Abstract
Ultrasound imaging is a vital imaging tool in musculoskeletal medicine, with the number of publications on ultrasound-guided surgery increasing in recent years, especially in minimally invasive procedures of sports, foot and ankle, and hand surgery. However, ultrasound imaging has drawbacks, such as operator dependency and image obscurity. Artificial intelligence (AI) and deep learning (DL), a subset of AI, can address these issues. AI/DL can enhance screening practices for hip dysplasia and osteochondritis dissecans (OCD) of the humeral capitellum, improve diagnostic accuracy for carpal tunnel syndrome (CTS), and provide physicians with better prognostic prediction tools for patients with knee osteoarthritis. Building on these advancements, DL methods, including segmentation, detection, and localization of target tissues and medical instruments, also have the potential to allow physicians and surgeons to perform ultrasound-guided procedures more accurately and efficiently. This review summarizes recent advances in ultrasound-guided procedures for musculoskeletal diseases and provides a comprehensive overview of the utilization of AI/DL in ultrasound for musculoskeletal medicine, particularly focusing on ultrasound-guided surgery.
1. Introduction
Musculoskeletal (MSK) ultrasound is an indispensable imaging modality, first developed in diagnostics [1,2,3] and now in the management of various MSK disorders [4,5]. Its ability to provide real-time, dynamic assessment of musculoskeletal structures makes it particularly valuable in clinical practice. MSK ultrasound offers numerous advantages, including high spatial resolution, absence of ionizing radiation, and the ability to perform bedside examinations, thus facilitating prompt clinical decision making. Additionally, its cost-effectiveness compared to other imaging modalities, such as MRI, has further cemented its role in the routine evaluation of MSK conditions. The versatility of MSK ultrasound enables clinicians to visualize soft tissue structures, such as muscles, tendons, ligaments, and nerves, as well as to guide therapeutic procedures with precision and safety [6].
However, despite these advantages, MSK ultrasound is not without its limitations. The modality is highly operator-dependent, requiring significant expertise to acquire and interpret images accurately. This dependency can lead to variability in diagnostic accuracy and procedural outcomes. Furthermore, the quality of the images can be compromised by factors such as patient body habitus, presence of soft tissue artifacts, and the inherent limitations of the ultrasound technology itself [7]. These challenges underscore the need for advanced techniques and tools to enhance the reliability and utility of MSK ultrasound in clinical practice.
Artificial intelligence (AI) encompasses several branches of data science that specialize in various domains. Deep learning (DL), a subset of machine learning which itself is a subset of AI, consists of frameworks of neural networks that accomplish data processing and are designed to mimic human cognitive abilities [8]. It depends on the availability of large amounts of data and algorithms to be efficient. Specifically, two-dimensional and three-dimensional convoluted neural networks (CNNs) along with other architectures can detect and analyze visual features with a high degree of accuracy that is often faster and more efficient than traditional methods. Furthermore, other forms of AI and semi-supervised learning can provide proofreading capabilities for image analyses performed by DL [6].
Ultrasound imaging is not viewed from standardized planes like other medical imaging technology, making it vulnerable to unclear graphics that are difficult and time-consuming to interpret manually [6]. As DL has gained traction in the past two decades as a useful tool, its application in clinical settings and MSK medicine has become important to remedy the disadvantages of ultrasound imaging mentioned previously. It has proven to be helpful for anatomical segmentation, localization and removal, release and cutting, and the repair of targeted afflictions [5]. The integration of AI/DL into MSK ultrasound represents a significant advancement in medical imaging.
This review aims to address the following key questions: (1) How extensively has ultrasound-guided surgery been explored in MSK medicine and orthopedics? (2) In what ways are AI and DL technologies being utilized to enhance the diagnostic capabilities and outcomes in MSK ultrasound-guided surgeries? By investigating these questions, the paper seeks to provide a comprehensive overview of the current state of research and the potential future impact of AI/DL on ultrasound-guided procedures in MSK medicine.
2. Ultrasound-Guided Surgery for Musculoskeletal Diseases
2.1. Literature Search
In the present review of ultrasound-guided surgery, we used the PubMed database to conduct a comprehensive literature search covering studies published from inception to June 2024. The search algorithm included combinations of the following keywords: “ultrasound-guided”, “sonographically-guided”, “ultrasonography-guided”, “ultrasound-assisted”, “sonographically-assisted”, “ultrasonography-assisted”, or “intraoperative ultrasound”. These terms were paired with “orthopedic”, “musculoskeletal”, “ligament”, “tendon”, or “nerve”. We excluded studies related to “biopsy”, “block”, “injection”, “anesthesia”, “pain”, “electromyography”, “catheter”, “aspiration”, “dry needling”, “radiofrequency ablation”, “electrolysis”, or “hydrodistension”, as well as those focused on pre-operative evaluation by ultrasound, to maintain a focus on intraoperative applications. Boolean operators (AND, OR) were used to combine search terms appropriately. Additionally, to ensure comprehensive coverage, a snowball approach was also conducted, manually searching references from relevant articles (Figure 1).
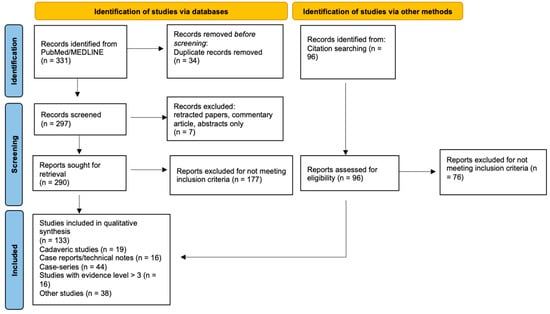
Figure 1.
Flowchart depicting the literature search methodology for ultrasound-guided surgery in musculoskeletal disease.
2.2. Classification of Studies
After removing duplicates, retracted papers, and narrative reviews, two independent reviewers screened titles and abstracts, followed by full-text review of potentially eligible studies. Disagreements were resolved through discussion or by consulting a third reviewer.
A total of 133 studies met the inclusion criteria for the literature search. Data extracted from these studies included study design (e.g., cadaveric studies, case report/technical note, case series, comparative studies, randomized control trial, and meta-analysis/systematic review), procedure type performed (e.g., localization, release, repair, etc.), pathology (e.g., carpal tunnel syndrome, Achilles tendon rupture, plantar fasciitis, etc.), targeted tissues (e.g., nerve, tendon, fascia, etc.), sample size, and key findings of each study.
2.3. Definition of Ultrasound-Guided Surgery
It is important to note the difference between ultrasound-guided and ultrasound-aided/assisted surgery. Ultrasound-guided surgeries included those where the entire surgical procedure was performed with ultrasound guidance. Examples included ultrasound-guided carpal tunnel release and trigger finger release. Ultrasound-assisted surgeries included those where ultrasound was used for specific parts of a surgical procedure, for instance, using ultrasound to assist in identifying portal entry sites for arthroscopic surgery. In Achilles tendon repair, the procedure was classified as ultrasound-assisted surgery when intraoperative ultrasound was used solely to identify the course of the sural nerve [9]. It was considered ultrasound-guided surgery when ultrasound was utilized to detect the sural nerve as well as the sutures and/or needle within the Achilles tendon during the repair [10].
2.4. Ultrasound-Assisted Surgery
Ultrasound-assisted surgery composed 38 out of the 133 studies reviewed. The ultrasound capacity to accurately detect targeted anatomical/pathological structures was utilized for a portion of the surgical procedures. During arthroscopic/endoscopic and even trauma surgery, ultrasound was used to accurately identify a joint space or critical landmarks and/or nerves and arteries for accuracy and safety [11,12,13,14,15,16,17]. Tumors, small ossicles, and calcifications, which were not possible to detect with conventional C-arm X-ray, could be localized with intraoperative ultrasound to facilitate open or arthroscopic removal [18,19,20]. Of note, ultrasound was used to localize and/or confirm decompression during spine surgery with a systematic review that validated its efficacy [21].
2.5. Classification of Ultrasound-Guided Surgery
The procedure types of ultrasound-guided surgery can be classified into 3 categories: 1. localization and removal/debridement, 2. release or cutting (partial or complete), 3. repair. Ultrasound allows for accurately localizing and removing (completely or partially) pathological tissues, and these procedures are referred to as the “first generation” of ultrasound-guided surgery [5]. Release or cutting of targeted structures including tendon, fascia, retinaculum, etc. is known as the “second generation” while ultrasound-guided repairs are called the “third generation” [22,23,24,25].
The review of 95 studies on ultrasound-guided surgery revealed that five “first generation” surgeries involved removing/debriding foreign bodies [26,27], hematoma [28,29], and excessive bone and bursa in Haglund deformity [30,31,32].
