Abstract
Preterm labour (PTL) affects about 11% of all deliveries world-wide. It is a major cause of perinatal morbidity and mortality. Although the precise cause is unknown in about 50% of cases, infections are thought to be a major contributing factor. These infections are more common in earlier preterm deliveries. While some women with these infections will manifest the classical features of fever, tachycardia (maternal and/or fetal), leucocytosis, raised biomarkers of infections, and uterine tenderness/irritation, others will be asymptomatic. Some of the women may develop a short/dilating cervix without any obvious contractions. Identifying such women is potentially challenging. Evidence has shown that a condensation of echogenic particles just above the cervix—amniotic fluid (AF) sludge, identified by ultrasound—is a marker for microbial invasion of the amniotic cavity (MIAC) and preterm birth (PTB) in both asymptomatic and symptomatic women (including those with a short or normal cervix). Those with a short cervix with AF sludge have a significantly greater risk of progression to PTB. Treatment with antibiotics has been shown in some but not all case series to result in a resolution of the sludge and either a delay or prevention of PTB. The widely varied results from treatment could be related to the antibiotics used and the route of administration. The use of the parenteral combination of clindamycin, a cephalosporin, and metronidazole has been shown to be more effective compared to azithromycin. Here we review the literature on the relationship between the sludge and PTB and conclude (1) that the AF sludge is an ultrasound marker of MIAC and PTL and (2) that following its diagnosis, appropriate counselling should be offered and the triple antibiotic combination offered. We suggest that randomised trials should be undertaken to determine the most efficacious antibiotic combination.
1. Introduction
Preterm birth (PTB), defined as delivery before 37 completed weeks of gestation, is the leading cause of severe neonatal morbidity and mortality world-wide and accounts for about 50% of neonatal deaths [1,2]. Of the estimated 15 million babies born preterm globally, 5% are before 28 weeks, 10% are between 28 and 32 weeks, and 84% are between 32 and 36 weeks [3]. It is estimated that of all the deaths in the first 5 years of life, about a million (18%) are in children born preterm [4]. The overall PTB rate is about 11%, but this varies from 4–20% depending on the WHO region/country, with the highest rates being in low-income countries. [3,5,6]
Although PTB has been defined by gestational age, there is increasingly a drive to change its taxonomy to phenotypes that incorporate variables such as risk factors, causal conditions, clinical presentation, nutritional status, laboratory features, and mechanisms of action [7,8]. The traditional definition of PTB as a single clinical entity based on gestational age alone fails to acknowledge its syndromic characteristics [9,10]. Furthermore, there has also been a drive to incorporate into this new approach taxonomy biomarkers that reflect maternal vascular malperfusion (e.g., placental growth factor—P1GF, soluble fms-like tyrosine-kinase 1—sFlt-1, and pregnancy-associated plasma protein-A—PAPP-A) [11,12,13]. It must, however, still be acknowledged that setting an upper limit of 36+6 weeks’ gestation for defining PTB, whilst it is arbitrary and there is no strong biological reason for doing so, remains a crucial summary marker for neonatal outcomes because it reflects organ immaturity, i.e., increased risk of death, as well as short- and long-term complications [14].
The exact cause of spontaneous preterm birth (SPTB) is unknown in as many as 50% of cases, and it is generally accepted that it is multifactorial in a large number of cases [3,15]. Infections appear to be a common pathway in approximately 25% of cases (and it is probably a common pathway in a much higher percentage in deliveries before 23 weeks, at 79%, with the percentage declining to about 11% for deliveries between 28 and 34 weeks) [16,17]. The presentation of these infections varies from obvious features of chorioamnionitis (such as fever, uterine tenderness, maternal leucocytosis and tachycardia, fetal tachycardia, and foul-smelling/malodourous vaginal discharge) to no obvious clinical features. Confirmation of microbial invasion of the amniotic cavity (MIAC) has traditionally been either from cultures of amniotic fluid and tissues and/or histologically on examination of fetal membranes, the umbilical cord (funisitis), and the decidua [18,19,20]. Several studies have demonstrated the presence of infections in the amniotic fluid, fetal membranes, and cervico-vaginal secretions in women presenting in spontaneous preterm labour (SPTL) [19,20,21]. In those with a short cervix, the evidence is overwhelming [22,23], with typical organisms identified including Ureaplasma urealyticum, Gardnerella vaginalis, Candida albicans, and Fusibacterium spp. [24,25]. For diagnosing intra-amniotic infections, opinions vary on whether this should be by amniocentesis routinely performed on all those presenting in SPTL [21] or only by screening the vagina/cervix for infections. Tests on the amniotic fluid, when obtained, include (a) those able to generate rapid results (such as the quantification of glucose, interleukin-6, and MMP-8 levels, white blood cells, and microscopy that could guide management) and (b) cultures that may require time to generate results. The increasing use of molecular biology diagnostic approaches (such as the multiplex PCR test) has improved the rapidity of diagnosis and increased the isolation of micro-organisms that would otherwise not have been identified from routine cultures [25,26].
While the evidence is robust for the role of infections in those in SPTL with or without a short cervix [24], this is less so in those who are asymptomatic and with a normal cervix, who may eventually progress to deliver preterm. Some of these women may have subclinical chorioamnionitis. Early identification of these women and timely institution of interventions (such as antibiotics) may reduce the risk of progression to preterm labour, not only in the symptomatic but also in the asymptomatic women. A key challenge for clinicians is whether there are features that may be indicative of a possible infection prior to shortening and dilatation of the cervix or contractions in this population. An ideal setup will be one where these women at risk are identified and timely interventions to interrupt the process of preterm labour are instituted. This can only be achieved with reliable and specific markers of the causes of SPTB. Ultrasound scans can identify a population with cervical changes and allow/enable interruptions (e.g., cervical cerclage) that have indeed been shown to reduce/delay SPTB. Another possible ultrasound marker, for MIAC—a process that has been shown to predispose mothers to SPTL—is the amniotic fluid sludge [27]. This review brings together the evidence for considering AF sludge as a marker for MIAC and the need to treat this with antibiotics.
