Generational Leaps in Intrapartum Fetal Surveillance
Abstract
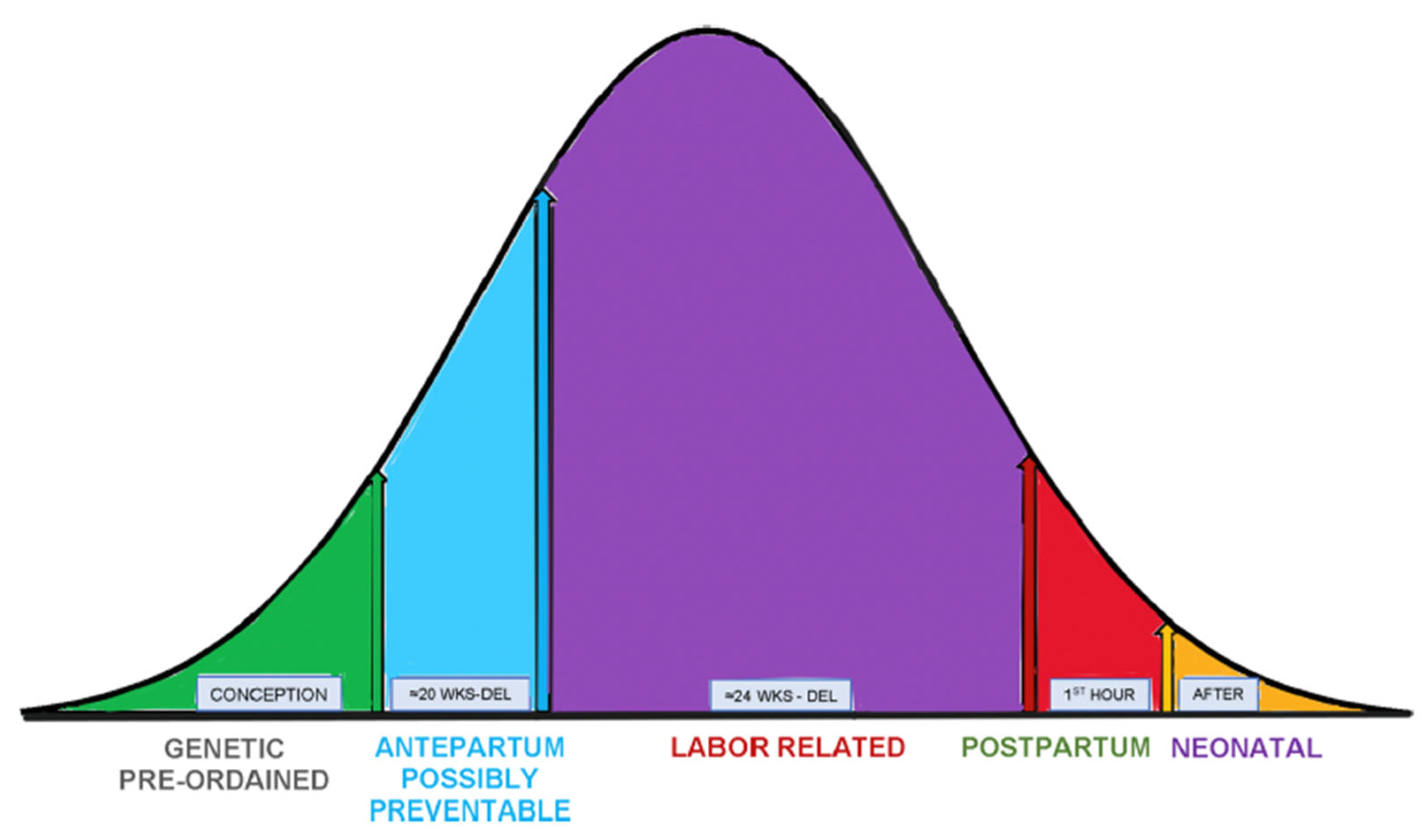
1. Introduction
2. Previous Attempts to Improve EFM
2.1. Standardization Efforts
2.2. Ancillary Supportive Technologies
2.2.1. Fetal Pulse Oximetry
2.2.2. STAN System
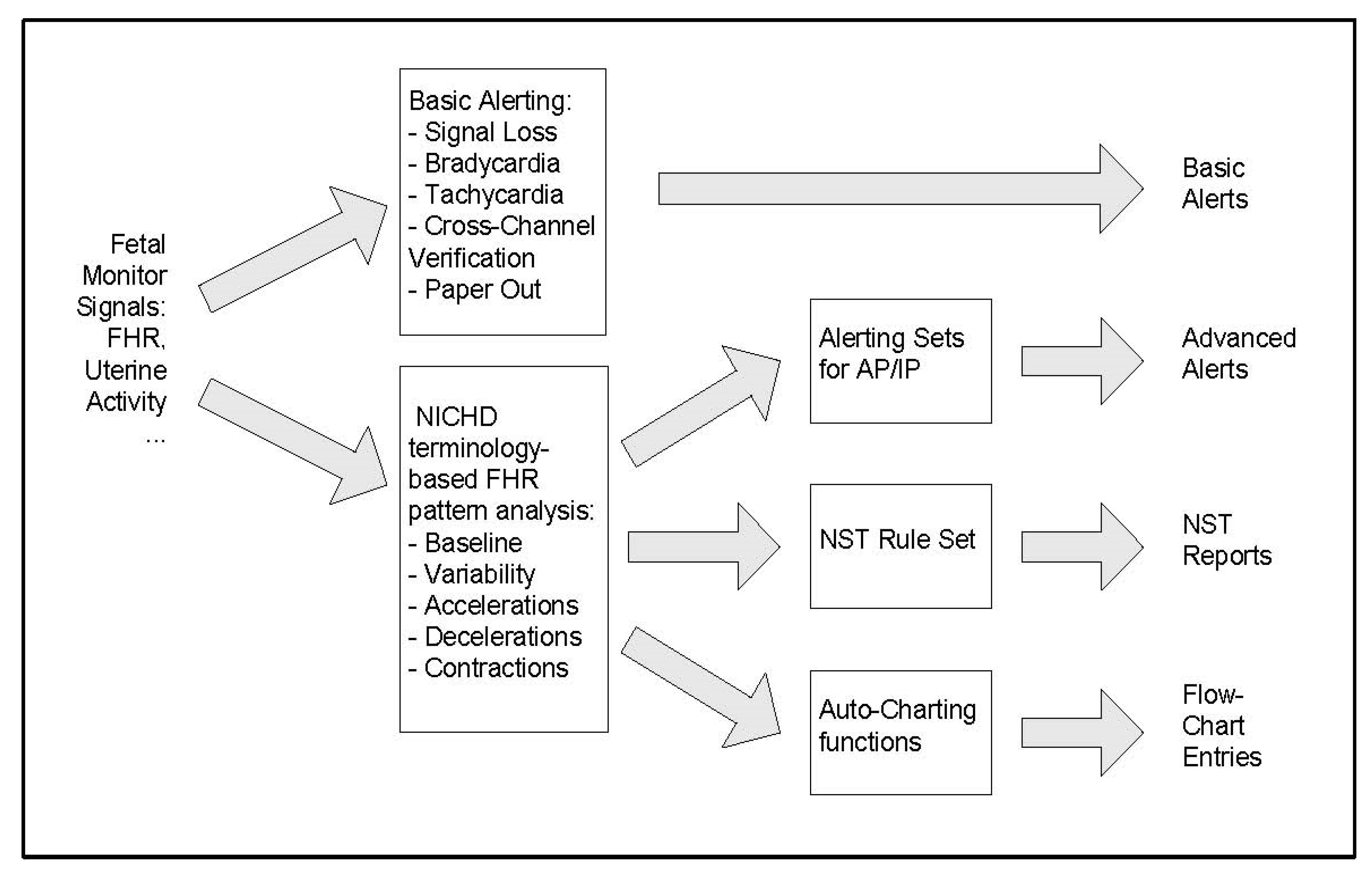
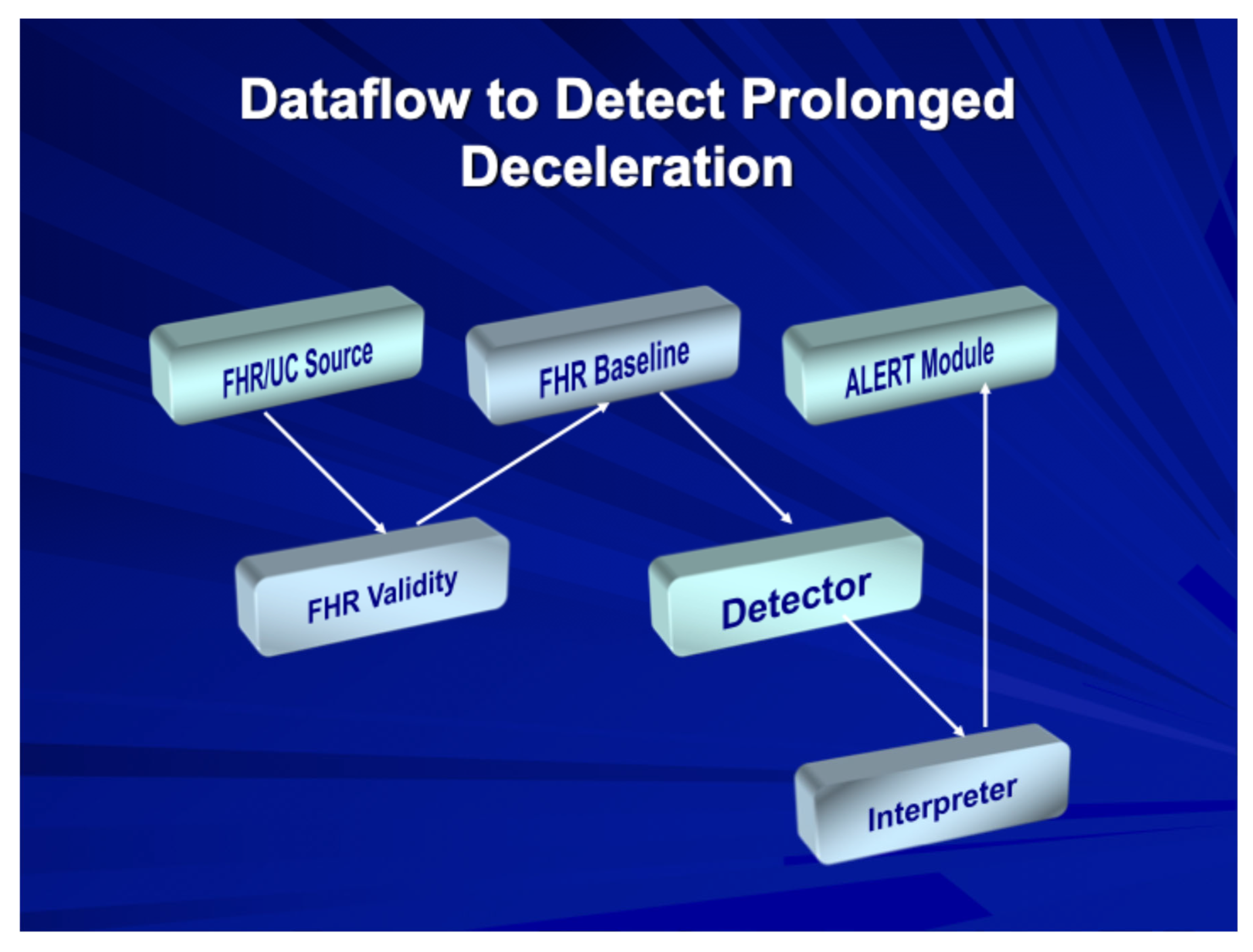
2.3. Automated FHR Analysis Systems
The INFANT Study
3. Future Directions for Fetal Surveillance Systems
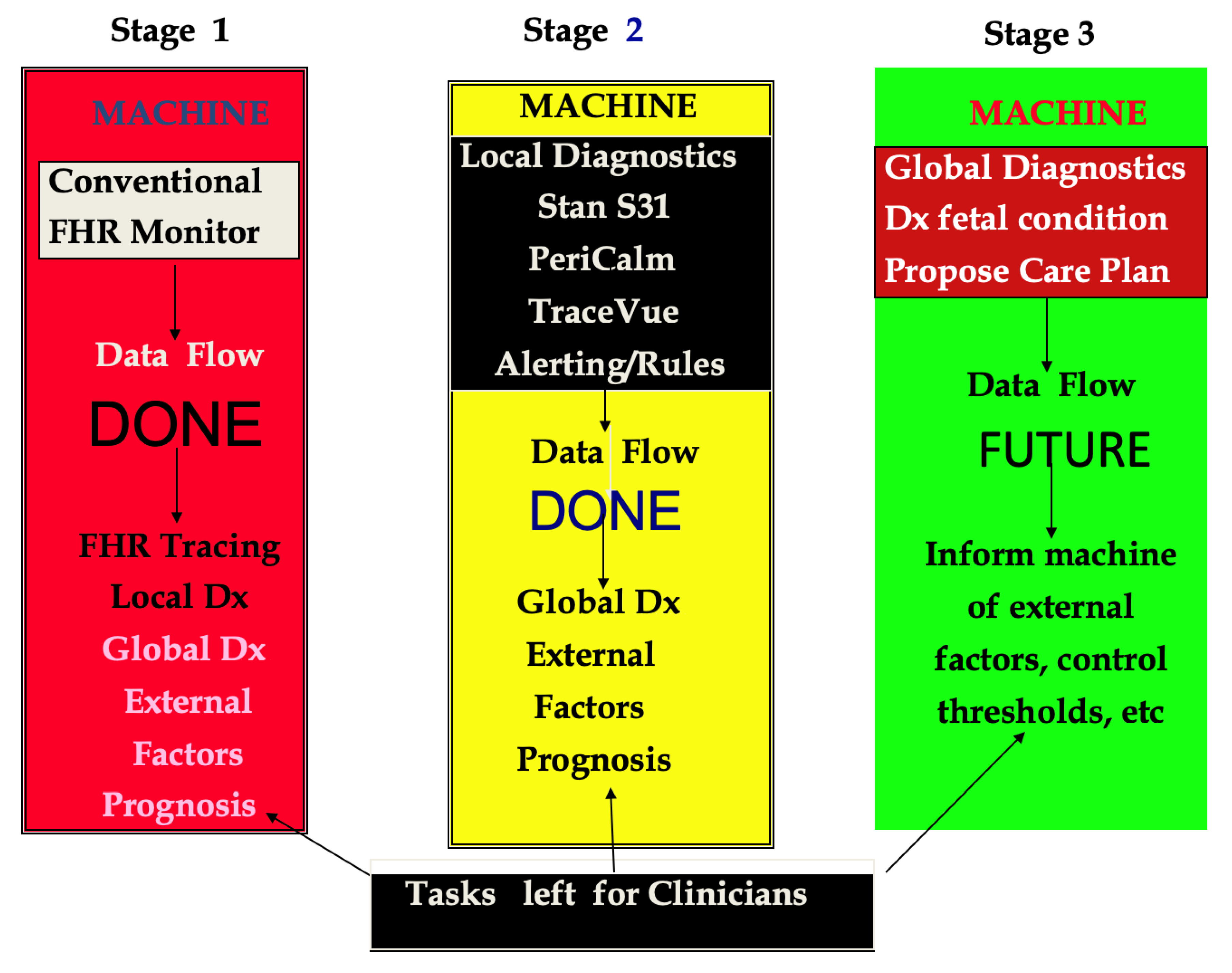
3.1. Developmental Stages of Surveillance Systems
3.2. Intelligent Intrapartum Surveillance Systems
3.3. Artificial Intelligence Applications
3.4. Risk Assessment Systems
