permeability disruption and neuroinflammation (Table 1) [21,22].
The systemic impact of oral–gut dysbiosis is extensive. In cardiovascular disease, metabolites such as trimethylamineN-oxide (TMAO) generated by the dysbiotic gut microbiota accelerate atherosclerosis [23]. Oral commensal Klebsiella spp., which is usually harmless in the oral cavity, can act as gut pathobionts when intestinal conditions are permissive. In murine models, colonization with Klebsiella pneumoniae induces strong Th1 responses, leading to colitis and systemic immune activation [24]. Furthermore, IBD is aggravated by oral bacteria colonizing the gut, which induce Th17-mediated immune responses. For example, ectopic gut colonization by Haemophilus parainfluenzae has been associated with IBD (Table 1) [25].
Interestingly, certain strains of Streptococcus mutans are a potential risk factor for aggravating ulcerative colitis (UC). This aggravation occurs when virulent oral strains, specifically those expressing a collagen-binding protein (CBP) and belonging to minor serotypes such as k or f, enter the bloodstream, often after dental procedures. These specific bacteria can resist phagocytosis and travel through the blood to the liver, where they are taken up by hepatocytes. The infection then stimulates the liver to produce the inflammatory cytokine interferon-gamma, which travels to the colon and intensifies existing inflammation. This blood-borne mechanism is significant, as oral administration of the bacteria does not produce the same effect, and patients with UC show a higher detection frequency of these specific, virulent S. mutans strains. Furthermore, Research has revealed a strong association between collagen-binding protein (Cnm) produced by certain Streptococcus mutans strains and the aggravation of hemorrhagic stroke. A 2011 Nature Communications study demonstrated that Cnm-positive serotype k strains can enter the bloodstream, adhere to exposed collagen in cerebral vessels, and disrupt platelet aggregation, thereby worsening intracerebral hemorrhage. The fact that recombinant Cnm protein alone induces hemorrhagic aggravation highlights its direct pathogenic role (Table 1) [26,27].
Furthermore, a group of bacterial species possessing genes that encode the enzymes β-glucuronidase and β-galactosidase, which are responsible for metabolizing conjugated estrogens, is collectively known as the “estrobolome.” Growing research interest has focused on this concept due to its potential role in influencing various diseases, including oral cancer. Although estrobolome-associated bacteria are predominantly found within the gut microbiota, emerging experimental evidence indicates a crosstalk between the oral and gut microbiomes. Notably, several oral bacterial species are also prevalent in the gut, where they may contribute to the activation of the estrobolome [28].
Collectively, these findings highlight the capacity of oral microorganisms not only to survive gastrointestinal passage but also to establish themselves as influential drivers of intestinal and systemic disease.
3. Experimental Models Used to Study Oral–Gut–Systemic Axis
To study the complex relationships within the oral–gut–systemic axis, researchers employ a variety of experimental models, most commonly using mice. These models allow for the manipulation of the oral microbiome and the observation of systemic health outcomes in a controlled setting (Figure 1).
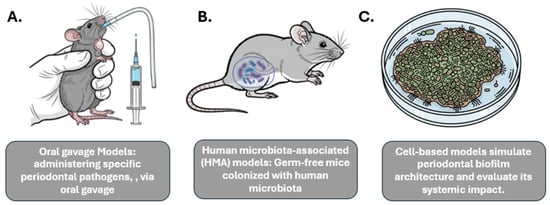
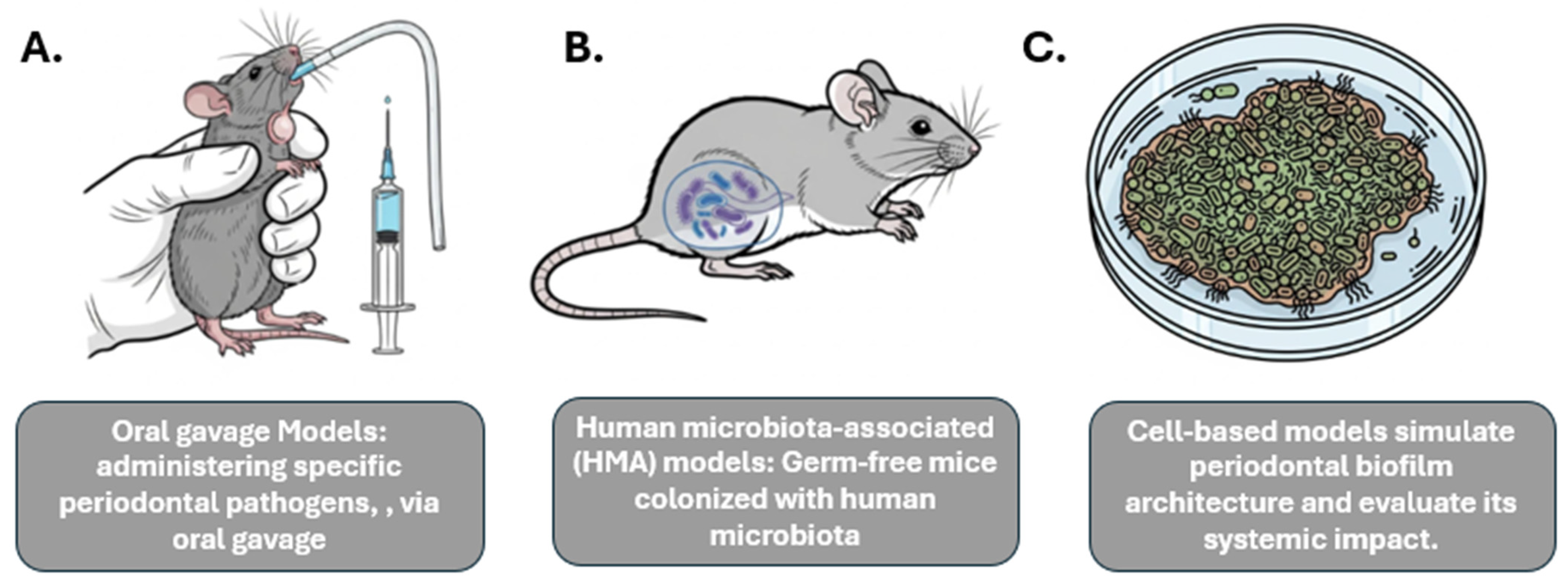
Figure 1.
