Infective Complications of Endobronchial Ultrasound-Transbronchial Needle Aspiration (EBUS-TBNA) and Clinical Biomarkers: A Concise Review
Highlights
- EBUS-TBNA is a minimally invasive procedure performed globally on thousands of patients and major complications, especially infective ones, may be underestimated
- Infective complications can be abrupt or encountered for up to 4 weeks after the procedure
- Patients’ comorbidities and radiological characteristics of the lesions can have a prognostic role in predicting, preventing and managing these complications
- Preventive measures like periprocedural administration of antibiotics and preprocedural sterilization mouth rinses to reduce infective complications have not been prospectively evaluated
Abstract
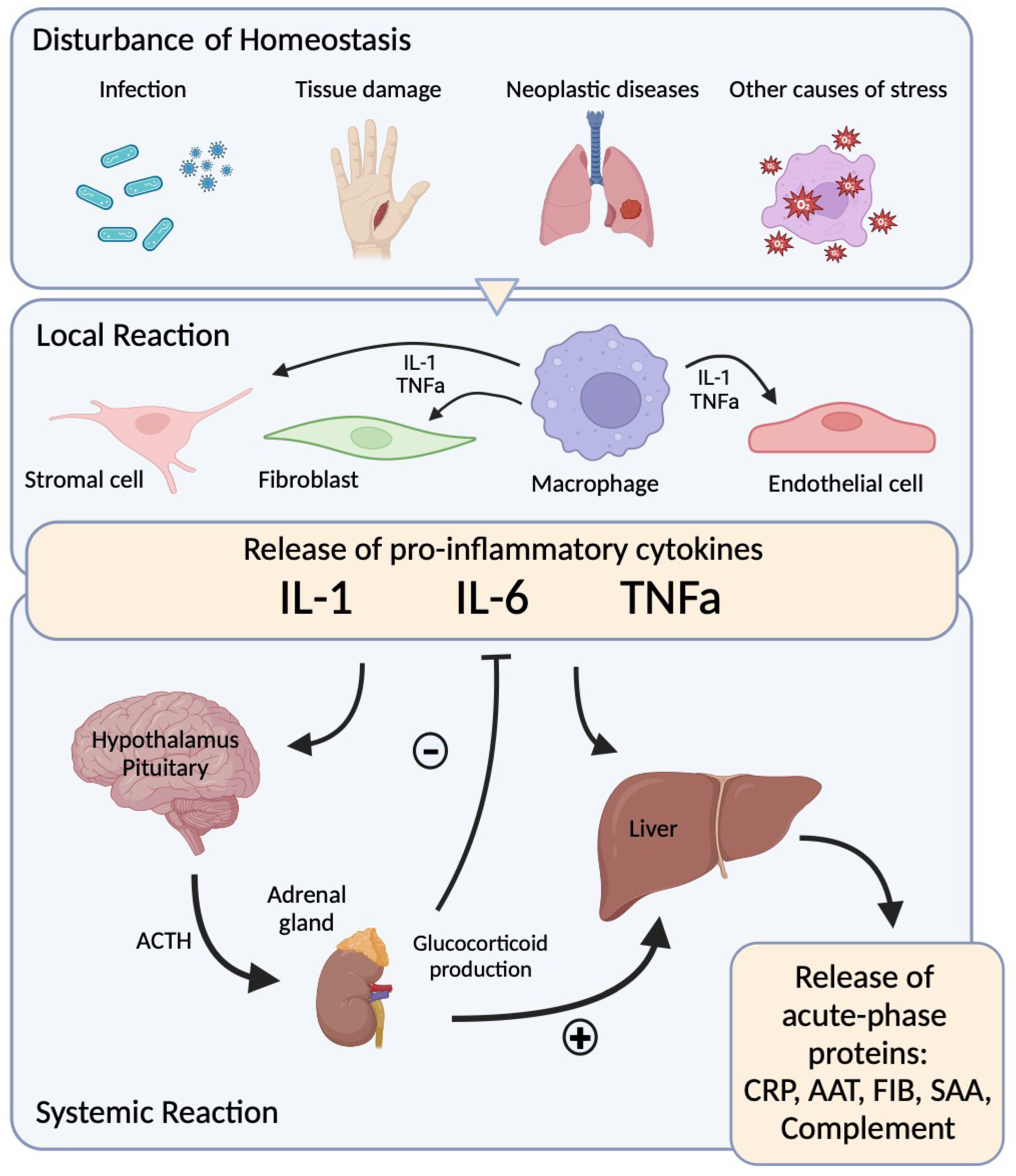
1. Introduction
2. EBUS-TBNA and Targeted Therapy in Lung Cancer
3. Infective Complications of EBUS-TBNA
4. Laboratory Biomarkers That Can Be Used to Predict EBUS-TBNA Related Infective Complications
5. Interleukin 6
6. Procalcitonin
7. C-Reactive Protein
8. Conclusions
Funding
Conflicts of Interest
References
- Sehgal, I.S.; Dhooria, S.; Aggarwal, A.N.; Behera, D.; Agarwal, R. Endosonography Versus Mediastinoscopy in Mediastinal Staging of Lung Cancer: Systematic Review and Meta-Analysis. Ann. Thorac. Surg. 2016, 102, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Madan, K.; Hadda, V.; Mittal, S.; Suri, T.; Shekh, I.; Guleria, R.; Khader, A.; Chhajed, P.; Christopher, D.J.; et al. Guidelines for Endobronchial Ultrasound-transbronchial Needle Aspiration (EBUS-TBNA): Joint Indian Chest Society (ICS)/Indian Association for Bronchology (IAB) Recommendations. Lung India 2023, 40, 368–400. [Google Scholar] [CrossRef] [PubMed]
- Serra Mitjà, P.; García-Cabo, B.; Garcia-Olivé, I.; Radua, J.; Rami-Porta, R.; Esteban, L.; Barreiro, B.; Call, S.; Centeno, C.; Andreo, F.; et al. EBUS-TBNA for Mediastinal Staging of Centrally Located T1N0M0 Non-Small Cell Lung Cancer Clinically Staged with PET/CT. Respirology 2024, 29, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Sampsonas, F.; Kakoullis, L.; Lykouras, D.; Karkoulias, K.; Spiropoulos, K. EBUS: Faster, Cheaper and Most Effective in Lung Cancer Staging. Int. J. Clin. Pract. 2018, 72, e13053. [Google Scholar] [CrossRef] [PubMed]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of Lung Cancer. Wspolczesna Onkol. 2021, 25, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Bosgana, P.; Nikou, S.; Dimitrakopoulos, F.I.; Bravou, V.; Kalophonos, C.; Kourea, E.; Tzelepi, V.; Zolota, V.; Sampsonas, F. Expression of Pluripotency Factors OCT4 and LIN28 Correlates with Survival Outcome in Lung Adenocarcinoma. Med. Lith. 2024, 60, 870. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.R.; Yatabe, Y.; Beer, D.G.; Powell, C.A.; Riely, G.J.; Van Schil, P.E.; et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J. Thorac. Oncol. 2011, 6, 244–285. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.; Yatabe, Y.; Ishikawa, Y.; Wistuba, I.; Flieder, D.B.; Franklin, W.; et al. Diagnosis of Lung Cancer in Small Biopsies and Cytology: Implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Classification. Arch. Pathol. Lab. Med. 2013, 137, 668–684. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.; Yatabe, Y.; Ishikawa, Y.; Wistuba, I.; Flieder, D.B.; Franklin, W.; et al. Diagnosis of Lung Adenocarcinoma in Resected Specimens: Implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Classification. Arch. Pathol. Lab. Med. 2012, 137, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.E.; Minna, J.D. Molecular Biology of Lung Cancer: Clinical Implications. Clin. Chest Med. 2011, 32, 703–740. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, G.A.; Gonzalez, A.V.; Jantz, M.A.; Margolis, M.L.; Gould, M.K.; Tanoue, L.T.; Harris, L.J.; Detterbeck, F.C. Diagnosis and Management of Lung Cancer, 3rd ed: ACCP Guidelines. Chest 2013, 143, e211S–e250S. [Google Scholar] [CrossRef]
