Clinician Perspectives on Digital and Computational Pathology: Clinical Benefits, Concerns, and Willingness to Adopt
Abstract
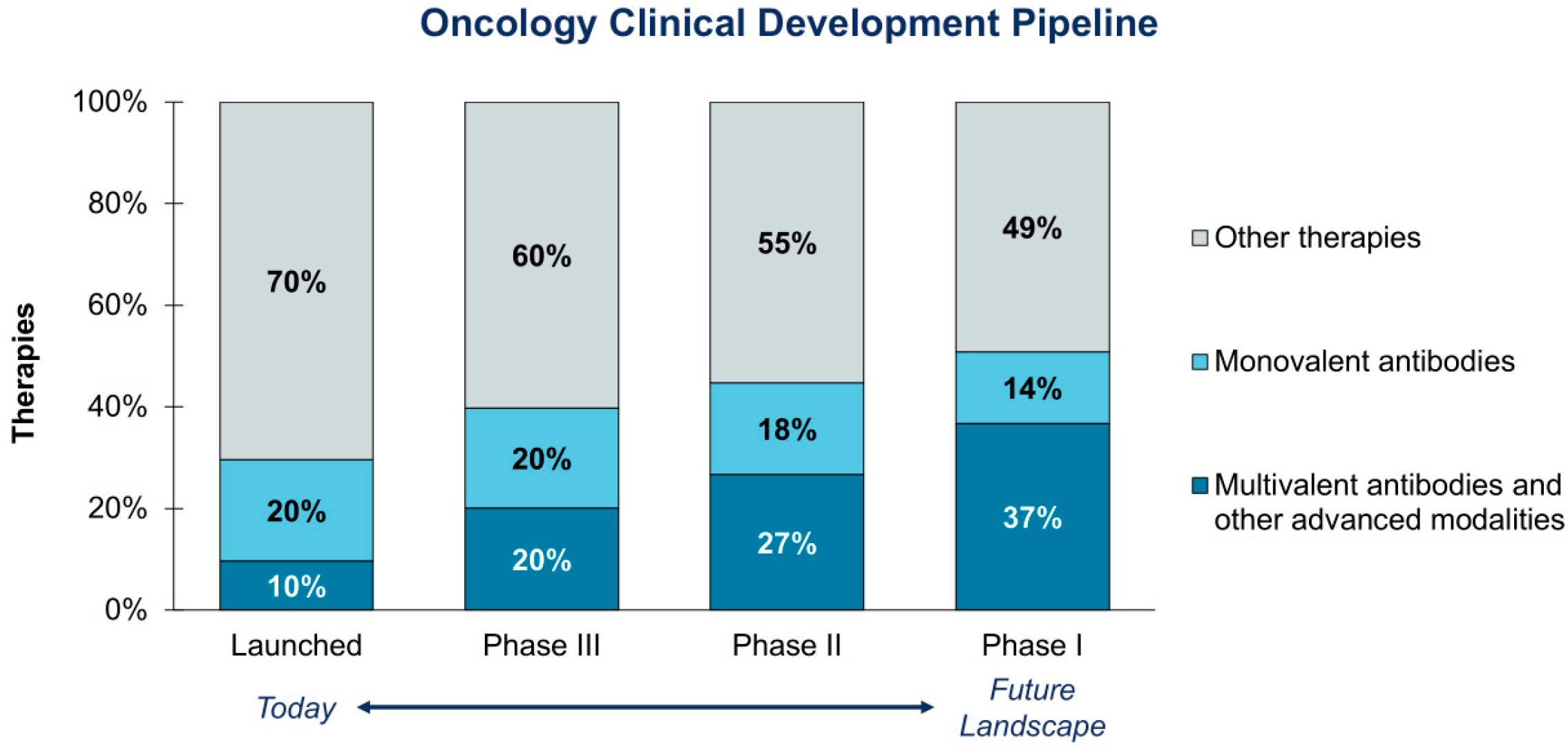
1. Introduction
2. Materials and Methods
2.1. Survey Methodology
Digital pathology encompasses the acquisition, management, analysis, and interpretation of pathology information generated from digitized glass-slide images.
Computational pathology is a subset of digital pathology that uses AI-guided computer-based quantification and classification of tissue morphology to generate more precise diagnoses and expand the recognition of pathologic changes beyond the limits of conventional visual assessment. This could include automatic detection, localization, or quantification of histological parameters and structural changes.