The second generation procedures were most common and involved release/cutting of soft tissues, including transverse carpal ligament release for carpal tunnel syndrome [1,33,34,35,36,37,38,39,40,41,42,43,44,45,46], flexor retinaculum release and septum for tarsal tunnel syndrome [47,48,49,50], shoulder capsule/coracohumeral ligament for adhesive capsulitis [51,52,53,54], cutting of gastrocnemius aponeurosis to lengthen Achilles tendon [55,56,57], tendon sheath release for trigger fingers and DeQuervain tenosynovitis [58,59,60,61,62,63,64,65], fasciotomy (complete cutting) for chronic exertional compartment syndrome [66,67,68], fasciotomy (partial cutting) for Dupuytren contracture [69,70,71], tenotomy (complete cutting) of long head biceps and plantaris tendon for shoulder pain and Achilles tendinopathy, respectively [72,73,74,75,76,77,78,79,80,81,82,83,84,85,86], and partial tenotomy/fasciotomy including Tenex® Lake forest, CA, USA for tendinopathy/fasciopathy [87,88,89,90,91,92,93,94,95].
Ultrasound-guided repairs are the “third generation” due to the novelty of their techniques. They comprised ultrasound-guided anterior talofibular ligament of the ankle [22,96,97], Achilles tendon [10,23,24,25,98,99], and medial collateral ligament and medial patellofemoral ligament of the knee [100,101].
2.6. Cadaveric Studies, Case Reports/Technical Notes, and Case Series
Among ultrasound-guided surgery, 19 cadaveric studies, 16 case reports/technical notes, and 44 case series were included. There were overlaps between the procedures in cadaveric studies and case series, indicating the advent and evolution of these techniques. Researchers initially conducted cadaveric studies to verify accuracy and feasibility, then progressed to a case series of their ultrasound-guided procedures on patients.
The second generation ultrasound-guided surgery, release or cutting of targeted structures, composed 86% of the 44 case series (the specific procedures are not clear in one case series), followed by localization and removal/debridement (the first generation) at 7% and repair (the third generation) at 7%.
2.7. Clinical Studies above Evidence Level 3
In 16 studies with an evidence level higher than 3 [102], targeted tissues of ultrasound-guided surgery included nerves, tendons, bursae, bone, and ligaments (Table 1). All of them were not evaluated or hard to identify with intraoperative C-arm X-ray.

Table 1.
Clinical Studies of ultrasound-guided surgery above Evidence Level 3.
Tendinopathy surgery was most common (N = 6) [84,93,103,104,105,106], followed by foot and ankle surgery (N = 5) [10,31,92,97,99] and hand surgery (N = 4) [44,45,46,64], among studies with higher evidence.
2.8. Randomized Control Trials and Meta-Analysis
The randomized controlled trials (RCTs) and meta-analysis on tendinopathy and fasciopathy suggested that US-guided procedures resulted in minimal complications. Across the studies, pain scores typically decreased significantly in the ultrasound-guided groups. Functional improvements were noted for ultrasound-guided procedures. General well-being, sleep quality, and function all showed positive trends. However, most of these results were not significant between ultrasound-guided and control groups.
The RCTs on carpal tunnel syndrome and trigger finger release collectively indicate that ultrasound-guided procedures exhibit favorable safety with no significant complications compared to control groups. Pain levels were significantly lower in the ultrasound-guided group [45]. Functional improvement was noted in every study, although the functional improvements were not significant between the ultrasound-guided and control groups in some studies [46,64]. Notably, ultrasound guidance led to earlier functional recovery in one study [45] and demonstrated a quicker return to normal activities and better cosmetic outcomes in the other study [64].
Another RCT on shoulder capsule/coracohumeral ligament release for adhesive capsulitis found that ultrasound-guided coracohumeral ligament release with Tenex® improved shoulder range of motion, pain, and function compared with local anesthetic injection group [54].
2.9. Strength of Ultrasound-Guided Surgery for Musculoskeletal Pathologies
As shown in the studies above, ultrasound-guided procedures demonstrated a strong safety profile and efficacy comparable to traditional methods, offering benefits in pain reduction and expeditious functional improvements. Intraoperative ultrasound can assist physicians and surgeons in accurately and effectively performing minimally invasive surgeries for soft tissue pathologies, particularly those not visible with intraoperative fluoroscopy (Figure 2).
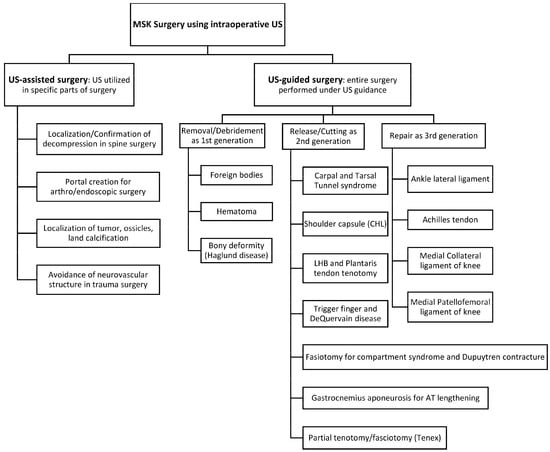
Figure 2.
Summary of Ultrasound-guided and -assisted surgery for targeted pathologies. MSK, musculoskeletal; US, ultrasound; CHL, coracohumeral ligament; LHB, long head of biceps; AT, Achilles tendon.
3. Artificial Intelligence and Musculoskeletal Ultrasound
3.1. Literature Search
We searched the PubMed database from inception to June 2024 using a search strategy including combinations of the following keywords: “ultrasound-guided”, “ultrasonography-guided”, “ultrasound-assisted”, “sonography-assisted”, “ultrasonography-assisted”. These terms were paired with “orthopedic” and “musculoskeletal”, in combination with “deep learning”, “artificial intelligence”, “convolutional neural networks”, and “machine learning”. To focus on studies with diagnostic or screening potential, “diagnosis” and “screening” were added where relevant. The asterisk (*) symbol was used to include all variations of the above words. Boolean operators (AND, OR) were used to combine search terms appropriately (Figure 3).
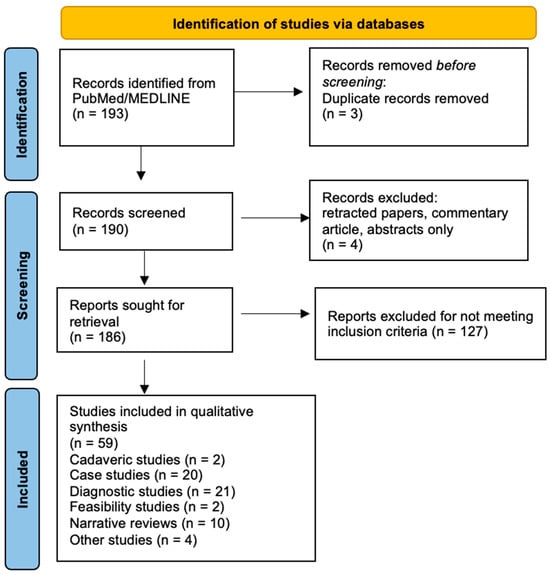
Figure 3.
Flowchart depicting the literature search methodology for artificial intelligence and musculoskeletal ultrasound.
3.2. Classification of Studies
After removing duplicates, retracted papers, and narrative reviews, two independent reviewers screened titles and abstracts, followed by full-text review of potentially eligible studies. Disagreements were resolved through discussion or by consulting a third reviewer.
A total of 59 studies were included for the investigation of how AI and DL are used with ultrasound in MSK medicine and orthopedics. From these studies the following data were extracted: type of imaging used (e.g., ultrasound, MRI, X-ray), the role of AI in the study (e.g., detection, segmentation, classification, etc.), the state of the images or subjects used (e.g., abnormal, healthy), investigated anatomy or pathology (e.g., tendinopathy, hip dysplasia, carpal tunnel syndrome), reference standard (if any), type of study (e.g., narrative review, diagnostic, cadaveric), level of evidence (if any), and key findings of each study.
3.3. Inclusion Criteria and Definitions of Artificial Intelligence, Deep Learning, and Convolutional Neural Network
The inclusion criteria for these studies necessitated that the computer model conformed to the established definitions of artificial intelligence (AI), deep learning (DL), or convolutional neural networks (CNNs). AI was defined as machines that are programmed to think and learn in a simulation of human intelligence. It encompasses a variety of technologies and applications, including machine learning, natural language processing, and robotics. DL was defined as a subset of machine learning that involves neural networks with many layers that are capable of automatically extracting and learning features from data. CNNs were defined as a type of DL algorithm specifically designed for processing structured grid data, like images that consist of multiple layers, that applies convolutional operations to learn features from input images.