Experimental Models Used to Study Oral–Gut–Systemic Axis. This figure illustrates diverse experimental models utilized to explore the intricate connections within the oral–gut–systemic axis. (A) Oral gavage Models depict the administration of specific periodontal pathogens via oral gavage in an animal model (e.g., mouse) to study their systemic impact. (B) Human microbiota-associated (HMA) models involve germ-free mice colonized with human microbiota to investigate host-microbiome interactions and their systemic effects. (C) Cell-based models demonstrate in vitro systems simulating periodontal biofilm architecture and evaluating its systemic implications through analysis of secreted factors or interactions with host cells. These models provide valuable tools for understanding the complex interplay between oral health, gut microbiota, and systemic conditions.
Oral gavage models: This method involves administering specific periodontal pathogens, such as P. gingivalis, via oral gavage to observe their systemic effects. This approach has been shown to induce gut barrier disruption, lead to metabolic endotoxemia (the presence of LPS in the bloodstream), and elevate systemic pro-inflammatory cytokines [29].
Human microbiota-associated (HMA) models: This approach uses germ-free mice that are colonized with human microbiota, allowing for the study of the effects of a human-relevant microbiome. Studies have shown that mice receiving salivary microbiota from periodontitis patients develop systemic pathologies, such as hepatic steatosis, adipose inflammation, and worsened NAFLD and colitis [19].
In addition to animal models, in vitro models are used to investigate specific mechanisms, such as those related to inflammatory signaling pathways. For example, cell-based models can simulate periodontal biofilm architecture and evaluate its systemic impact.
While useful for high-throughput screening, a key limitation is their inability to fully replicate the complexity of the oral–gut–systemic axis [30].
4. Immunological Mechanisms
The oral–gut immune axis represents a critical bidirectional inflammatory pathway, where the primary immunological mediators are often Th17 cells. During active oral pathologies like periodontitis, oral pathobionts such as Klebsiella, Enterobacter, and P. gingivalis expand and are ingested. These bacteria translocate to the gut where they activate the inflammasome within lamina propria macrophages. Concurrently, periodontal inflammation generates oral pathobiont-reactive Th17 effector cells in the oral cavity that are imprinted with gut-specific homing markers. Upon reaching the intestine, these oral-origin Th17 cells are reactivated by the translocated oral pathobionts and amplify the gut’s inflammatory response, driving the onset and progression of colitis. Conversely, gut-primed Th17 cells can also migrate to the oral mucosa and exacerbate periodontal inflammation, with studies showing that ectopic gut colonization by oral pathogens like P. gingivalis can enhance Th17 differentiation in Peyer’s patches, with these cells subsequently accumulating in the mouth and worsening periodontitis. This highlights how localized dysbiosis in one mucosal site can trigger systemic immune responses that fuel inflammation in the other, establishing a dynamic and reciprocal inflammatory cycle [31,32,33,34].
Beyond the Th17-centric T-cell migration, other immune mechanisms contribute to the complexities of the oral–gut axis. An imbalance between pro-inflammatory Th17 cells and anti-inflammatory regulatory T cells (Tregs) is a key feature, as the gut microbiota and its metabolites, such as short-chain fatty acids, are known to shape the Th17/Treg balance. Oral bacteria can also be transported to the gut by invading and utilizing immune cells like macrophages and dendritic cells as “Trojan horses”. This mechanism bypasses digestive barriers, delivering pathogens directly to the intestinal immune system where they can influence macrophage polarization. For example, specific oral pathobionts can activate pro-inflammatory M1 macrophages and drive inflammation. Furthermore, the oral–bone–gut axis illustrates how oral inflammation leaves a lasting mark on the immune system, specifically on hematopoietic progenitor cells in the bone marrow. Oral infection can program these progenitors to produce a more inflammatory phenotype of immune cells, retaining an “inflammatory memory” that can circulate and contribute to systemic inflammation, including in the gut, even after the initial oral infection has subsided. These diverse pathways reveal a multi-layered, interconnected system where oral health profoundly impacts systemic immune function [35,36,37,38,39].