4. The Fetal Reserve Index: A Novel Approach
4.1. Conceptual Framework
4.2. Initial Validation Studies
4.3. Clinical Applications
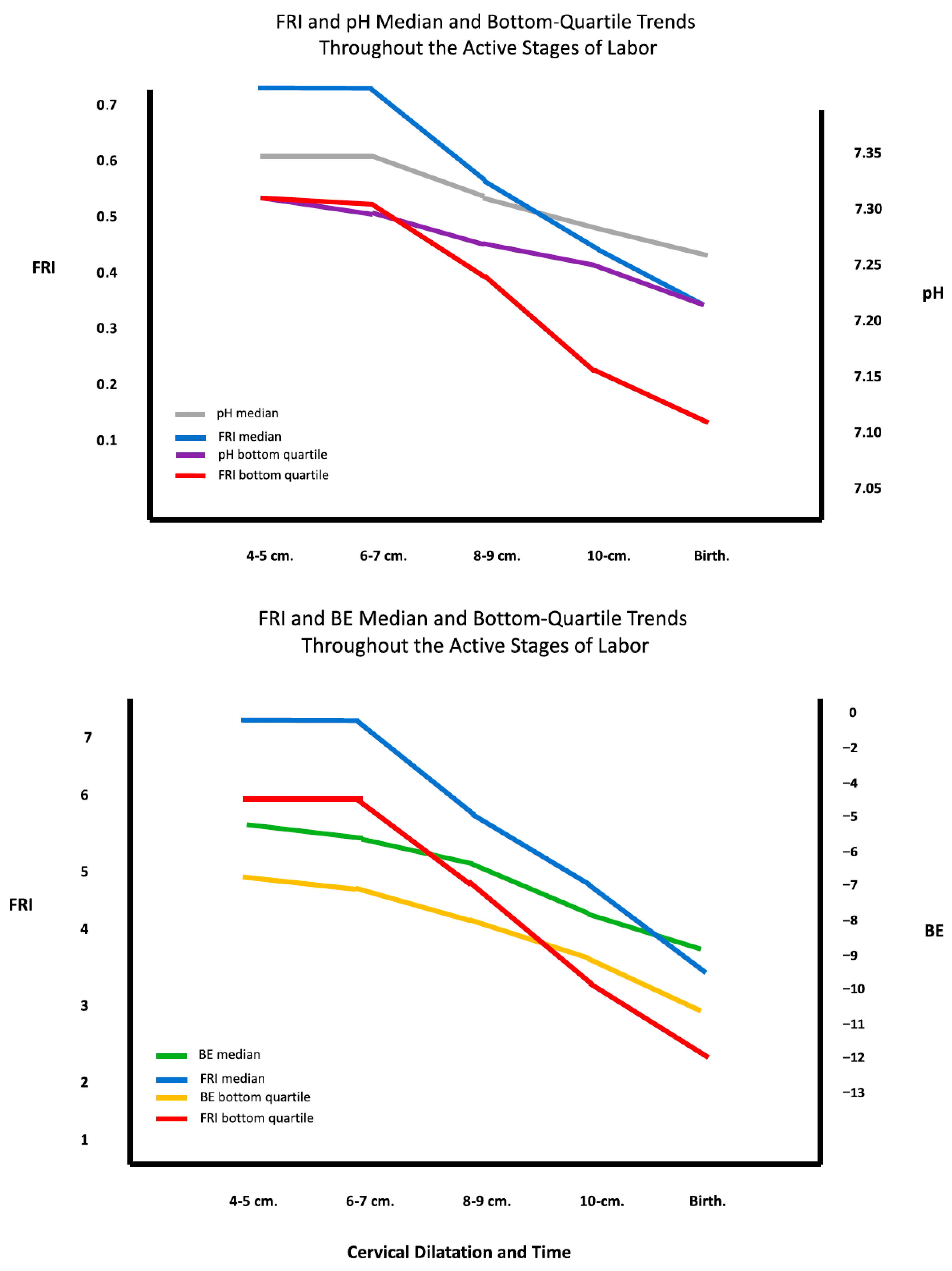
4.4. Correlation with Acid–Base Status
5. Future Horizons for Improving Intrapartum Monitoring Systems
5.1. Limitations of Current Approaches
5.2. AI-Enhanced FRI Development
- Automated FHR Analysis: Enable extraction of the four features (baseline FHR, variability, accelerations, and decelerations) and uterine activity quantification to determine uterine contraction frequencies.
- Electronic Health Record Integration: Incorporation of maternal, fetal, and obstetrical risk factors directly from electronic health records.
- Machine Learning: Weighting of risk factors with machine learning to create AI programs, most likely in the form of neural networks.
- Real-time Scoring: Automated FRI score generation updated every 10 to 20 min, generating clinical alerts when necessary (e.g., fetal status changes as colorimetric zones transition from green to yellow or from yellow to red).
- Intervention Tracking: When IR is being performed, associated FRI scores would track its impact on fetal status at 20 min intervals.
- Cloud Accessibility: FRI system output would be accessible in secure cloud servers that could notify care providers at any location.
- Remote Monitoring: The FRI system would provide the backbone for remote fetal monitoring at home with the capability of notifying providers of changes in status.
- Postnatal Surveillance: Postnatal fetal surveillance using FRI principles for continuous FHR monitoring and transcutaneous pulse oximetry for the first hour of neonatal life. The need for such surveillance is suggested by patterns of abnormal neonatal physiologic adaptation for compromised infants that were observed in one of our earlier studies [36].
- Resource-Adapted Versions: Versions of the FRI system would be developed for low-resource settings.