Other inclusion criteria were developed based on these definitions and required the role of a computer in the study to detect an anatomical area or pathology from ultrasound, classify and/or diagnose a detected anatomical area or pathology from ultrasound, enhance ultrasound images for interpretation, or segment ultrasound images.
3.4. Types of Studies
Between 2017 and 2020, no diagnostic studies were performed, and most studies focused on developing algorithms and utilizing AI/DL/CNN for segmenting and tracking ultrasound images. The subjects of these studies were mostly healthy and/or cadaver models. Of the 59 studies included, 2 were categorized as cadaveric, 2 as feasibility-based, 10 as narrative reviews, and 20 as miscellaneous case studies, most of which were looking at segmentation and tracking.
From 2021, however, the number of clinical studies increased, and 21 of the 58 studies were categorized as a “diagnostic study” and given an evidence level based on the guideline from Journal of Bone and Joint Surgery published in 2003 [102]. This categorization was performed by an experienced orthopedic surgeon.
3.5. Non-Diagnostic Studies
Studies that were not classified as diagnostic looked at the use of computers in US imaging for tracking, segmentation, and measurement of cross-section area and echo texture. The two tracking studies looking at tendon and cartilage found excellent tracking results with AI [107,108]. One study even reported knee cartilage tracking results comparable to those of experienced surgeons [108]. Bone was primarily researched regarding segmenting as seen in three studies which reported automatic bone segmentation was accurate and comparable to existing techniques [109,110,111]. Seven studies investigated muscle for the purposes of measurement and segmentation [112,113,114,115,116,117,118]. Two studies looked at the gastrocnemius and reported they were able to automatically label ultrasound images and estimate neural output, length, and tension [115,116]. One study found it was possible to segment and track muscle on ultrasound images in real time, suggesting a potential usage for diagnosis [112].
3.6. Diagnostic Studies
Twenty-two “diagnostic studies” could be further divided into screening, diagnosis, and prediction of prognosis, depending upon the role of AI/DL/CNN-based ultrasound in a clinical setting (Table 2).

Table 2.
Diagnostic studies utilizing AI/DL in musculoskeletal ultrasound imaging.
3.6.1. Screening
Ultrasound is inherently an ideal imaging modality for screening due to its portability, cost-effectiveness, safety, and accessibility. AI can enhance the value of ultrasound as a screening tool by improving its diagnostic accuracy.
Screening infants’ hips for hip dysplasia was the most studied use, with five diagnostic studies focusing on it. The results of these studies demonstrated that computer algorithms could successfully differentiate between diseased and healthy hips at a rate comparable to that of medical experts and the conventional Graf method [120,123,130,131,132].
Osteochondritis dissecans (OCD) of the humeral capitellum was another well-studied pathology, featuring in three diagnostic studies [135,138,139]. These studies indicated that DL-assisted ultrasound has a high accuracy for identifying and classifying OCD lesions. These studies’ results highlighted the potential use of DL-based ultrasound in screening baseball players for OCD.
Another screening-based diagnostic study focused on osteoporosis and found a multichannel CNN-based ultrasound may be more accurate than a conventional quantitative ultrasound [124].
3.6.2. Diagnosis
Carpal tunnel syndrome (CTS) was a frequent subject of investigation, with three studies, including one systematic review [119,121,126,133,134]. Two of these studies showed that the diagnosis of CTS could be performed with greater accuracy than that of radiologists [119,121].
Similarly, three studies investigating tendinopathy diagnosis with computer-guided ultrasound found that AI was able to detect Achilles, lateral elbow, and supraspinatus calcific tendinopathy with high diagnostic accuracy [122,129,136].
3.6.3. Prediction
Two studies focused on the prediction of prognosis. In prognosis studies the machine learning software is trained on data (including clinical data, ultrasound images, laboratory data, etc.) to identify patterns and risk factors that may indicate the risk of developing disease.
One study found that DL was effective at predicting total knee replacement in patients with knee osteoarthritis [125]. Another study showed that machine learning was effective at predicting rheumatoid arthritis relapse [127].
3.7. Benefits of Utilizing AI/DL in Ultrasound Evaluation
Ultrasound is inherently operator-dependent, which can lead to variability in diagnostic accuracy. However, as demonstrated in recent diagnostic studies above, the integration of AI/DL into ultrasound evaluation significantly enhances the screening, diagnosis, and prediction of various MSK pathologies. These advancements underscore AI’s potential to transform ultrasound imaging into a more precise, reliable, and predictive modality in medical practice.
3.8. Limitations of AI/DL-Assisted Ultrasound
3.8.1. Image Quality Dependency
AI algorithms are highly sensitive to image quality. As observed in the hip dysplasia studies, low-quality images could significantly impact the accuracy of AI interpretations [130,132]. The variability in ultrasound image acquisition techniques and equipment across different clinical settings posed a challenge for developing robust AI models. Consistent, high-quality ultrasound images across different operators and machines remain a hurdle that needs to be overcome.
3.8.2. Region of Interest (ROI) Sensitivity
The accuracy of AI algorithms can be affected by variations in the selected region of interest. In osteochondritis dissecans of the humeral capitellum and carpal tunnel syndrome, adjusting the ROI improved consistency [133,138]. Optimal ROI selection across different pathologies and anatomical structures is crucial for reliable results.
4. Utility of Artificial Intelligence in Ultrasound-Guided Surgery
4.1. Literature Search
Given the relatively novel and specialized nature of this section, initial structured database searches yielded limited relevant results. Therefore, we employed a snowball sampling approach to identify literature. This method involved identifying key papers in the field and systematically exploring their references (backward snowballing) and citations (forward snowballing). This approach allowed for the discovery of highly specific and relevant studies that might have been missed through conventional search strategies. While this method uncovered valuable research, it is important to note that it may not capture the entire breadth of available literature.
4.2. AI in Ultrasound-Guided Surgery
Our comprehensive literature review revealed no studies meeting the criteria for fully AI-integrated ultrasound-guided surgery as defined in our methodology. Currently, the field appears to be taking a staged approach, focusing on AI-enhanced assistive technologies rather than fully autonomous systems.
4.3. Applications in Spine Surgery
In spine surgery, AI-augmented ultrasound guidance has shown remarkable potential. Baka et al. developed an AI-based method to identify vertebral levels using ultrasound imaging. The method achieved 92–95% accuracy in correctly identifying vertebral levels in a test set of 19 patients, significantly outperforming traditional manual palpation techniques [140]. By combining pre-operative X-rays with intraoperative ultrasound, their method could offer a promising alternative to C-arm imaging, potentially reducing radiation exposure and improving workflow in operating rooms.
4.4. Current State and Future Direction
Real-time incorporation of AI while performing ultrasound-guided surgery is currently limited, mostly due to the nascency of both components. However, this does not preclude the use of AI techniques with ultrasound for the improvement of the perioperative experience. As the history of ultrasound evolved from diagnostic applications to interventional uses, the integration of AI in ultrasound technology is expected to transition from its current use in screening, diagnosis, and prediction to broader utilization in intervention and surgery (Figure 4).
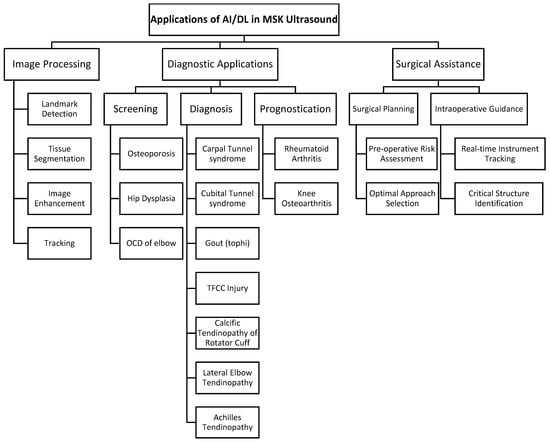
Figure 4.
Summary of AI/DL applications in musculoskeletal ultrasound. AI/DL, artificial intelligence/deep learning; MSK, musculoskeletal; OCD, osteochondritis dissecans; TFCC, triangular fibrocartilage complex.
5. Conclusions
Recent studies on ultrasound-guided surgery, particularly for soft tissue pathologies, have demonstrated a strong safety profile and efficacy comparable to traditional methods, with additional benefits such as pain reduction and quicker functional recovery. Despite ultrasound’s inherent operator dependency, which can lead to variability in diagnostic and therapeutic accuracy, our review demonstrated that integrating AI and deep learning into ultrasound imaging significantly improved the screening, diagnosis, and prediction of various musculoskeletal pathologies. These advancements underscore the potential of AI and deep learning to transform ultrasound, especially in ultrasound-guided procedures, into a more precise and reliable tool in musculoskeletal medicine. Further development of specialized devices for ultrasound-guided surgery, such as Tenex®, can further enhance the effectiveness of ultrasound as a tool for surgical guidance.