2. Amniotic Fluid (AF) Sludge and Its Constitution
Particulate materials in the amniotic cavity are a common ultrasound scan finding. They are in general evenly distributed in the amniotic cavity and are thought to represent desquamated fetal cells, vernix caseosa, meconium, and, in some cases, blood [28,29,30,31]. This can also be pathological, where there is excessive desquamation, as in the case of congenital ichthyosis [32]. These particulate materials have been reported in about 4% of scans performed between the first and second trimesters [33,34], a percentage that rises to about 88% by 35 weeks [35]. AF sludge, on the other hand, is a dense aggregate of highly echogenic material accumulating above the cervix, which is present in about 1% of uncomplicated term pregnancies [19], with the percentage rising to about 23.5% in high-risk populations (spontaneous preterm labouring women with intact membranes) [36]. Espinoza and colleagues proposed this term to describe this free-floating hyperechogenic material and associated it with an increased risk of SPTB [27].
The location of the AF sludge and its association with microbial invasion of the amniotic cavity is highly suggestive of an infective/inflammatory process (involved in its formation) [37]. Micro-organisms may reach the amniotic cavity by breaching the membranes (when they ascend from the lower genital tract) or transplacentally, reaching through a haematogenous spread [38]. On reaching the amniotic cavity, the micro-organisms provoke an inflammatory response in the epithelial cells of the fetal skin, the membranes, and the umbilical cord [39]. As a result, there is a significant increase in pro-inflammatory cytokines (typically IL-1, IL-6, and IL-8 and TNF-α), prostaglandins, chemokines, and an increased expression of matrix metalloproteinases [37,39]. The increased expression of cytokines/chemokines in the amniotic cavity stimulates the migration of neutrophils across the decidua and the chorioamniotic membrane into the amniotic cavity, resulting in an increased white cell population and enhanced anti-microbial activity [37,39,40]. The facts that some studies have failed to isolate micro-organisms and that the AF sludge has also been found in pregnancies that progress to term suggest that the infective pathogenesis may not be applicable in all cases. Some have suggested that intra-amniotic inflammation can be a result of exposure to “danger signals” produced by cells undergoing stress/damage or death [41,42,43,44]. This is supported by the fact that sterile inflammation is more common in women presenting with SPTL and preterm premature rupture of fetal membranes (PPROM) compared to the classical microbiological inflammation [41,42,43,44]. Of note is also the fact that conventional cultures are only able to accurately identify infection in a proportion of cases. The increasing use of modern approaches to identify infectious organisms (PCR techniques) has increased isolation rates [26,27].
In an interesting report, Romero et al. not only performed an amniocentesis from the sludge but were able to physically observe it in vitro. The sludge was described as resembling pus on naked-eye examination. Gram staining from this and other studies has shown the presence of a variety of organisms (Mycoplasma hominis, Streptococcus mutans, and Aspergillus flavus). The key question, therefore, is what is the precise composition of this AF sludge? It has been postulated that progressive infection induces an intense inflammatory response and that the inflammatory cells from this response (neutrophils) combine with the micro-organisms to form the particulate material that is visible as the AF sludge [44].
When micro-organisms breach the feto-maternal barrier, the consequence depends on how large the dose of micro-organisms reaching the fetal membranes is—with the risk of infection/inflammation greatest with the highest micro-organism load [44]. These micro-organisms (bacteria) can exist in one of two forms—singly (in the planktonic form) or organised in biofilms or both [44]. Following invasion of the amniotic cavity, bacteria change their phenotype to protect themselves from the host response (which includes the generation of inflammatory cells (neutrophils and monocytes) and the production of anti-microbial peptides and other mediators which can kill or injure the bacteria [44]. The bacteria achieve this by aggregating themselves in building-like structures known as biofilms. These biofilms make the micro-organisms resistant to attack by the macrophages, natural or synthetic antibiotics, and anti-inflammatory mediators [37,38,39,40]. The bacteria in these biofilms are less likely to elicit an inflammatory response [28]. The formation of these intra-amniotic biofilms would partly explain why the intra-amniotic infection tends to be chronic and perhaps why they are difficult to treat, as these biofilms are relatively resistant to antibiotic treatment [45,46,47,48,49]. The balance between the proportion of bacteria that are in the planktonic form and those in the biofilms likely determines the course of the infection and the likelihood of positive cultures from amniotic fluid sampling. Planktonic bacteria are more likely to be cultured positive as opposed to those in the biofilm [44,47]. In some cases, no organisms have been isolated.
The concept of sterile inflammation, as has been found in some cases with amniotic fluid sludge, is supported by the recent findings by Wu et al. [50]. It would appear that there may be complex potential links between placental dysfunction and inflammation [51]. Both infection and sterile inflammation mechanisms of immune origin (including maternal antifetal rejection) may lead to infiltration of the placenta by lymphocytes, plasma cells, and/or macrophages, leading to chronic placental inflammatory lesions, which may be responsible for both abnormal placental function and perhaps alterations in maternal white blood cell distributions, as shown in the study by Wu et al. [50]. This could perhaps explain the failure to identify organisms in some cases of amniotic fluid sludge, but underscores the potential mechanisms by which these women progress to PTB.
3. Imaging for AF Sludge
The best approach to identifying AF sludge is with a transvaginal ultrasound scan with the woman lying in the lithotomy position after emptying her bladder. The probe should be placed in the anterior fornix and adjusted to obtain a sagittal view of the entire cervical length. The length of the cervix should be measured preferably thrice and the average obtained. AF sludge is diagnosed from the presence of a dense aggregate of highly echogenic particulate matter in close proximity to the internal cervical os [27,52]. This material may scatter with fetal movements or abdominal pressure, but should re-accumulate within a few seconds. Where facilities are available, VOCALTM software (manufactured by GE Healthcare Technologies, Inc., Chicago, IL, USA) can be used to measure the volume of the AF sludge. We recommend that at least 3 min be spent assessing the sludge because of the dynamic nature of this marker. Figure 1 shows the AF sludge in four of our patients.