5. Bidirectional Influence
Emerging evidence indicates that the interaction between the oral and gut microbiota is bidirectional. While oral microorganisms can translocate to the gastrointestinal tract and contribute to gut dysbiosis and systemic pathologies, alterations in the gut microbiome have likewise been shown to modulate the immune response, metabolic status, and microbial ecology of the oral cavity, thereby influencing the onset and progression of oral diseases. For example, dysbiosis within the gut exacerbates periodontal inflammation by disrupting the host’s systemic metabolic and immune balance, particularly through diet-induced changes. Furthermore, the close association between periodontitis and IBD is evidenced by the increased prevalence of IBD among patients with periodontitis and the greater incidence and severity of periodontitis in individuals with IBD. This bidirectional relationship suggests that periodontitis and IBD are interconnected rather than independent entities, forming an oral–gut axis through which each condition exacerbates the other, thereby establishing a self-perpetuating pathogenic cycle [40]. In high-fat-diet models, an altered gut microbiota leads to elevated levels of serum uric acid, a key metabolite derived from the purine degradation pathway. This systemic hyperuricemia promotes inflammation and drives excessive alveolar bone resorption, demonstrating a direct mechanistic link between diet-induced gut dysbiosis and periodontal tissue destruction [Figure 2] [40,41]. Conversely, a healthy gut microbiota contributes to bone and systemic health through the production of short-chain fatty acids (SCFAs), which play a pivotal role in maintaining bone homeostasis and intestinal integrity. SCFAs such as butyrate and propionate promote osteoblast activity, inhibit osteoclast differentiation, and enhance bone mineral density by modulating immune cell differentiation and systemic metabolism. Beyond their skeletal benefits, SCFAs—particularly butyrate—serve as the primary energy source for colonocytes, the epithelial cells lining the colon [42]. A reduction in SCFA-producing bacteria leads to diminished SCFA levels, resulting in impaired gut barrier function. This weakening of the intestinal epithelium increases permeability, a condition commonly referred to as “leaky gut,” which facilitates the translocation of bacterial endotoxins such as lipopolysaccharide (LPS) into the systemic circulation. The resulting endotoxemia triggers chronic, low-grade inflammation that has been strongly implicated in the onset and progression of IBD, colorectal cancer, and other systemic inflammatory disorders. Beyond the gut, low SCFA levels can have detrimental effects on metabolic and immunological health. Acetate and propionate are SCFAs that regulate glucose and lipid metabolism in tissues like the liver and fat cells. A decrease in their production can contribute to metabolic disorders, including obesity, insulin resistance, and type 2 diabetes. Furthermore, SCFAs influence the gut–brain axis and immune system regulation. Specifically, butyrate promotes the development of regulatory T cells, which help to suppress excessive immune responses and prevent autoimmunity. Without sufficient SCFAs, this critical immunomodulatory function is impaired, leading to a state of chronic inflammation that can affect multiple systems throughout the body [43,44,45,46].