Author Contributions
Conceptualization, S.H., C.L.B., and M.V.H.; methodology, R.S.; validation, S.H., C.L.B., and M.V.H.; investigation, R.S., E.H., A.Q., J.M., and S.H.; writing—original draft preparation, R.S., E.H., A.Q., J.M., and B.F.; writing—review and editing, S.H.; supervision, C.L.B., S.P.C., D.S., and M.V.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest in the writing of the manuscript.
References
- McDonald, D.G.; Leopold, G.R. Ultrasound B-scanning in the differentiation of Baker’s cyst and thrombophlebitis. Br. J. Radiol. 1972, 45, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Mayer, V. Ultrasonography of the rotator cuff. J. Ultrasound Med. 1985, 4, 608. [Google Scholar] [CrossRef] [PubMed]
- Cooperberg, P.L.; Tsang, I.; Truelove, L.; Knickerbocker, W.J. Gray scale ultrasound in the evaluation of rheumatoid arthritis of the knee. Radiology 1978, 126, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Finnof, J.T.; Hall, M.M.; Adams, E.; Berkoff, D.; Concoff, A.L.; Dexter, W.; Smith, J. American Medical Society for Sports Medicine (AMSSM) position statement: Interventional musculoskeletal ultrasound in sports medicine. PM R 2015, 7, 151–168. [Google Scholar] [CrossRef]
- Hattori, S.; Onishi, K. Ultrasound-guided surgery in Musculoskeletal medicine. J. Med. Ultrasound 2022, 49, 513–515. [Google Scholar] [CrossRef]
- Masoumi, N.; Rivaz, H.; Hacihaliloglu, I.; Ahmad Reinertsen, I.; Xiao, Y. The Big Bang of Deep Learning in Ultrasound-Guided Surgery: A Review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2023, 70, 909–919. [Google Scholar] [CrossRef]
- Jacobson, J.A. Introduction. In Fundamentals of Musculoskeletal Ultrasound, 3rd ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2017; Volume 1, pp. 1–15. [Google Scholar]
- Lee, J.-G.; Jun, S.; Cho, Y.-W.; Lee, H.; Kim, G.B.; Seo, J.B.; Kim, N. Deep Learning in Medical Imaging: General overview. Korean J. Radiol. 2017, 18, 570–584. [Google Scholar] [CrossRef]
- Samy, A.M. Intra-operative ultrasound: Does it improve the results of percutaneous repair of acute Achilles tendon rupture? Eur. J. Trauma. Emerg. Surg. 2022, 48, 4061–4068. [Google Scholar] [CrossRef]
- Paczesny, Ł.; Zabrzyński, J.; Domżalski, M.; Gagat, M.; Termanowski, M.; Szwedowski, D.; Łapaj, Ł.; Kruczyński, J. Mini-Invasive, Ultrasound Guided Repair of the Achilles Tendon Rupture-A Pilot Study. J. Clin. Med. 2021, 10, 2370. [Google Scholar] [CrossRef]
- Rowe, N.M.; Joseph Michaels, V.; Soltanian, H.; Dobryansky, M.; Peimer, C.A.; Gurtner, G.C. Sonographically guided percutaneous carpal tunnel release: An anatomic and cadaveric study. Ann. Plast. Surg. 2005, 55, 52–56. [Google Scholar] [CrossRef]
- Knörr, J.; Soldado, F.; Menendez, M.E.; Domenech, P.; Sanchez, M.; Sales de Gauzy, J. Arthroscopic Talocalcaneal Coalition Resection in Children. Arthroscopy 2015, 31, 2417–2423. [Google Scholar] [CrossRef] [PubMed]
- Moses, V.; Daniel, R.T.; Chacko, A.G. The value of intraoperative ultrasound in oblique corpectomy for cervical spondylotic myelopathy and ossified posterior longitudinal ligament. Br. J. Neurosurg. 2010, 24, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, H.; Torres, R.J.; Shinga, K.; Ichikawa, K.; Kato, Y.; Hattori, S.; Yamada, S. Ultrasound-assisted posteromedial portal placement of the elbow joint to prevent ulnar nerve injury. Arthrosc. Tech. 2017, 6, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, N.; Koga, H.; Nakamura, T.; Nakagawa, Y.; Ohara, T.; An, J.S.; Sekiya, I. Ultrasound-assisted arthroscopic all-inside repair technique for posterior lateral meniscus tear. Arthrosc. Tech. 2022, 11, e929–e935. [Google Scholar] [CrossRef] [PubMed]
- Chryssikos, T.; Wessell, A.; Pratt, N.; Cannarsa, G.; Sharma, A.; Olexa, J.; Han, N.; Schwartzbauer, G.; Sansur, C.; Crandall, K. Enhanced safety of pedicle subtraction osteotomy using intraoperative ultrasound. World Neurosurg. 2021, 152, 523–531. [Google Scholar] [CrossRef]
- Ge, X.; Ge, X.; Wang, C.; Liu, Q.; Wang, B.; Chen, L.; Cheng, K.; Qin, M. Application of ultrasound in avoiding radial nerve injury during elbow arthroscopy: A retrospective follow-up study. BMC Musculoskelet. Disord. 2022, 23, 1126. [Google Scholar] [CrossRef]
- Farfalli, G.L.; Aponte-Tinao, L.A.; Rasumoff, A.; Ayerza, M.A.; Muscolo, D.L. Intraoperative ultrasound assistance for excision of impalpable musculoskeletal soft tissue tumors. Orthopedics 2011, 34, 570–573. [Google Scholar] [CrossRef]
- Levin, R.S.; Vasiliev, S.A.; Aslanukov, M.N.; Zuev, A.A.; Oshchepkov, S.K. Intraoperative ultrasound-assisted surgery of spinal tumors. Burdenko’s J. Neurosurg. Zhurnal Voprosy Neirokhirurgii Imeni N.N. Burdenko 2022, 86, 56–65. [Google Scholar] [CrossRef]
- Martinel, V.; Bonnevialle, N.; Maltes Fermandois, P. Does intraoperative ultrasound help the surgeon in arthroscopic excision of rotator cuff tendon calcifications? Eur. J. Orthop. Surg. Traumatol. 2022, 32, 939–944. [Google Scholar] [CrossRef]
- Tat, J.; Tat, J.; Yoon, S.; Yee, A.J.; Larouche, J. Intraoperative ultrasound in spine decompression surgery: A systematic review. Spine 2022, 47, 73–85. [Google Scholar] [CrossRef]
- Hattori, S.; Onishi, K.; Chan, C.K.; Yamakawa, S.; Yano, Y.; Winkler, P.W.; Hogan, M.V.; Debski, R.E. Ultrasound-Guided Anterior Talofibular Ligament Repair with Augmentation Can Restore Ankle Kinematics: A Cadaveric Biomechanical Study. Orthop. J. Sports Med. 2022, 10, 23259671221111397. [Google Scholar] [CrossRef] [PubMed]
- Zappia, M.; Berritto, D.; Oliva, F.; Maffulli, N. High resolution real time ultrasonography of the sural nerve after percutaneous repair of the Achilles tendon. Foot Ankle Surg. 2018, 24, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Wu, T.C.; Yang, K.C.; Li, Y.C.; Wang, C.C. Ultrasonography-Guided Minimally Invasive Surgery for Achilles Sleeve Avulsions. Foot Ankle Int. 2021, 42, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Giannetti, S.; Patricola, A.A.; Stancati, A.; Santucci, A. Intraoperative ultrasound assistance for percutaneous repair of the acute Achilles tendon rupture. Orthopedics 2014, 37, 820–824. [Google Scholar] [CrossRef]
- White, R.Z.; Rezaian, P.; Parasuramar, A.; Sampson, M.J. Ultrasound-assisted foreign body extraction (U-SAFE): Review of technique and technical pearls. J. Med. Imaging Radiat. Oncol. 2022, 66, 362–369. [Google Scholar] [CrossRef]
- Shiels, W.E.; Babcock, D.S.; Wilson, J.L.; Burch, R.A. Localization and guided removal of soft-tissue foreign bodies with sonography. AJR Am. J. Roentgenol. 1990, 155, 1277–1281. [Google Scholar] [CrossRef]
- Quiñones, P.K.; Hattori, S.; Yamada, S.; Kato, Y.; Ohuchi, H. Ultrasonography-Guided Muscle Hematoma Evacuation. Arthrosc. Tech. 2019, 8, 721–725. [Google Scholar] [CrossRef]
- Michalski, Ł.; Paczesny, Ł.; Zabrzyński, J.; Kruczyński, J. Ultrasound-assisted Endoscopic Evacuation of Recurrent Calf Hematoma Following Anterior Cruciate Ligament Reconstruction. Case Study. Ortop. Traumatol. Rehabil. 2019, 21, 357–365. [Google Scholar] [CrossRef]