2.2. Interviews
2.3. Study Participant Selection Criteria
3. Results
3.1. Clinician Awareness, Utilization, and General Comfort with DP/CP
3.2. Benefits and Barriers to DP/CP Adoption
3.3. Clinicians’ Role in DP/CP Adoption
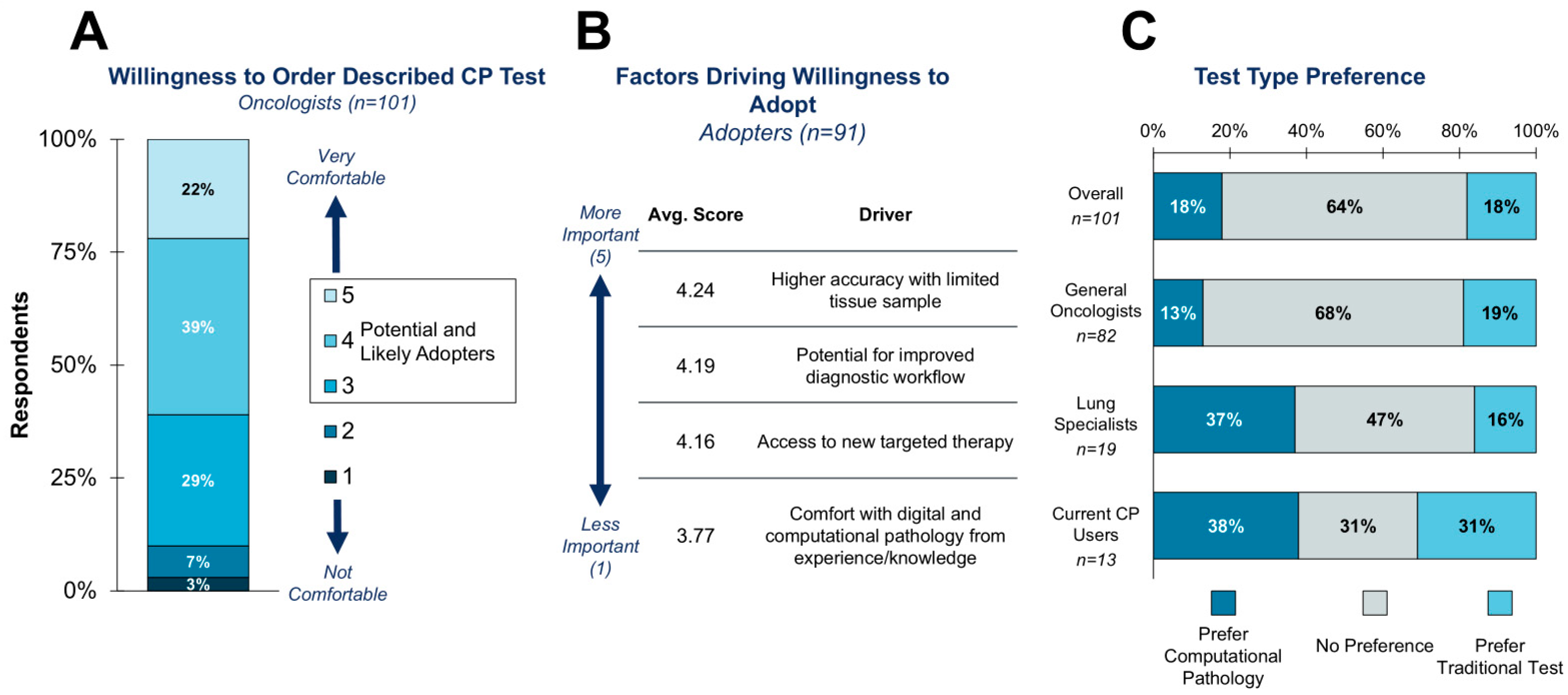
3.4. Willingness to Adopt a Theoretical CP-Based CDx Test
Assume there is a companion diagnostic for a newly approved targeted therapy in lung cancer. The companion diagnostic test uses computational pathology to precisely quantify the level of a biomarker in tumor cell slide images (i.e., quantifying a human-interpretable feature) to determine positivity and eligibility for the targeted therapy. Assume this is the only test available for this therapy.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DP/CP | Digital pathology and computational pathology |
| OOP | Out of pocket |
| CDx | Companion diagnostic |
| TROP2 | Trophoblast cell surface antigen 2 |
References
- Sheffield, B.S. Immunohistochemistry as a Practical Tool in Molecular Pathology. Arch. Pathol. Lab. Med. 2016, 140, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.; Orsi, N.M. The current troubled state of the global pathology workforce: A concise review. Diagn. Pathol. 2024, 19, 163. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA A Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Li, X.; Gan, Y.; Han, S.; Rong, P.; Wang, W.; Li, W.; Zhou, L. Artificial intelligence assists precision medicine in cancer treatment. Front. Oncol. 2023, 12, 998222. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Hsiao, T.-H.; Lin, C.-H.; Fann, Y.C. Unlocking precision medicine: Clinical applications of integrating health records, genetics, and immunology through artificial intelligence. J. Biomed. Sci. 2025, 32, 16. [Google Scholar] [CrossRef] [PubMed]
- Niazi, M.K.K.; Parwani, A.V.; Gurcan, M.N. Digital pathology and artificial intelligence. Lancet Oncol. 2019, 20, e253–e261. [Google Scholar] [CrossRef] [PubMed]
- Bera, K.; Schalper, K.A.; Rimm, D.L.; Velcheti, V.; Madabhushi, A. Artificial intelligence in digital pathology—New tools for diagnosis and precision oncology. Nat. Rev. Clin. Oncol. 2019, 16, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Gupta, R.; Gupta, S. Whole Slide Imaging (WSI) in Pathology: Current Perspectives and Future Directions. J. Digit. Imaging 2020, 33, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Baxi, V.; Edwards, R.; Montalto, M.; Saha, S. Digital pathology and artificial intelligence in translational medicine and clinical practice. Mod. Pathol. 