5.3. Regulatory and Implementation Considerations
5.4. Medico-Legal Considerations
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paul, R.H.; Hon, E.H. Clinical fetal monitoring. V. Effect on perinatal outcome. Am. J. Obstet. Gynecol. 1974, 118, 529–533. [Google Scholar] [CrossRef]
- Alfirevic, Z.; Devane, D.; Gyte, G.M.; Cuthbert, A. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst. Rev. 2017, 2, CD006066. [Google Scholar] [CrossRef]
- Devoe, L.; Golde, S.; Kilman, Y.; Morton, D.; Shea, K.; Waller, J. A comparison of visual analyses of intrapartum fetal heart rate tracings according to the new National Institute of Child Health and Human Development guidelines with computer analyses by an automated fetal heart rate monitoring system. Am. J. Obstet. Gynecol. 2001, 183, 361–366. [Google Scholar] [CrossRef]
- National Institute of Child Health and Human Development Research Planning Workshop. Electronic fetal heart rate monitoring: Research guidelines for interpretation. Am. J. Obstet. Gynecol. 1997, 177, 1385–1390. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin no. 106:Intrapartum fetal heart rate monitoring: Nomenclature, interpretation, and general management principles. Obstet. Gynecol. 2009, 114, 192–202. [Google Scholar] [CrossRef]
- Dildy, G.A.; Clark, S.L.; Loucks, C.A. Intrapartum fetal pulse oximetry: Past, present, and future. Am. J. Obstet. Gynecol. 1996, 175, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bloom, S.L.; Spong, C.Y.; Thom, E.; Varner, M.W.; Rouse, D.J.; Weininger, S.; Ramin, S.M.; Caritis, S.N.; Peaceman, A.; Sorokin, Y.; et al. Fetal pulse oximetry and cesarean delivery. N. Engl. J. Med. 2006, 355, 2195–2202. [Google Scholar] [CrossRef] [PubMed]
- Amer-Wåhlin, I.; Yli, B.; Arulkumaran, S. Foetal ECG and STAN technology—A review. Eur. Clin. Obstet. Gynaecol. 2005, 1, 61–73. [Google Scholar] [CrossRef]
- Norén, H.; Carlsson, A. Reduced prevalence of metabolic acidosis at birth: An established STAN usage in the total population of deliveries in a Swedish district hospital. Am. J. Obstet. Gynecol. 2010, 202, 546.e1–546.e7. [Google Scholar] [CrossRef]
- Belfort, M.A.; Saade, G.R.; Thom, E.; Blackwell, S.C.; Reddy, U.M.; Thorp, J.M.J.; Tita, A.T.; Miller, R.S.; Peaceman, A.M.; McKenna, D.S.; et al. A randomized trial of intrapartum fetal ECG ST-segment analysis. N. Engl. J. Med. 2015, 373, 632–641. [Google Scholar] [CrossRef]
- Alonso-Betanzos, A.; Moret-Bonillo, V.; Devoe, L.; Searle, J.; Banias, B.; Ramos, E. Computerized antenatal assessment: The NST-Expert Project. Automedica 1992, 14, 3–22. [Google Scholar]
- Dawes, G.S.; Lobb, M.; Moulden, M.; Redman, C.W.; Wheeler, T.G. Antenatal cardiotocography quality and interpretation using computers. Int. J. Obstet. Gynaecol. 1992, 99, 787–791. [Google Scholar]
- Keith, R.D.; Beckley, S.; Garibaldi, J.M.; Westgate, J.A.; Ifeachor, E.C.; Greene, K.R. A multicentre comparative study of 17 experts and an intelligent computer system for managing labour using the cardiotocogram. Br. J. Obstet. Gynaecol. 1995, 102, 688–700. [Google Scholar] [CrossRef]
- Bernardes, J.; Costa-Pereira, A.; Ayres-de-Campos, D.; van Geijn, H.P.; Pereira-Leite, L. Evaluation of interobserver agreement of cardiotocograms. Int. J. Gynaecol. Obstet. 1997, 57, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.F.; Warrick, P.A. New perspectives in electronic fetal surveillance. J. Perinat. Med. 2012, 40, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Pereira, J.; Costa, A.; Ayres-De-Campos, D.; Costa-Santos, C.; Amaral, J.; Bernardes, J. Computerized analysis of cardiotocograms and ST signals is associated with significant reductions in hypoxic-ischemic encephalopathy and cesarean delivery: An observational study in 38,466 deliveries. Am. J. Obstet. Gynecol. 2019, 220, 269.e1–269.e8. [Google Scholar] [CrossRef] [PubMed]
- Brocklehurst, P.; Field, D.; Greene, K.; Juszczak, E.; Keith, R.; Kenyon, S.; Linsell, L.; Mabey, C.; Newburn, M.; Plachcinski, R.; et al. Computerised interpretation of fetal heart rate during labour (INFANT): A randomised controlled trial. Lancet 2017, 389, 1719–1729. [Google Scholar] [CrossRef]
- Devoe, L.D.; Muhanna, M.; Maher, J.; Evans, M.I. Current state of artificial intelligence model development in obstetrics. Obstet. Gynecol. 2025, 146, 233–243. [Google Scholar] [CrossRef]
- Georgieva, A.; Payne, S.J.; Moulden, M.; Redman, C.W. Artificial neural networks applied to fetal monitoring in labor. Neural Comput. Appl. 2013, 22, 85–93. [Google Scholar] [CrossRef]
- Al-Yousif, S.; Naim, I.A.; Talab, H.S.; Al Qahtani, N.H.; Alfiras, M.; Al-Rawi, O.Y.; Al-Dayyeni, W.S.; Alrawi, A.A.A.; Mnati, M.J.; Jarrar, M.; et al. Intrapartum cardiotocography pattern pre-processing features extraction and fetal health condition based on RCOG guideline. PeerJ Comput. Sci. 2022, 8, e1050. [Google Scholar] [CrossRef]
- Park, T.J.; Chang, H.J.; Choi, B.J.; Jung, J.A.; Kang, S.; Yoon, S.; Kim, M.; Yoon, D. Machine learning model for classifying the results of fetal cardiotocography conducted in high-risk pregnancies. Yonsei Med. J. 2022, 63, 692–700. [Google Scholar] [CrossRef]