- Rakovac, I.; Madarevic, T.; Tudor, A.; Prpic, T.; Sestan, B.; Mihelic, R.; Santic, V.; Jurkovic, H.; Ruzic, L. The “cello technique”: A new technique for ultrasound-assisted calcaneoplasty. Arthrosc. Tech. 2012, 1, 91. [Google Scholar] [CrossRef]
- Wang, C.L.; Chen, P.Y.; Yang, K.C.; Wu, H.C.; Wang, C.C. Ultrasound-Guided Minimally Invasive Surgical Resection of Retrocalcaneal Bursitis: A Preliminary Comparison with Traditional Open Surgery. J. Foot Ankle Surg. 2019, 58, 855–860. [Google Scholar] [CrossRef]
- Madarevic, T.; Rakovac, I.; Ruzic, L.; Tudor, A.; Gudac Madarevic, D.; Prpic, T.; Sestan, B. Ultrasound-assisted calcaneoplasty. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2250–2253. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Guo, J.; Malone, D.G.; Wei, N.; McCool, L.C. A Cadaveric Study for the Improvement of Thread Carpal Tunnel Release. J. Hand Surg. Am. 2016, 41, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Hebbard, P.D.; Hebbard, A.I.; Tomka, J.; Appleyard, R. Ultrasound-guided microinvasive carpal tunnel release using a novel retractable needle-mounted blade: A cadaveric study. J. Ultrasound Med. 2018, 37, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Dekimpe, C.; Andreani, O.; Camuzard, O.; Raffaelli, C.; Petrover, D.; Foti, P.; Amoretti, N. Ultrasound-guided percutaneous release of the carpal tunnel: Comparison of the learning curves of a senior versus a junior operator. A cadaveric study. Skeletal Radiol. 2019, 48, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Sangha, H.; Flannery, J.; Robinson, L.R.; Agur, A. Ultrasound-Guided Incisionless Carpal Tunnel Release Using a Hook Knife: A Cadaveric Study. PM R 2019, 11, 1101–1106. [Google Scholar] [CrossRef]
- Guo, D.; Guo, J.; Schmidt, S.C.; Lytie, R.M. A Clinical Study of the Modified Thread Carpal Tunnel Release. Hand 2017, 12, 453–460. [Google Scholar] [CrossRef]
- McShane, J.M.; Slaff, S.; Gold, J.E.; Nazarian, L.N. Sonographically guided percutaneous needle release of the carpal tunnel for treatment of carpal tunnel syndrome: Preliminary report. J. Ultrasound Med. 2012, 31, 1341–1349. [Google Scholar] [CrossRef]
- Henning, P.T.; Yang, L.; Awan, T.; Lueders, D.; Pourcho, A.M. Minimally Invasive Ultrasound-Guided Carpal Tunnel Release: Preliminary Clinical Results. J. Ultrasound Med. 2018, 37, 2699–2706. [Google Scholar] [CrossRef]
- Joseph, A.E.; Leiby, B.M.; Beckman, J.P. Clinical Results of Ultrasound-Guided Carpal Tunnel Release Performed by a Primary Care Sports Medicine Physician. J. Ultrasound Med. 2020, 39, 441–452. [Google Scholar] [CrossRef]
- Leiby, B.M.; Beckman, J.P.; Joseph, A.E. Long-term Clinical Results of Carpal Tunnel Release Using Ultrasound Guidance. Hand 2021, 17, 1558944720988080. [Google Scholar] [CrossRef]
- David, I. Sonography-Guided Carpal Tunnel Release. Hand Clin. 2022, 38, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Krogh, T.P.; Isaksen, C.; Rojo-Manaute, J.; Damkier, H.H.; Jensen, P.; Fredberg, U.; Brix, L. Implementation of ultrasound-guided carpal tunnel release. Dan. Med. J. 2023, 70, A11220689. [Google Scholar] [PubMed]
- Capa-Grasa, A.; Rojo-Manaute, J.; Rodríguez, F.C.; Martín, J.V. Ultra minimally invasive sonographically guided carpal tunnel release: An external pilot study. Orthop. Traumatol. Surg. Res. 2014, 100, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Manaute, J.; Capa-Grasa, A.; Chana-Rodríguez, F.; Perez-Mañanes, R.; Rodriguez-Maruri, G.; Sanz-Ruiz, P.; Muñoz-Ledesma, J.; Aburto-Bernardo, M.; Esparragoza-Cabrera, L.; Cerro-Gutiérrez, M.D.; et al. Ultra-Minimally Invasive Ultrasound-Guided Carpal Tunnel Release: A Randomized Clinical Trial. J. Ultrasound Med. 2016, 35, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Aramendi, J.F.; Ibañez, J.M.; Blasi, M.; Vazquez, A.; Aurrekoetxea, J.J.; Dávila, F. Minimally invasive ultrasound-guided vs open release for carpal tunnel syndrome in working population: A randomized controlled trial. J. Clin. Ultrasound 2021, 49, 693–703. [Google Scholar] [CrossRef]
- Fernández-Gibello, A.; Moroni, S.; Camuñas, G.; Montes, R.; Zwierzina, M.; Tasch, C.; Starke, V.; Sañudo, J.; Vazquez, T.; Konschake, M. Ultrasound-guided decompression surgery of the tarsal tunnel: A novel technique for the proximal tarsal tunnel syndrome-Part II. Surg. Radiol. Anat. 2019, 41, 43–51. [Google Scholar] [CrossRef]
- Iborra, Á.; Villanueva-Martínez, M.; Barrett, S.L.; Rodríguez-Collazo, E.R.; Sanz-Ruiz, P. Ultrasound-Guided Release of the Tibial Nerve and Its Distal Branches: A Cadaveric Study. J. Ultrasound Med. 2019, 38, 2067–2079. [Google Scholar] [CrossRef]
- Iborra, A.; Villanueva, M.; Sanz-Ruiz, P. Results of ultrasound-guided release of tarsal tunnel syndrome: A review of 81 cases with a minimum follow-up of 18 months. J. Orthop. Surg. Res. 2020, 15, 30. [Google Scholar] [CrossRef]
- Iborra Marcos, A.; Villanueva Martinez, M.; Sanz-Ruiz, P.; Barrett, S.L.; Zislis, G. Ultrasound-Guided Proximal and Distal Tarsal Decompression: An Analysis of Pressures in the Tarsal, Medial Plantar, and Lateral Plantar Tunnels. Foot Ankle Spec. 2021, 14, 133–139. [Google Scholar] [CrossRef]
- Wahezi, S.; Yerra, S.; Rivelis, Y.; Sitapara, K.; Gonzalez, D.; Downie, S.; Jain, R.; Deer, T.; Abd-Elsayed, A.; Gulati, A. Sonographically guided percutaneous sectioning of the coracohumeral ligament for the treatment of refractory adhesive capsulitis: Proof of concept. Pain Med. 2020, 21, 3314–3319. [Google Scholar] [CrossRef]
- Ahn, K.; Jhun, H.J.; Choi, K.M.; Lee, Y.S. Ultrasound-guided interventional release of rotator interval and posteroinferior capsule for adhesive capsulitis of the shoulder using a specially designed needle. Pain Physician 2011, 14, 531–537. [Google Scholar] [PubMed]
- Yukata, K.; Goto, T.; Sakai, T.; Fujii, H.; Hamawaki, J.; Yasui, N. Ultrasound-guided coracohumeral ligament release. Orthop. Traumatol. Surg. Res. 2018, 104, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Wahezi, S.E.; Naeimi, T.; Yerra, S.; Gruson, K.; Hossack, M.; Alvarez, E.T.; Vydyanathan, A.; Voleti, P.; Malhotra, R.; Martinez, E. Percutaneous ultrasound-guided coracohumeral ligament release for refractory adhesive capsulitis: A prospective, randomized, controlled, crossover trial demonstrating one-year efficacy. Pain Physician 2023, 26, 509–516. [Google Scholar]
- Moroni, S.; Fernández-Gibello, A.; Nieves, G.C.; Montes, R.; Zwierzina, M.; Vazquez, T.; Garcia-Escudero, M.; Duparc, F.; Moriggl, B.; Konschake, M. Anatomical basis of a safe mini-invasive technique for lengthening of the anterior gastrocnemius aponeurosis. Surg. Radiol. Anat. 2021, 43, 53–61. [Google Scholar] [CrossRef]
- Iborra Marcos, Á.; Villanueva Martínez, M.; Fahandezh-Saddi Díaz, H. Needle-based gastrocnemius lengthening: A novel ultrasound-guided noninvasive technique. J. Orthop. Surg. Res. 2022, 17, 435. [Google Scholar] [CrossRef]
- Villanueva, M.; Iborra, Á.; Ruiz, M.D.M.; Sanz-Ruiz, P. Proximal Ultrasound-Guided Gastrocnemius Recession: A New Ultra-Minimally Invasive Surgical Technique. J. Foot Ankle Surg. 2019, 58, 870–876. [Google Scholar] [CrossRef]