- Sequist, L.V.; Neal, J.W. Personalized, Genotype-Directed Therapy for Advanced Non-Small Cell Lung Cancer. 2018. Available online: https://www.uptodate.com/contents/personalized-genotype-directed-therapy-for-advanced-non-small-cell-lung-cancer (accessed on 1 December 2024).
- Russell, P.A.; Wainer, Z.; Wright, G.M.; Daniels, M.; Conron, M.; Williams, R.A. Does Lung Adenocarcinoma Subtype Predict Patient Survival?: A Clinicopathologic Study Based on the New International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Lung Adeno. J. Thorac. Oncol. 2011, 6, 1496–1504. [Google Scholar] [CrossRef]
- KRAS Mutation as a Biomarker for Survival in Patients with Non-Small Cell Lung Cancer, A Meta-Analysis of 12 Randomized Trials. Asian Pac. J. Cancer Prev. 2015, 16, 4439–4445. [CrossRef] [PubMed]
- Purandare, N.C.; Rangarajan, V. Imaging of Lung Cancer: Implications on Staging and Management. Indian J. Radiol. Imaging 2015, 25, 109–120. [Google Scholar] [CrossRef]
- Kerr, K.M.; Bubendorf, L.; Edelman, M.J.; Marchetti, A.; Mok, T.; Novello, S.; O’Byrne, K.; Stahel, R.; Peters, S.; Felip, E.; et al. Second ESMO Consensus Conference on Lung Cancer: Pathology and Molecular Biomarkers for Non-Small-Cell Lung Cancer. Ann. Oncol. 2014, 25, 1681–1690. [Google Scholar] [CrossRef]
- Shigematsu, H.; Lin, L.; Takahashi, T.; Nomura, M.; Suzuki, M.; Wistuba, I.I.; Fong, K.M.; Lee, H.; Toyooka, S.; Shimizu, N.; et al. Clinical and Biological Features Associated with Epidermal Growth Factor Receptor Gene Mutations in Lung Cancers. J. Natl. Cancer Inst. 2005, 97, 339–346. [Google Scholar] [CrossRef]
- Sehgal, I.S.; Agarwal, R.; Dhooria, S.; Prasad, K.; Aggarwal, A.N. Role of EBUS TBNA in Staging of Lung Cancer: A Clinician’s Perspective. J. Cytol. 2019, 36, 61–64. [Google Scholar] [CrossRef]
- Zappa, C.; Mousa, S.A. Non-Small Cell Lung Cancer: Current Treatment and Future Advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef]
- Mok, T.S.K. Personalized Medicine in Lung Cancer: What We Need to Know. Nat. Rev. Clin. Oncol. 2011, 8, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-Small Cell Lung Cancer, Version 3.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef]
- Braga, S.; Costa, R.; Magalhães, A.; Fernandes, G. EBUS-TBNA in Mediastinal Staging of Non-Small Cell Lung Cancer: Comparison with Pathological Staging. J. Bras. Pneumol. 2024, 50, e20230353. [Google Scholar] [CrossRef]
- Aravena, C.; Patel, J.; Goyal, A.; Jaber, W.; Khemasuwan, D.; MacHuzak, M.; Cicenia, J.; Gildea, T.; Sethi, S.; Mehta, A.C.; et al. Role of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration in the Diagnosis and Management of Mediastinal Cyst. J. Bronchol. Interv. Pulmonol. 2020, 27, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Vilmann, P.; Clementsen, P.F.; Colella, S.; Siemsen, M.; De Leyn, P.; Dumonceau, J.M.; Herth, F.J.; Larghi, A.; Vasquez-Sequeiros, E.; Hassan, C.; et al. Combined Endobronchial and Esophageal Endosonography for the Diagnosis and Staging of Lung Cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in Cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Endoscopy 2015, 47, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zheng, X.; Mao, X.; Zhao, R.; Ye, J.; Zhang, Y.; Sun, J. Next-Generation Sequencing for Genotyping of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration Samples in Lung Cancer. Ann. Thorac. Surg. 2019, 108, 219–226. [Google Scholar] [CrossRef]