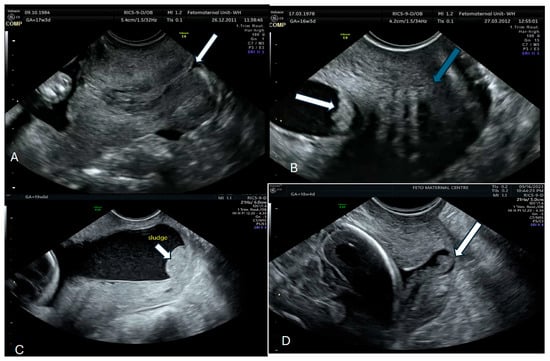
Figure 1.
Normal cervix with no sludge arrow (A); Amniotic sludge (arrow with normal cervix-green arrow) (B,C) Sludge (white arrow) and Sludge with a dilated cervix-white arrow (D).
4. AF Sludge and Intra-Amniotic Infections
An investigation of AF sludge aspirated by amniocentesis showed this to be positive for the micro-organisms Streptococcus mutans, Mycoplasma hominis, Ureplasma urealyticum, and Aspergillus flavus [53]. Interestingly, in a study by Yoneda and colleagues of women presenting in preterm labour at 20–29 weeks, [54] using polymerase chain reaction (PCR), the AF “sludge” was present in 18.1% (19/105) of patients. However, there was a similar positive micro-organism rate in the women with sludge and those without (31.6% versus 38.4%), but a significantly higher level of amniotic fluid interleukin-8 (15.2 ng/mL vs. 5.8 ng/mL; p = 0.005) and a higher frequency of histological chorioamnionitis in those with sludge (52.6% vs. 23.3%; p = 0.01). In another study of 25 women with AF sludge, examination of amniotic fluid collected after amniotomy showed that it was frequently associated with an inflammatory process [55]. Gill et al., in a cohort of 62 women with a short cervix and AF sludge (AFS) who had undergone amniocentesis, showed the rate of intra-amniotic inflammation to be 31.4% vs. 3.7% in those who delivered <32 weeks compared to those who delivered after, and furthermore, histological chorioamnionitis was significantly more common in the former group (75% vs. 32%) [56]. Interleukin-8 was shown to have the highest sensitivity and specificity for intra-amniotic inflammation and histological chorioamnionitis in this cohort. In an earlier study, Kusanovic and colleagues [36] found a higher positive culture rate in those with sludge (33.3% vs. 2.5%; p = 0.003), and a higher frequency of histological chorioamnionitis (77.8% vs. 19%; p < 0.001). The findings of histological chorioamnionitis and funisitis were in four cases presenting with cervical insufficiency and AF sludge [57]. Despite all these studies showing a high frequency of infection/inflammation in the presence of AF sludge, Ventura et al., in a study of 16 cases, failed to show any difference in these parameters, although the women with AF sludge delivered earlier [58]. Taken together, these case series appear to suggest that AF sludge is a proxy or indeed a marker for microbial invasion of the amniotic cavity, which in some cases may manifest as intra-amniotic infection and/or inflammation and, therefore, is a risk factor for PTB. There is a need for more studies to investigate just how good a marker of preterm birth AF sludge is. Table 1 summarises the studies that have investigated the association between AF sludge and intra-amniotic infection/inflammation [27,36,54,57,58,59,60].

Table 1.
Summary of studies that have investigated intra-amniotic infection/inflammation in women with amniotic sludge.
5. AF Sludge and an Ultrasound Marker for Spontaneous Preterm Labour?
Since intra-amniotic infections are generally associated with SPTB, and AF sludge is thought to represent MIAC, the key question is whether AF sludge could be an ultrasound marker of PTB. Identification of AF sludge in the first half of pregnancy has been shown to be associated with inflammation/infection, while in late pregnancy, it is thought to reflect maturation of the fetus (i.e., dominated by vernix caseosa, fetal squames, and meconium) [28,29,61,62,63]. Espinoza et al. [27], in a retrospective study of 84 women (19, or 22.4%) with intact membranes and spontaneous preterm labour, and 298 (1% with AF sludge) at term with intact membranes, concluded that the presence of AF “sludge” in the spontaneous preterm labour and intact membranes group was a risk factor for MIAC, histological chorioamnionitis, and impending spontaneous preterm delivery. Several other case series (mostly retrospective) have not only shown that those with AF sludge are at greater risk of SPTL but that this risk is much greater in those with a short cervix. In a large retrospective study of 281 asymptomatic women who underwent cervical length measurement and screening for AF sludge, Kusanovic et al. [36] showed AF sludge to be present in 66 cases. The shorter the cervical length, the higher the presence of AF sludge. The SPTB rate was higher in those with AF sludge, and this significant difference was maintained for PTB < 28, <32, and <35 weeks. The combination of a cervical length of <25 mm and the presence of AF sludge conferred odds ratios of 14.8 and 9.9 for spontaneous delivery at <28 and <32 weeks, respectively [36]. Although some studies have concluded that the presence of AF sludge is an independent risk factor for the occurrence of SPTB, these observations have not been universal. There has, to the best of our knowledge, been only one prospective study [59]. Table 2 is a summary of the reports on the association between amniotic fluid sludge and SPTB [27,33,34,36,52,58,64,65,66,67].

Table 2.
Summary of studies that have investigated the association between amniotic fluid sludge and preterm labour.