- Chern, T.; Jou, I.; Yen, S.; Lai, K.; Shao, C. Cadaveric study of sonographically assisted percutaneous release of the A1 pulley. Plast. Reconstr. Surg. 2005, 115, 811–822. [Google Scholar] [CrossRef]
- Kuo, L.C.; Su, F.C.; Tung, W.L.; Lai, K.Y.; Jou, I.M. Kinematical and functional improvements of trigger digits after sonographically assisted percutaneous release of the A1 pulley. J. Orthop. Res. 2009, 27, 891–896. [Google Scholar] [CrossRef]
- Rojo-Manaute, J.M.; Rodríguez-Maruri, G.; Capa-Grasa, A.; Chana-Rodríguez, F.; Soto Mdel, V.; Martín, J.V. Sonographically guided intrasheath percutaneous release of the first annular pulley for trigger digits, part 1: Clinical efficacy and safety. J. Ultrasound Med. 2012, 31, 417–424. [Google Scholar] [CrossRef]
- Lapègue, F.; André, A.; Meyrignac, O.; Pasquier-Bernachot, E.; Dupré, P.; Brun, C.; Bakouche, S.; Chiavassa-Gandois, H.; Sans, N.; Faruch, M. US-guided Percutaneous Release of the Trigger Finger by Using a 21-gauge Needle: A Prospective Study of 60 Cases. Radiology 2016, 280, 493–499. [Google Scholar] [CrossRef]
- Pan, M.; Sheng, S.; Fan, Z.; Lu, H.; Yang, H.; Yan, F.; E, Z. Ultrasound-Guided Percutaneous Release of A1 Pulley by Using a Needle Knife: A Prospective Study of 41 Cases. Front. Pharmacol. 2019, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Colberg, R.E.; Jurado Vélez, J.A.; Garrett, W.H.; Hart, K.; Fleisig, G.S. Ultrasound-guided microinvasive trigger finger release technique using an 18-gauge needle with a blade at the tip: A prospective study. PM R 2022, 14, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, V.S.; Malahias, M.A.; Kaseta, M.K.; Sourlas, I.; Babis, G.C. Comparative clinical study of ultrasound-guided A1 pulley release. World J. Orthop. 2017, 8, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Colberg, R.E.; Henderson, R.G. Ultrasound-Guided First Dorsal Compartment Release for Refractory de Quervain Tenosynovitis: A Case Report. PM R 2019, 11, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Lueders, D.R.; Sellon, J.L.; Smith, J.; Finnoff, J.T. Ultrasound-Guided Fasciotomy for Chronic Exertional Compartment Syndrome: A Cadaveric Investigation. PM R 2017, 9, 683–690. [Google Scholar] [CrossRef]
- Balius, R.; Bong, D.A.; Ardèvol, J.; Pedret, C.; Codina, D.; Dalmau, A. Ultrasound-Guided Fasciotomy for Anterior Chronic Exertional Compartment Syndrome of the Leg. J. Ultrasound Med. 2016, 35, 823–829. [Google Scholar] [CrossRef]
- Finnoff, J.T.; Johnson, W. Ultrasound-Guided Fasciotomy for Chronic Exertional Compartment Syndrome: A Case Report. Clin. J. Sports Med. 2020, 30, 231–233. [Google Scholar] [CrossRef]
- Sakellariou, V.I.; Brault, J.; Rizzo, M. Ultrasound-assisted percutaneous needle fasciotomy for Dupuytren’s contracture. Orthopedics 2015, 38, 299–303. [Google Scholar] [CrossRef]
- Villanueva, M.; Iborra, Á.; Fahandezh-Saddi, H.; Sanz-Ruiz, P.; Noriega, C. Ultrasound-guided aponeurotomy and interphalangeal joint capsular release for treatment of Dupuytren’s disease. J. Hand Surg. Eur. Vol. 2022, 47, 742–749. [Google Scholar] [CrossRef]
- Patel, M.M. A novel treatment for refractory plantar fasciitis. Am. J. Orthop. (Belle Mead NJ) 2015, 44, 107–110. [Google Scholar]
- McShane, J.M.; Nazarian, L.N.; Harwood, M.I. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow. J. Ultrasound Med. 2006, 25, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Testa, V.; Capasso, G.; Benazzo, F.; Maffulli, N. Management of Achilles tendinopathy by ultrasound-guided percutaneous tenotomy. Med. Sci. Sports Exerc. 2002, 34, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.; Ellis, M.B.; Johnson, K.; Haddon, T.B. Fasciotomy and Surgical Tenotomy for Chronic Achilles Insertional Tendinopathy. J. Am. Podiatr. Med. Assoc. 2019, 109, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Oliva, F.; Testa, V.; Capasso, G.; Del Buono, A. Multiple percutaneous longitudinal tenotomies for chronic Achilles tendinopathy in runners: A long-term study. Am. J. Sports Med. 2013, 41, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Vohra, P.K.; Japour, C.J. Ultrasound-guided plantar fascia release technique: A retrospective study of 46 feet. J. Am. Podiatr. Med. Assoc. 2009, 99, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Iborra, A.; Villanueva, M.; Sanz-Ruiz, P.; Martin, A.; Noriega, C. A novel closed technique for ultrasound-guided plantar fascia release with a needle: Review of 107 cases with a minimum follow-up of 24 months. J. Orthop. Surg. Res. 2021, 16, 153. [Google Scholar] [CrossRef]
- Malahias, M.; Roumeliotis, L.; Tyrpenou, E.; Kazas, S.; Sourlas, I.; Kaseta, M. Ultrasound-Guided Partial Plantar Fascia Release with the Use of a Fine Cutting Device for the Treatment of Persistent Plantar Fasciitis: A Case Series. J. Am. Podiatr. Med. Assoc. 2022, 112. [Google Scholar] [CrossRef]
- Lévy, B.; Ducat, A.; Gaudin, P.; Maqdés, A.; Brasseur, J.L.; Klouche, S.; Hardy, P. Ultrasound-guided percutaneous tenotomy of the long head of the biceps tendon: A non-reliable technique. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1027–1030. [Google Scholar] [CrossRef]
- Aly, A.R.; Rajasekaran, S.; Mohamed, A.; Beavis, C.; Obaid, H. Feasibility of ultrasound-guided percutaneous tenotomy of the long head of the biceps tendon--A pilot cadaveric study. J. Clin. Ultrasound 2015, 43, 361–366. [Google Scholar] [CrossRef]
- Atlan, F.; Werthel, J.D. Ultrasound-guided intra-articular tenotomy of the long head of the biceps: A cadaveric feasibility study. Int. Orthop. 2016, 40, 2567–2573. [Google Scholar] [CrossRef]
- Sconfienza, L.M.; Mauri, G.; Messina, C.; Aliprandi, A.; Secchi, F.; Sardanelli, F.; Randelli, P.S. Ultrasound-Guided Percutaneous Tenotomy of Biceps Tendon: Technical Feasibility on Cadavers. Ultrasound Med. Biol. 2016, 42, 2513–2517. [Google Scholar] [CrossRef] [PubMed]
- Greditzer, H.G.; Kaplan, L.D.; Lesniak, B.P.; Jose, J. Ultrasound-guided percutaneous long head of the biceps tenotomy: A novel technique with case report. HSS J. 2014, 10, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, J.S.; Cheng, J.; Hurwitz, N.; Santiago, K.; Lin, E.; Beatty, N.; Kingsbury, D.; Wendel, I.; Milani, C. Ultrasound-guided percutaneous needle tenotomy (PNT) alone versus PNT plus platelet-rich plasma injection for the treatment of chronic tendinosis: A randomized controlled trial. PM R 2021, 13, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Hickey, B.; Lee, J.; Stephen, J.; Antflick, J.; Calder, J. It is possible to release the plantaris tendon under ultrasound guidance: A technical description of ultrasound guided plantaris tendon release (UPTR) in the treatment of non-insertional Achilles tendinopathy. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2858–2862. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Alfredson, H.; Masci, L.; Sellon, J.L.; Woods, C.D. Sonographically Guided Plantaris Tendon Release: A Cadaveric Validation Study. PM R 2019, 11, 56–63. [Google Scholar] [CrossRef]