- Puri, S.; Saltos, A.; Perez, B.; Le, X.; Gray, J.E. Locally Advanced, Unresectable Non-Small Cell Lung Cancer. Curr. Oncol. Rep. 2020, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Mithoowani, H.; Febbraro, M. Non-Small-Cell Lung Cancer in 2022: A Review for General Practitioners in Oncology. Curr. Oncol. 2022, 29, 1828–1839. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takezawa, K.; Pirazzoli, V.; Arcila, M.E.; Nebhan, C.A.; Song, X.; de Stanchina, E.; Ohashi, K.; Janjigian, Y.Y.; Spitzler, P.J.; Melnick, M.A.; et al. HER2 Amplification: A Potential Mechanism of Acquired Resistance to EGFR Inhibition in EGFR-Mutant Lung Cancers That Lack the Second-Site EGFRT790M Mutation. Cancer Discov. 2012, 2, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Qiu, L.-X.; Liao, R.-Y.; Du, F.-B.; Ding, H.; Yang, W.-C.; Li, J.; Chen, Q. KRAS Mutations and Resistance to EGFR-TKIs Treatment in Patients with Non-Small Cell Lung Cancer: A Meta-Analysis of 22 Studies. Lung Cancer 2010, 69, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Tuminello, S.; Sikavi, D.; Veluswamy, R.; Gamarra, C.; Lieberman-Cribbin, W.; Flores, R.; Taioli, E. PD-L1 as a Prognostic Biomarker in Surgically Resectable Nonsmall Cell Lung Cancer: A Meta-Analysis. Transl. Lung Cancer Res. 2020, 9, 1343–1360. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Boyle, T.A.; Zhou, C.; Rimm, D.L.; Hirsch, F.R. PD-L1 Expression in Lung Cancer. J. Thorac. Oncol. 2016, 11, 964–975. [Google Scholar] [CrossRef]
- Santini, F.C.; Hellmann, M.D. PD-1/PD-L1 Axis in Lung Cancer. Cancer J. 2018, 24, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Muriana, P.; Rossetti, F. The Role of EBUS-TBNA in Lung Cancer Restaging and Mutation Analysis. Mediastinum 2020, 4, 23. [Google Scholar] [CrossRef]
- Sandler, J.E.; D’Aiello, A.; Halmos, B. Changes in Store for Early-Stage Non-Small Cell Lung Cancer. J. Thorac. Dis. 2019, 11, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Kalemkerian, G.P.; Narula, N.; Kennedy, E.B.; Biermann, W.A.; Donington, J.; Leighl, N.B.; Lew, M.; Pantelas, J.; Ramalingam, S.S.; Reck, M.; et al. Association for Molecular Pathology Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 911–919. [Google Scholar] [CrossRef]
- Ahmetoğlu, E.; Karadoğan, D.; Gündoğdu, H.; Kostakoğlu, U.; Kara, B.Y.; Rakıcı, Z.; Kazdal, H.; Bedir, R.; Türüt, H.; Şahin, Ü. Mediastinitis and Subcutaneous Abscess Complicated after EBUS-TBNA of 2R Mediastinal Lymph Node. Tuberk. Toraks. 2023, 71, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, J.; Wang, D.; Wang, H.; Liang, J.; Li, Z. Analysis of Fever Following Bronchoscopy and Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration. Altern. Ther. Health Med. 2024, 30, 254–259. [Google Scholar] [PubMed]
- Moon, K.M.; Choi, C.M.; Ji, W.; Lee, J.S.; Lee, S.W.; Jo, K.W.; Song, J.W.; Lee, J.C. Clinical Characteristics of and Risk Factors for Fever after Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: A Retrospective Study Involving 6336 Patients. J. Clin. Med. 2020, 9, 152. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, J.W.; Park, Y.S.; Lee, C.H.; Lee, S.M.; Yim, J.J.; Kim, Y.W.; Han, S.K.; Yoo, C.G. Incidence of Fever Following Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration. Tuberc. Respir. Dis. Seoul 2017, 80, 45–51. [Google Scholar] [CrossRef]
- Asano, F.; Aoe, M.; Ohsaki, Y.; Okada, Y.; Sasada, S.; Sato, S.; Suzuki, E.; Semba, H.; Fukuoka, K.; Fujino, S.; et al. Complications Associated with Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: A Nationwide Survey by the Japan Society for Respiratory Endoscopy. 2013. Available online: https://pubmed.ncbi.nlm.nih.gov/23663438/ (accessed on 1 December 2024).