6. Will Treatment Improve Outcomes?
Since there is strong evidence associating AF sludge with infection/inflammation, it follows that treatment with antibiotics may reduce the risk of PTB. This was the basis of several studies that have investigated the effect of antibiotics in women at risk of SPTB based on the ultrasound finding of AF sludge. The results from various studies on the efficacy of antibiotics in this regard have been inconclusive. These studies (reviewed below) include case reports and retrospective and prospective studies. The first report of the use of antibiotics in women with AF sludge was reported by Himaya et al. [59]. They describe a woman diagnosed with the sludge at 15+6 weeks with a cervical length of 33 mm treated with an intravenous ampicillin–gentamicin and oral azithromycin combination following an amniocentesis and culture of Staphylococcus warneri at 22 weeks. Following treatment, a second amniocentesis was performed, and the culture was negative. She progressed to deliver at term. A historical controlled observational study by Hatanaka et al. [68] was reported in which women with AF sludge diagnosed before 2012 and who were not treated were compared with those diagnosed after 2012–2015 and treated with antibiotics. The women were divided into two groups—those at low risk were given oral clindamycin (300 mg every 6 h) and cephalexin (500 mg every 6 h) for 7 days, while the high-risk group was given intravenous clindamycin (600 mg every 8 h) and cefazolin (1 g every 8 h) for 5 days followed by oral treatment for 5 days. They showed that there was a reduction in the PTB rate in the treated group compared to the untreated group (13.2% vs. 38.5%). Cuff et al. [67], in a retrospective cohort study of 97 women with sludge, compared outcomes between those who had either been given oral azithromycin 500 mg on day 1 followed by 250 mg orally for 4 days or moxifloxacin 400 mg orally taken daily for 5 days and those who had not been treated. They showed that sonographic resolution of the AF sludge occurred in 34% of those who had been treated and 43% of those who had not—a difference that was not statistically significant. Furthermore, there were no differences in the rates of SPTB in both groups. Pustotina [69] undertook a prospective study of 29 women with AF sludge (some with a short cervix and symptomatic) in which they offered vaginal clindamycin and other combinations (cefoperazone, clavulonate + amoxicillin, and, in some cases, and in some, combined with indomethacin, progesterone, and IV sulbactam) and showed that antibiotic treatment eliminated the AF sludge and prevented SPTB in all the cases. Jin et al. [70], in a retrospective study of 58 women with a sludge diagnosed at 15–23 weeks and treated with a combination of IV ceftriaxone, clarithromycin, and metronidazole, showed a lower level of preterm birth following treatment, with disappearance of the sludge in 51.7% of cases. More recently, Giles et al. [71] reported on a retrospective cohort of women with AF sludge who were treated and compared the outcomes to those not treated. The antibiotic used was azithromycin. Interestingly, the overall spontaneous preterm birth rate was higher in the treatment group, but there were no differences in neonatal morbidity. Table 3 summarises all these studies [67,68,69,70,71,72,73]. Two meta-analyses [74,75] and a review of the literature by Luca et al. [76] concluded that while AF sludge is a marker of preterm birth, there are no robust data on the benefit of antibiotics in this group. More recently, there have been reports on cases where AF sludge was identified and antibiotics administered and followed until it disappeared, and the pregnancy progressed to term [73].

Table 3.
Summary of studies that have investigated antibiotics in women with amniotic fluid and its impact on the risk of preterm birth.
A major factor that has been suggested as a potential confounder responsible for the variable results is the different antibiotic regimens used and the routes of their administration. Additionally, the studies have been very heterogenous, varying from those on asymptomatic low-risk/high-risk women to those on women with symptoms (i.e., presenting with uterine activity/contractions) or a combination of both. The two studies that showed no difference in treatment used azithromycin as the main antibiotic compared to the others that showed a difference (all of which used a variety of antibiotics, including intravenous clindamycin and a cephalosporin). From these data, it would seem that the biofilm is less likely to be penetrated by antibiotics administered orally as these may not achieve high levels in the blood. It would therefore be reasonable to recommend that these women are offered intravenous antibiotics. We have treated a number of cases in our unit (n = 21) over the last 18 months with AF sludge with a combination of intravenous clindamycin, metronidazole, and ceftriaxone (given for one week) and in 14 out of the 21 (67%) cases, the AF sludge resolved, and pregnancies progressed to term, or delivery was delayed by an average of 2 weeks in those who delivered preterm. We believe that considering how common this finding is in women at risk of SPTL, there should be randomised controlled trials on the efficacy of intravenous clindamycin combined with a cephalosporin and metronidazole to determine if such a regimen will reduce the risk of SPTB and therefore neonatal morbidity and mortality. This is the combination that appears to be most effective from the case series and covers most of the spectrum of organisms that have been isolated from the various studies. Clindamycin, for example, covers Gram-positive organisms and many anaerobes, including most strains of B. fragilis and β-lactam—resistant strains of S. pneumoniae and Staphylococcus. Metronidazole, on the other hand, is used because it is also effective against anaerobic bacteria and certain protozoa. Specifically, it is active against various anaerobic bacteria such as Bacteroides, Fusobacterium, Clostridium, Gardnerella vaginalis, Prevotella, Porphyromonas, and Peptostreptococcus species. Ceftriaxone is effective against Gram-positive (Streptococcus and staphylococcus species) and Gram-negative bacteria (especially Enterobacteriaceae, E. coli, and Klebsiella). We recommend this combination because most studies showed it to be the most effective, covering most of the pathogens that have been isolated in the amniotic fluid (Table 1). There are no studies that show a close relationship between cervical pathogens and the pathogens from the amniotic fluid sludge, hence the recommendation to use a broad-spectrum combination. One study investigated [77] the cervical inflammatory markers (IL-8) and showed them to be higher in those with sludge, but no organisms were cultured.
Finally, it could be argued that leaving the fetus in utero with MIAC would increase morbidity. The fact that the antibiotics led to resolution of the AF sludge in many cases and that the case series reviewed have not reported increased morbidity in the neonates (if anything, delaying birth was associated with better outcomes) is reassuring in this context. Prior to commencing women on broad spectrum antibiotics, the potential of bacteria resistance must be discussed.