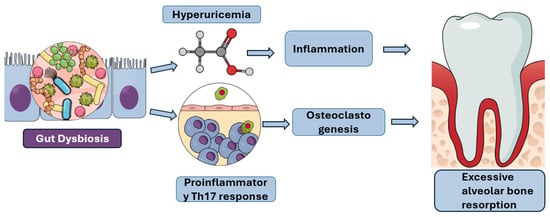
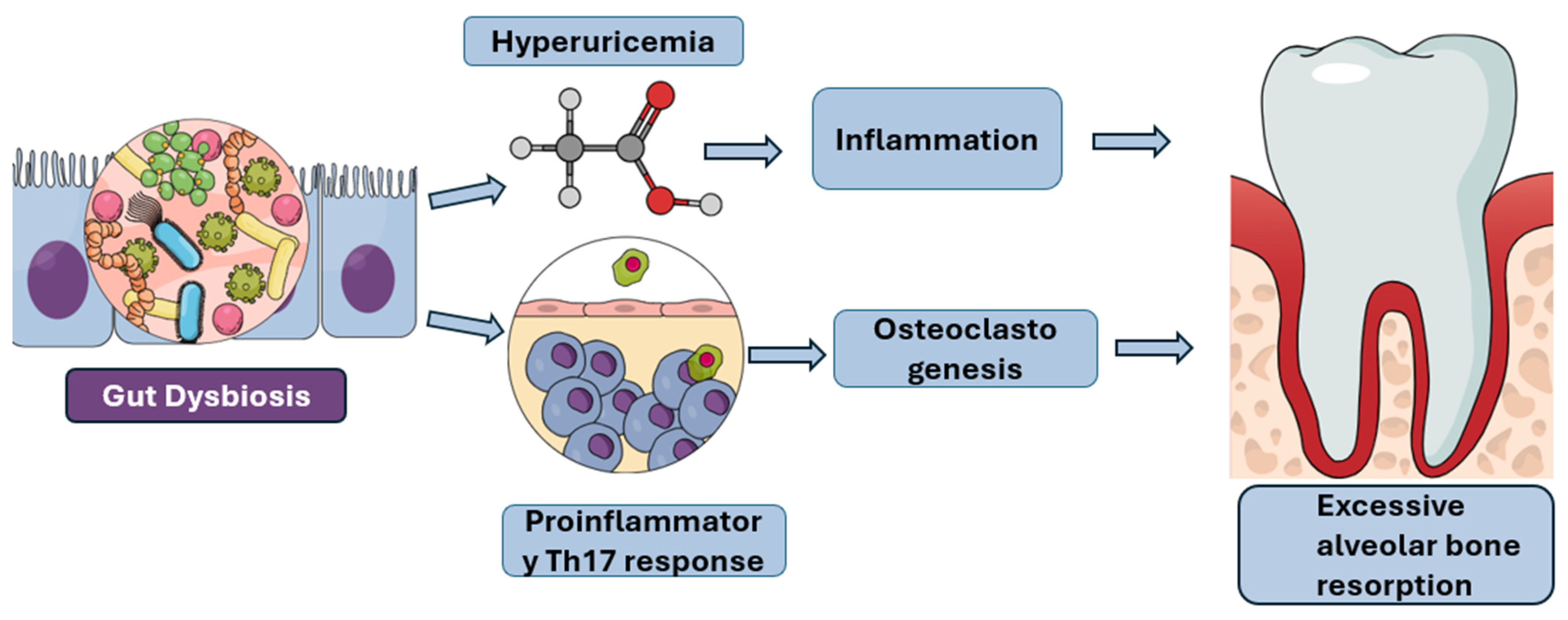
Figure 2.
Pathways Linking Gut Dysbiosis to Excessive Alveolar Bone Resorption: This figure illustrates the proposed mechanisms by which gut dysbiosis contributes to excessive alveolar bone resorption, a hallmark of periodontal disease. Gut dysbiosis can lead to hyperuricemia, which in turn promotes inflammation. Concurrently, gut dysbiosis can also induce a proinflammatory Th17 response and stimulate osteoclastogenesis. These interconnected pathways ultimately culminate in the accelerated breakdown of alveolar bone.Furthermore, gut dysbiosis shifts the immune landscape by promoting pro-inflammatory Th17 responses at the expense of protective regulatory T cells (Tregs), thereby inducing Th17-driven osteoclastogenesis and bone loss. When this Th17/Treg imbalance occurs in the gut due to dysbiosis, the pro-inflammatory immune cells are primed to migrate to distant sites, including the oral cavity, where they worsen local inflammation and accelerate periodontal bone destruction [Figure 2] [47]. In addition to Th17 activation, obesity and metabolic dysfunction contribute to systemic inflammation through adipose tissue-derived inflammatory mediators, which perpetuates a vicious cycle. This cycle links gut dysbiosis to heightened systemic inflammation, which in turn impairs the periodontium’s ability to resist bacterial challenge, ultimately accelerating periodontal disease and bone loss [48].
The intricate interplay of these microbial, metabolic, and immunological pathways reveals how gut health profoundly influences periodontal disease progression, offering a potential target for therapeutic intervention through managing obesity and metabolic disorders.
6. Clinical Implications
The oral–gut axis represents a critical frontier in understanding systemic health, reflecting a bidirectional communication network where microbial homeostasis is pivotal to preventing disease. Oral pathogens, such as P. gingivalis and F. nucleatum, can translocate to the gut, disrupting the delicate intestinal microbiome and exacerbating systemic inflammation, which is implicated in metabolic and autoimmune pathologies. This dysbiotic crosstalk can be harnessed for novel diagnostic and therapeutic strategies. For diagnostics, the analysis of salivary and fecal microbiota offers a non-invasive approach to identifying predictive biomarkers for diseases like colorectal cancer and IBD, reflecting systemic changes. Therapeutically, interventions aim to restore microbial balance; these include the use of prebiotics and probiotics, fecal microbiota transplantation (FMT) for severe dysbiosis, and pharmaceuticals such as metformin and allopurinol, which have been shown to modulate the gut microbiota. Crucially, preventative oral care, including diligent hygiene practices, serves as an essential and accessible intervention to disrupt this pathological loop, particularly for patients with metabolic and autoimmune conditions, thereby mitigating systemic inflammatory burden. Unraveling the complex dynamics of this oral–gut continuum is key to developing precise diagnostic tools and effective treatments that address disease at its source [39,49,50,51,52,53,54].