- Kang, N.; Shin, S.H.; Yoo, H.; Jhun, B.W.; Lee, K.; Um, S.W.; Kim, H.; Jeong, B.H. Infectious Complications of EBUS-TBNA: A Nested Case-Control Study Using 10-Year Registry Data. Lung Cancer 2021, 161, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Steinfort, D.P.; Johnson, D.F.; Irving, L.B. Incidence of Bacteraemia Following Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration. Eur. Respir. J. 2010, 36, 28–32. [Google Scholar] [CrossRef]
- Magnini, D.; Sotgiu, G.; Bello, G.; Puci, M.; Livi, V.; Dell’Anna, A.M.; De Santis, P.; Dell’Ariccia, R.; Viscuso, M.; Flore, M.C.; et al. Thirty-Day Complications, Unplanned Hospital Encounters, and Mortality after Endosonography and/or Guided Bronchoscopy: A Prospective Study. Cancers 2023, 15, 4531. [Google Scholar] [CrossRef] [PubMed]
- Souma, T.; Minezawa, T.; Yatsuya, H.; Okamura, T.; Yamatsuta, K.; Morikawa, S.; Horiguchi, T.; Maeda, S.; Goto, Y.; Hayashi, M.; et al. Risk Factors of Infectious Complications After Endobronchial Ultrasound-Guided Transbronchial Biopsy. Chest 2020, 158, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Minami, D.; Takigawa, N.; Oki, M.; Saka, H.; Shibayama, T.; Kiura, K. Needle Wash Solution Cultures Following EBUS-TBNA with or without Endobronchial Intubation. Respir. Investig. 2018, 56, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Park, J.H.; Park, J.; Kwak, N.; Choi, S.M.; Park, Y.S.; Lee, C.H.; Cho, J. Effect of Chlorhexidine Mouthrinse on Prevention of Microbial Contamination during EBUS-TBNA: A Randomized Controlled Trial. BMC Cancer 2022, 22, 1334. [Google Scholar] [CrossRef]
- Serra Mitjà, P.; Gonçalves Dos Santos Carvalho, F. Incidence and Risk Factors for Infectious Complications of EBUS-TBNA: Prospective Multicenter Study. Arch. Bronconeumol. 2023, 59, 84–89. [Google Scholar] [CrossRef]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.J.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-Treated Sepsis Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.H.; Sibbald, W.J. Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef]
- Gruys, E.; Toussaint, M.J.M.; Niewold, T.A.; Koopmans, S.J. Acute Phase Reaction and Acute Phase Proteins. J. Zhejiang Univ. Sci. 2005, 6B, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann-Struzek, C.; Rudd, K. Challenges of Assessing the Burden of Sepsis. Med. Klin.—Intensivmed. und Notfallmedizin 2023, 118, 68–74. [Google Scholar] [CrossRef]
- Nathan, C. Nonresolving Inflammation Redux. Immunity 2022, 55, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Castellt, J.V.; Andust, T. Interleukin-6 and the Acute Phase Response. Biochem J. 1990, 265, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. The Spectrum of Inflammatory Responses. Science 1979 2021, 374, 1070–1075. [Google Scholar] [CrossRef]
- Mantovani, A.; Garlanda, C. Humoral Innate Immunity and Acute-Phase Proteins. N. Engl. J. Med. 2023, 388, 439–452. [Google Scholar] [CrossRef]
- Speelman, T.; Dale, L.; Louw, A.; Verhoog, N.J.D. The Association of Acute Phase Proteins in Stress and Inflammation-Induced T2D. Cells 2022, 11, 2163. [Google Scholar] [CrossRef] [PubMed]
- Roytblat, L.; Rachinsky, M.; Fisher, A.; Greemberg, L.; Shapira, Y.; Douvdevani, A.; Gelman, S. Raised Interleukin-6 Levels in Obese Patients. Obes. Res. 2000, 8, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Ehlting, C.; Wolf, S.D.; Bode, J.G. Acute-Phase Protein Synthesis: A Key Feature of Innate Immune Functions of the Liver. Biol. Chem. 2021, 402, 1129–1145. [Google Scholar] [CrossRef]
- Strimbu, K.; Tavel, J.A. What Are Biomarkers? Curr. Opin. HIV AIDS 2010, 20, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Mayne, E.S.; George, J.A.; Louw, S. Assessing Biomarkers in Viral Infection. Adv. Exp. Med. Biol. 2023, 1412, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Morley, D.; Torres, A.; Cillóniz, C.; Martin-Loeches, I. Predictors of Treatment Failure and Clinical Stability in Patients with Community Acquired Pneumonia. In Annals of Translational Medicine; AME Publishing Company: Hong Kong, China, 2017. [Google Scholar] [CrossRef]
- Salluh, J.I.F.; Souza-Dantas, V.C.; Póvoa, P. The Current Status of Biomarkers for the Diagnosis of Nosocomial Pneumonias. Curr. Opin. Crit. Care 2017, 23, 391–397. [Google Scholar] [CrossRef]
- Menzel, A.; Samouda, H.; Dohet, F.; Loap, S.; Ellulu, M.S.; Bohn, T. Common and Novel Markers for Measuring Inflammation and Oxidative Stress Ex Vivo in Research and Clinical Practice—Which to Use Regarding Disease Outcomes? Antioxidants 2021, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Lodha, R. Biomarkers for Diagnosing Ventilator Associated Pneumonia: Is That the Way Forward? Indian J. Pediatr. 2018, 85, 411–412. [Google Scholar] [CrossRef] [PubMed]