7. Conclusions
There is no doubt that intra-amniotic infections are central to a significant proportion of preterm births. Some of these manifest as obvious clinical infections, and in most cases, with uterine contractions and a short/dilated cervix. In some cases, however, the infections may be subclinical/asymptomatic. Identifying such cases is challenging, but the presence of AF sludge has been shown in case series to be a marker of such infections. The evidence linking amniotic sludge with SPTB, although predominantly from case series, is moderately robust, although more data are required. What is uncertain is whether treatment with antibiotics does indeed lead to the resolution of AF sludge and prevention/delay of PTB. The potency of the antibiotics depends on the type of antibiotics and how they are administered. The data reviewed here suggests that oral azithromycin is not effective, while a combination of parenteral ceftriaxone, metronidazole, and clindamycin appears to be effective. While we feel there is enough to suggest treating these women with this combination, there is a need for randomised controlled trials on the efficacy of this regimen to generate robust evidence. Such studies ideally should include other factors such as maternal characteristics and inflammatory biomarkers (e.g., IL-1 and -6, TNF-α, IL-8, and MMP-9) in amniotic fluid, plasma, and vaginal secretions [67] that increase the risk of preterm birth to allow for a better generation of algorithms for the prevention of PTB. Until such studies are undertaken, clinicians must continue to counsel women diagnosed with AF sludge on the pros and cons of antibiotics and the limited data on efficacy.
Author Contributions
J.C.K. conceived the idea. M.A.B. wrote the first draft, which was reviewed by B.A. and J.C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Blencowe, H.; Cousens, S.; Chou, D.; Oestergaard, M.; Say, L.; Moller, A.-B.; Kinney, M.; Lawn, J.; on behalf of the Born Too Soon Preterm Birth Action Group. Born too soon: The global epidemiology of 15 million preterm births. Reprod. Health 2013, 10 (Suppl. S1), S2. [Google Scholar] [CrossRef]
- World Health Organization. Children: Improving Survival and Well-Being; World Health Organization: Geneva, Switzerland, 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/children-reducing-mortality (accessed on 23 December 2024).
- Ahmed, B.; Abushama, M.; Konje, J.C. Prevention of spontaneous preterm delivery—An update on where we are today. J. Matern. Fetal Neonatal Med. 2023, 36, 2183756. [Google Scholar] [CrossRef]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.-B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, E37–E46. [Google Scholar] [CrossRef] [PubMed]
- Ohuma, E.O.; Moller, A.B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271, Erratum in Lancet 2024, 403, 618. [Google Scholar] [CrossRef] [PubMed]
- Muglia, L.J.; Katz, M. The enigma of spontaneous preterm birth. N. Engl. J. Med. 2010, 362, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Cavoretto, P.I.; Candiani, M.; Farina, A. Spontaneous Preterm Birth Phenotyping Based on Cervical Length and Immune-Mediated Factors. JAMA Netw. Open 2024, 7, e244559. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Cavoretto, P.I.; Barros, F.C.; Romero, R.; Papageorghiou, A.T.; Kennedy, S.H. Etiologically Based Functional Taxonomy of the Preterm Birth Syndrome. Clin. Perinatol. 2024, 51, 475–495. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Villar, J.; Restrepo-Méndez, M.C.; McGready, R.; Barros, F.C.; Victora, C.G.; Munim, S.; Papageorghiou, A.T.; Ochieng, R.; Craik, R.; Barsosio, H.C.; et al. Association Between Preterm-Birth Phenotypes and Differential Morbidity, Growth, and Neurodevelopment at Age 2 Years: Results From the INTERBIO-21st Newborn Study. JAMA Pediatr. 2021, 175, 483–493. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frenquelli, R.; Ratcliff, M.; Villar de Onis, J.; Fernandes, M.; Barros, F.C.; Hirst, J.E.; Papageorghiou, A.T.; Kennedy, S.H.; Villar, J. Complex Perinatal Syndromes Affecting Early Human Growth and Development: Issues to Consider to Understand Their Aetiology and Postnatal Effects. Front. Neurosci. 2022, 16, 856886. [Google Scholar] [CrossRef]
- Romero, R.; Jung, E.; Chaiworapongsa, T.; Erez, O.; Gudicha, D.W.; Kim, Y.M.; Kim, J.S.; Kim, B.; Kusanovic, J.P.; Gotsch, F.; et al. Toward a new taxonomy of obstetrical disease: Improved performance of maternal blood biomarkers for the great obstetrical syndromes when classified according to placental pathology. Am. J. Obstet Gynecol. 2022, 227, 615.e1–615.e25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sovio, U.; Gaccioli, F.; Cook, E.; Charnock-Jones, D.S.; Smith, G.C.S. Association between adverse pregnancy outcome and placental biomarkers in the first trimester: A prospective cohort study. BJOG 2024, 131, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Cavoretto, P.I.; Farina, A.; Salmeri, N.; Syngelaki, A.; Tan, M.Y.; Nicolaides, K.H. First trimester risk of preeclampsia and rate of spontaneous birth in patients without preeclampsia. Am. J. Obstet. Gynecol. 2024, 231, 452.e1–452.e7. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Knight, H.E.; de Onis, M.; Bertino, E.; Gilli, G.; Papageorghiou, A.T.; Ismail, L.C.; Barros, F.C.; Bhutta, Z.A.; International Fetal and Newborn Growth Consortium (INTERGROWTH-21st). Conceptual issues related to the construction of prescriptive standards for the evaluation of postnatal growth of preterm infants. Arch. Dis. Child. 2010, 95, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Preterm Labour and Birth. NICE Guideline (NG25). 2015. Available online: www.nice.org.uk/guidance/ng25 (accessed on 6 August 2025).