7. Future Directions
Prospective research necessitates the integration of multi-omics strategies, including metagenomics, metabolomics, and immunomics, to systematically delineate the intricate molecular interactions governing the oral–gut–systemic axis. The complexity of these datasets requires advanced bioinformatic platforms and systems biology approaches for effective integration, moving beyond traditional analyses that often fail to capture the holistic interplay between different biological layers. A critical next step is the execution of rigorous, randomized clinical trials to validate the efficacy of microbiome-modulating therapies, such as probiotics or prebiotics, in patients with periodontitis and concurrent systemic comorbidities. For example, a recent study demonstrated that paraprobiotic-based toothpaste and mouthwash, used alongside scaling and root planning, significantly improved clinical periodontal parameters and reduced pathogenic bacterial counts more effectively than conventional chlorhexidine treatment [55]. Such trials are essential for transitioning promising mechanistic findings into clinically actionable interventions. Ultimately, the synthesis of these multi-omics data and clinical evidence will inform the development of precision dentistry, where individualized patient care is guided by systemic health profiles, thereby ushering in a new standard of preventative and therapeutic dental practice. This paradigm shift will leverage biomarker identification and predictive modeling to mitigate disease progression and improve overall patient outcomes in the era of precision medicine
8. Conclusions
The oral–gut–systemic axis offers a unifying mechanistic framework linking periodontitis and systemic diseases. The bidirectional relationship between oral and gut dysbiosis underscores the importance of oral health in systemic disease prevention and management. Clinical translation of these insights holds promise for diagnostics, prognostics, and microbiome-targeted therapies
The robust mechanistic framework of the oral–gut–systemic axis demands a paradigm shift from reactive treatment to proactive, integrated healthcare. Multidisciplinary teams, including dental, medical, and nutritional specialists, must collaborate to implement comprehensive prevention and management strategies. By leveraging advanced diagnostics and precision microbiome-targeted therapies, such as specific prebiotics and probiotics, clinicians can actively modulate dysbiosis to prevent the onset and progression of chronic disease. This integrative approach not only improves patient outcomes but also redefines systemic health by placing oral and gut microbial balance at the forefront of holistic care.
Funding
This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| P. gingivalis | Porphyromonas gingivalis |
| F. nucleatum | Fusobacterium nucleatum |
| CRC | Colorectal Cancer |
| FadA | Fusobacterium adhesin A (protein) |
| Fap2 | Fusobacterium protein involved in immune evasion |
| TIGIT | T-cell Immunoreceptor with Ig and ITIM domains |
| IL | Interleukin (e.g., IL-17) |
| TNF-α | Tumor Necrosis Factor alpha |
| ZO-1 | Zonula Occludens-1 (tight junction protein) |
| Ocln | Occludin (tight junction protein) |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| RA | Rheumatoid Arthritis |
| UC | Ulcerative Colitis |
| CBP | Collagen-Binding Protein |
| Cnm | Collagen-binding protein of Streptococcus mutans |
| TMAO | Trimethylamine N-oxide |
| IBD | Inflammatory Bowel Disease |
| HMA | Human Microbiota-Associated (mouse models) |
| LPS | Lipopolysaccharide |
| Th17 | T helper 17 cells |
| Tregs | Regulatory T cells |
| SCFAs | Short-Chain Fatty Acids |
| FMT | Fecal Microbiota Transplantation |
References
- Escapa, I.F.; Chen, T.; Huang, Y.; Gajare, P.; Dewhirst, F.E.; Lemon, K.P. New Insights into Human Nostril Microbiome from the Expanded Human Oral Microbiome Database (eHOMD): A Resource for the Microbiome of the Human Aerodigestive Tract. mSystems 2018, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Koren, N.; Zubeidat, K.; Saba, Y.; Horev, Y.; Barel, O.; Wilharm, A.; Heyman, O.; Wald, S.; Eli-Berchoer, L.; Shapiro, H.; et al. Maturation of the neonatal oral mucosa involves unique epithelium-microbiota interactions. Cell Host Microbe 2021, 29, 197–209.e195. [Google Scholar] [CrossRef]