- Prucha, M.; Bellingan, G.; Zazula, R. Sepsis Biomarkers. Clin. Chim. Acta 2015, 440, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Sankar, V.; Webster, N.R. Clinical Application of Sepsis Biomarkers. J. Anesth. 2013, 27, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, K.; Meisner, M.; Brunkhorst, F.M. Markers for Sepsis Diagnosis: What Is Useful? Crit. Care Clin. 2006, 22, 503–519. [Google Scholar] [CrossRef]
- Bowcock, A.M.; Kidd, J.R.; Lathrop, G.M.; Daneshvar, L.; May, L.T.; Ray, A.; Sehgal, P.B.; Kidd, K.K.; Cavalli-Sforza, L.L. The human “interferon-beta 2/hepatocyte stimulating factor/interleukin-6” gene: DNA polymorphism studies and localization to chromosome 7p21. Genomics 1988, 3, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, L.H.; Rose-John, S. IL-6 Biology: Implications for Clinical Targeting in Rheumatic Disease. Nat. Rev. Rheumatol. 2014, 10, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Kontarakis, Z.; Gerri, C.; Nolte, H.; Hölper, S.; Krüger, M.; Stainier, D.Y.R. Genetic Compensation Induced by Deleterious Mutations but Not Gene Knockdowns. Nature 2015, 524, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Il-6 in Inflammation, Immunity, And Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J. Structure-function relationships. Protein Sci. 1997, 6, 929–955. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S. The Soluble Interleukin 6 Receptor: Advanced Therapeutic Options in Inflammation. Clin. Pharmacol. Ther. 2017, 102, 591–598. [Google Scholar] [CrossRef]
- Wolf, J.; Prüss-Ustün, A.; Cumming, O.; Bartram, J.; Bonjour, S.; Cairncross, S.; Clasen, T.; Colford, J.M.; Curtis, V.; De France, J.; et al. Systematic Review: Assessing the Impact of Drinking Water and Sanitation on Diarrhoeal Disease in Low- and Middle-Income Settings: Systematic Review and Meta-Regression. Trop. Med. Int. Health 2014, 19, 928–942. [Google Scholar] [CrossRef]
- Robak, T.; Gladalska, C.A.; Stepień, H.S.; Robak, E. Serum Levels of Interleukin-6 Type Cytokines and Soluble Interleukin-6 Receptor in Patients with Rheumatoid Arthritis. Mediat. Inflamm. 1998, 7, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Garbers, C.; Rose-John, S. Interleukin-6: From Basic Biology to Selective Blockade of Pro-Inflammatory Activities. Semin. Immunol. 2014, 26, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Sage, A.T.; del Sorbo, L.; Cypel, M.; Martinu, T.; Juvet, S.C.; Mariscal, A.; Wright, J.; Chao, B.T.; Shamandy, A.A.; et al. A Biomarker Assay to Risk-Stratify Patients with Symptoms of Respiratory Tract Infection. Eur. Respir. J. 2022, 60, 2200459. [Google Scholar] [CrossRef] [PubMed]
- Sage, A.T.; Richard-Greenblatt, M.; Zhong, K.; Bai, X.H.; Snow, M.B.; Babits, M.; Ali, A.; Baciu, C.; Yeung, J.C.; Liu, M.; et al. Prediction of Donor Related Lung Injury in Clinical Lung Transplantation Using a Validated Ex Vivo Lung Perfusion Inflammation Score. J. Heart Lung Transplant. 2021, 40, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Andreasson, A.S.I.; Karamanou, D.M.; Gillespie, C.S.; Özalp, F.; Butt, T.; Hill, P.; Jiwa, K.; Walden, H.R.; Green, N.J.; Borthwick, L.A.; et al. Profiling Inflammation and Tissue Injury Markers in Perfusate and Bronchoalveolar Lavage Fluid during Human Ex Vivo Lung Perfusion. Eur. J. Cardio-Thorac. Surg. 2017, 51, 577–586. [Google Scholar] [CrossRef]
- Machuca, T.N.; Cypel, M.; Hsin, M.K.; Zamel, R.; Yeung, J.C.; Chen, M.; Saito, T.; Guan, Z.; Waddell, T.K.; Liu, M.; et al. Protein Expression Profiling Predicts Graft Performance in Clinical Ex Vivo Lung Perfusion. J. Heart Lung Transplant. 2013, 32, S47. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Xu, S.; Nie, J. Role of Interleukin-6 Family Cytokines in Organ Fibrosis. Kidney Dis. 2023, 9, 239–253. [Google Scholar] [CrossRef]
- Deftos, L.J.; Roos, B.A.; Parthemore, J.G.; Jolla, L. Medical Progress. Calcium and Skeletal Metabolism. West J. Med. 1975, 123, 447–458. [Google Scholar]
- Müller, B.; Müller, M.; White, J.C.; Nylé, N.E.S.; Snider, R.H.; Becker, K.L.; Habener, J.F. Ubiquitous Expression of the Calcitonin-I Gene in Multiple Tissues in Response to Sepsis. J. Clin. Endocrinol. Metab. 2001, 86, 396–404. [Google Scholar] [CrossRef]
- Samsudin, I.; Vasikaran, S.D. Clinical Utility and Measurement of Procalcitonin. Clin. Biochem. Rev. 2017, 38, 59–68. [Google Scholar] [PubMed]