- Watts, D.H.; Krohn, M.A.; Hiler, S.L.; Eschenbach, D.A. The association of occult amniotic Fluid. infection with gestational age and neonatal outcome in women in preterm labor. Obstet. Gynecol. 1992, 79, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Hirsch, E. Intrauterine infection and preterm labor. Semin. Fetal Neonatal Med. 2012, 17, 12–19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romero, R.; Sirtori, M.; Oyarzun, E.; Avila, C.; Mazor, M.; Callahan, R.; Sabo, V.; Athanassiadis, A.P.; Hobbins, J.C. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am. J. Obstet. Gynecol. 1989, 161, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Andrews, W.W. Intrauterine infection and why preterm prevention programs have failed. Am. J. Public Health 1996, 86, 781–783. [Google Scholar] [CrossRef]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Chaiyasit, N.; Yoon, B.H.; Kim, Y.M. Acute chorioamnionitis and funisitis: Definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 2015, 213 (Suppl. 4), S29–S52. [Google Scholar] [CrossRef]
- Romero, R.; Salafia, C.M.; Athanassiadis, A.P.; Hanaoka, S.; Mazor, M.; Sepulveda, W.; Bracken, M.B. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am. J. Obstet. Gynecol. 1992, 166, 1382–1388. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, K.H.; Jung, E.Y.; Jang, J.A.; Yoo, H.N. Frequency and clinical significance of short cervix in patients with preterm premature rupture of membranes. PLoS ONE 2017, 12, e0174657. [Google Scholar] [CrossRef]
- Kiefer, D.G.; Keeler, S.M.; Rust, O.A.; Wayock, C.P.; Vintzileos, A.M.; Hanna, N. Is midtrimester short cervix a sign of intraamniotic inflammation? Am. J. Obstet. Gynecol. 2009, 200, 374.e1–374.e5. [Google Scholar] [CrossRef]
- Cassell, G.H.; Davis, R.O.; Waites, K.B.; Brown, M.B.; Marriott, P.A.; Stagno, S.; Davis, J.K. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16–20 weeks of gestation: Potential effect on outcome of pregnancy. Sex. Transm. Dis. 1983, 10 (Suppl. 4), 294–302. [Google Scholar] [PubMed]
- Daskalakis, G.; Psarris, A.; Koutras, A.; Fasoulakis, Z.; Prokopakis, I.; Varthaliti, A.; Karasmani, C.; Ntounis, T.; Domali, E.; Theodora, M.; et al. Maternal Infection and Preterm Birth: From Molecular Basis to Clinical Implications. Children 2023, 10, 907. [Google Scholar] [CrossRef]
- Romero, R.; Miranda, J.; Chaiworapongsa, T.; Chaemsaithong, P.; Gotsch, F.; Dong, Z.; Ahmed, A.I.; Yoon, B.H.; Hassan, S.S.; Kim, C.J.; et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am. J. Reprod. Immunol. 2014, 71, 330–358. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.; Gonçalves, L.F.; Romero, R.; Nien, J.K.; Stites, S.; Kim, Y.M.; Hassan, S.; Gomez, R.; Yoon, B.H.; Chaiworapongsa, T.; et al. The prevalence and clinical significance of amniotic fluid ‘sludge’ in patients with preterm labor and intact membranes. Ultrasound Obstet. Gynecol. 2005, 25, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Benacerraf, B.R.; Gatter, M.A.; Ginsburgh, F. Ultrasound diagnosis of meconium-stained amniotic fluid. Am. J. Obstet. Gynecol. 1984, 149, 570–572. [Google Scholar] [CrossRef] [PubMed]
- DeVore, G.R.; Platt, L.D. Ultrasound appearance of particulate matter in amniotic cavity: Vernix or meconium? J. Clin. Ultrasound 1986, 14, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, W.H.; Quiroz, V.H. Sonographic detection of echogenic amniotic fluid and its clinical significance. J. Perinat. Med. 1989, 17, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Sherer, D.M.; Abramowicz, J.S.; Smith, S.A.; Woods, J.R., Jr. Sonographically homogeneous echogenic amniotic fluid in detecting meconium-stained amniotic fluid. Obstet. Gynecol. 1991, 78, 819–822. [Google Scholar]
- Vohra, N.; Rochelson, B.; Smith-Levitin, M. Three-dimensional sonographic findings in congenital (harlequin) ichthyosis. J. Ultrasound Med. 2003, 22, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Bujold, E.; Pasquier, J.C.; Simoneau, J.; Arpin, M.H.; Duperron, L.; Morency, A.M.; Audibert, F. Intra-amniotic sludge, short cervix, and risk of preterm delivery. J. Obstet. Gynaecol. Can. 2006, 28, 198–202. [Google Scholar] [CrossRef]
- Adanir, I.; Ozyuncu, O.; Gokmen Karasu, A.F.; Onderoglu, L.S. Amniotic fluid “sludge”; prevalence and clinical significance of it in asymptomatic patients at high risk for spontaneous preterm delivery. J. Matern. Fetal Neonatal Med. 2018, 31, 135–140. [Google Scholar] [CrossRef]
- Parulekar, S.G. Ultrasonographic demonstration of floating Ultrasonographic demonstration of floating particles in amniotic fluid. J. Ultrasound Med. 1983, 2, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Kusanovic, J.P.; Espinoza, J.; Romero, R.; Gonçalves, L.F.; Nien, J.K.; Soto, E.; Khalek, N.; Camacho, N.; Hendler, I.; Mittal, P.; et al. Clinical significance of the presence of amniotic fluid ‘sludge’ in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet. Gynecol. 2007, 30, 706–714. [Google Scholar] [CrossRef]