- Ogasawara, J.; Ikenoue, S.; Yamamoto, H.; Sato, M.; Kasuga, Y.; Mitsukura, Y.; Ikegaya, Y.; Yasui, M.; Tanaka, M.; Ochiai, D. Deep neural network-based classification of cardiotocograms outperformed conventional algorithms. Sci. Rep. 2021, 11, 13367. [Google Scholar] [CrossRef]
- Daydulo, Y.D.; Thamineni, B.L.; Dasari, H.K.; Aboye, G.T. Deep learning based fetal distress detection from time frequency representation of cardiotocogram signal using Morse wavelet: Research study. BMC Med. Inform. Decis. Mak. 2022, 22, 329. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, Z.; Shao, H.; Sun, C.; He, X.; Zhang, J.; Wang, T.; Yang, X.; Wang, Y.; Bin, Y.; et al. Intelligent classification of cardiotocography based on a support vector machine and convolutional network: Multiscene research. Int. J. Gynaecol. Obstet. 2024, 165, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Choi, E.S.; Nam, Y.J.; Liu, N.W.; Yang, Y.S.; Kim, H.Y.; Ahn, K.H.; Hong, S.C. Real-time classification of fetal status based on deep learning and cardiotocography data. J. Med. Syst. 2023, 47, 82. [Google Scholar] [CrossRef]
- McCoy, J.A.; Levine, L.D.; Wan, G.; Chivers, C.; Teel, J.; La Cava, W.G. Intrapartum electronic fetal heart rate monitoring to predict acidemia at birth with use of deep learning. Am. J. Obstet. Gynecol. 2025, 232, 116.e1–116.e9. [Google Scholar] [CrossRef] [PubMed]
- Aeberhard, J.L.; Radan, A.-P.; Delgado-Gonzalo, R.D.; Strahm, K.M.; Sigurthorsdottir, H.B.; Schneider, S.; Surbek, D. Artificial intelligence and machine learning in cardiotocography: A scoping review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 281, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Devoe, L.D. Future perspectives in intrapartum fetal surveillance. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 30, 98–106. [Google Scholar] [CrossRef]
- Evans, M.I.; Britt, D.W.; Evans, S.M.; Devoe, L.D. Improving the interpretation of electronic fetal monitoring: The fetal reserve index. Am. J. Obstet. Gynecol. 2023, 228, S1129–S1143. [Google Scholar] [CrossRef]
- Eden, R.D.; Evans, M.I.; Evans, S.M.; Schifrin, B.S. The Fetal Reserve Index: Re-engineering the interpretation and responses to fetal heart rate patterns. Fetal Diagn. Ther. 2018, 43, 90–104. [Google Scholar] [CrossRef]
- Eden, R.D.; Evans, M.I.; Evans, S.M.; Schifrin, B.S. Reengineering electronic fetal monitoring interpretation: Using the fetal reserve index to anticipate the need for emergent operative delivery. Reprod. Sci. 2018, 25, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Eden, R.D.; Evans, M.I.; Britt, D.W.; Evans, S.M.; Schifrin, B.S. Safely lowering the emergency cesarean and operative vaginal delivery rates using the “fetal reserve index”. J. Matern. Fetal Neonatal Med. 2020, 33, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Britt, D.W.; Evans, M.I.; Schifrin, B.S.; Eden, R.D. Refining the prediction and prevention of emergency operative deliveries with the fetal reserve index. Fetal Diagn. Ther. 2019, 46, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.I.; Britt, D.W.; Worth, J.; Evans, S.M.; Mussalli, G.; Devoe, L.D. Uterine contraction frequency in the last hour of labor: How many contractions are too many? J. Matern. Fetal Neonatal Med. 2022, 35, 8698–8705. [Google Scholar] [CrossRef]
- Evans, M.I.; Britt, D.E.; Eden, R.D.; Evans, S.M.; Schifrin, B.S. Early and improved screening for impending fetal compromise. J. Matern. Fetal Neonatal Med. 2022, 35, 2895–2903. [Google Scholar] [CrossRef]
- Eden, R.D.; Evans, M.I.; Britt, D.W.; Evans, S.M.; Gallagher, P.; Schifrin, B.S. Combined prenatal and postnatal prediction of early neonatal compromise. J. Matern. Fetal Neonatal Med. 2021, 34, 2996–3007. [Google Scholar] [CrossRef]
- Devoe, L.D.; Britt, D.W.; Macedonia, C.R.; Worth, J.M.; Mussalli, G.M.; Modestin-Sorrentino, M.; Evans, M.I. Reconceptualizing intrauterine resuscitation and its short-term impact. Diagnostics 2025, 15, 255. [Google Scholar] [CrossRef]
- ACOG. Neonatal Encephalopathy and Neurologic Outcome; D’Alton, M., Ed.; ACOG: Washington, DC, USA, 2014. [Google Scholar]
- Evans, M.I.; Britt, D.W.; Devoe, L.D. Etiology and ontogeny of cerebral palsy: Implications for practice and research. Reprod. Sci. 2024, 31, 1179–1189. [Google Scholar] [CrossRef]
- Evans, M.I.; Eden, R.D.; Britt, D.W.; Evans, S.M.; Schifrin, B.S. Re-engineering the interpretation of electronic fetal monitoring to identify reversible risk for cerebral palsy: A case control series. J. Matern. Fetal Neonatal Med. 2018, 31, 3144–3152. [Google Scholar] [CrossRef]
- Evans, M.I.; Britt, D.W.; Eden, R.D.; Gallagher, P.; Evans, S.M.; Schifrin, B.S. The fetal reserve index significantly outperforms ACOG Category system in predicting cord blood base excess and pH: A methodological failure of the category system. Reprod. Sci. 2019, 26, 858–863. [Google Scholar] [CrossRef]
- Miller, D.A. Intrapartum Fetal Heart Rate Assessment. UpToDate 2019. Available online: https://www.uptodate.com/contents/table-of-contents/obstetrics-gynecology-and-womens-health (accessed on 1 March 2025).