- Wiedermann, F.J.; Kaneider, N.; Egger, P.; Tiefenthaler, W.; Wiedermann, C.J.; Lindner, K.H.; Schobersberger, W. Migration of human monocytes in response to procalcitonin. Crit. Care. Med. 2002, 30, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, K.; Karzai, W.; Meisner, M. Procalcitonin as a Marker of the Systemic Inflammatory Response to Infection. Intens. Care Med. 2000, 26, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Albrich, W.; Mueller, B. Procalcitonin for Diagnosis of Infection and Guide to Antibiotic Decisions: Past, Present and Future. BMC Med. 2011, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Lee, H. Procalcitonin as a Biomarker of Infectious Diseases. Korean J. Intern. Med. 2013, 28, 285–291. [Google Scholar] [CrossRef]
- Meisner, M. Procalcitonin: Erfahrungen Mit Einer Neuen Meßgröße Für Bakterielle Infektionen Und Systemische Inflammation Procalcitonin: Experience with a New Diagnostic Tool for Bacterial Infection and Systemic Inflammation. J. Lab. Med. 1999, 23, 263–272. [Google Scholar] [CrossRef]
- Schuetz, P.; Briel, M.; Christ-Crain, M.; Stolz, D.; Bouadma, L.; Wolff, M.; Luyt, C.E.; Chastre, J.; Tubach, F.; Kristoffersen, K.B.; et al. Procalcitonin to Guide Initiation and Duration of Antibiotic Treatment in Acute Respiratory Infections: An Individual Patient Data Meta-Analysis. Clin. Infect. Dis. 2012, 55, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Matthaiou, D.K.; Ntani, G.; Kontogiorgi, M.; Poulakou, G.; Armaganidis, A.; Dimopoulos, G. An ESICM Systematic Review and Meta-Analysis of Procalcitonin-Guided Antibiotic Therapy Algorithms in Adult Critically Ill Patients. Intens. Care Med. 2012, 38, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Brunkhorst, F.M.; Heinz, U.; Forycki, Z.E. Kinetics of Procalcitonin in Iatrogenic Sepsis. Intens. Care Med. 1998, 24, 888–889. [Google Scholar] [CrossRef]
- Meisner, M.; Tschaikowsky, K.; Palmaers, T.; Schmidt, J.; Meisner, M. Comparison of Procalcitonin (PCT) and C-Reactive Protein (CRP) Plasma Concentrations at Different SOFA Scores during the Course of Sepsis and MODS. Crit Care 1999, 3, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Velissaris, D.; Zareifopoulos, N.; Lagadinou, M.; Platanaki, C.; Tsiotsios, K.; Stavridis, E.L.; Kasartzian, D.I.; Pierrakos, C.; Karamouzos, V. Procalcitonin and sepsis in the Emergency Department: an update. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Nix, D.; Wilson, M.F.; Aljada, A.; Love, J.; Assicot, M.; Bohuon, C.; Pharmacokinetics, C.; Hospitals, M.F. Procalcitonin Increase after Endotoxin Injection in Normal Subjects. J. Clin. Endocrinol. Metab. 1994, 79, 1605–1608. [Google Scholar] [PubMed]
- Póvoa, P. C-Reactive Protein: A Valuable Marker of Sepsis. Intens. Care Med. 2002, 28, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Acutephase Proteins and Other Systemic Responses to Inflammation. N. Engl. J. Med. 1999, 340, 448–454. [CrossRef]
- Luna, C.M. C-Reactive Protein in Pneumonia: Let Me Try Again. Chest 2004, 125, 1192–1195. [Google Scholar] [CrossRef] [PubMed]
- Seeger, A.; Rohde, G. Ambulant erworbene Pneumonie [Community-acquired pneumonia]. Dtsch. Med. Wochenschr. 2023, 148, 335–341, Epub 2023 Mar 6. [Google Scholar] [CrossRef] [PubMed]
- Petel, D.; Winters, N.; Gore, G.C.; Papenburg, J.; Beltempo, M.; Lacroix, J.; Fontela, P.S. Use of C-Reactive Protein to Tailor Antibiotic Use: A Systematic Review and Meta-Analysis. BMJ Open 2018, 8, e022133. [Google Scholar] [CrossRef] [PubMed]
- Póvoa, P.; Coelho, L.; Almeida, E.; Fernandes, A.; Mealha, R.; Moreira, P.; Sabino, H. C-Reactive Protein as a Marker of Ventilator-Associated Penumonia Resolution: A Pilot Study. Eur. Respir. J. 2005, 25, 804–812. [Google Scholar] [CrossRef]
- Póvoa, P.; Coelho, L.; Almeida, E.; Fernandes, A.; Mealha, R.; Moreira, P.; Sabino, H. Early Identification of Intensive Care Unit-Acquired Infections with Daily Monitoring of C-Reactive Protein: A Prospective Observational Study. Crit. Care 2006, 10, R63. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.; Agrawal, A. Evolution of C-Reactive Protein. Front. Immunol. 2019, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, G.; Huang, L. Association of the NLR, BNP, PCT, CRP, and D-D with the Severity of Community-Acquired Pneumonia in Older Adults. Clin. Lab. 2023, 69. [Google Scholar] [CrossRef] [PubMed]
| Diagnostic |
|
| |
| |
| |
| |
| |
| Therapeutic |
|
| |
| Special situations |
|
| |
| |
| |
| |
|
| Study Authors | Study Details | Objective of Study | Main Findings | EBUS-Induced Inflammation Clinically Significant | EBUS-Induced Inflammation Correlated with Biomarkers | EBUS-Induced Inflammation Correlated with Infective Element |