- Bearfield, C.; Davenport, E.S.; Sivapathasundaram, V.; Allaker, R.P. Possible association between amniotic fluid microorganism infection and microflora in the mouth. BJOG Int. J. Obstet. Gynaecol. 2002, 109, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; Romero, R.; Xu, Y.; Miller, D.; Unkel, R.; Shaman, M.; Jacques, S.M.; Panaitescu, B.; Garcia-Flores, V.; Hassan, S.S. Neutrophil Extracellular Traps in the Amniotic Cavity of Women with Intra-Amniotic Infection: A New Mechanism of Host Defense. Reprod. Sci. 2017, 24, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; Romero, R.; Garcia-Flores, V.; Xu, Y.; Leng, Y.; Alhousseini, A.; Hassan, S.S.; Panaitescu, B. Amniotic fluid neutrophils can phagocytize bacteria: A mechanism for microbial killing in the amniotic cavity. Am. J. Reprod. Immunol. 2017, 78, e12723. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Nunez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romero, R.; Miranda, J.; Chaiworapongsa, T.; Korzeniewski, S.J.; Chaemsaithong, P.; Gotsch, F.; Dong, Z.; Ahmed, A.I.; Yoon, B.H.; Hassan, S.S.; et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am. J. Reprod. Immunol. 2014, 72, 458–474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romero, R.; Miranda, J.; Chaemsaithong, P.; Chaiworapongsa, T.; Kusanovic, J.P.; Dong, Z.; Ahmed, A.I.; Shaman, M.; Lannaman, K.; Yoon, B.H.; et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J. Matern. Fetal Neonatal Med. 2014, 28, 1394–1409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romero, R.; Miranda, J.; Chaiworapongsa, T.; Chaemsaithong, P.; Gotsch, F.; Dong, Z.; Ahmed, A.I.; Yoon, B.H.; Hassan, S.S.; Kim, C.J.; et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: Prevalence and clinical significance. J. Matern. Fetal Neonatal Med. 2015, 28, 1343–1359. [Google Scholar] [CrossRef]
- Romero, R.; Schaudinn, C.; Kusanovic, J.P.; Gorur, A.; Gotsch, F.; Webster, P.; Nhan-Chang, C.L.; Erez, O.; Kim, C.J.; Espinoza, J.; et al. Detection of a microbial biofilm in intraamniotic infection. Am. J. Obstet. Gynecol. 2008, 198, 135.e1–135.e5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Costerton, W.; Veeh, R.; Shirtliff, M.; Pasmore, M.; Post, C.; Ehrlich, G. The application of biofilm science to the study and control of chronic bacterial infections. J. Clin. Investig. 2003, 112, 1466–1477. [Google Scholar] [CrossRef] [PubMed Central]
- Donlan, R.M. Role of biofilms in antimicrobial resistance. ASAIO J. 2000, 46, S47–S52. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.T.; Kharazmi, A.; Lam, K.; Costerton, J.W.; Hoiby, N. Human polymorphonuclear leukocyte response to Pseudomonas aeruginosa grown in biofilms. Infect. Immun. 1990, 58, 2383–2385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jensen, E.T.; Kharazmi, A.; Hoiby, N.; Costerton, J.W. Some bacterial parameters influencing the neutrophil oxidative burst response to Pseudomonas aeruginosa biofilms. APMIS 1992, 100, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Li, S.; Gong, X.; Li, J.; Li, X.; Zhai, Y.; Huang, J.; Li, X.; Li, L.; Yang, J.; et al. Longitudinal cervical length measurements and spontaneous preterm birth in singleton and twin pregnancies. JAMA Netw Open. 2024, 7, e244592. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Romero RChaemsaithong, P.; Kim, J.S. Chronic inflammation of the placenta.: Definition classification, pathogenesis and clinical significance. Am. J. Obstet. Gynecol. 2015, 213, S53–S69. [Google Scholar] [CrossRef]
- Hatanaka, A.R.; Mattar, R.; Kawanami, T.E.; França, M.S.; Rolo, L.C.; Nomura, R.M.; Araujo Júnior, E.; Nardozza, L.M.; Moron, A.F. Amniotic fluid “sludge” is an independent risk factor for preterm delivery. J. Matern. Fetal Neonatal Med. 2016, 29, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Kusanovic, J.P.; Espinoza, J.; Gotsch, F.; Nhan-Chang, C.L.; Erez, O.; Kim, C.J.; Khalek, N.; Mittal, P.; Goncalves, L.F.; et al. What is amniotic fluid sludge? Ultrasound Obstet. Gynecol. 2007, 30, 793–798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoneda, N.; Yoneda, S.; Niimi, H.; Ito, M.; Fukuta, K.; Ueno, T.; Ito, M.; Shiozaki, A.; Kigawa, M.; Kitajima, I.; et al. Sludge reflects intra-amniotic inflammation with or without microorganisms. Am. J. Reprod. Immunol. 2018, 79, e12807. [Google Scholar] [CrossRef]
- Kusanovic, J.P.; Jung, E.; Romero, R.; Green, P.M.; Nhan-Chang, C.-L.; Vaisbuch, E.; Erez, O.; Kim, C.J.; Gonçalves, L.F.; Espinoza, J.; et al. Characterization of amniotic fluid sludge in preterm and term gestations. J. Matern. Fetal Neonatal Med. 2022, 35, 9770–9779. [Google Scholar] [CrossRef] [PubMed]
- Gill, N.; Romero, R.; Pacora, P.; Tarca, A.L.; Benshalom-Tirosh, N.; Pacora, P.; Kabiri, D.; Tirosh, D.; Jung, E.J.; Yeo, L.; et al. 467: Patients with Short Cervix and Amniotic Fluid Sludge Delivering ≤32 Weeks Have Stereotypic Inflammatory Signature. Am. J. Obstet. Gynecol. 2019, 220, S312. [Google Scholar] [CrossRef]
- Paules, C.; Moreno, E.; Gonzales, A.; Fabre, E.; González de Agüero, R.; Oros, D. Amniotic fluid sludge as a marker of intra-amniotic infection and histological chorioamnionitis in cervical insufficiency: A report of four cases and literature review. J. Matern. Fetal Neonatal Med. 2016, 29, 2681–2684. [Google Scholar] [CrossRef] [PubMed]