- Koh, J.S.; Mohan, P.C.; Howe, T.S.; Lee, B.P.; Chia, S.L.; Yang, Z.; Morrey, B.F. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: Early clinical experience with a novel device for minimally invasive percutaneous microresection. Am. J. Sports Med. 2013, 41, 636–644. [Google Scholar] [CrossRef]
- Seng, C.; Mohan, P.C.; Koh, S.B.; Howe, T.S.; Lim, Y.G.; Lee, B.P.; Morrey, B.F. Ultrasonic Percutaneous Tenotomy for Recalcitrant Lateral Elbow Tendinopathy: Sustainability and Sonographic Progression at 3 Years. Am. J. Sports Med. 2016, 44, 504–510. [Google Scholar] [CrossRef]
- Stover, D.; Fick, B.; Chimenti, R.L.; Hall, M.M. Ultrasound-guided tenotomy improves physical function and decreases pain for tendinopathies of the elbow: A retrospective review. J. Shoulder Elbow Surg. 2019, 28, 2386–2393. [Google Scholar] [CrossRef]
- Sanchez, P.J.; Grady, J.F.; Saxena, A. Percutaneous Ultrasonic Tenotomy for Achilles Tendinopathy Is a Surgical Procedure with Similar Complications. J. Foot Ankle Surg. 2017, 56, 982–984. [Google Scholar] [CrossRef]
- Pourcho, A.M.; Hall, M.M. Percutaneous Ultrasonic Fasciotomy for Refractory Plantar Fasciopathy After Failure of a Partial Endoscopic Release Procedure. PM R 2015, 7, 1194–1197. [Google Scholar] [CrossRef]
- Turner, A.; Wang, J.; Liu, G.; Wukich, D.; VanPelt, M. Retrospective Evaluation of Ultrasound Guided Percutaneous Plantar Fasciotomy with and without Platelet Rich Plasma. J. Foot Ankle Surg. 2024, 63, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Boden, A.L.; Scott, M.T.; Dalwadi, P.P.; Mautner, K.; Mason, R.A.; Gottschalk, M.B. Platelet-rich plasma versus Tenex in the treatment of medial and lateral epicondylitis. J. Shoulder Elbow Surg. 2019, 28, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Fick, B.; Stover, D.W.; Chimenti, R.L.; Hall, M.M. The Safety of Ultrasound Guided Tenotomy and Debridement for Upper and Lower Extremity Tendinopathies: A Retrospective Study. Iowa Orthop. J. 2021, 41, 82–90. [Google Scholar]
- Chimenti, R.L.; Stover, D.W.; Fick, B.S.; Hall, M.M. Percutaneous Ultrasonic Tenotomy Reduces Insertional Achilles Tendinopathy Pain with High Patient Satisfaction and a Low Complication Rate. J. Ultrasound Med. 2019, 38, 1629–1635. [Google Scholar] [CrossRef]
- Hattori, S.; Alvarez, C.A.D.; Canton, S.; Hogan, M.V.; Onishi, K. Ultrasound-Guided Ankle Lateral Ligament Stabilization. Curr. Rev. Musculoskelet. Med. 2019, 12, 497–508. [Google Scholar] [CrossRef]
- Hattori, S.; Onishi, K.; Yano, Y.; Kato, Y.; Ohuchi, H.; Hogan, M.V.; Kumai, T. Sonographically Guided Anchor Placement in Anterior Talofibular Ligament Repair Is Anatomic and Accurate. Orthop. J. Sports Med. 2020, 8, 2325967120967322. [Google Scholar] [CrossRef]
- Chavez, J.; Hattori, S.; Kato, Y.; Takazawa, S.; Yamada, S.; Ohuchi, H. The use of ultrasonography during minimally invasive Achilles tendon repair to avoid sural nerve injury. J. Med. Ultrason. 2019, 46, 513–514. [Google Scholar] [CrossRef]
- Lee, J.K.; Kang, C.; Hwang, D.S.; Kang, D.H.; Lee, G.S.; Hwang, J.M.; Song, J.H.; Lee, C.W. A comparative study of innovative percutaneous repair and open repair for acute Achilles tendon rupture: Innovative usage of intraoperative ultrasonography. J. Orthop. Surg. 2020, 28, 2309499020910274. [Google Scholar] [CrossRef]
- Hirahara, A.M.; Mackay, G.; Andersen, W.J. Ultrasound-Guided Suture Tape Augmentation and Stabilization of the Medial Collateral Ligament. Arthrosc. Tech. 2018, 7, 205–210. [Google Scholar] [CrossRef]
- Hirahara, A.M.; Andersen, W.J. Ultrasound-Guided Percutaneous Repair of Medial Patellofemoral Ligament: Surgical Technique and Outcomes. Am. J. Orthop. 2017, 46, 152–157. [Google Scholar]
- Wright, J.G.; Swiontkowski, M.F.; Heckman, J.D. Introducing Levels of Evidence to The Journal. J. Bone Jt. Surg. 2003, 85, 1–3. [Google Scholar] [CrossRef]
- Altahawi, F.; Li, X.; Demarest, B.; Forney, M.C. Percutaneous ultrasonic tenotomy with the TX-1 device versus surgical tenotomy for the treatment of common extensor tendinosis. Skeletal Radiol. 2021, 50, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Alfredson, H. Ultrasound and Doppler-guided mini-surgery to treat midportion Achilles tendinosis: Results of a large material and a randomised study comparing two scraping techniques. Br. J. Sports Med. 2011, 45, 407–410. [Google Scholar] [CrossRef]
- Shomal Zadeh, F.; Shafiei, M.; Shomalzadeh, M.; Pierce, J.; Thurlow, P.C.; Chalian, M. Percutaneous ultrasound-guided needle tenotomy for treatment of chronic tendinopathy and fasciopathy: A meta-analysis. Eur. Radiol. 2023, 33, 7303–7320, Erratum in Eur. Radiol. 2023, 33, 7353–7354. [Google Scholar] [CrossRef]
- Jacobson, J.A.; Yablon, C.M.; Henning, P.T.; Kazmers, I.S.; Urquhart, A.; Hallstrom, B.; Bedi, A.; Parameswaran, A. Greater Trochanteric Pain Syndrome: Percutaneous Tendon Fenestration Versus Platelet-Rich Plasma Injection for Treatment of Gluteal Tendinosis. J. Ultrasound Med. 2016, 35, 2413–2420. [Google Scholar] [CrossRef]
- Chuang, B.I.; Hsu, J.H.; Kuo, L.C.; Jou, I.M.; Su, F.C.; Sun, Y.N. Tendon-motion tracking in an ultrasound image sequence using optical-flow-based block matching. Biomed. Eng. Online 2017, 16, 47. [Google Scholar] [CrossRef]
- Dunnhofer, M.; Antico, M.; Sasazawa, F.; Takeda, Y.; Camps, S.; Martinel, N.; Micheloni, C.; Carneiro, G.; Fontanarosa, D. Siam-U-Net: Encoder-decoder siamese network for knee cartilage tracking in ultrasound images. Med. Image Anal. 2020, 60, 101631. [Google Scholar] [CrossRef]
- Alsinan, A.Z.; Patel, V.M.; Hacihaliloglu, I. Automatic segmentation of bone surfaces from ultrasound using a filter-layer-guided CNN. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 775–783. [Google Scholar] [CrossRef]
- Alsinan, A.Z.; Patel, V.M.; Hacihaliloglu, I. Bone shadow segmentation from ultrasound data for orthopedic surgery using GAN. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 1477–1485. [Google Scholar] [CrossRef]
- Quader, N.; Hodgson, A.J.; Mulpuri, K.; Schaeffer, E.; Abugharbieh, R. Automatic Evaluation of Scan Adequacy and Dysplasia Metrics in 2-D Ultrasound Images of the Neonatal Hip. Ultrasound Med. Biol. 2017, 43, 1252–1262. [Google Scholar] [CrossRef]
- Rosa, L.G.; Zia, J.S.; Inan, O.T.; Sawicki, G.S. Machine learning to extract muscle fascicle length changes from dynamic ultrasound images in real-time. PLoS ONE 2021, 16, e0246611. [Google Scholar] [CrossRef] [PubMed]
- Kuok, C.P.; Yang, T.H.; Tsai, B.S.; Jou, I.M.; Horng, M.H.; Su, F.C.; Sun, Y.N. Segmentation of finger tendon and synovial sheath in ultrasound image using deep convolutional neural network. Biomed. Eng. Online 2020, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Li, B.; Wang, D.; Chen, W.; Yue, S.; Meng, D.; Qiao, X.; Zhang, Y. Deep learning for the rapid automatic segmentation of forearm muscle boundaries from ultrasound datasets. Front. Physiol. 2023, 14, 1166061. [Google Scholar] [CrossRef] [PubMed]