|---|---|---|---|---|---|---|
| Chen M. et al. [37] | Retrospective Single-centered 512 patients Three study groups:
Inspection only
Conventional sampling
Inflammatory biomarkers utilized: pre- and post-procedural WBC neutrophils IL-6 Post-procedural fever (>38.5 °C) Blood cultures (when fever observed) | Assessment of procedural-related inflammatory reactions and identification of infective component | All three groups had raised inflammatory biomarkers post procedure Less fever episodes with inspection-only bronchoscopy Conventional bronchoscopy sampling and EBUS sampling induced fever but with no statistically significant difference EBUS sampling had higher levels of inflammatory markers but not statistically significant Post-procedural cultures obtained in fever cases but positivity was poorly correlated to clinical significance | NO | YES Increased: WBC, Neutrophils, IL-6 Not statistically significant | NO |
| Moon K. et al. [38] | Retrospective Single-centered 6336 patients EBUS-TBNA cases +/− other interventions Post-procedural fever (>37.8 °C) within 24 h Inflammatory biomarkers utilized: WBC neutrophils CRP | Post-procedural fever assessed within 24 h and correlation with EBUS-TBNA sampling was evaluated | 665 cases developed fever (10.5%) Mean peak temperature: 38.3 °C Fever was more frequent when samples obtained were >4/caseTB diagnosis was more frequent in the fever group 72 cases had received prophylactic antibiotics in both the fever and non-fever groups Prophylactic antibiotics had been used more frequently (5.7%) for the fever group vs. the non-fever group (0.6%), p < 0.001 EBUS-TBNA + other interventions induced fever more frequently (bronchial washing, endobronchial biopsies, bronchial washing, core needle biopsy, transbronchial biopsy) | YES
| YES Increased CRP, WBC, Neutrophils were significantly higher, in the fever group, pre-procedurally | YES
|
| Kim S.Y. et al. [39] | Retrospective Single-centered 684 patients Post-procedural fever Inflammatory biomarker utilized: WBC neutrophils | Incidence of post-procedural fever within 24 h | 552 patients met criteria Incidence of fever: 110 cases, 20% Median peak temperature: 38.3 °C Median time of fever presentation: 7 h Median time of fever duration: 7 h | YES
| YES
| NO
|
| Asano F. et al. [40] | Retrospective multi-centered 7345 patients EBUS-TBNA related complications reported in 90 cases Post-procedural fever assessed Inflammatory biomarkers utilized: none | Identification of most common EBUS-TBNA related complications | Infectious-related complications were the second most common (14 cases, 0.19%) after hemorrhage (50 cases, 0.68%) Prophylactic antibiotics were used in only 3 out of the 14 cases | YES
| NO
described in the study | YES
|
| Kang N. et al. [41] | Retrospective Single-centered 6826 patients Nested case-control study Two-month follow-up after procedure Inflammatory biomarkers utilized: none | Identification of infectious complications and of the relevant risk factors | Infectious incidence: 33 cases, 0.48% Infectious complications primarily occurred after sampling target lesions with necrotic features Infectious complications comprised of pneumonia and mediastinal infections Median days of infectious-related clinical findings, warranting antibiotic initiation: 7 | YES
| NO
| YES
|
| Steinfort D.P. et al. [42] | Prospective study Single-centered 43 patients Post-procedural blood sampling in 60 min for blood cultures Sample of the EBUS TBNA needle was also obtained Inflammatory biomarkers utilized: none | Identification of the incidence of bacteremia and infectious complications associated with EBUS-TBNA | Bacteremia incidence: 3 cases, 7% None of them experienced any significant complications No significant correlation of the bacteremia to the size of the sampled lesion or to the underlying pathology The bacteremia rate was comparable to that induced by conventional bronchoscopy | NO | NO
| NO
|
| Magnini et al. [43] | Prospective study Single-centered 697 patients Inflammatory biomarkers utilized: none | Evaluation of major complications and outcomes post-EBUS TBNA and EUS-B TBNA within 30 d
Bleeding Infection Respiratory Failure
| Severe complications were identified in 17 (2.4%) cases. Late complications (8.47%) occurred Median time of presentation: 14 d. Infectious complications primarily in malignancy cases Patients with low density areas in their lesions had increased likelihood to develop infectious complications. | YES
2.5% of the cases.