- Ventura, W.; Nazario, C.; Ingar, J.; Huertas, E.; Limay, O.; Castillo, W. Risk of impending preterm delivery associated with the presence of amniotic fluid sludge in women in preterm labor with intact membranes. Fetal Diagn. Ther. 2011, 30, 116–121. [Google Scholar] [CrossRef]
- Himaya, E.; Rhalmi, N.; Girard, M.; Tétu, A.; Desgagné, J.; Abdous, B.; Gekas, J.; Giguère, Y.; Bujold, E. Midtrimester intra-amniotic sludge and the risk of spontaneous preterm birth. Am. J. Perinatol. 2011, 28, 815–820. [Google Scholar] [CrossRef]
- Pedregosa, J.P.; Ruiz, C.M.; Medina, T.B.; Rascin, A.G.; del Gallo, J.; de la Fuente, J.L.; Alonso, M.J.T. Amniotic sludge and short cervix as inflammation and intraamniotic infection markers. Obstet. Gynecol. Int. J. 2017, 7, 215–218. [Google Scholar] [CrossRef][Green Version]
- Buyuk, G.N.; Oskovi-Kaplan, Z.A.; Kahyaoglu, S.; Engin-Ustun, Y. Echogenic particles in the amniotic fluid of term low-risk pregnant women: Does it have a clinical significance? J. Obstet. Gynaecol. 2021, 41, 1048–1052. [Google Scholar] [CrossRef]
- Kaluarachchi, A.; Jayawardena, G.R.M.U.G.P.; Ranaweera, A.K.P.; Rishard, M.R.M. Hyperechoic amniotic fluid in a term pregnancy. J. Fam. Med. Prim. Care 2018, 7, 635–637. [Google Scholar] [CrossRef]
- Zimmer, E.Z.; Bronshtein, M. Ultrasonic features of intraamniotic ‘unidentified debris’ at 14–16 weeks’ gestation. Ultrasound Obstet. Gynecol. 1996, 7, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, Y.; Fukami, T.; Yoneyama, K.; Kawabata, I.; Takeshita, T. The presence of amniotic fluid sludge in pregnant women with a short cervix: An independent risk of preterm delivery. J. Matern. Fetal Neonatal Med. 2020, 33, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Tanaka, M.; Kyozuka, H.; Suzuki, S.; Yamaguchi, A.; Nomura, Y.; Fujimori, K. Association of amniotic fluid sludge with preterm labor and histologic chorioamnionitis in pregnant Japanese women with intact membranes: A retrospective study. J. Obstet. Gynaecol. Res. 2020, 46, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Pahlavan, F.; Niknejad, F.; Irani, S.; Niknejadi, M. Does Amniotic Fluid Sludge Result in Preterm Labor in Pregnancies after Assisted Reproduction Technology? A Nested Case—Control Study. J. Matern. Fetal Neonatal Med. 2022, 35, 7153–7157. [Google Scholar] [CrossRef] [PubMed]
- Cuff, R.D.; Carter, E.; Taam, R.; Bruner, E.; Patwardhan, S.; Newman, R.B.; Chang, E.Y.; Sullivan, S.A. Effect of Antibiotic Treatment of Amniotic Fluid Sludge. Am. J. Obstet. Gynecol. MFM 2020, 2, 100073. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, A.R.; Franca, M.S.; Hamamoto, T.E.N.K.; Rolo, L.C.; Mattar, R.; Moron, A.F. Antibiotic treatment for patients with amniotic fluid “sludge” to prevent spontaneous preterm birth: A historically controlled observational study. Acta Obstet. Gynecol. Scand. 2019, 98, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Pustotina, O. Effects of antibiotic therapy in women with the amniotic fluid “sludge” at 15–24 weeks of gestation on pregnancy outcomes. J. Matern. Fetal Neonatal Med. 2020, 33, 3016–3027. [Google Scholar] [CrossRef]
- Jin, W.H.; Ha Kim, Y.; Kim, J.W.; Kim, T.Y.; Kim, A.; Yang, Y. Antibiotic treatment of amniotic fluid “sludge” in patients during the second or third trimester with uterine contraction. Int. J. Gynaecol. Obstet. 2021, 153, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Giles, M.L.; Krishnaswamy, S.; Metlapalli, M.; Roman, A.; Jin, W.; Li, W.; Mol, B.W.; Sheehan, P.; Said, J. Azithromycin treatment for short cervix with or without amniotic fluid sludge: A matched cohort study. Aust. N. Z. J. Obstet. Gynaecol. 2023, 63, 384–390. [Google Scholar] [CrossRef]
- Fuchs, F.; Boucoiran, I.; Picard, A.; Dube, J.; Wavrant, S.; Bujold, E.; Audibert, F. Impact of amniotic fluid “sludge” on the risk of preterm delivery. J. Matern. Fetal Neonatal Med. 2015, 28, 1176–1180. [Google Scholar] [CrossRef]
- Yeo, L.; Romero, R.; Chaiworapongsa, T.; Para, R.; Johnson, J.; Kmak, D.; Jung, E.; Yoon, B.H.; Hsu, C.D. Resolution of acute cervical insufficiency after antibiotics in a case with amniotic fluid sludge. J. Matern. Fetal Neonatal Med. 2022, 35, 5416–5426. [Google Scholar] [CrossRef] [PubMed]
- Sapantzoglou, I.; Pergialiotis, V.; Prokopakis, I.; Douligeris, A.; Stavros, S.; Panagopoulos, P.; Theodora, M.; Antsaklis, P.; Daskalakis, G. Antibiotic therapy in patients with amniotic fluid sludge and risk of preterm birth: A meta-analysis. Arch. Gynecol. Obstet. 2024, 309, 347–361. [Google Scholar] [CrossRef]
- Pannain, G.D.; Pereira, A.M.G.; Rocha, M.L.T.L.F.D.; Lopes, R.G.C. Amniotic Sludge and Prematurity: Systematic Review and Meta-analysis. Rev. Bras. Ginecol. Obstet. 2023, 45, e489–e498. [Google Scholar] [CrossRef]
- Luca, S.T.; Săsăran, V.; Muntean, M.; Mărginean, C. A Review of the Literature: Amniotic Fluid “Sludge”-Clinical Significance and Perinatal Outcomes. J. Clin. Med. 2024, 13, 5306. [Google Scholar] [CrossRef] [PubMed]
- Karampitsakos, T.; Mavrogianni, D.; Machaiotis, N.; Potiris, A.; Pangagopoulos, P.; Stavros, S.; Antsaklis, P.; Drakakis, P. The impact of amniotic fluid interleukin-6, interleukin -8 and metalloproteinase -9 on preterm labor: A narrative reveiw. Biomedicines 2025, 13, 118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).