- Ross, M.G. Misinformation and junk science in obstetrics medical malpractice. O&G Open 2025, 2, e073. [Google Scholar] [CrossRef]
- Castel, A.; Frank, Y.S.; Feltner, J.; Karp, F.B.; Albright, C.M.; Frasch, M.G. Monitoring fetal electroencephalogram intrapartum: A systematic literature review. Front. Pediatr. 2020, 8, 584. [Google Scholar] [CrossRef]
- Zourabian, A.; Siegel, A.; Chance, B.; Ramanujan, N.; Rode, M.; Boas, D.A. Trans-abdominal monitoring of fetal arterial blood oxygenation using pulse oximetry. J. Biomed. Opt. 2000, 5, 391–405. [Google Scholar] [CrossRef]
- Blumenthal, D.; Patel, B. The regulation of clinical artificial intelligence. Nejm AI 2024, 1, AIpc2400545. [Google Scholar] [CrossRef]
- Richards, E.P. The Supreme Court Rules on Medical Device Liability—Or Does It? IEEE Eng. Med. Biol. Mag. 1997, 16, 87. [Google Scholar] [CrossRef]

| Authors | System Name | Design | Data Analyzed | Year |
|---|---|---|---|---|
| Dawes GS et al. [12] | Oxford Sonicaid | Rule-based | Antepartum FHR | 1996 |
| Alonzo-Betanzos A et al. [11] | NST-Expert | Rule-based | Antepartum FHR | 1992 |
| Keith RDF et al. [13] | INFANT | RB + AI * | Intrapartum FHR | 1994 |
| Bernades J et al. [14] | SIS-Porto | Rule-based | Intrapartum FHR | 2000 |
| Hamilton et al. [15] | Peri-Calm | RB + AI | Intrapartum FHR | 2010 |
| Amer-Wahlin et al. [8] | STAN | Rule-based | IP FHR + fetal ECG | 2014 |
| Reference | Study Design | Study Population | AI Model | Accuracy Metric | Category | Benefits |
|---|---|---|---|---|---|---|
| Georgieva et al., 2013 [19] | Retrospective | 124 patients: training set 252 patients: testing set 7568 patients: validation set | NN | PPV: 84.8% NPV: 81.5% Sensitivity: 80% Specificity: 85% | EFM | Highly accurate model for predicting adverse outcomes |
| McCoy et al., 2025 [26] | Retrospective | 10,182 patients, time-stamped matched with cord blood gases with pH values | NN | AUROC: 0.89 | EFM Clinical Care | Correlation of pH values < 7.05 and BE of <−10 meq/L With EFM patterns |
| Lee et al., 2023 [25] | Retrospective | 5249 cardiotocography traces | NN | Harmonic means 0.94–0.98 | Analyze EFM | Efficient model for fetal status classification in real time |
| Zhang et al., 2024 [24] | Retrospective | 2542 CTG records | SVM NN | AUROC: 0.95 | Analyze EFM | Effectively assists obstetricians in classifying CTG i |
| Daydulo et al., 2022 [23] | Retrospective | 552 recording | NN | AUROC: 0.78 | EFM Clinical Care | Can be used as a decision-making aid system for obstetricians |
| Ogasawara et al., 2021 [22] | Retrospective | 5406 deliveries | NN | AUROC: 0.73 | EFM Clinical Care | Reliably predicted infant outcomes from 30 min of CTG just before delivery |
| Park et al., 2022 [21] | Retrospective | 1456 recordings | NN, LGBM | AUROC: 0.73 | Analyze EFM | Precise FHR signal classifying as a screening tool to monitor fetal status |
| Al-Yousif et al., 2022 [20] | Retrospective | 150 CTG signals | Fuzzy logic | Kappa scores ranging from 0.607 to 0.926 | Analyze EFM | Good prediction of the visual classification results by three expert panelists |
| Representative FRI Studies | Population | Study Type | Objective | Results |
|---|---|---|---|---|
| Eden RD, Evans MI, Evans SM, Schifrin BS. The Fetal Reserve Index: Re-engineering the interpretation and responses to fetal heart rate patterns Fetal Diagn Ther. 2018; 43(2):90–104 [30] | 50 CP cases 200 controls All cases normal on admission | Retrospective | Compare FRI to ACOG Categories and CP Monograph to identify CP | For CP cases FRI scores identified 100%, ACOG Category III identified 44%, ACOG CP Monograph criteria found 30%. |
| Eden RD, Evans MI, Evans SM, Schifrin BS. Re-engineering electronic fetal monitoring interpretation: Using the Fetal Reserve Index to anticipate the need for emergent operative delivery. Reprod Sci. 2018 Apr; 25(4): 487–97 [31] | 300 cases with normal neonatal outcomes | Retrospective | Compare FRI scores and zones for EOD cases with non-EOD cases | EOD cases << FRI scores than non-EOD cases. “Red zone” more often and longer for EOD cases with sensitivity of 92%, PPV of 64%, and false positive rate of 10%. |
| Eden RD, Evans MI, Britt DW, Evans SM, Schifrin BS. Safely lowering the emergency cesarean and operative vaginal delivery rates using the Fetal Reserve Index. J Matern Fetal Med 2020 May;33(9):1473–1479 [32] | 400 control; 400 using FRI All normal outcomes | Prospective | Predict risk of EOD if FRI principles used in management | Comparable incidence of red zone tracings (25%). IR in 1st group (20%), in 2nd group (47%). EODs reduced from 17.3% to 4%. |
| Britt DW, Evans MI, Schifrin BS, Eden RD. Refining the prediction and prevention of emergency operative deliveries with the Fetal Reserve Index. Fetal Diag Ther 2019; 46:159–165 [33] | 1402 term singletons in labor with normal outcomes | Retrospective | Predict EOD risk in FRI Red zone ≥ 1 h and if IR performed | Reaching Red zone early and remaining > 1 h increases EOD probability. When these risk factors are paired with IR in Stage 1, EOD probability is reduced from 0.93 to 0.15. |