| NO
| YES
|
| Souma T. et al. [44] | Retrospective study Single centered 1045 patients Criteria for infectious element: respiratory exacerbation for >24 h: fever >37 °C cough sputum, chest pain, dyspnea elevation of WBC or CRP compared with pre-bronchoscopy levels imaging findings with accompanying need of antibiotics Inflammatory biomarkers utilized: pre- and post-procedural WBC, CRP | Identification of infectious complications, after EBUS-TBNA sampling for peripheral lesions via a guide sheath, within 4 weeks | Infectious complications incidence: 47 (4.47%) cases Need of antibiotics Main risk factors identified: cavitation of lesion low-density areas in the lesion bronchial stenosis. Use of prophylactic antibiotics—before or after the procedure, in 102 patients—could not provide reliable results regarding the efficacy in preventing post-procedural infectious complications | YES
| YES Increased CRP, WBC | YES
27 normal flora |
| Minami D. et al. [45] | Retrospective Single-centered 80 patients’ EBUS TBNA sampling Two groups of patients: 60 intubated 20 non-intubated Fever was assessed EBUS TBNA needle wash cultures processed Inflammatory biomarkers utilized: none | Identification of procedure-related infectious reactions and correlation with EBUS-TBNA needle wash cultures comparing the intubated and non-intubated groups | Positive EBUS-TBNA needle wash cultures: Intubated group: only 2 cases (3.3%) Non-intubated group: all 20 cases (100%). Fever occurred: Intubated group: 6 (10%) Non-intubated group: only 2 cases (10%). Fever development was equal in both groups despite the fact that contamination of EBUS-TBNA needle is less likely with the use of intubation | NO Fever was not associated with any clinical features | NO
| NO
|
| Mitja S. et al. [47] | Prospective Multi-centered 370 patients EBUS TBNA sampling Two groups of patients: 245 with risk factors 125 without risk factors (control group) 30-day follow up: days 2, 14, 21, 30 Fever (>38 °C) assessed initially, every 8 h, for 48 h Blood samples obtained 30 min post-procedure Inflammatory biomarkers utilized: WBC, ESR, CRP, procalcitonin Blood cultures and bronchial aspirate cultures processed | Identification of EBUS TBNA –related infectious complications and of potential risk factors | Infectious incidence: 15 cases (4.05%) 14 cases (5.71%) with risk factors 1 case (0.8%), from the control group Stronger risk factors:
>10 | YES Fever presentation:
6 of the 9 cases had self-limited fever 3 of the 9 cases developed infectious complication (pneumonia)
self-limited 7 of the 14 cases had self-limited fever 7 of the 14 cases developed infectious complication related to infectious complication: mediastinitis (1) pneumonia (1) obstructive pneumonia (2) respiratory tract infection (2) All required antibiotics and infectious complication resolved without any more severe complication | NO
(despite mention within the data collection) | YES
|
| Biomarker | Specificity Bacterial Infection | Sensitivity Inflammation | Advantages | Disadvantages |
|---|---|---|---|---|
| WBC | low | high | Simple and non expensive | Sensitivity for bacteria Non-specific for bacterial infection All inflammation & infections Disease states |
| CRP | moderate | moderate | Non expensive Moderately specific | All inflammation and infections Slow induction (peak > 24 h) No correlation with severity |
| Lactate | low | low | Inexpensive Reliable marker of perfusion Prognosis > Sepsis | Must be in sepsis to be elevated Very poor specificity for bacterial infection |
| Fever | low | low | Inexpensive Readily available | No specificity to bacteria Affected by >1180 drugs and/or disease states |
| Procalcitonin | high | low | Specificity for bacteria Favorable kinetics Rise/half-life Correlates with severity of illness Antibiotic use | Education Instrument for Lab More expensive than WBC, CRP, and lactate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosgana, P.; Ampazis, D.; Vlachakos, V.; Tzouvelekis, A.; Sampsonas, F. Infective Complications of Endobronchial Ultrasound-Transbronchial Needle Aspiration (EBUS-TBNA) and Clinical Biomarkers: A Concise Review. Diagnostics 2025, 15, 145. https://doi.org/10.3390/diagnostics15020145
Bosgana P, Ampazis D, Vlachakos V, Tzouvelekis A, Sampsonas F. Infective Complications of Endobronchial Ultrasound-Transbronchial Needle Aspiration (EBUS-TBNA) and Clinical Biomarkers: A Concise Review. Diagnostics. 2025; 15(2):145. https://doi.org/10.3390/diagnostics15020145
Chicago/Turabian StyleBosgana, Pinelopi, Dimitrios Ampazis, Vasileios Vlachakos, Argyrios Tzouvelekis, and Fotios Sampsonas. 2025. "Infective Complications of Endobronchial Ultrasound-Transbronchial Needle Aspiration (EBUS-TBNA) and Clinical Biomarkers: A Concise Review" Diagnostics 15, no. 2: 145. https://doi.org/10.3390/diagnostics15020145
APA StyleBosgana, P., Ampazis, D., Vlachakos, V., Tzouvelekis, A., & Sampsonas, F. (2025). Infective Complications of Endobronchial Ultrasound-Transbronchial Needle Aspiration (EBUS-TBNA) and Clinical Biomarkers: A Concise Review. Diagnostics, 15(2), 145. https://doi.org/10.3390/diagnostics15020145