- Radaic, A.; Kapila, Y.L. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef]
- Benítez-Páez, A.; Álvarez, M.; Belda-Ferre, P.; Rubido, S.; Mira, A.; Tomás, I. Detection of transient bacteraemia following dental extractions by 16S rDNA pyrosequencing: A pilot study. PLoS ONE 2013, 8, e57782. [Google Scholar] [CrossRef]
- Castillo, D.M.; Sánchez-Beltrán, M.C.; Castellanos, J.E.; Sanz, I.; Mayorga-Fayad, I.; Sanz, M.; Lafaurie, G.I. Detection of specific periodontal microorganisms from bacteraemia samples after periodontal therapy using molecular-based diagnostics. J. Clin. Periodontol. 2011, 38, 418–427. [Google Scholar] [CrossRef]
- Marin, M.J.; Figuero, E.; Gonzalez, I.; O’Connor, A.; Diz, P.; Alvarez, M.; Herrera, D.; Sanz, M. Comparison of the detection of periodontal pathogens in bacteraemia after tooth brushing by culture and molecular techniques. Med. Oral Patol. Oral Cir. Bucal. 2016, 21, e276–e284. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Sheng, H.; Zhen, Y.; Wu, B.; Li, Z.; Chen, D.; Zhou, H. Oral Fusobacterium nucleatum resists the acidic pH of the stomach due to membrane erucic acid synthesized via enoyl-CoA hydratase-related protein FnFabM. J. Oral. Microbiol. 2025, 17, 2453964. [Google Scholar] [CrossRef]
- Chopra, A.; Shiheido-Watanabe, Y.; Eberhard, J. Editorial: Porphyromonas gingivalis: Molecular mechanisms of invasion, immune evasion, and dysbiosis. Front. Cell Infect Microbiol. 2023, 13, 1289103. [Google Scholar] [CrossRef]
- Olsen, I.; Yamazaki, K. Can oral bacteria affect the microbiome of the gut? J. Oral Microbiol. 2019, 11, 1586422. [Google Scholar] [CrossRef]
- Khor, B.; Snow, M.; Herrman, E.; Ray, N.; Mansukhani, K.; Patel, K.A.; Said-Al-Naief, N.; Maier, T.; Machida, C.A. Interconnections Between the Oral and Gut Microbiomes: Reversal of Microbial Dysbiosis and the Balance Between Systemic Health and Disease. Microorganisms 2021, 9, 496. [Google Scholar] [CrossRef]
- Sohn, J.; Li, L.; Zhang, L.; Settem, R.P.; Honma, K.; Sharma, A.; Falkner, K.L.; Novak, J.M.; Sun, Y.; Kirkwood, K.L. Porphyromonas gingivalis indirectly elicits intestinal inflammation by altering the gut microbiota and disrupting epithelial barrier function through IL9-producing CD4(+) T cells. Mol. Oral Microbiol. 2022, 37, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Nagao, J.I.; Kishikawa, S.; Tanaka, H.; Toyonaga, K.; Narita, Y.; Negoro-Yasumatsu, K.; Tasaki, S.; Arita-Morioka, K.I.; Nakayama, J.; Tanaka, Y. Pathobiont-responsive Th17 cells in gut-mouth axis provoke inflammatory oral disease and are modulated by intestinal microbiome. Cell Rep. 2022, 40, 111314. [Google Scholar] [CrossRef] [PubMed]
- Velsko, I.M.; Chukkapalli, S.S.; Rivera-Kweh, M.F.; Chen, H.; Zheng, D.; Bhattacharyya, I.; Gangula, P.R.; Lucas, A.R.; Kesavalu, L. Fusobacterium nucleatum Alters Atherosclerosis Risk Factors and Enhances Inflammatory Markers with an Atheroprotective Immune Response in ApoE(null) Mice. PLoS ONE 2015, 10, e0129795. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Baik, J.E.; Lagana, S.M.; Han, R.P.; Raab, W.J.; Sahoo, D.; Dalerba, P.; Wang, T.C.; Han, Y.W. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/β-catenin modulator Annexin A1. EMBO Rep. 2019, 20, e47638. [Google Scholar] [CrossRef]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef]
- Nakajima, M.; Arimatsu, K.; Kato, T.; Matsuda, Y.; Minagawa, T.; Takahashi, N.; Ohno, H.; Yamazaki, K. Oral Administration of P. gingivalis Induces Dysbiosis of Gut Microbiota and Impaired Barrier Function Leading to Dissemination of Enterobacteria to the Liver. PLoS ONE 2015, 10, e0134234. [Google Scholar] [CrossRef]
- Kashiwagi, Y.; Aburaya, S.; Sugiyama, N.; Narukawa, Y.; Sakamoto, Y.; Takahashi, M.; Uemura, H.; Yamashita, R.; Tominaga, S.; Hayashi, S.; et al. Porphyromonas gingivalis induces entero-hepatic metabolic derangements with alteration of gut microbiota in a type 2 diabetes mouse model. Sci. Rep. 2021, 11, 18398. [Google Scholar]