2022, 35, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, V.S.; Toro, P.; Corredor, G.; Mukhopadhyay, S.; Madabhushi, A. The state of the art for artificial intelligence in lung digital pathology. J. Pathol. 2022, 257, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.; Li, Z.; Parwani, A.V. Artificial intelligence’s impact on breast cancer pathology: A literature review. Diagn. Pathol. 2024, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Yousif, M.; van Diest, P.J.; Laurinavicius, A.; Rimm, D.; van der Laak, J.; Madabhushi, A.; Schnitt, S.; Pantanowitz, L. Artificial intelligence applied to breast pathology. Virchows Arch. 2022, 480, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, N.; Kaakinen, M.; Eklund, L.; Heikkilä, J. Towards Virtual H&E Staining of Hyperspectral Lung Histology Images Using Conditional Generative Adversarial Networks. In Proceedings of the 2017 IEEE International Conference on Computer Vision Workshops (ICCVW), Venice, Italy, 22–29 October 2017; pp. 64–71. [Google Scholar] [CrossRef]
- Kapil, A.; Spitzmüller, A.; Brieu, N.; Haneder, S.; Shumilov, A.; Meier, A.; Cecchi, F.; Barkell, A.; Harder, N.; Mittermaier, K.; et al. HER2 quantitative continuous scoring for accurate patient selection in HER2 negative trastuzumab deruxtecan treated breast cancer. Sci. Rep. 2024, 14, 12129. [Google Scholar] [CrossRef] [PubMed]