| Evans MI, Britt DW, Worth J, Mussali G, Evans SM, Devoe LD. Uterine contraction frequency in the last hour of labor: how many contractions are too many? J Matern Fetal Neonatal Med 2022 Dec; 35 (25):8698–8705 [34] | 475 patients monitored in labor and neonatally | Retrospective | Evaluate CB BE, and pH; 1′ Apgar, non-NSVDs, NHR@16′ postnatal | UCF > 4: higher sensitivity to detect decreased Apgar-1′ and 5′, NHR above 160 bpm, higher BE, and non-NSVD than UCF > 5, earlier fetal compromise detected. |
| Evans MI, Britt DW, Eden RD, Evans SM, Schifrin BS. Earlier and improved screening for impending fetal compromise. J Matern Fetal & Neonatal Med 2022 Dec, 35 (15): 2895–2903 [35] | 475 high-risk patients monitored in labor and neonatally | Retrospective | Assess FRI score as a proxy for fetal pH and BE values from fetal scalp sampling (FSS) | FSS-obtained pH and BE worsens during 1st stage of labor. Trajectory of FRI provides reasonable approximation of FSSpHBE trajectory to enable earlier intervention as needed. |
| Eden RD, Evans MI, Britt DW, Evans SM, Gallagher P, Schifrin BS. Combined prenatal and postnatal prediction of early neonatal compromise risk. J Maternal-Fetal & Neonatal Medicine 2021, 34 (18); 2996–3007 [36] | 251 high-risk singleton term pregnancies | Retrospective | Last FRI score to immediate NHR pattern and umbilical/NN acid-base balance | FRI successfully predicted neonates with suboptimal adaptation and need for additional support. |
| Devoe LD, Britt DW, Macedonia CR, Worth JM, Mussalli GM, Mondestin-Sorrentino M, Evans MI: Reconceptualizing intrauterine resuscitation and its short-term impact. Diagnostics 2025; 15: 255. doi.org/10.3390/diagnostics15030255 [37] | 118 patients receiving Pitocin to induce or augment labor and who had IR | Retrospective | Derived 2 measures of IR effectiveness: (1) Improvement (2) Stabilization based on FRI score change | 71% improved; 78% stabilized with IR by FRI score changes. However, wide variation in clinician practices for using IR were noted and did not necessarily correlate with FRI-calculated fetal risk. |
| Maternal Risk Factors | Obstetric Risk Factors | Fetal Risk Factors |
|---|---|---|
| Decreased cardiac output/vascular perfusion of the placenta - Cardiac disease with risk of reduced cardiac output in pregnancy - Hypertension (chronic, pregnancy-induced - SLE | IUGR/Macrosomia. | Abnormal Dopplers/BPP |
| Oxygen carrying capacity - Pulmonary disorders (e.g., asthma) - Anemia and hemoglobinopathy | Oligohydramnios | Genetic Disorders |
| Infection (chronic and acute) | Polyhydramnios | Fetal arrhythmia |
| Chronic debilitating disease | Bleeding and abruption | Meconium passage |
| Malabsorption/poor weight gain | Previous cesarean section | Chorioamnionitis |
| Endocrine—diabetes and thyroid disorders | Placental and umbilical cord anomalies | Second stage of labor-pushing |
| Advanced maternal age | Rupture of membranes PPROM, SROM, AROM | Amnioinfusion |
| Drug abuse, addiction and smoking | Dystocia (protraction, arrest labor disorders) | Discontinuation of Pitocin due to fetal intolerance |
| Obesity—BMI > 35 | Malpresentation | Conversion pattern (acute prolonged tachycardia > 170 bpm) |
| Short stature ≤ 5′2″ | Ominous overshoots | |
| Bradycardia (<100 bpm) | ||
| Missing important data in labor (e.g., lack of EFM in second stage) |
| Uterine Contraction Frequency (No./Min.) | Apgar1′ * Sensitivity (Specificity) [PPV] PLR | Apgar5′ * Sensitivity (Specificity) [PPV] PLR | NHR 16 * Sensitivity (Specificity) [PPV] PLR | Non-NSVD * Sensitivity (Specificity) [PPV] PLR | BEcg * Sensitivity (Specificity) [PPV] PLR | pHcg * Sensitivity (Specificity) [PPV] PLR |
|---|---|---|---|---|---|---|
| UCF > 4 ** | 79% (28%) [17%] 1.10 | 74% (28%) [27%] 0.97 | 81% (31%) [52%] 1.19 | 78% (31%) [41%] 1.12 | 82% (29%) [16%] 1.16 | 84% (29%) [15%] 1.18 |
| UCF > 5 ** | 38% (67%) [18%] 1.17 | 34% (67%) [8%] 1.02 | 38% (68%) [52%] 1.17 | 37% (68%) [42%] 1.17 | 37% (28%) [16%] 1.12 | 32% (66%) [13%] 1.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devoe, L.D. Generational Leaps in Intrapartum Fetal Surveillance. Diagnostics 2025, 15, 2482. https://doi.org/10.3390/diagnostics15192482
Devoe LD. Generational Leaps in Intrapartum Fetal Surveillance. Diagnostics. 2025; 15(19):2482. https://doi.org/10.3390/diagnostics15192482
Chicago/Turabian StyleDevoe, Lawrence D. 2025. "Generational Leaps in Intrapartum Fetal Surveillance" Diagnostics 15, no. 19: 2482. https://doi.org/10.3390/diagnostics15192482
APA StyleDevoe, L. D. (2025). Generational Leaps in Intrapartum Fetal Surveillance. Diagnostics, 15(19), 2482. https://doi.org/10.3390/diagnostics15192482