- Feng, Y.K.; Wu, Q.L.; Peng, Y.W.; Liang, F.Y.; You, H.J.; Feng, Y.W.; Li, G.; Li, X.J.; Liu, S.H.; Li, Y.C.; et al. Oral P. gingivalis impairs gut permeability and mediates immune responses associated with neurodegeneration in LRRK2 R1441G mice. J. Neuroinflammation 2020, 17, 347. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Hao, M.; Shi, N.; Wang, X.; Yuan, L.; Yuan, H.; Wang, X. Porphyromonas gingivalis: A potential trigger of neurodegenerative disease. Front. Immunol. 2025, 16, 1482033. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Huang, L.; Zhou, X.; Zhao, D.; Wang, Y.; Min, H.; Song, S.; Sun, W.; Gao, Q.; Hu, Q.; et al. Experimental Periodontitis Deteriorated Atherosclerosis Associated with Trimethylamine N-Oxide Metabolism in Mice. Front. Cell. Infect. Microbiol. 2021, 11, 820535. [Google Scholar]
- Atarashi, K.; Suda, W.; Luo, C.; Kawaguchi, T.; Motoo, I.; Narushima, S.; Kiguchi, Y.; Yasuma, K.; Watanabe, E.; Tanoue, T.; et al. Ectopic colonization of oral bacteria in the intestine drives T(H)1 cell induction and inflammation. Science 2017, 358, 359–365. [Google Scholar] [CrossRef]
- Sohn, J.; Li, L.; Zhang, L.; Genco, R.J.; Falkner, K.L.; Tettelin, H.; Rowsam, A.M.; Smiraglia, D.J.; Novak, J.M.; Diaz, P.I.; et al. Periodontal disease is associated with increased gut colonization of pathogenic Haemophilus parainfluenzae in patients with Crohn’s disease. Cell Rep. 2023, 42, 112120. [Google Scholar] [CrossRef]
- Nakano, K.; Hokamura, K.; Taniguchi, N.; Wada, K.; Kudo, C.; Nomura, R.; Kojima, A.; Naka, S.; Muranaka, Y.; Thura, M.; et al. The collagen-binding protein of Streptococcus mutans is involved in haemorrhagic stroke. Nat. Commun. 2011, 2, 485. [Google Scholar] [CrossRef]
- Kojima, A.; Nakano, K.; Wada, K.; Takahashi, H.; Katayama, K.; Yoneda, M.; Higurashi, T.; Nomura, R.; Hokamura, K.; Muranaka, Y.; et al. Infection of specific strains of Streptococcus mutans, oral bacteria, confers a risk of ulcerative colitis. Sci. Rep. 2012, 2, 332. [Google Scholar] [CrossRef]
- Tatullo, M.; Nor, J.; Orru, G.; Piattelli, A.; Cascardi, E.; Spagnuolo, G. Oral-Gut-Estrobolome Axis May Exert a Selective Impact on Oral Cancer. J. Dent. Res. 2024, 103, 461–466. [Google Scholar] [CrossRef]
- Huang, L.; Ge, S.; Yang, K.; Duan, L.; Gao, L.; Li, Y.Z.; Yi, Y.S. Effects of oral gavage with periodontal pathogens and plaque biofilm on gut microbiota ecology and intestinal tissue architecture in mice: A mechanistic study. Front. Cell. Infect. Microbiol. 2025, 15, 1589055. [Google Scholar] [CrossRef]
- Montgomery, M.L.; Fuller, K.K. Experimental Models for Fungal Keratitis: An Overview of Principles and Protocols. Cells 2020, 9, 1713. [Google Scholar] [CrossRef]
- Kitamoto, S.; Nagao-Kitamoto, H.; Jiao, Y.; Gillilland, M.G., 3rd; Hayashi, A.; Imai, J.; Sugihara, K.; Miyoshi, M.; Brazil, J.C.; Kuffa, P.; et al. The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell 2020, 182, 447–462.e414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, N.; Wang, Z.; Zeng, X.; Ji, N.; Chen, Q. Targeting Th17 cells: A promising strategy to treat oral mucosal inflammatory diseases. Front. Immunol. 2023, 14, 1236856. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, Y.; Huang, Y.; Jin, Z.; Li, Z.; Chen, J.; Hu, F.; Zhang, X.; Rausch-Fan, X. Periodontitis-induced oral microbiome alterations provide clues on how periodontitis exacerbates colitis. J. Clin. Periodontol. 2023, 50, 627–641. [Google Scholar] [CrossRef]
- Tanwar, H.; Gnanasekaran, J.M.; Allison, D.; Chuang, L.-s.; He, X.; Aimetti, M.; Baima, G.; Costalonga, M.; Cross, R.K.; Sears, C.; et al. Unravelling the Oral–Gut Axis: Interconnection Between Periodontitis and Inflammatory Bowel Disease, Current Challenges, and Future Perspective. J. Crohn’s Colitis 2024, 18, 1319–1341. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, W.; Li, Y.; Cui, J.; Zhu, M.; Liu, Y.; Liu, Y. The oral-gut microbiota axis: A link in cardiometabolic diseases. NPJ Biofilms Microbiomes 2025, 11, 11. [Google Scholar] [CrossRef]
- Kitamoto, S.; Kamada, N. The oral-gut axis: A missing piece in the IBD puzzle. Inflamm. Regen. 2023, 43, 54. [Google Scholar] [CrossRef]