- Abele, N.; Tiemann, K.; Krech, T.; Wellmann, A.; Schaaf, C.; Länger, F.; Peters, A.; Donner, A.; Keil, F.; Daifalla, K.; et al. Noninferiority of Artificial Intelligence–Assisted Analysis of Ki-67 and Estrogen/Progesterone Receptor in Breast Cancer Routine Diagnostics. Mod. Pathol. 2023, 36, 100033. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Casanova, R.; Machiraju, D.; McKee, T.D.; Weder, W.; Beck, A.H.; Soltermann, A. Computationally-Guided Development of a Stromal Inflammation Histologic Biomarker in Lung Squamous Cell Carcinoma. Sci. Rep. 2018, 8, 3941. [Google Scholar] [CrossRef] [PubMed]
- Prelaj, A.; Miskovic, V.; Zanitti, M.; Trovo, F.; Genova, C.; Viscardi, G.; Rebuzzi, S.E.; Mazzeo, L.; Provenzano, L.; Kosta, S.; et al. Artificial intelligence for predictive biomarker discovery in immuno-oncology: A systematic review. Ann. Oncol. 2024, 35, 29–65. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.; Paz-Ares, L.; Lin, C.; Herbet, S.; Yang, T.-Y.; Tolcher, A.W.; Lou, Y.; Zenke, Y.; Cortinovis, D.; Felip, E.; et al. TROPION-Lung02: Datopotamab deruxtecan (Dato-DXd) plus pembrolizumab (pembro) with or without platinum chemotherapy (Pt-CT) as first-line (1L) therapy for advanced non-small cell lung cancer (aNSCLC). J. Clin. Oncol. 2025, 43, 8501. [Google Scholar] [CrossRef]