- Cronin, N.J.; Finni, T.; Seynnes, O. Using deep learning to generate synthetic B-mode musculoskeletal ultrasound images. Comput. Methods Programs Biomed. 2020, 196, 105583. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, R.J.; Loram, I.D. Estimation of absolute states of human skeletal muscle via standard B-mode ultrasound imaging and deep convolutional neural networks. J. R. Soc. Interface 2020, 17, 20190715. [Google Scholar] [CrossRef]
- Lee, K.S.; Jung, S.H.; Kim, D.H.; Chung, S.W.; Yoon, J.P. Artificial intelligence- and computer-assisted navigation for shoulder surgery. J. Orthop. Surg. 2024, 32, 10225536241243166. [Google Scholar] [CrossRef]
- Marzola, F.; van Alfen, N.; Doorduin, J.; Meiburger, K.M. Deep learning segmentation of transverse musculoskeletal ultrasound images for neuromuscular disease assessment. Comput. Biol. Med. 2021, 135, 104623. [Google Scholar] [CrossRef]
- Faeghi, F.; Ardakani, A.A.; Acharya, U.R.; Mirza-Aghazadeh-Attari, M.; Abolghasemi, J.; Ejtehadifar, S.; Mohammadi, A. Accurate automated diagnosis of carpal tunnel syndrome using radiomics features with ultrasound images: A comparison with radiologists’ assessment. Eur. J. Radiol. 2021, 136, 109518. [Google Scholar] [CrossRef]
- Lee, S.W.; Ye, H.U.; Lee, K.J.; Jang, W.Y.; Lee, J.H.; Hwang, S.M.; Heo, Y.R. Accuracy of New Deep Learning Model-Based Segmentation and Key-Point Multi-Detection Method for Ultrasonographic Developmental Dysplasia of the Hip (DDH) Screening. Diagnostics 2021, 11, 1174. [Google Scholar] [CrossRef]
- Chiu, P.H.; Boudier-Revéret, M.; Chang, S.W.; Wu, C.H.; Chen, W.S.; Özçakar, L. Deep Learning for Detecting Supraspinatus Calcific Tendinopathy on Ultrasound Images. J. Med. Ultrasound 2022, 30, 196–202. [Google Scholar] [CrossRef]
- Droppelmann, G.; Tello, M.; García, N.; Greene, C.; Jorquera, C.; Feijoo, F. Lateral elbow tendinopathy and artificial intelligence: Binary and multilabel findings detection using machine learning algorithms. Front. Med. 2022, 9, 945698. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cui, L.; Chen, T.; Lyu, X.; Yu, J.; Guo, W.; Wang, D.; Qin, X.; Zhao, Y.; Zhang, S. Study on multiplanar measurements of infant hips with three-dimensional ultrasonography. J. Clin. Ultrasound 2022, 50, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Chen, Z.; Zhang, Q.; Lei, B.; Chen, Z.; Fu, Y.; Guo, P.; Li, C.; Ma, T.; Liu, J.; et al. Osteoporosis Diagnostic Model Using a Multichannel Convolutional Neural Network Based on Quantitative Ultrasound Radiofrequency Signal. Ultrasound Med. Biol. 2022, 48, 1590–1601. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Kamada, M.; Imamura, A.; Shimizu, M.; Inagaki, M.; Tsuji, Y.; Hashimoto, M.; Tanaka, M.; Ito, H.; Fujii, Y. Machine learning-based prediction of relapse in rheumatoid arthritis patients using data on ultrasound examination and blood test. Sci. Rep. 2022, 12, 7224. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, I.; Inui, A.; Mifune, Y.; Nishimoto, H.; Yamaura, K.; Mukohara, S.; Yoshikawa, T.; Kato, T.; Furukawa, T.; Hoshino, Y.; et al. Using deep learning for ultrasound images to diagnose carpal tunnel syndrome with high accuracy. Ultrasound Med. Biol. 2022, 48, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, I.; Inui, A.; Mifune, Y.; Nishimoto, H.; Yamaura, K.; Mukohara, S.; Yoshikawa, T.; Kato, T.; Furukawa, T.; Hoshino, Y.; et al. Diagnosis of cubital tunnel syndrome using deep learning on ultrasonographic images. Diagnostics 2022, 12, 632. [Google Scholar] [CrossRef]
- Shinohara, I.; Yoshikawa, T.; Inui, A.; Mifune, Y.; Nishimoto, H.; Mukohara, S.; Yoshikawa, T.; Furukawa, T.; Tanaka, S.; Kusunose, M.; et al. Ultrasound with artificial intelligence models predicted Palmer 1B triangular fibrocartilage complex injuries. Arthroscopy 2022, 38, 2417–2424. [Google Scholar] [CrossRef]
- Tiulpin, A.; Saarakkala, S.; Mathiessen, A.; Hammer, H.B.; Furnes, O.; Nordsletten, L.; Englund, M.; Magnusson, K. Predicting total knee arthroplasty from ultrasonography using machine learning. Osteoarthr. Cartil. Open 2022, 4, 100319. [Google Scholar] [CrossRef]
- Atalar, H.; Üreten, K.; Tokdemir, G.; Tolunay, T.; Çiçeklidağ, M.; Atik, O. The diagnosis of developmental dysplasia of the hip from hip ultrasonography images with deep learning methods. J. Pediatr. Orthop. 2023, 43, e132–e137. [Google Scholar] [CrossRef]
- Jaremko, J.L.; Hareendranathan, A.; Bolouri, S.E.S.; Frey, R.F.; Dulai, S.; Bailey, A.L. AI aided workflow for hip dysplasia screening using ultrasound in primary care clinics. Sci. Rep. 2023, 13, 9224. [Google Scholar] [CrossRef]
- Kinugasa, M.; Inui, A.; Satsuma, S.; Kobayashi, D.; Sakata, R.; Morishita, M.; Komoto, I.; Kuroda, R. Diagnosis of Developmental Dysplasia of the Hip by Ultrasound Imaging Using Deep Learning. J. Pediatr. Orthop. 2023, 43, e538–e544. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.; Zhang, Y.; Zhang, M.; Jiang, M.; Yu, J.; Zhu, J.; Zhang, B. Ultrasound-based radiomics in the diagnosis of carpal tunnel syndrome: The influence of regions of interest delineation method on mode. J. Clin. Ultrasound 2023, 51, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.T.; Lin, C.Y.; Shu, Y.C.; Shen, P.C.; Lin, T.Y.; Chang, K.V.; Ozcakar, L. The Potential of Ultrasound Radiomics in Carpal Tunnel Syndrome Diagnosis: A Systematic Review and Meta-Analysis. Diagnostics 2023, 13, 3280. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, I.; Yoshikawa, T.; Inui, A.; Mifune, Y.; Nishimoto, H.; Mukohara, S.; Yoshikawa, T.; Kato, T.; Furukawa, T.; Tanaka, S.; et al. Degree of accuracy with which deep learning for ultrasound images identifies osteochondritis dissecans of the humeral capitellum. Am. J. Sports Med. 2023, 51, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wen, D.; Yin, Y.; Zhang, P.; Wen, W.; Gao, J.; Jiang, Z. Musculoskeletal Ultrasound Image-Based Radiomics for the Diagnosis of Achilles Tendinopathy in Skiers. J. Ultrasound Med. 2023, 42, 363–371. [Google Scholar] [CrossRef]
- Yu, L.; Li, Y.; Wang, X.F.; Zhang, Z.Q. Analysis of the value of artificial intelligence combined with musculoskeletal ultrasound in the differential diagnosis of pain rehabilitation of scapulohumeral periarthritis. Medicine 2023, 102, e33125. [Google Scholar] [CrossRef]
- Sasaki, K.; Fujita, D.; Takatsuji, K.; Kotoura, Y.; Minami, M.; Kobayashi, Y.; Sukenari, T.; Kida, Y.; Takahashi, K.; Kobashi, S. Deep learning-based osteochondritis dissecans detection in ultrasound images with humeral capitellum localization. Int. J. Comput. Assist. Radiol. Surg. 2024, 51, 1–10. [Google Scholar] [CrossRef]
- Takatsuji, K.; Kida, Y.; Sasaki, K.; Fujita, D.; Kobayashi, Y.; Sukenari, T.; Kotoura, Y.; Minami, M.; Kobashi, S.; Takahashi, K. Deep Learning-Based Computer-Aided Diagnosis of Osteochondritis Dissecans of the Humeral Capitellum Using Ultrasound Images. J. Bone Joint Surg. Am. 2024. [Google Scholar] [CrossRef]
- Baka, N.; Leenstra, S.; van Walsum, T. Ultrasound Aided Vertebral Level Localization for Lumbar Surgery. IEEE Trans. Med. Imaging 2017, 36, 2138–2147. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).