- Wang, A.; Zhai, Z.; Ding, Y.; Wei, J.; Wei, Z.; Cao, H. The oral-gut microbiome axis in inflammatory bowel disease: From inside to insight. Front. Immunol. 2024, 15, 1430001. [Google Scholar] [CrossRef]
- Lun, H.; Li, P.; Li, J.; Liu, F. The effect of intestinal flora metabolites on macrophage polarization. Heliyon 2024, 10, e35755. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, W.; Wang, Q.; Jiang, C.; Li, H.; Chao, Y.; Sun, Y.; A, L. The effect of the “Oral-Gut” axis on periodontitis in inflammatory bowel disease: A review of microbe and immune mechanism associations. Front. Cell Infect Microbiol. 2023, 13, 1132420. [Google Scholar] [CrossRef]
- Reytor-Gonzalez, C.; Parise-Vasco, J.M.; Gonzalez, N.; Simancas-Racines, A.; Zambrano-Villacres, R.; Zambrano, A.K.; Simancas-Racines, D. Obesity and periodontitis: A comprehensive review of their interconnected pathophysiology and clinical implications. Front. Nutr. 2024, 11, 1440216. [Google Scholar] [CrossRef]
- Sato, K.; Yamazaki, K.; Kato, T.; Nakanishi, Y.; Tsuzuno, T.; Yokoji-Takeuchi, M.; Yamada-Hara, M.; Miura, N.; Okuda, S.; Ohno, H.; et al. Obesity-Related Gut Microbiota Aggravates Alveolar Bone Destruction in Experimental Periodontitis through Elevation of Uric Acid. mBio 2021, 12, e0077121. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Chiba, T.; Tousen, Y. Short-chain fatty acids, acetate and propionate, directly upregulate osteoblastic differentiation. Int. J. Food Sci. Nutr. 2022, 73, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Nireeksha; Maniangat Luke, A.; Kumari, N.S.; Hegde, M.N.; Hegde, N.N. Metabolic interplay of SCFA’s in the gut and oral microbiome: A link to health and disease. Front. Oral Health 2025, 6, 1646382. [Google Scholar] [CrossRef] [PubMed]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 2325–2340. [Google Scholar] [CrossRef]
- Münte, E.; Hartmann, P. The Role of Short-Chain Fatty Acids in Metabolic Dysfunction-Associated Steatotic Liver Disease and Other Metabolic Diseases. Biomolecules 2025, 15, 469. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Dutzan, N.; Kajikawa, T.; Abusleme, L.; Greenwell-Wild, T.; Zuazo, C.E.; Ikeuchi, T.; Brenchley, L.; Abe, T.; Hurabielle, C.; Martin, D.; et al. A dysbiotic microbiome triggers T(H)17 cells to mediate oral mucosal immunopathology in mice and humans. Sci. Transl. Med. 2018, 10, eaat0797. [Google Scholar] [CrossRef]
- Jia, X.; Yang, R.; Li, J.; Zhao, L.; Zhou, X.; Xu, X. Gut-Bone Axis: A Non-Negligible Contributor to Periodontitis. Front. Cell Infect Microbiol. 2021, 11, 752708. [Google Scholar] [CrossRef]
- Gao, Y.; Bi, D.; Xie, R.; Li, M.; Guo, J.; Liu, H.; Guo, X.; Fang, J.; Ding, T.; Zhu, H.; et al. Fusobacterium nucleatum enhances the efficacy of PD-L1 blockade in colorectal cancer. Signal Transduct. Target Ther. 2021, 6, 398. [Google Scholar] [CrossRef]
- Rong, J.; Chen, X.; Li, Z.; Li, B.; Sun, Y.; Miao, Y. Dysregulation of saliva and fecal microbiota as novel biomarkers of colorectal cancer. Front. Oncol. 2024, 14, 1498328. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, Z.; Li, Y.; Lv, C.; Li, C.; Hu, Y.; Fu, M.; Song, L. Salivary and fecal microbiota: Potential new biomarkers for early screening of colorectal polyps. Front. Microbiol. 2023, 14, 1182346. [Google Scholar] [CrossRef]
- Acharya, C.; Sahingur, S.E.; Bajaj, J.S. Microbiota, cirrhosis, and the emerging oral-gut-liver axis. JCI Insight 2017, 2, e94416. [Google Scholar] [CrossRef]
- Azzolino, D.; Carnevale-Schianca, M.; Santacroce, L.; Colella, M.; Felicetti, A.; Terranova, L.; Castrejon-Perez, R.C.; Garcia-Godoy, F.; Lucchi, T.; Passarelli, P.C. The Oral-Gut Microbiota Axis Across the Lifespan: New Insights on a Forgotten Interaction. Nutrients 2025, 17, 2538. [Google Scholar] [CrossRef]
- Tortora, S.C.; Agurto, M.G.; Martello, L.A. The oral-gut-circulatory axis: From homeostasis to colon cancer. Front. Cell Infect Microbiol. 2023, 13, 1289452. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Maiorani, C.; Milone, A.; Alovisi, M.; Scribante, A. Paraprobiotics in Non-Surgical Periodontal Therapy: Clinical and Microbiological Aspects in a 6-Month Follow-Up Domiciliary Protocol for Oral Hygiene. Microorganisms 2022, 10, 337. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).