- Schroll, M.M.; Quinn, E.; Pritchard, D.; Chang, A.; Garner Amanti, K.; Perez, O.; Agarwal, A.; Gustavsen, G. Perspectives on Clinical Adoption Barriers to Blood-Based Multi-Cancer Early Detection Tests across Stakeholders. J. Pers. Med. 2024, 14, 593. [Google Scholar] [CrossRef] [PubMed]

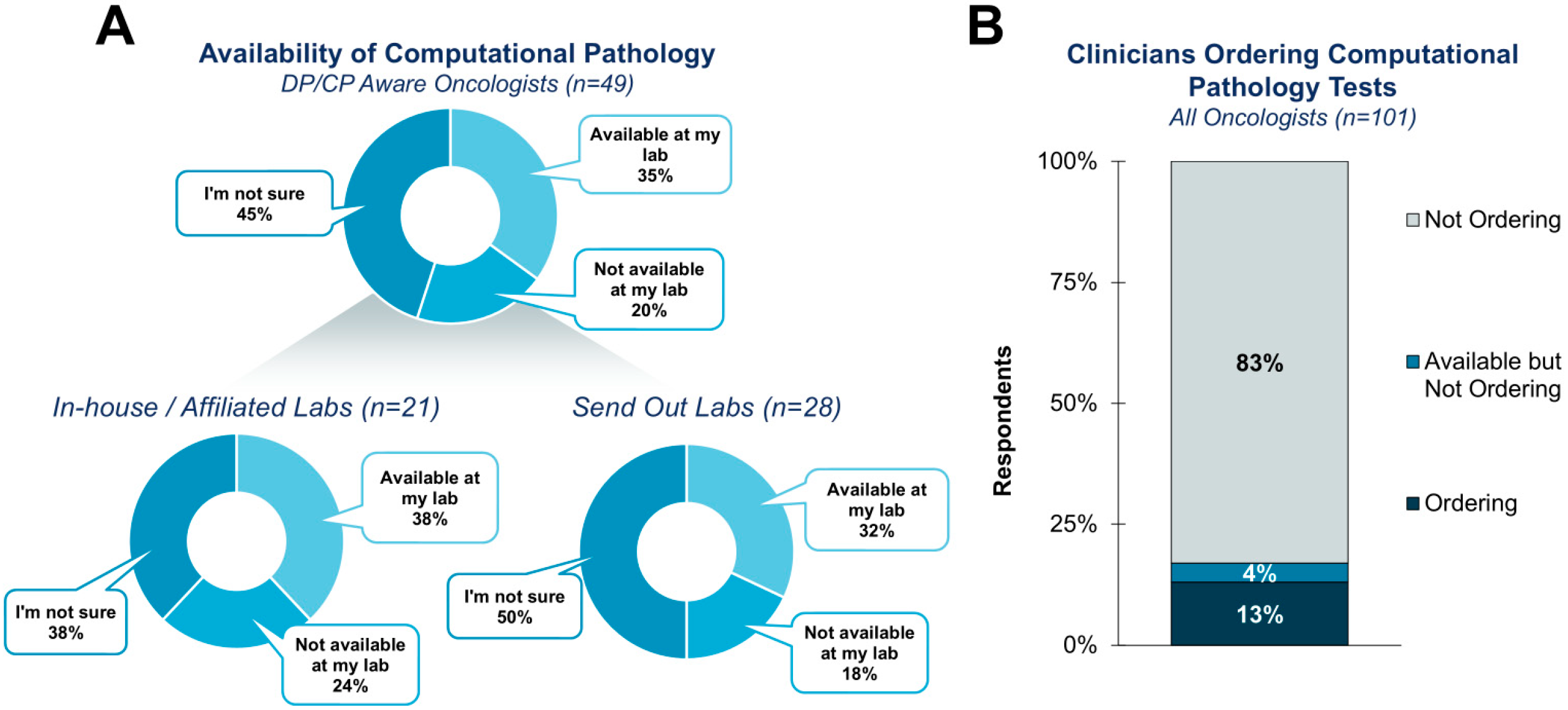
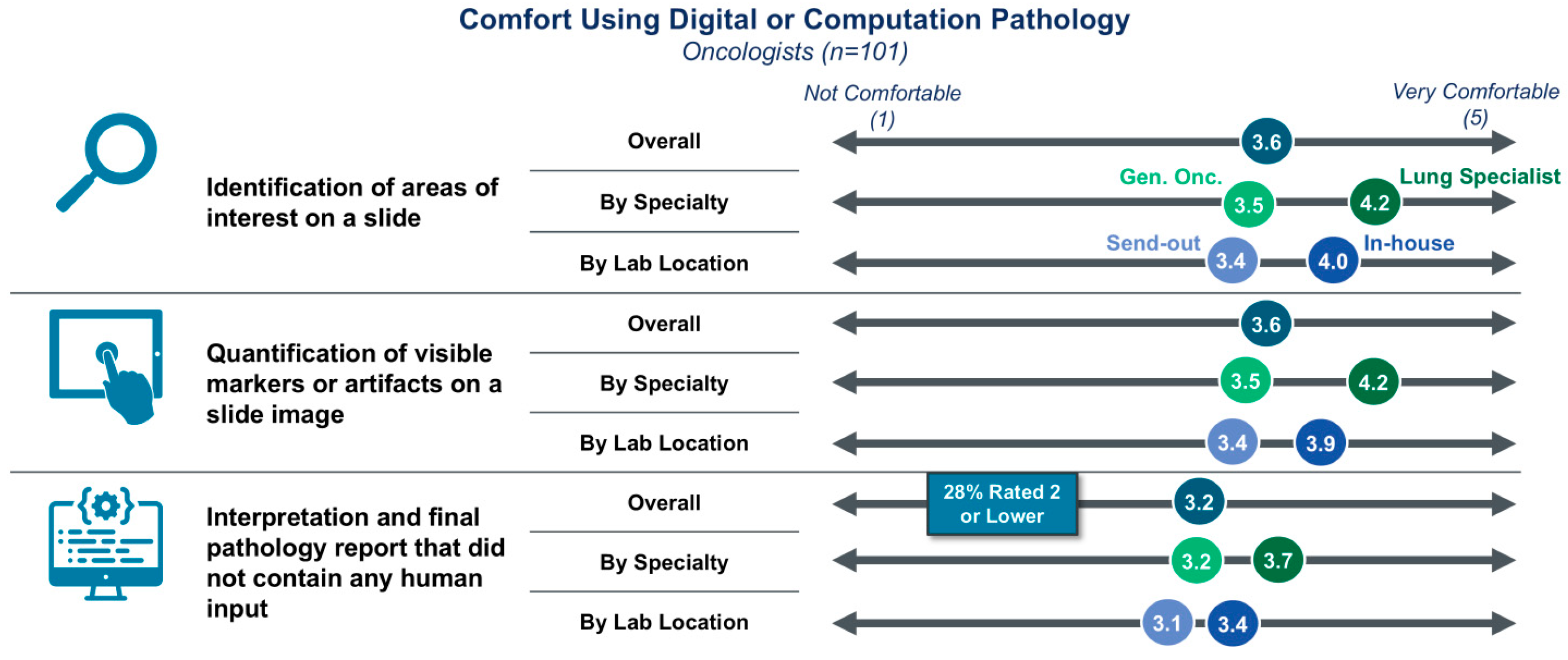
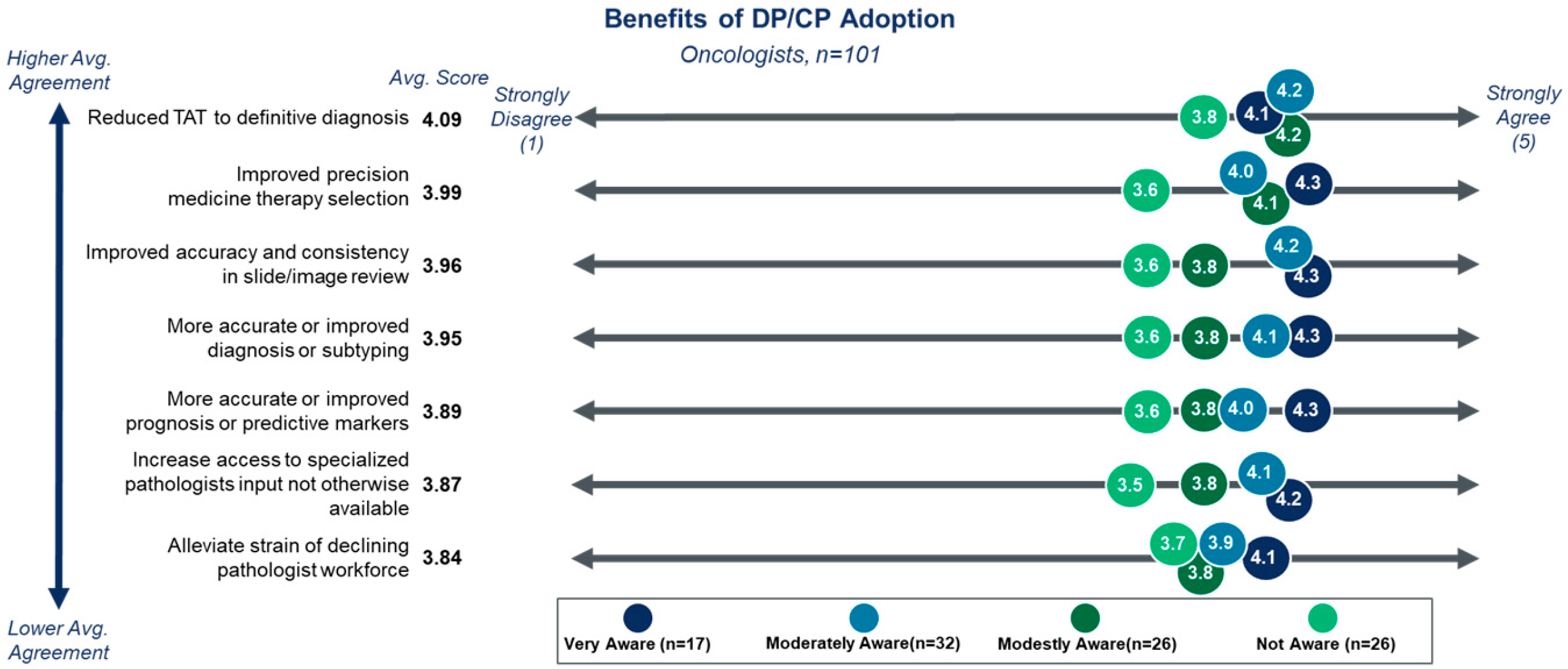
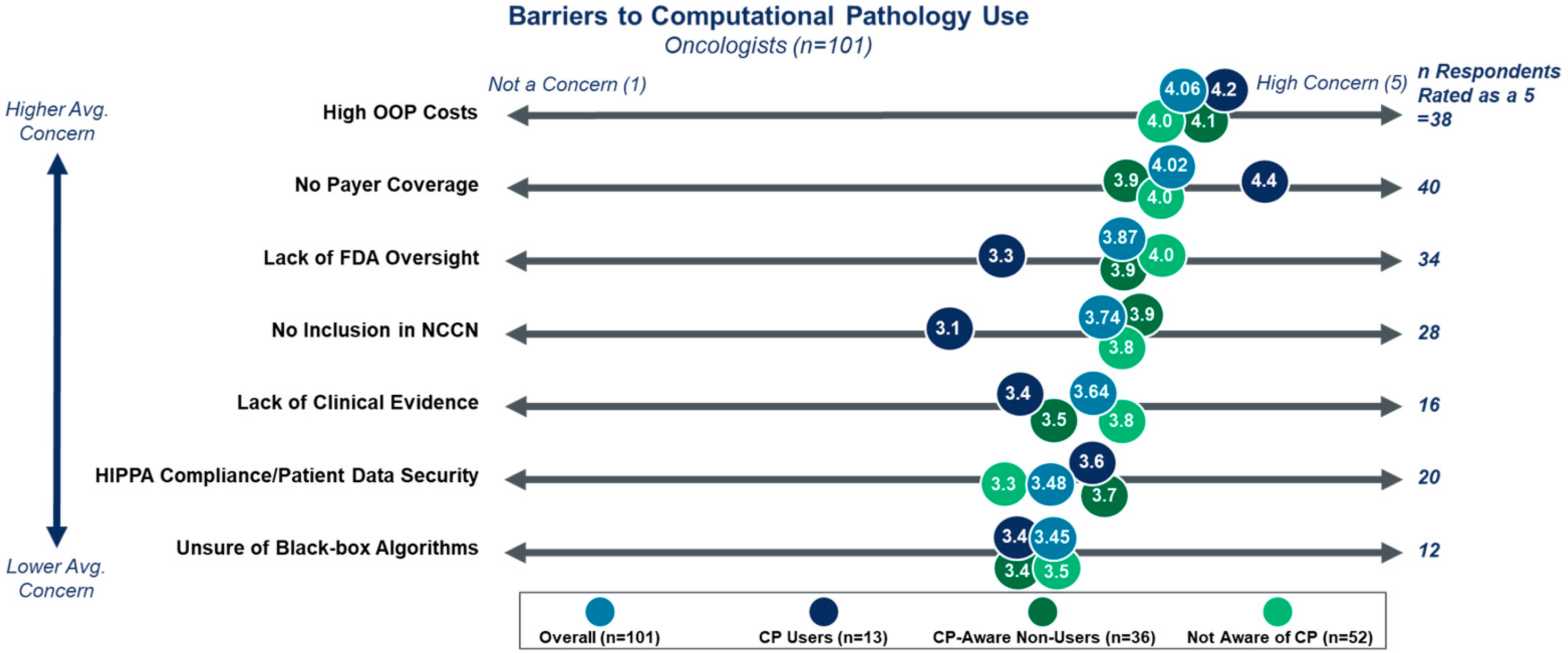
| Cohort | Segment | Percentage of Respondents |
|---|---|---|
| All Clinicians | All | 100% |
| Geography | Northeast | 21% |
| Midwest | 22% | |
| South | 36% | |
| West | 22% | |
| Practice Type | Academic Hospital | 35% |
| Community Hospital with Academic Affiliation | 10% | |
| Community Hospital | 20% | |
| Private Practice, Hospital, or Network-Affiliated | 14% | |
| Independent Private Practice | 22% | |
| Years in Practice | 0–1 | 6% |
| 2–10 | 36% | |
| 11–20 | 30% | |
| 21–40 | 29% | |
| Anatomic Pathology Lab Most Commonly Used | In-House Lab | 29% |
| Affiliated Hospital Lab | 5% | |
| Commercial Reference Lab | 22% | |
| Specialty Reference Lab 1 | 45% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aggarwal, C.; Desai, A.; McConnell, N.; Cadirov, N.; Gustavsen, G.; Agarwal, A.; Chehab, N.; Kotapati, S.; Patel, N. Clinician Perspectives on Digital and Computational Pathology: Clinical Benefits, Concerns, and Willingness to Adopt. Diagnostics 2025, 15, 2527. https://doi.org/10.3390/diagnostics15192527
Aggarwal C, Desai A, McConnell N, Cadirov N, Gustavsen G, Agarwal A, Chehab N, Kotapati S, Patel N. Clinician Perspectives on Digital and Computational Pathology: Clinical Benefits, Concerns, and Willingness to Adopt. Diagnostics. 2025; 15(19):2527. https://doi.org/10.3390/diagnostics15192527
Chicago/Turabian StyleAggarwal, Charu, Aakash Desai, Nicholas McConnell, Nicholas Cadirov, Gary Gustavsen, Arushi Agarwal, Nabil Chehab, Srividya Kotapati, and Nikunj Patel. 2025. "Clinician Perspectives on Digital and Computational Pathology: Clinical Benefits, Concerns, and Willingness to Adopt" Diagnostics 15, no. 19: 2527. https://doi.org/10.3390/diagnostics15192527
APA StyleAggarwal, C., Desai, A., McConnell, N., Cadirov, N., Gustavsen, G., Agarwal, A., Chehab, N., Kotapati, S., & Patel, N. (2025). Clinician Perspectives on Digital and Computational Pathology: Clinical Benefits, Concerns, and Willingness to Adopt. Diagnostics, 15(19), 2527. https://doi.org/10.3390/diagnostics15192527






